Abstract
Introduction
Concomitant risk factors challenge the mechanistic understanding of cardiac aging. We determined the degree to which the left atrial function could be distinguished by advanced cardiac magnetic resonance (CMR) imaging in older adults and assessed associations between the left atrial function and the plasma biomarkers related to biological aging and cardiovascular disease [serum monocyte chemoattractant protein-1 (MCP1), matrix metallopeptidase 9 (MMP-9), B-type natriuretic peptides (BNPs), galectin-3 (Gal-3), high-sensitivity cardiac troponin I (hsTn1), high-sensitivity C-reactive protein (hs-CRP), and soluble urokinase plasminogen activator receptor (sUPAR)].
Methods
Among a cross-sectional population-based cohort of older adults, longitudinal LA strain including reservoir strain (ε<sub>s</sub>), conduit strain (ε<sub>e</sub>), and booster strain (ε<sub>a</sub>) as well as peak strain rates (SRs, SRe, SRa) were determined using CMR and studied in association with blood biomarkers.
Results
We studied 243 community adults (42.8% female, mean age 70.3 ± 9.5 years). In bivariate analysis, ε<sub>e</sub> and SRe were reduced in gradation with increasing risk factors (all p values <0.0001). Corresponding levels of sUPAR (ng/mL) were quantitatively higher in older adults with <2 risk factors (2.5 ± 1.6 vs. 1.7 ± 1.3, p = 0.0005), in those with ≥2 risk factors (3.3 ± 2.4 vs. 1.7 ± 1.3, p < 0.0001), compared to young adults; including between older adults with ≥2 risk factors and older adults with <2 risk factors (3.3 ± 2.4 vs. 2.5 ± 1.6, p = 0.017). Based on multivariate analysis, sUPAR was significantly associated with both ε<sub>e</sub> (OR 1.52, p = 0.006) and SRe decline (OR 1.5, p = 0.019). The associations between Gal-3 and ε<sub>e</sub> reduction (OR 1.2, p = 0.022) and between BNP and SRe decline were generally weaker (OR 1.03, p = 0.027). The addition of sUPAR to a model consisting of age, risk factors, Gal-3, and BNPs increased the area under the curve of ε<sub>e</sub> from 0.72 to 0.77 (p = 0.015).
Conclusion
By advanced CMR imaging, a panel of circulating biomarkers comprising galectin, MMP-9 and sUPAR were associated with left atrial dysfunction in older adults. Higher levels of Gal-3 and MMP-9 may be suggestive of fibrotic mechanisms in left atrial aging while impairments in left atrial strain seen in association with circulating sUPAR may be related to immune activation in the left atrium in response to left atrial remodeling and fibrotic processes.
Keywords: Cardiovascular, Left atrium, Mechanisms, Biomarkers, Aging
Introduction
Aging is a major risk factor for cardiovascular diseases. Aging produces the substrate that leads to the occurrence of atherosclerosis, myocardial infarction, other systemic endothelial dysfunction, and onward to the development of heart failure [1, 2, 3].
Yet, aging does not exist in isolation. In tandem with chronological aging, the accumulation of vascular risk factors, such as hypertension, dyslipidemia, and diabetes mellitus, occurs in aggregate with aging. The current understanding of aging as a risk factor is often indistinct from its accompanying vascular risk factors. Ideally, biomarkers that could assess the degree of deleterious aging within the cardiovascular system, independent of confounding risk factors, would be informative.
In addition, modern imaging techniques in the realm of cardiovascular imaging have enhanced the ability to detect cardiovascular aging changes in structure and function that occurs in older adults. These alterations which were previously only detectable on postmortem histopathology are now detected with sensitive noninvasive imaging techniques.
Heart left atrium changes with aging. In terms of structure, left atrial volume may increase as a result of aging [4, 5, 6]. In terms of function, our earlier investigations detected alterations in the left atrial phasic function by advanced cardiac magnetic resonance (CMR) imaging, among older adults without prevalent cardiovascular disease, demonstrating impairments in specific phases of left atrial function that occurred with age independent of risk factors [7]. Overall, as a well-validated marker of clinical and subclinical cardiovascular disease, the left atrium is associated with an increased risk of cardiovascular events and poor prognostic outcomes [8, 9, 10]. As CMR imaging allows for superior spatial resolution and reproducibility, functional assessment of the left atrium by CMR enhances detection of left atrial aging beyond structural changes [11, 12].
Hence, in this current study, we evaluated the degree of left atrial dysfunction by CMR among a prospective cohort of adults across age groups and a range of vascular risk factors. In addition, hypothesizing that morphological changes correspond to changes in biomarkers, we tested the performance of novel and traditional biomarkers in association with left atrial function.
Methods
Study Population
The subjects were recruited from the Cardiac Ageing Study (CAS) [7], a prospective study initiated in 2014 that examines characteristics and determinants of cardiovascular function in older adults. CAS participants were recruited from the prospective, population-based cohort, the Singapore Chinese Health Study (SCHS) [13], and directly from the local community. The current study sample is a cross-sectional analysis of men and women who participated in the baseline CAS 2014–2017 examination who had no self-reported history of physician-diagnosed cardiovascular disease (such as coronary heart disease, atrial fibrillation), stroke, or cancer. Written informed consent was obtained from participants upon enrolment. The SingHealth Centralised Institutional Review Board (CIRC/2014/628/C) had approved the study protocol.
Data Acquisition
All participants were examined and interviewed in 1 study visit by trained study coordinators. Participants completed a standardized questionnaire that included medical history and coronary risk factors. Hypertension was defined by current use of antihypertensive drugs or physician-diagnosed hypertension. Diabetes mellitus was defined by current use of antidiabetic agents or physician-diagnosed diabetes mellitus. Dyslipidemia was defined by current use of lipid-lowering agents or physician-diagnosed dyslipidemia. Smoking history was defined as ever smokers (former or current smoking) or never smokers. Body mass index was calculated as weight in kilograms divided by the square of height in meters. Sinus rhythm status was ascertained by resting electrocardiogram. Clinical data were obtained on the same day as assessment of echocardiography and serum collection.
Cine CMR scans were performed using a balanced steady-state free precession sequence. All subjects were imaged on a 3T magnetic resonance imaging system (Ingenia, Philips Healthcare, The Netherlands) with a dStream Torso coil (maximal number of channels 32). BFFE end-expiratory breath hold cine images were acquired in multiplanar long-axis views (2–4-chamber views) and a stack of parallel short-axis views to cover the left ventricle (LV) from base to apex. Typical parameters were as follows: TR/TE 3/1 ms; flip angle, 45°; in-plane spatial resolution, 1.0 mm × 1.0 mm to 1.5 mm × 1.5 mm; slice thickness, 8 mm; pixel bandwidth, 1,797 Hz; field of view, 300 mm; frame rate, 30 or 40 per cardiac cycle. Dedicated Qstrain software (version 2.0; Medis, North Ryde NSW, Australia) was used in deriving LV longitudinal strain including LV global longitudinal strain, circumferential strain, radial strain, and right ventricular global longitudinal strain [14].
Left Atrial Function Assessment
We developed an in-house semi-automatic algorithm to track the distance (L) between the left atrioventricular junction and a user-defined point at the mid posterior LA wall on standard CMR 2- and 4-chamber views [7, 15, 16, 17]. Both 2- and 4-chamber views were used to generate the average strain and strain rate (SR) results. Longitudinal strain (ε) at any time point (t) in the cardiac cycle from end-diastole (time 0) was calculated as ε (t) = (L [t]−L0)/L0. LA reservoir strain (εS), conduit strain (εe), and booster strain (εa) were calculated at t equals left ventricular end-systole, diastasis, and pre-LA systole, respectively. Peak values of the first time derivative of the strain-time curve at systole, diastasis, and LA contraction corresponded to the respective peak SRs. Strain and SR parameters from both 2- and 4-chamber views were averaged to obtain mean results for analysis.
Antecubital venous blood samples (20–30 mL) were taken from consenting participants in the morning; fasting was not required before blood collection. After collection, the blood samples were immediately placed on ice for transportation and were processed within 6 h to obtain serum samples, which were subsequently stored at −80°C. Plasma levels of soluble uPAR were measured using the Human uPAR Quantikine ELISA kit (R&D Systems, cat No. DUP00), according to the manufacturer's assay procedures, as previously described [18].
Serum levels of monocyte chemoattractant protein-1 (MCP1) and matrix metallopeptidase 9 (MMP-9) were measured using commercial ELISA kits (R&D Systems; Minneapolis, MN, USA) according to the manufacturer's instructions. High-sensitivity C-reactive protein (hs-CRP) was measured using standard assays. Plasma levels of galectin-3 (Gal-3) and B-type natriuretic peptide (BNP) were measured by chemiluminescent microparticle immunoassay (ARCHITECT galectin-3 and ARCHITECT BNP; produced by Fujirebio Diagnostics Inc for Abbott Laboratories) using the Abbott ARCHITECT i2000SR analyzer.
Statistical Analysis
Clinical characteristics are presented as mean and standard deviation for continuous data and frequency and percentage for categorical data. Participants were categorized into 3 groups for comparison: Young adults below age 70 years, older adults aged 70 years and above with less than two vascular risk factors [19] (dyslipidemia, hypertension, smoking) (group 1), and older adults aged 70 years and above with two or more vascular risk factors (dyslipidemia, hypertension, smoking) (group 2). Diabetes was studied as an independent risk factor [20, 21]. We assessed the statistical significance of the differences between the groups. Student's t test was used for continuous data and χ2 test was used for categorical data.
To predict risk of cardiac function in the left atrium (based on mean values of εe ≤17, SRe≥−1.8), univariate logistic regression was performed on predictors − age, gender, two or more vascular risk factors, MCP1, MMP-9, BNPs, hsTn1, Gal-3, sUPAR, and hs-CRP. Age was categorized into 2 categories <76 and ≥76 years (based on mean age of group 2). Predictors with p < 0.05 in the univariate analysis were subsequently included in the multivariate logistic regression. The relationships between sUPAR with left atrium conduit strain (εe) and left atrium conduit strain rate (SRe) were assessed using Pearson's correlations and displayed in the scatter plots with linear fitted line. We derived receiver operating curves of the final model for εe and SRe, and compared area under the curve (AUC) with and without sUPAR.
All statistical analyses were performed using STATA 15 (College Station, TX, USA). For all analysis, a two-tailed p value of <0.05 was considered significant.
Results
Baseline Characteristics
We studied a total of 243 participants (women 43%, n = 104) with a mean age 70 ± 9.5 years. Participants were categorized into 3 groups comprising young adults (n = 76), older adults with less than two vascular risk factors (n = 91) (group 1), and older adults with two or more vascular risk factors (n = 76) (group 2). Baseline clinical characteristics of the 3 groups are shown in Table 1. Young adults were younger in age compared to group 1 and group 2 (61 ± 12.7 vs. 74 ± 2.2 and 75 ± 2.7 years, all p values <0.001). Group 2 had fewer female participants (31.6% vs. 49.4% and 46.7% compared to young adults and group 1, respectively, p ≤ 0.048).
Table 1.
Baseline clinical characteristics
| Clinical covariates | Young (n = 77) | Group 1: old and CV risk <2 (n = 90) | Group 2: old and CV risk ≥2 (n = 76) | Total (n = 243) | p value young versus group 1 | p value young versus group 2 | p value group 1 versus group 2 |
|---|---|---|---|---|---|---|---|
| Age, years | 61 (12.6) | 74 (2.2) | 75 (2.7) | 70 (9.5) | <0.0001 | <0.0001 | 0.026 |
| Female gender, n (%) | 38 (49.4) | 42 (46.7) | 24 (31.6) | 104 (42.8) | 0.73 | 0.025 | 0.048 |
| Body mass index, kg/m2 | 24 (2.8) | 23 (3.4) | 24 (3.4) | 24 (3.2) | 0.35 | 0.47 | 0.12 |
| Systolic blood pressure, mm Hg | 140 (14.5) | 152 (37.0) | 152 (14.9) | 148 (25.8) | 0.013 | <0.0001 | 0.97 |
| Diastolic blood pressure, mm Hg | 75 (10.3) | 76 (11.2) | 74 (10.0) | 75 (10.6) | 0.44 | 0.56 | 0.18 |
| Heart rate, beats per min | 73 (12.7) | 74 (12.5) | 73 (12.6) | 73 (12.6) | 0.57 | 0.76 | 0.37 |
| Hypertension, n (%) | 29 (37.7) | 30 (33.3) | 72 (94.7) | 131 (53.9) | 0.56 | <0.0001 | <0.0001 |
| Dyslipidemia, n (%) | 30 (39.0) | 16 (17.8) | 71 (93.4) | 117 (48.2) | 0.002 | <0.0001 | <0.0001 |
| Diabetes, n (%) | 10 (13.0) | 9 (10.0) | 27 (35.5) | 46 (18.9) | 0.55 | 0.001 | <0.0001 |
| Ever smoked, n (%) | 9 (12.3) | 11 (12.2) | 22 (29.0) | 42 (17.6) | 0.98 | 0.012 | 0.007 |
| Cardiovascular risk factors >2, n (%) | 20 (26.0) | 0 | 76 (100.0) | 96 (39.5) | <0.0001 | <0.0001 | <0.0001 |
Cardiovascular Measurements
Table 2 summarizes the cardiac structural and functional characteristics of the cohort. Markers of left ventricular strain were similar between the groups. Right ventricular global longitudinal strain was reduced in group 2, compared to young adults (−32 ± 5.3 vs. −30 ± 4.7, p = 0.028).
Table 2.
Cardiac structure and function
| Measurements | Young (n = 77) | Group 1: old and CV risk <2 (n = 90) | Group 2: old and CV risk ≥2 (n = 76) | Total (n = 243) | p value young versus group 1 | p value young versus group 2 | p value group 1 versus group 2 |
|---|---|---|---|---|---|---|---|
| LVGLS | –21 (2.7) | –21 (3.1) | –21 (2.8) | –21 (2.9) | 0.80 | 0.41 | 0.58 |
| LVGCS | –23 (3.6) | –22 (4.0) | –22 (3.4) | –22 (3.7) | 0.31 | 0.59 | 0.60 |
| LVGRS | 100 (24.0) | 100 (32.4) | 105 (21.7) | 102 (26.9) | 0.90 | 0.13 | 0.23 |
| RVGLS | –30 (4.7) | –32 (5.9) | –32 (5.3) | –31 (5.4) | 0.087 | 0.028 | 0.67 |
| Left atrium εs (%) | 33 (7.9) | 31 (7.9) | 29 (5.4) | 31 (7.3) | 0.067 | 0.0003 | 0.10 |
| Left atrium εe (%) | 16 (5.9) | 13 (4.0) | 12 (3.7) | 14 (5.0) | <0.0001 | <0.0001 | 0.025 |
| Left atrium εa (%) | 16 (4.6) | 17 (5.2) | 17 (4.0) | 17 (4.7) | 0.66 | 0.65 | 0.99 |
| Left atrium SRs (1/s) | 1.7 (0.5) | 1.6 (0.5) | 1.5 (0.4) | 1.6 (0.5) | 0.30 | 0.011 | 0.13 |
| Left atrium SRe (1/s) | –1.8 (0.7) | –1.3 (0.5) | –1.1 (0.4) | –1.4 (0.6) | <0.0001 | <0.0001 | 0.027 |
| Left atrium SRa (1/s) | –2.2 (0.7) | –2.2 (0.7) | –2.2 (0.6) | –2.2 (0.7) | 0.94 | 0.81 | 0.87 |
| SRe/SRa ratio | 0.9 (0.4) | 0.7 (0.6) | 0.6 (0.2) | 0.7 (0.4) | 0.027 | <0.0001 | 0.067 |
| Left atrium volume (minimum), mL | 30 (10.3) | 32 (18.8) | 31 (11.2) | 31 (14.3) | 0.50 | 0.79 | 0.63 |
| Left atrium volume (maximum), mL | 65 (14.2) | 63 (22.3) | 62 (17.1) | 63 (18.5) | 0.63 | 0.27 | 0.66 |
| LA ejection fraction (%) | 54 (9.3) | 52 (10.3) | 51 (8.3) | 52 (9.4) | 0.19 | 0.051 | 0.059 |
LVGLS, left ventricle global longitudinal strain; LVGCS, left ventricle circumferential strain; LVGRS, left ventricle radial strain; RVGLS, right ventricle global longitudinal strain; εs, reservoir strain; εa, booster strain; SRs, reservoir strain rate; SRa, booster strain rate.
Most of the cardiovascular differences were observed in the left atrium, specifically in left atrial phasic function. In bivariate analysis, the left atrium εe and left atrium SRe were reduced in group 1 and group 2, all p values <0.0001). Moreover, left atrium εe and left atrium SRe were markedly reduced in group 2 when compared to group 1 (p ≤ 0.027). The SRe/SRa ratio was also significantly reduced in both groups 1 and 2 when compared to young adults (p = 0.027 and p < 0.0001, respectively), however, no significant difference was observed between group 1 and group 2. Finally, left atrium reservoir strain (ɛs) was impaired in group 2 compared to young adults (29 ± 5.4 vs. 33 ± 7.9,% p = 0.0003), corresponding to observed impairments in group 2 versus young adults as well (1.5 ± 0.4 vs. 1.7 ± 0.5, p = 0.011).
Biomarker Measurements
Biomarker levels were compared between the groups as shown in Table 3. Bivariate comparisons consistently showed higher levels of sUPAR across the groups from young adults, group 1 to group 2. The level of sUPAR was significantly higher in group 1 compared to young adults (2.5 ± 1.6 vs. 1.7 ± 1.3, p = 0.0005), in group 2 compared to young adults (3.3 ± 2.4 vs. 1.7 ± 1.3, p < 0.0001), and between group 2 and group 1 (3.3 ± 2.4 vs. 2.5 ± 1.6, p = 0.017). Similarly, for MMP-9, the highest levels were observed in group 2, which were significantly higher when compared to young adults (114 ± 134 vs. 66 ± 55.4, p = 0.020), and group 1 (114 ± 134 vs. 72 ± 61, p = 0.013). There were no observed differences in the levels of BNP and hs-CRP across the groups. Marginal differences were observed in the levels of MCP1 and Gal-3, in group 2 compared to young adults.
Table 3.
Biomarker distribution between the groups
| Biomarkers | Young (n = 77) | Group 1: old and CV risk<2 (n = 90) | Group 2: old and CV risk ≥2 (n = 76) | Total (n = 243) | p value young versus group 1 | p value young versus group 2 | p value group 1 versus group 2 |
|---|---|---|---|---|---|---|---|
| MCP1, pg/mL | 353 (99.2) | 436 (354) | 433 (254) | 416 (277) | 0.12 | 0.040 | 0.95 |
| MMP-9, ng/mL | 66 (55.0) | 72 (61.7) | 114 (134) | 86 (95.3) | 0.54 | 0.020 | 0.013 |
| BNP, pg/mL | 35 (35.3) | 47 (50.4) | 44 (39.0) | 42 (42.4) | 0.088 | 0.17 | 0.62 |
| Gal-3, ng/mL | 15 (3.7) | 16 (5.2) | 17 (3.6) | 16 (4.3) | 0.20 | 0.0017 | 0.18 |
| hsTnl, ng/L | 39 (123) | 13 (27.7) | 23 (70.0) | 24 (81.4) | 0.057 | 0.34 | 0.21 |
| sUPAR, ng/mL | 1.7 (1.3) | 2.5 (1.6) | 3.3 (2.4) | 2.5 (1.9) | 0.0005 | <0.0001 | 0.017 |
| hs-CRP, mg/L | 1.3 (1.8) | 4.3 (13.9) | 1.9 (2.9) | 2.7 (8.8) | 0.071 | 0.12 | 0.15 |
hsTnl, high-sensitivity cardiac troponin I.
Relationship between Baseline Characteristics, Cardiovascular Characteristics, and Biomarker Measurements
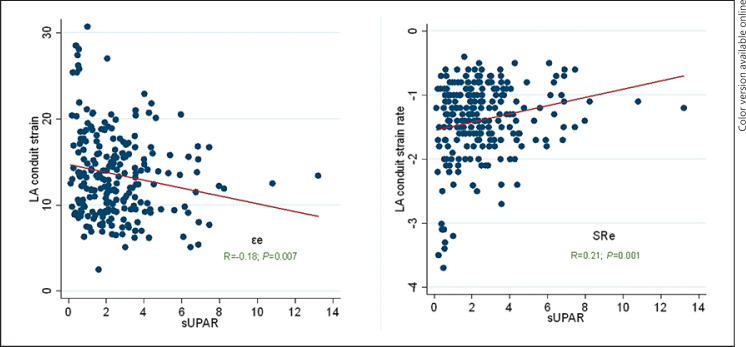
Based on univariate analysis, CV risk factors, age, sUPAR, BNPs, and Gal-3 levels were independently associated with εe and SRe reduction (Table 4). However, based on multivariate analysis, only sUPAR remained significantly associated with both εe (OR 1.52, CI 95% 1.1–2.1, p = 0.006) and SRe decline (OR 1.5, CI 95% 1.1–2.1, p = 0.019). There were positive albeit weaker associations between Gal-3 and εe reduction (OR 1.2, CI 95% 1.02–1.3, p = 0.022) and between BNPs and SRe decline (OR 1.03, CI 95% 1.003–1.1, p = 0.027). Based on the correlational analysis, there was a positive relationship between sUPAR and εe (r = −0.18, p = 0.007) and SRe (r = 0.21, p = 0.001) (Fig. 1).
Table 4.
Predictors of cardiac function in the left atrium
| Variables | Univariate regression analysis |
Multivariate regression analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| left atrium εe reduction |
SRe reduction |
left atrium εe reduction |
SRe reduction |
|||||
| unadjusted OR (95% CI) | p value | unadjusted OR (95% CI) | p value | adjusted OR (95% CI) | p value | adjusted OR (95% CI) | p value | |
| Age | 6.6 (1.5–28) | 0.011 | 9.5 (1.3–71) | 0.029 | 3.6 (0.8–16) | 0.096 | 4.7 (0.6–37) | 0.14 |
| Diabetes mellitus | 1.0 (0.5–2.2) | 0.99 | 1.1 (0.5–2.7) | 0.83 | ||||
| Cardiovascular risk factors >2 | 1.7 (0.9–3.4) | 0.10 | 2.8 (1.2–6.4) | 0.015 | 1.6 (0.6–4.2) | 0.29 | ||
| Female | 0.9 (0.5–1.6) | 0.62 | 0.9 (0.4–1.7) | 0.65 | ||||
| MCP1 (pg/mL) | 1.0 (1.0–1.01) | 0.24 | 1.0 (1.0–1.004) | 0.65 | ||||
| MMP-9 (ng/mL) | 1.0 (1.0–1.01) | 0.43 | 1.0 (1.0–1.01) | 0.50 | ||||
| BNP (pg/mL) | 1.02 (1.01–1.04) | 0.011 | 1.03 (1.01–1.06) | 0.004 | 1.0 (1.0–1.03) | 0.12 | 1.03 (1.003–1.1) | 0.027 |
| Gal-3 (ng/mL) | 1.20 (1.08–1.34) | 0.001 | 1.2 (1.03–1.3) | 0.015 | 1.2 (1.02–1.3) | 0.022 | 1.1 (0.9–1.2) | 0.37 |
| hsTnl (ng/L) | 1.0 (1.0–1.001) | 0.28 | 1.0 (1.0–1.003) | 0.77 | ||||
| sUPAR (ng/mL) | 1.5 (1.2–2.0) | 0.001 | 1.7 (1.3–2.3) | 0.001 | 1.52 (1.1–2.1) | 0.006 | 1.5 (1.1–2.1) | 0.019 |
| hs-CRP (mg/L) | 1.1 (0.9–1.3) | 0.20 | 1.1 (0.9–1.2) | 0.38 | ||||
Fig. 1.
Correlation of sUPAR with left atrium εe (left) and left atrium SRe (right). sUPAR, soluble urokinase plasminogen activator receptor.
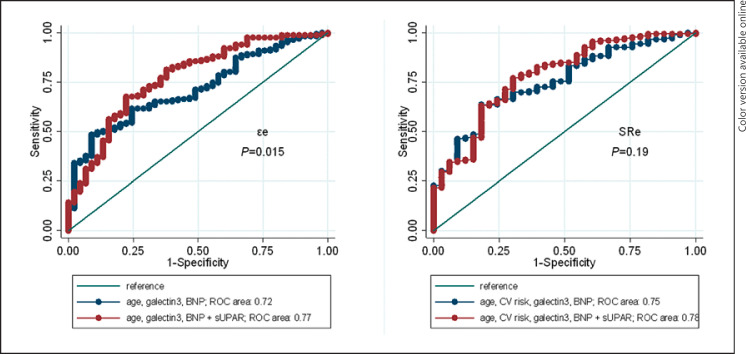
Finally, based on receiver operating curve analysis, the addition of sUPAR to a model consisting of age, CV risk factors, Gal-3, and BNPs increased the AUC of εe from 0.72 to 0.77 (p = 0.015). The AUC increased when sUPAR was added to the model for SRe as well, although the increase was not statistically significant (0.75–0.78, p = 0.19) (Fig. 2).
Fig. 2.
ROC curves for discriminating between εe (left) or SRe (right) using either age, CV risks, Gal-3, and BNP variables (blue line) or the same model plus sUPAR (red line).
Discussion
In summary, left atrial phasic function by advanced cardiac imaging distinguished the left atrium in a population-based cohort of older adults, and further differentiated those with risk factors. Specifically, conduit strain functions in the left atrium were progressively impaired with aging, and further exacerbated in the presence of risk factors. These cardiac functional impairments were associated with circulating levels of sUPAR, quantitatively higher in older adults with aging, as well as with accumulation of risk factors. As a circulating biomarker, sUPAR enhanced prediction of left atrial conduit strain function.
In cardiovascular disease, the use of circulating biomarkers to predict risks of disease in older adults is fraught with challenges. A plethora of studies have evaluated biomarkers either singly or in multiples for their clinical value in cardiovascular disease [22]. However, most of the studies have focused on middle-aged populations with limited representation from older adults [23]. Moreover, multiple pathophysiological mechanisms, including inflammation, oxidative stress, and dysmetabolism, have been implicated with cardiovascular pathology in older adults [24], resulting in less optimal performance from singular biomarkers. In addition, there are problems posed by age-related alterations in the renal clearance of circulating biomarkers.
For the evaluation of possible biomarkers specific to cardiovascular aging, prespecified cohorts are necessary for accurate assessment. This was a strength of our study design as it had been prespecified to study characteristics and determinants of cardiovascular aging in the community. First, our study cohort had captured older adults at a crucial window without clinical cardiovascular disease, hence circulating biomarkers represent aging processes as opposed to studying disease processes based on sampling from clinical patients with cardiovascular disease. Our cohort of community-dwelling older adults do not have clinical heart failure, supported by relatively low BNP levels. Levels of high-sensitivity troponin I assays in the cohort were additionally low, and below upper reference limits suggested in other studies and those that rule out coronary artery disease [25]. Furthermore, the lack of an association between traditional biomarkers of inflammation such as high sensitivity C-reactive protein (hs-CRP) between our groups and in association with left atrial function, points to alternative processes in the aging heart that may not be represented by standard markers of inflammation. Therefore, traditional cardiovascular biomarkers in the setting of cardiovascular aging may not reflect risks of aging-related cardiovascular dysfunction. Second, by prespecifying older adults as a priority in our cohort, we had performed detailed imaging using advanced CMR to delineate key features of the aged heart, without confounding by ongoing disease. As an important chamber that reflects the burden of aging and associated systemic risk factors [26], the alterations observed in our study reflect disturbances in left atrial strain, namely conduit strain functions, which have been previously associated with aging [7].
These disturbances reflect impairments in myocardial diastolic function [27] with fibrotic mechanisms suggested as contributing factors [28]. Higher levels of Gal-3 that were observed in older adults, and those with risk factors, may reflect increased collagen production and cellular proliferation in the myocardium with aging [29, 30]. Notably, Gal-3 contains collagen-like domains which are substrates for cleavage by matrix metalloproteinases, a group of endopeptidases responsible for matrix protein degradation [31, 32]. Higher levels of MMP-9 among older adults and those with risk factors in our study, in conjunction with Gal-3 levels in this cohort, are jointly supportive of fibrotic mechanisms in left atrial aging. Impairments in the left atrial strain seen in association with circulating sUPAR in our study may be related to increased immune activation in the left atrium in response to left atrial remodeling and fibrotic processes [31]. Taken together, enhanced fibrosis [33], immune activation, and chronic inflammation [34] may explain the association observed between chief biomarkers in the final model (sUPAR, Gal-3, age) that were seen in association with left atrial function.
Our observations regarding sUPAR are novel in the context of cardiovascular aging, expanding our previous observations which associated impairments in myocardial relaxation in the LV [18] with increases in sUPAR level. In contrast to hs-CRP [35], sUPAR is considered a marker of low-grade inflammation [36], where it is released by activated immune cells at the sites of inflammation to mediate chemotactic effects [37, 38]. These observations have linked increased levels of sUPAR to cardiovascular diseases such as heart failure [39, 40]. In the study by Koller et al. [39], sUPAR levels were a strong and independent predictor of mortality among patients with chronic heart failure. In addition, our observations between left atrial function and sUPAR may explain links between sUPAR and left atrial myopathy, underscoring studies that have demonstrated clinical risks between sUPAR and atrial fibrillation [41].
Overall, the role of sUPAR as a clinical biomarker for cardiovascular aging-related conditions is intriguing and warrants more study. However, the stability of sUPAR in blood samples, including in settings of acute vascular events, supports its role as a biomarker specific to chronic processes such as chronic inflammation, where timing of sample collection is less critical for its interpretation. The quantitative levels of sUPAR measured in our study, would be invaluable in defining specific cut-off values of sUPAR based on age and clinical risk groups. Our results should form the basis for future studies to characterize similar populations in order to confirm our observations.
Our study has limitations. The clinical implication of these results is currently uncertain in the absence of clinical outcome data among this community cohort of older adults. However, our focus on left atrial function was guided by prior work that had established the prognostic value of left atrial strain in disease cohorts, extending beyond contrast-enhanced imaging markers [16]. Therefore, the value of left atrial strain might only be underestimated, and not overestimated in this low-risk community cohort. In addition, the current results may be limited to cohorts of similar Asian demographics and may not apply to cohorts of varying risk profiles, or ethnic composition. However, the assessments of circulating biomarkers were obtained in a contemporary cohort, reflective of general population characteristics, thus expanding on future generalizability of the results. As an observational study, we are unable to ascertain if the biomarkers are drivers of the aging process or merely bystanders involved in cardiovascular aging. However, in aggregate, the spectrum of biomarkers evaluated in this study should aid in prioritizing candidate biomarkers relevant to cardiovascular aging. Finally, our work supports an approach where early detection of cardiovascular aging by a combination of modern imaging and biomarkers [42] may be a fresh approach to identify and prevent onset of cardiovascular disease in aging populations.
Conclusion
Left atrial dysfunction in older adults as detected by advanced CMR imaging was associated with a panel of circulating biomarkers comprising of Gal-3, MMP-9, and sUPAR. As a cardiac chamber frequently altered by aging, higher levels of Gal-3 and MMP-9 seen in association with left atrial aging may be suggestive of fibrotic mechanisms in left atrial aging. Impairments in left atrial strain seen in association with circulating sUPAR may be related to immune activation and inflammation. These findings may be used by future work to prioritize older adults at higher risks of aging-related cardiovascular diseases.
Statement of Ethics
Written informed consent was obtained from participants upon enrolment. The SingHealth Centralised Institutional Review Board (CIRC/2014/628/C) had approved the study protocol.
Conflict of Interest Statement
No conflicts of interest.
Funding Sources
The Cardiac Aging Study has received funding support from the National Medical Research Council of Singapore (MOH-000153), Hong Leong Foundation, Duke-NUS Medical School, Estate of Tan Sri Khoo Teck Puat and Singhealth Foundation. Those participants recruited from the Singapore Chinese Health Study were supported by the United States National Institutes of Health (NIH R01 CA144034 and UM1 CA182876). The CMR imaging analysis was partially supported by National Medical Research Council of Singapore (NMRC/OFIRG/0018/2016). The Nanyang Assistant Professorship from Nanyang Technological University funded the work done by Dr. Christine Cheung. W.-P. Koh is supported by the National Medical Research Council, Singapore (MOH-CSASI19nov-0001). The funders had no role in the design and conduct of the study; collection; management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Author Contributions
A.S.K., A.S., F.G., R.S.T., and C.C.: analyses, paper drafting, and revision; A.S., F.W.J.C., S.L., X.Z., L.Z., R.S.T., P.L.K., J.-P.K., W.S.L., G.S.L., and W.-P.K.: acquisition, imaging, analyses, and clinical data preparation. A.S.K., F.G., W.-P.K., C.C., and A.S.: study design, substantial revision, and paper drafting.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank the staff of the laboratories involved for participating in the conduct of the study, and Abbott for providing reagents and kits for certain biomarker measurements.
Funding Statement
The Cardiac Aging Study has received funding support from the National Medical Research Council of Singapore (MOH-000153), Hong Leong Foundation, Duke-NUS Medical School, Estate of Tan Sri Khoo Teck Puat and Singhealth Foundation. Those participants recruited from the Singapore Chinese Health Study were supported by the United States National Institutes of Health (NIH R01 CA144034 and UM1 CA182876). The CMR imaging analysis was partially supported by National Medical Research Council of Singapore (NMRC/OFIRG/0018/2016). The Nanyang Assistant Professorship from Nanyang Technological University funded the work done by Dr. Christine Cheung. W.-P. Koh is supported by the National Medical Research Council, Singapore (MOH-CSASI19nov-0001). The funders had no role in the design and conduct of the study; collection; management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
References
- 1.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I − aging arteries − a “set upˮ for vascular disease. Circulation. 2003;107((1)):139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 2.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises − part II − the aging heart in health − links to heart disease. Circulation. 2003;107((2)):346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 3.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises − part III − cellular and molecular clues to heart and arterial aging. Circulation. 2003;107((3)):490. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 4.Triposkiadis F, Tentolouris K, Androulakis A, Trikas A, Toutouzas K, Kyriakidis M, et al. Left atrial mechanical function in the healthy elderly: new insights from a combined assessment of changes in atrial volume and transmitral flow velocity. J Am Soc Echocardiogr. 1995;8((6)):801–809. doi: 10.1016/s0894-7317(05)80004-5. [DOI] [PubMed] [Google Scholar]
- 5.Aurigemma GP, Gottdiener JS, Arnold AM, Chinali M, Hill JC, Kitzman D. Left atrial volume and geometry in healthy aging: the Cardiovascular Health Study. Circ Cardiovasc Imaging. 2009;2((4)):282–289. doi: 10.1161/CIRCIMAGING.108.826602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyd AC, Schiller NB, Leung D, Ross DL, Thomas L. Atrial dilation and altered function are mediated by age and diastolic function but not before the eighth decade. JACC Cardiovasc Imaging. 2011;4((3)):234–242. doi: 10.1016/j.jcmg.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Koh AS, Gao F, Leng S, Kovalik JP, Zhao X, Tan RS, et al. Dissecting clinical and metabolomics associations of left atrial phasic function by cardiac magnetic resonance feature tracking. Sci Rep. 2018;8((1)):8138–26456. doi: 10.1038/s41598-018-26456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung DY, Chi C, Allman C, Boyd A, Ng AC, Kadappu KK, et al. Prognostic implications of left atrial volume index in patients in sinus rhythm. Am J Cardiol. 2010;105((11)):1635–1639. doi: 10.1016/j.amjcard.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 9.Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol. 2003;1941((6)):1036–1043. doi: 10.1016/s0735-1097(02)02981-9. [DOI] [PubMed] [Google Scholar]
- 10.Moller JE, Hillis GS, Oh JK, Seward JB, Reeder GS, Wright RS, et al. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation. 2003;107((17)):2207–2212. doi: 10.1161/01.CIR.0000066318.21784.43. [DOI] [PubMed] [Google Scholar]
- 11.Evin M, Redheuil A, Soulat G, Perdrix L, Ashrafpoor G, Giron A, et al. Left atrial aging: a cardiac magnetic resonance feature-tracking study. Am J Physiol Heart Circ Physiol. 2016;310((5)):H542–9. doi: 10.1152/ajpheart.00504.2015. [DOI] [PubMed] [Google Scholar]
- 12.Pellett AA, Myers L, Welsch M, Jazwinski SM, Welsh DA. Left atrial enlargement and reduced physical function during aging. J Aging Phys Act. 2013;21((4)):417–432. doi: 10.1123/japa.21.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hankin JH, Stram DO, Arakawa K, Park S, Low SH, Lee HP, et al. Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer. 2001;39((2)):187–195. doi: 10.1207/S15327914nc392_5. [DOI] [PubMed] [Google Scholar]
- 14.Leng S, Tan RS, Zhao X, Allen JC, Koh AS, Zhong L. Fast long-axis strain: a simple, automatic approach for assessing left ventricular longitudinal function with cine cardiovascular magnetic resonance. Eur Radiol. 2020;30((7)):3672–3683. doi: 10.1007/s00330-020-06744-6. [DOI] [PubMed] [Google Scholar]
- 15.Leng S, Tan RS, Zhao X, Allen JC, Koh AS, Zhong L. Validation of a rapid semi-automated method to assess left atrial longitudinal phasic strains on cine cardiovascular magnetic resonance imaging. J Cardiovasc Magn Reson. 2018;20((1)):71. doi: 10.1186/s12968-018-0496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leng S, Ge H, He J, Kong L, Yang Y, Yan F, et al. Long-term prognostic value of cardiac MRI left atrial strain in ST-segment elevation myocardial infarction. Radiology. 2020;296((2)):299–309. doi: 10.1148/radiol.2020200176. [DOI] [PubMed] [Google Scholar]
- 17.Leng S, Zhao XD, Huang FQ, Wong JI, Su BY, Allen JC, et al. Automated quantitative assessment of cardiovascular magnetic resonance-derived atrioventricular junction velocities. Am J Physiol Heart Circ Physiol. 2015;309((11)):H1923–35. doi: 10.1152/ajpheart.00284.2015. [DOI] [PubMed] [Google Scholar]
- 18.Koh AS, Velmurugan B, Gao F, Tan RS, Wong JI, Teo LLY, et al. Value of soluble urokinase plasminogen activator receptor over age as a biomarker of impaired myocardial relaxation. BMC Geriatr. 2017;17((1)):275–0668. doi: 10.1186/s12877-017-0668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lind L, Sundström J, Ärnlöv J, Lampa E. Impact of aging on the strength of cardiovascular risk factors: a longitudinal study over 40 years. J Am Heart Assoc. 2018;7((1)):e007061. doi: 10.1161/JAHA.117.007061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao F, Kovalik JP, Zhao X, Chow VJ, Chew H, Teo LL, et al. Exacerbation of cardiovascular ageing by diabetes mellitus and its associations with acyl-carnitines. Aging. 2021;13((11)):14785–805. doi: 10.18632/aging.203144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovalik JP, Zhao X, Gao F, Leng S, Chow V, Chew H, et al. Amino acid differences between diabetic older adults and non-diabetic older adults and their associations with cardiovascular function. J Mol Cell Cardiol. 2021;158:63–71. doi: 10.1016/j.yjmcc.2021.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Tan GJ, Han LN, Bai YY, He M, Liu HB. Novel biomarkers for cardiovascular risk prediction. J Geriatr Cardiol. 2017;14((2)):135. doi: 10.11909/j.issn.1671-5411.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bell SP, Giuseffi JL, Forman DE. Cardiovascular biomarkers and their utility in the older adult. Curr Cardiovasc Risk Rep. 2012;6((5)):397. doi: 10.1007/s12170-012-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodgers JL, Jones J, Bolleddu SI, Vanthenapalli S, Rodgers LE, Shah K, et al. Cardiovascular risks associated with gender and aging. J Cardiovasc Dev Dis. 2019;6((2)):19. doi: 10.3390/jcdd6020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Lemos JA, deFilippi CR. Prevalence and significance of detectable troponins as measured by highly sensitive assays in the general population. Coron Artery Dis. 2013;24((8)):705–709. doi: 10.1097/MCA.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 26.Kaminski M, Steel K, Jerosch-Herold M, Khin M, Tsang S, Hauser T, et al. Strong cardiovascular prognostic implication of quantitative left atrial contractile function assessed by cardiac magnetic resonance imaging in patients with chronic hypertension. J Cardiovasc Magn Reson. 2011;13((1)):42–13. doi: 10.1186/1532-429X-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyd AC, Richards DA, Marwick T, Thomas L. Atrial strain rate is a sensitive measure of alterations in atrial phasic function in healthy ageing. Heart. 2011;97((18)):1513–1519. doi: 10.1136/heartjnl-2011-300134. [DOI] [PubMed] [Google Scholar]
- 28.Nicoletti A, Michel JB. Cardiac fibrosis and inflammation: interaction with hemodynamic and hormonal factors. Cardiovasc Res. 1999;41((3)):532–543. doi: 10.1016/s0008-6363(98)00305-8. [DOI] [PubMed] [Google Scholar]
- 29.Keng BMH, Gao F, Ewe SH, Tan RS, Teo LLY, Xie BQ, et al. Galectin-3 as a candidate upstream biomarker for quantifying risks of myocardial ageing. ESC Heart Fail. 2019;6((5)):1068–1076. doi: 10.1002/ehf2.12495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martos R, Baugh J, Ledwidge M, OʼLoughlin C, Conlon C, Patle A, et al. Diastolic heart failure: evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation. 2007;115((7)):888–895. doi: 10.1161/CIRCULATIONAHA.106.638569. [DOI] [PubMed] [Google Scholar]
- 31.Ochieng J, Fridman R, Nangia-Makker P, Kleiner DE, Liotta LA, Stetler-Stevenson WG, et al. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and-9. Biochemistry. 1994;33((47)):14109–14. doi: 10.1021/bi00251a020. [DOI] [PubMed] [Google Scholar]
- 32.Puthenedam M, Wu F, Shetye A, Michaels A, Rhee KJ, Kwon JH. Matrilysin-1 (MMP7) cleaves galectin-3 and inhibits wound healing in intestinal epithelial cells. Inflamm Bowel Dis. 2011;17((1)):260–267. doi: 10.1002/ibd.21443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sjowall C, Martinsson K, Cardell K, Ekstedt M, Kechagias S. Soluble urokinase plasminogen activator receptor levels are associated with severity of fibrosis in nonalcoholic fatty liver disease. Transl Res. 2015;165((6)):658–666. doi: 10.1016/j.trsl.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita T, Sekiguchi A, Suzuki S, Ohtsuka T, Sagara K, Tanabe H, et al. Enlargement of the left atrium is associated with increased infiltration of immune cells in patients with atrial fibrillation who had undergone surgery. J Arrhythm. 2015;31((2)):78–82. doi: 10.1016/j.joa.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107((3)):363. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 36.Eugen-Olsen J, Andersen O, Linneberg A, Ladelund S, Hansen TW, Langkilde A, et al. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J Intern Med. 2010;268((3)):296–308. doi: 10.1111/j.1365-2796.2010.02252.x. [DOI] [PubMed] [Google Scholar]
- 37.Fazioli F, Resnati M, Sidenius N, Higashimoto Y, Appella E, Blasi F. A urokinase-sensitive region of the human urokinase receptor is responsible for its chemotactic activity. EMBO J. 1997;16((24)):7279. doi: 10.1093/emboj/16.24.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pliyev BK. Activated human neutrophils rapidly release the chemotactically active D2D3 form of the urokinase-type plasminogen activator receptor (uPAR/CD87) Mol Cell Biochem. 2009;321((1–2)):111. doi: 10.1007/s11010-008-9925-z. [DOI] [PubMed] [Google Scholar]
- 39.Koller L, Stojkovic S, Richter B, Sulzgruber P, Potolidis C, Liebhart F, et al. Soluble urokinase-type plasminogen activator receptor improves risk prediction in patients with chronic heart failure. JACC Heart Fail. 2017;5((4)):268–277. doi: 10.1016/j.jchf.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Gong KZ, Song G, Spiers JP, Kelso EJ, Zhang ZG. Activation of immune and inflammatory systems in chronic heart failure: novel therapeutic approaches. Int J Clin Pract. 2007;61((4)):611. doi: 10.1111/j.1742-1241.2007.01295.x. [DOI] [PubMed] [Google Scholar]
- 41.Ichihara N, Miyamura M, Maeda D, Fujisaka T, Fujita SI, Morita H, et al. Association between serum soluble urokinase-type plasminogen activator receptor and atrial fibrillation. J Arrhythm. 2017;33((5)):469–474. doi: 10.1016/j.joa.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koh AS, Kovalik JP. Metabolomics and cardiovascular imaging: a combined approach for cardiovascular ageing. ESC Heart Fail. 2021;8((3)):1738–1750. doi: 10.1002/ehf2.13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.