Abstract
Background
It is predicted that approximately two billion tourist trips to foreign countries will be taken worldwide each year by 2030. Germany has long been among the most active countries in tourism. The frequency of illness among persons returning from developing and newly industrialized countries is 43–79%. The appropriate diagnosis of fever in returning travelers is a clinically important matter, as it can be a sign of a life-threatening illness.
Methods
This review is based on publications (2001–2022) retrieved by a selective search in PubMed for studies on the epidemiology, diagnosis, and treatment of febrile illnesses in returning travelers, or on specific tropical diseases.
Results
Diarrhea, fever, and skin changes are the most common manifestations of disease after travel to tropical and subtropical areas. The diagnostic evaluation should be performed in a series of steps, beginning with a precise travel history and the identification of specific risk factors. Among travelers returning from sub-Saharan Africa, Plasmodium falciparum malaria is the most common cause of fever on presentation to centers for infectious diseases and tropical medicine, affecting approximately 50 per 1000 travelers. Among persons returning from travel to Southeast Asia, dengue fever is the most common infectious disease, affecting 50–160 per 1000 travelers. Further potentially dangerous diseases include chikungunya and zika fever, typhoid and paratyphoid fever, amoebic liver abscess, visceral leishmaniasis (kala-azar), leptospirosis, and, very rarely, imported cases of viral hemorrhagic fever. COVID-19 and influenza are important differential diagnoses.
Conclusion
The differential diagnosis can be narrowed by thorough history-taking with particular attention to the patient’s travel route, combined with a good knowledge of the geographic spread and incubation times of the main tropical diseases. Algorithms help clinicians to focus the diagnostic work-up and select the appropriate further laboratory tests and diagnostic procedures.
cme plus
This article has been certified by the North Rhine Academy for Continuing Medical Education. Participation in the CME certification program is possible only over the internet: cme.aerzteblatt.de. The deadline for submission is 6 June 2023.
According to the World Tourism Organization (UNWTO), an estimated two billion persons will travel each year by 2030 (1). As a result of the global increase in mobility, travel-related diseases are gaining in importance. Although travel medicine advice can minimize health risks even before a trip begins, physician contacts are common during and after a trip (2). Studies from Europe and the United States have reported that 43% to 79% of travelers fall ill during or after staying in developing and newly industrialized countries (3, 4). According to data from the EuroTravNet clinics, the European surveillance sub-network of GeoSentinel, acute diarrhea, viral syndromes with or without skin manifestation as well as malaria are the most common reasons for clinic visits (5). Furthermore, a significant increase in arbovirus infections (e.g., dengue fever, Chikungunya and Zika fever) was observed over a period of 20 years (1998–2018). The most frequently visited travel regions include sub-Saharan Africa, Southeast Asia and the Indian subcontinent. Among travelers returning from sub-Saharan Africa, P. falciparum malaria (Malaria tropica) is with about 50 cases per 1000 travelers the most common cause of fever in patients seen at centers for infectious disease and tropical medicine. In other tropical and subtropical regions, such as Southeast Asia, dengue fever is the dominant cause of fever (50–160 cases per 1000 travelers with fever) (5, 6). In 2019, 993 malaria cases were reported in Germany; in the previous years, the number of cases was at a similar level of around 1000 per year (7– 9). The number of dengue cases reported in 2019 was 1176, representing a threefold increase in ten years. In the years 2020/2021, a decrease in reported travel-related infectious diseases of 40–90% compared to 2019 occurred as a result of the pandemic-related decrease in long-distance travel (10). Yet, the number of tourist travels and travel-related diseases is expected to rise again (11).
Given its clinical relevance, the differential diagnostic work-up of fever as a sign of a potentially life-threatening disease is at the heart of the diagnostic process. In many cases, there is uncertainty in the management of febrile returning travelers outside of specialized facilities (12). The aim of this article is to support mainly primary care doctors by providing guidance for a structured diagnostic approach focusing on relevant diseases, i.e., conditions with high incidence, high transmissibility and/or high potential to pose a threat to the patient’s life.
Methods
This review is based on pertinent publications retrieved from a selective literature search in the PubMed database for studies on the epidemiology and diagnosis of fever in the returning traveler or specific tropical diseases, published between 2001 and 2022. In the absence of evidence or supporting literature, the management recommendations (including the algorithm in the Box) are based on the authors’ own experiences or best practices in a center for infectious diseases and tropical medicine.
Structured approach to fever after return from travel
Fever, defined as a body temperature ≥ 38.3 °C, is an important symptom in the returning traveler seeking medical attention, as it leads to hospitalization in about 30 % of cases (13) and may be indicative of severe disease (14). One-third of the fever-related clinic visits are diagnosed with a tropical infectious disease, with malaria accounting for the majority of cases (70%); 38% of the visits are due to non-tropical infections and in 23% of cases the cause is non-infectious or unspecified (13). SARS-CoV-2 infection (COVID-19) as a differential diagnosis is increasing in importance, as is influenza (11). Climate change and environmental changes are causing shifts in vector distribution areas, so that formerly “classic” tropical diseases, for example some arboviral infections, are becoming more likely to occur even during travel within Europe (15). Examples of autochthonous diseases with the potential to become endemic in the future include West Nile fever (also in Germany), dengue fever (Southern Europe) and Chikungunya fever (Southern Europe). In primary care, it is a key concern to identify diseases with high individual and societal health risk and to narrow down the differential diagnostic spectrum, taking a stepwise approach. By no means should rare (“exotic”) diseases be ruled out a priori. Special considerations apply to children, pregnant women and immunocompromised patients (eBox 1).
eBOX 2. Management of patients with suspected viral hemorrhagic fever as per standards in the authors’ department, adapted from (17, 22, e11-e13).
-
Primary risk assessment for the presence of viral hemorrhagic fever (VHF) (such as Ebola fever, Marburg fever, Lassa fever, hemorrhagic Crimean-Congo fever, South American hemorrhagic fever) in the returning traveler
On initial suspicion, a distance of >1.5 m from the patient, contact minimization and basic hygiene procedures are sufficient when taking the focused history. If immediate physical examination is required (e.g., clinical instability) or contact with body fluids is deemed likely (e.g., vomiting), personal protective equipment*1 should be worn.
-
Onset of symptoms*2 within 21 days of
-
Staying in a high-risk area*3 plus potentially:
Contact with an animal potentially infected with, ill with, and/or deceased from a VHF virus (contact with bats, monkeys, rodents, ticks, including consumption of „bush meat“ or food potentially contaminated with rodent feces; animal slaughter)
Contact with a case of suspected VHF (diseased or deceased)
(Laboratory) handling of VHF viruses/pathogen-containing material
-
*1 Personal protective equipment: double gloves, safety goggles or face shield, disposable protective gown, FFP3 mask, foot protection
*2 Initial suspicion in the presence of the following symptoms: Fever/increased body temperature in the last 24 h, potentially with accompanying symptoms (hemorrhages, diarrhea, nausea, vomiting, headache, muscle and joint aches, abdominal pain, rash, conjunctivitis)
*3 Endemic/epidemic areas for Ebola virus and Marburg Virus include: Central and West Africa; for Lassa virus: West Africa; for Crimean-Congo fever virus: Africa, Asia, South East Europe.
In addition, note current outbreaks (see internet resources on current outbreak events)
Information available online
-
Computer-based diagnostic algorithms, among others for febrile diseases in returning travelers
www.fevertravel.ch (free of charge)
www.itg.be/E/kabisa (free of charge)
www.gideononline.com (chargeable)
-
Internet resources on current outbreak events
-
Contacts for advice on infectious diseases and tropical medicine
German Competence and Treatment Centers for High Consequence Infectious Diseases (STAKOB)
-
(STAKOB centers in Hamburg, Berlin, Leipzig, Frankfurt/Main, Düsseldorf, Stuttgart, Munich)
Risk assessment for highly contagious diseases and clinical instability
Highly contagious, life-threatening diseases (high consequence infectious diseases, HCID) are very rarely imported from the tropics/subtropics (16, 17). In Germany, the last two cases of a viral hemorrhagic fever (VHF) transmissible from human to human were reported in 2016. One was a US citizen transferred from Togo to Germany for treatment of Lassa fever, which was only diagnosed post-mortem, the other a mortician with additional secondary infection (18, 19). Besides the Lassa virus, the group of highly contagious VHF pathogens include Ebola virus, Marburg virus and Crimean-Congo hemorrhagic fever virus (CCHFV), among others (17, 20). Even though imported VHF cases are very rare, the transmission risk of HCID makes it an important differential diagnosis to consider (ebox 2) (17, 20– 22).
Most notable in the group of severe highly contagious respiratory diseases are the Middle East Respiratory Syndrome (MERS coronavirus; Arabian Peninsula and neighboring countries, mainly Saudi Arabia) and pneumonic plague (mainly Madagascar and East/Central Africa, secondary South/Southeast Asia and South America, sporadic United States) (23, 24). These should be considered in patients with respiratory symptoms/pneumonia, above mentioned geographic exposure and potentially additional contact to animals, animal products or animal excrements (MERS: dromedary camels; pneumonic plague: fleas, rats, cats, guinea pigs and others) or contact with persons with proven or suspected infection (17, 20, 23, 24).
If a highly contagious disease is already suspected based on a patient’s history and clinical findings, the patient should be isolated immediately. The health authorities must be informed instantly pursuant to paragraph 6 of the German Protection against Infection Act (Infektionsschutzgesetz, IfSG). The decision on how to proceed in a case, especially with regard to the initial patient transfer, is made by the local health authority together with the treating physician and the responsible competence/treatment center of the Permanent Working Group of Competence and Treatment Centers for High Consequence Infectious Diseases (STAKOB, Ständiger Arbeitskreises der Kompetenz- und Behandlungszentren für Krankheiten durch hochpathogene Erreger) (ebox 2) as well as the responsible state health authority. Personal protective equipment should be worn while providing patient care until transfer (17, 22).
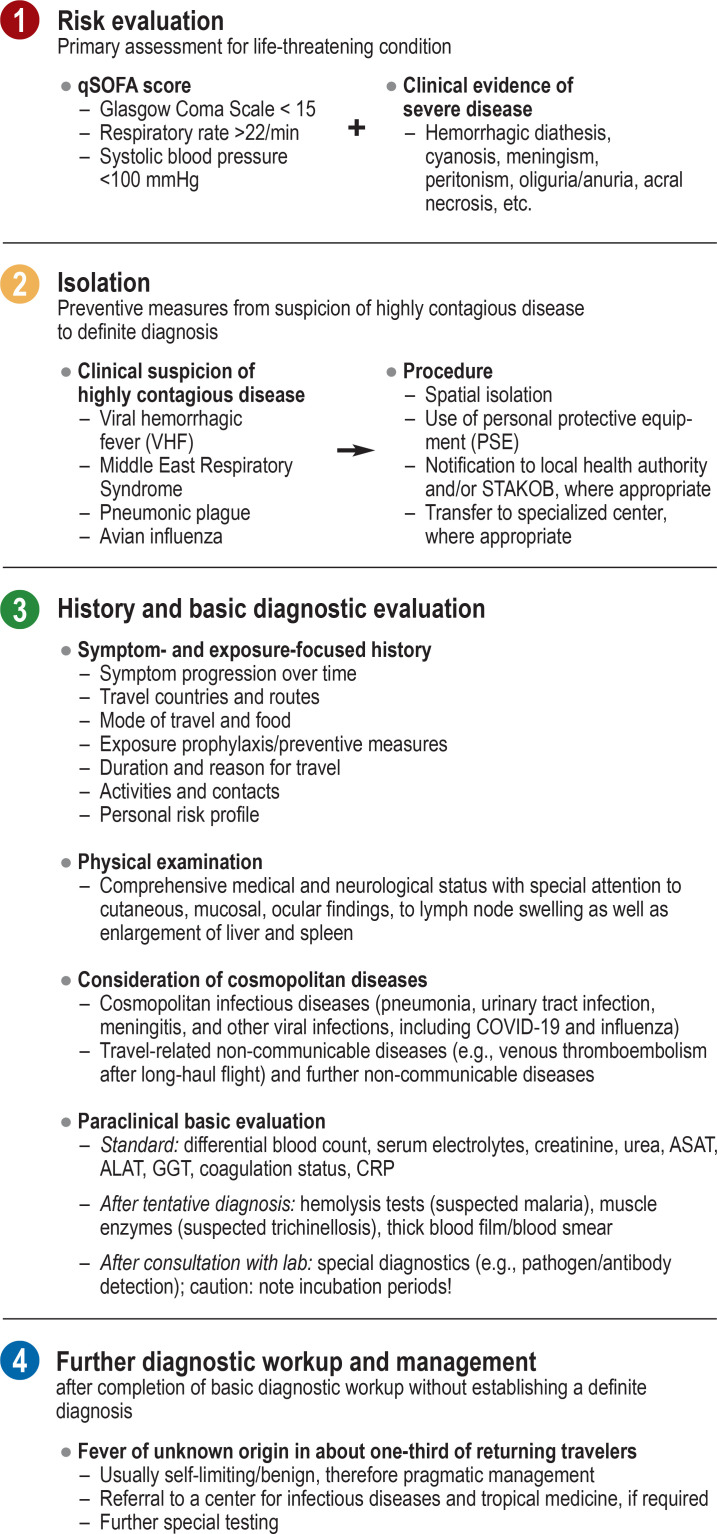
To identify patients with a greater risk of a lethal progression, all patients with suspected systemic infectious disease should be assessed using the quick Sequential Organ Failure Assessment“ (qSOFA) score (box) (4, 21, 25). Apart from fever, clinical findings indicative of severe disease include petechiae/purpura as a sign of hemorrhagic diathesis, cyanosis, meningism, peritonism, oliguria/anuria, and acral necrosis (17, 21). Any identified high-risk patient should be immediately hospitalized and, if necessary, admitted to ICU (4, 21, 26).
BOX.
Fever in the returning traveler: Initial assessment and establishing the diagnosis (according to procedure in the authors’ department), adapted from (4)
ALAT, alanine aminotransferase; ASAT, aspartate aminotransferase; CRP, C-reactive protein; GGT, gamma-glutamyl transferase; qSOFA, quick Sequential Organ Failure Assessment;
STAKOB, Permanent Working Group of Competence and Treatment Centers for High Consequence Infectious Diseases (Ständiger Arbeitskreis der Kompetenz- und Behandlungszentren für Krankheiten durch hochpathogene Erreger)
According to a systematic review, deaths are rare among febrile migrants and returning travelers (overall lethality, 0.22%; eight studies) (13). Nevertheless, urgent diagnosis is recommended, as delayed treatment may lead to rapid disease progression with poorer prognosis, especially in cases with P. falciparum malaria, the most common cause of death in the returning traveler (21). In Germany, three malaria deaths were reported in 2017 and 2018, respectively, two deaths in 2019 and one death in 2020, all after staying in sub-Saharan Africa (7– 10, 16).
Symptom-focused clinical history and physical examination
When taking a patient’s history, current symptoms as well as symptoms experienced before, during and after the travel should be recorded along the timeline with as much detail as possible. Temporary, self-limiting symptoms, such as a transient rash (e.g., associated with rickettsioses, dengue fever) (efigure) or a “typical“ biphasic disease course, e.g., with leptospirosis), can provide valuable clues (27). Based on the symptom timeline and familiarity with incubation times (Table 1, eFigure 1), it is already possible to narrow down the diagnosis. For example, if first symptoms occurred ≥ 21 days after the presumed or latest possible exposure (usually corresponds to the day of departure), VHF and dengue fever can be virtually ruled out given their maximum incubation periods (21, 26, 28, 29). Conversely, for example, P. falciparum malaria with a minimum incubation period of six days can be ruled out in the case of a three-day short stay in an endemic area and symptom onset immediately on the day of departure (26, 30). However, due to the highly variable incubation times, malaria infections can manifest after several months or even years (Table 1, eFigure 1). As a rule of thumb, malaria diagnostics should be performed within 24 hours (on an emergency basis) up to four months after a stay in an endemic area (28, 30). Caution should be used when making differential diagnostic discriminations based on incubation periods, as vague or incorrect patient information or lack of awareness of symptoms and their chronological progression may result in incorrect differential diagnostic conclusions. Rarer scenarios that equally thwart this approach are cases of non-endemic transmission of a tropical disease (e.g., airport malaria) (26).
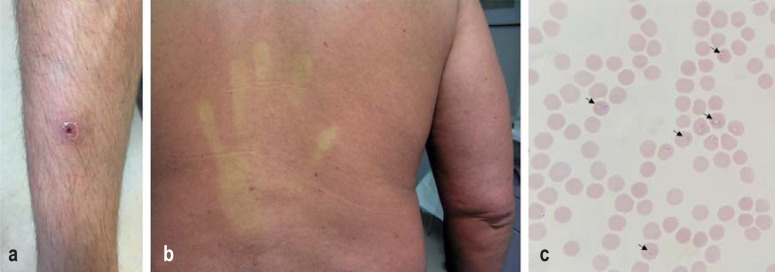
eFigure.
Important differential diagnoses:
a) African tick bite fever (eschar of lower leg),
b) Dengue fever (erythematous rash, especially on trunk, with white dermographism),
c) Blood smear for malaria tropica with detection of Plasmodium falciparum
(giemsa-stained, arrows mark parasitized red blood cells with trophozoites)
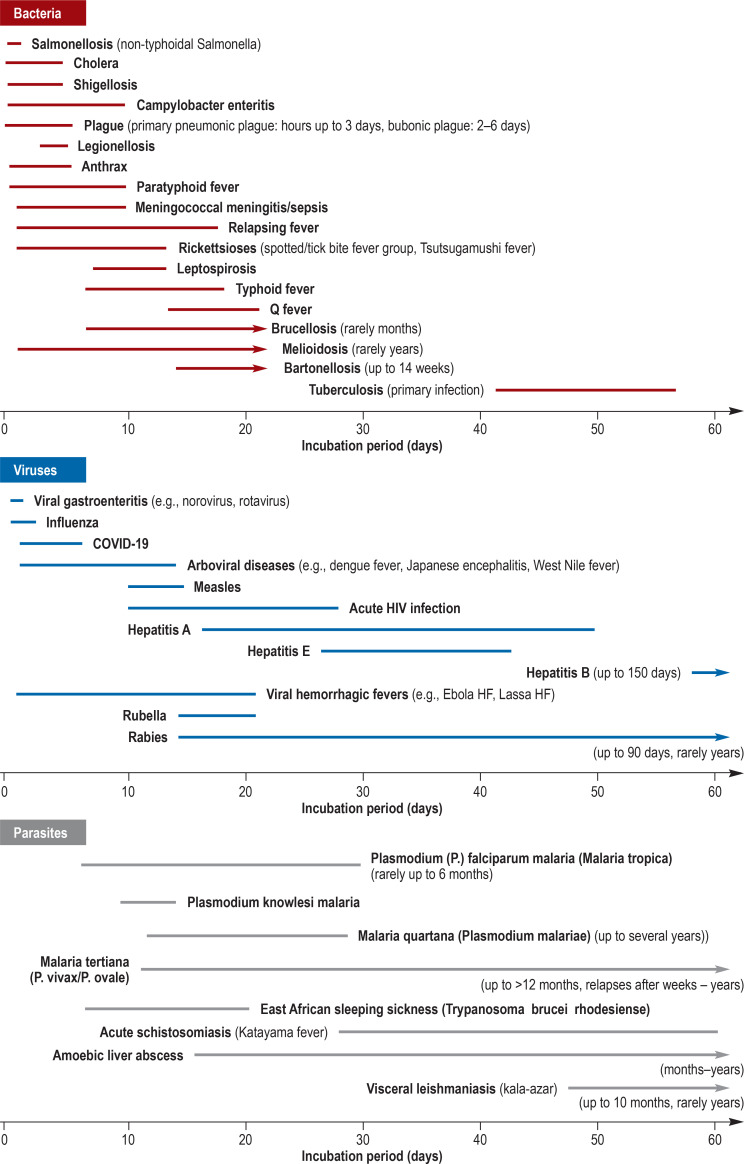
Table 1. Incubation periods of febrile travel-related infectious diseases in detail, adapted from (21, e5– e7).
| Incubation period | Bacteria | Viruses | Parasites |
| <14 days | ● Anthrax: 1–6 d ● Bacterial diarrhea: – Campylobacteriosis: 1–10 d – Salmonellosis (NTS): 12–48 hrs – Shigellosis: 1–5 d – Cholera: few hrs – 5 d ● Legionellosis: 5–6 d ● Leptospirosis: 7–12 d ● Melioidosis 2–21 d (rarely mths to yrs) ● Meningococcal meningitis/sepsis: 2–10 d ● Plaque: Bubonic plague: 2–6 d – Primary pneumonic plague: hrs to 2–3 d ● Rickettsioses (spotted fever and tick-bite fever group, Tsutsugamushi fever): 2–14 d ● Relapsing fever: 7 d (2–18 d) ● Typhoid fever: 7–18 d ● Paratyphoid fever: 1–10 d |
● Arboviral disease (dengue fever, Chikungunya fever, Zika fever, yellow fever, Japanese encephalitis, Rift Valley fever, West Nile fever): 1–14 d ● Measles: 10–14 d ● Rabies: rarely <14 d ● Viral hemorrhagic fevers (Ebola HF, Lassa HF, Marburg HF, Crimean–Congo HF, yellow fever): 2–21 d ● Viral diseases of the respiratory system: Influenza: 1–3 d, SARS-CoV-2 infection/COVID-19: 5–6 d ● Viral gastroenteritis (e.g., norovirus, rotavirus): 1–2 d |
● Malaria: Plasmodium (P.) falciparum: 6–30 d, P. knowlesi: 10–14 d ● East African trypanosomiasis (sleeping sickness): 7–21 d |
| 14 days to 6 weeks | ● Bartonellosis: 3 wks (2–14 mths) ● Brucellosis: 1–3 wks (to several mths) ● Melioidosis: 2–21 d (to several years) ● Q fever: 2–3 wks ● Typhoid fever: 7–18 d |
● Acute HIV infection: 10–28 d ● Hepatitis A: 15–50 d (usually 25–30 d) ● Hepatitis E: 26–42 d ● Rubella: 14–21 d ● Rabies: 20–90 d |
● Malaria: P. falciparum: 6–30 d, P. vivax/ovale: 12 d –12 Mo, P. malariae: 13–28 d ● Acute schistosomiasis (Katayama fever): 28–60 d |
| >6 weeks | ● Tuberculosis: 6–8 wks (primary infection), usually asymptomatic | ● Hepatitis B: 60–150 d ● Rabies: rarely up to years |
● Malaria: P. vivax/ovale: up to 12 mths ● Amoebic liver abscess: wks to mths ● Visceral leishmaniasis: 2–10 mths ● West African trypanosomiasis: wks to mths |
d, days; HF, hemorrhagic fever; hrs, hours; mths, months; NTS, non-typhoidal Salmonella; wks, weeks
eFigure 1.
Incubation periods of febrile, travel-related infectious diseases, adapted from (21, e57-e7)
Physical examination of all body systems should be performed with special attention to instability signs, skin and mucosal findings, including the anogenital region, ophthalmological findings (e.g., conjunctivitis, bleeding), swelling of lymph nodes and enlargement of the liver and spleen, as mentioned under step 1. The guiding findings obtained allow for further differentiation (table 2). Approximately 20% of febrile returning travelers experience undifferentiated fever (fever without concomitant symptoms other than headache, muscle and joint aches, fatigue) (29, 31). In 14–35% of the cases with undifferentiated fever, malaria is the underlying condition; in the subgroup of travelers from sub-Saharan Africa, this proportion is even higher (32–62%) (29). Other tropical disease with undifferentiated fever include dengue fever, typhoid/paratyphoid fever and rickettsioses (21, 28, 29). For some rickettsioses (e.g., African tick bite fever), a thorough inspection for a necrotic skin lesion (so-called eschar or “tache noire“, eFigure) is warranted.
Table 2. Symptom-based overview of travel-related infectious fever syndromes, adapted from (21, e5, e8).
| Fever syndrome | Common pathogens |
| Fever without local symptoms | ● Malaria (especially sub-Saharan Africa) ● Dengue fever (especially Southeast Asia, South and Central America) ● Typhoid and paratyphoid fever (especially Indian subcontinent) ● Rickettsioses (especially sub-Saharan Africa) |
|
Fever and rash (exanthema) |
● Arboviral diseases (especially dengue, Chikungunya, Zika, and West Nile fever) ● Viral HF, e.g. Ebola HF, Marburg HF ● Measles, rubella, chickenpox, herpes zoster generalisatus (worldwide) ● Acute HIV, EBV, CMV infection (mononucleosis syndrome) ● Rickettsioses (eschar, rash); relapsing fever (especially tick-borne relapsing fever) ● Typhoid fever (rose spots), syphilis (stage II) ● African trypanosomiasis; acute schistosomiasis (urticaria) ● Strongyloidiasis (urticaria, larva currens), trichinellosis (urticaria) |
| Fever and respiratory symptoms | ● Influenza, COVID-19 (SARS-CoV-2) (worldwide) ● MERS (MERS-CoV) (Arabian Peninsula) ● Legionellosis (e.g., after cruise trip) ● Melioidosis (especially Southeast Asia) ● Tuberculosis (VFR, HCW), Q fever, psittacosis, leptospirosis ● Acute histoplasmosis and coccidioidomycosis ● Löffler syndrome and tropical pulmonary eosinophilia (helminth infection) |
| Fever and diarrhea | ● Traveler‘s diarrhea, especially due to infection with ETEC, EAEC, EPEC, campylobacteriosis, salmonellosis, shigellosis, very rarely cholera ● Norovirus, rotavirus enteritis (e.g., outbreaks on cruise ships) ● Lambliasis/giardiasis, cryptosporidiosis (watery diarrhea) ● Amoebic colitis (bloody diarrhea) ● Malaria (in approx. 30% of cases watery diarrhea) ● Typhoid fever (diarrhea starting week 2–3 of the disease) |
| Fever and abdominal pain | ● Typhoid/paratyphoid fever ● Lambliasis/giardiasis, cryptosporidiosis (upper abdominal pain, meteorism, nausea) ● Pyogenic liver abscess or amebic liver abscess (hepatomegaly) ● Brucellosis, malaria, visceral leishmaniosis (kala-azar), infectious mononucleosis (splenomegaly) ● Relapsing fever (hepatosplenomegaly) |
| Fever and icterus | ● Severe malaria (hemolysis), hepatitis A-E ● Viral HF (yellow fever, Crimean–Congo HF, Rift Valley fever, severe dengue fever) ● Leptospirosis (Weil‘s disease); bartonellosis (Oroya fever), HUS (e.g., EHEC) ● Acute cholangitis (e.g., liver fluke infection) |
| Fever and hepatitis | ● Hepatitis A-E, dengue fever ● Acute CMV, EBV, HIV, toxoplasma gondii infection (mononucleosis syndrome) ● Q fever, brucellosis, leptospirosis |
| Fever and neurological symptoms | ● Bacterial meningitis (e.g., meningococcal, TB), typhoid/paratyphoid fever, neurosyphilis ● Viral encephalitis (e.g., due to HSV, VZV, JE, WNV, dengue virus, rabies virus, measles, enteroviruses) ● Cerebral malaria, African trypanosomiasis (sleeping sickness) ● Angiostrongyliasis, gnathostomiasis (eosinophilic meningitis) |
| Fever and signs of bleeding (purpura) | ● Sepsis (meningococcal, staphylococcal, streptococcal), rickettsioses, leptospirosis, plague ● HF (e.g., Crimean-Congo HF, Ebola HF, Marburg HF, dengue HF, yellow fever) |
| Fever and eosinophilia | ● Helminth infection, especially acute schistosomiasis, ascariasis, ancylostomiasis (hook worms), strongyloidiasis (Strongyloides stercoralis), fascioliasis (Fasciola hepatica), visceral larva migrans (toxocariasis), filariasis, trichinellosis |
| Fever and arthralgia or myalgia | ● Chikungunya fever, dengue fever, Zika fever, Ross River fever (Australia), muscular sarcocystosis (Southeast Asia), trichinellosis |
CMV, cytomegalovirus; EAEC, enteroaggregative E. coli; EBV, Epstein-Barr virus; EHEC, enterohemorrhagic E. coli; EPEC, enteropathogenic E. coli; ETEC, enterotoxigenic E. coli;
HCW, health care worker; HF, hemorrhagic fever; HIV, human immunodeficiency virus; HSV, herpes simplex virus; HUS, hemolytic uremic syndrome; JE, Japanese encephalitis;
MERS-CoV, Middle East Respiratory Syndrome coronavirus; SARS-CoV-2, Severe Acute Respiratory Syndromecoronavirus type 2; TB, tuberculosis; VFR, visiting friends and relatives; VZV, varicella zoster virus; WNV, West Nile virus
History focused on exposure risk
The travel history comprises information about all countries and regions of countries travelled (including transit countries), mode and duration of travel, reason for travel, activities (including sexual and drug history), contacts with animals/sick persons/health facilities, accommodations, food, potentially sick fellow travelers, and preventive measures (including travel vaccinations, vector protection, malaria chemoprophylaxis).
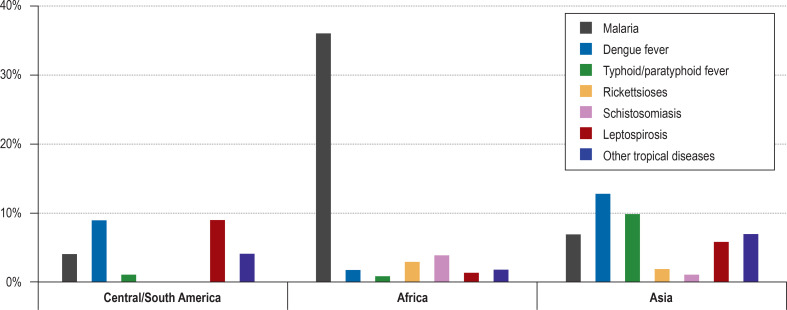
With the information about the geographical exposure (Table 3, eFigure 2), the differential diagnosis can be further narrowed down. For example, malaria is by far the most common specific diagnosis among travelers returning from sub-Saharan Africa, while among travelers to Asia dengue fever and typhoid fever and to South America dengue fever and leptospirosis are the predominant diagnoses (5, 13). As a rule, the longer the duration of the travel (i.e. with increasing risk of exposure), the greater the risk of contracting an infectious disease (26). The reason for travel, too, has an impact on the evaluation of differential diagnoses: According to a prospective, monocentric study from Antwerpen, P. falciparum malaria was significantly more common among returning travelers who travelled abroad for work (38%), migrants (26%) and VFRs (travelers visiting friends and relatives, migrants or their offspring visiting friends/family in their country of origin) (26%) compared to tourists (14%) (p<0,001, n = 1743), whereas rickettsioses, dengue fever and acute schistosomiasis were almost exclusively diagnosed in tourists and returning travelers who had been abroad for work (31).
Table 3. Overview of infection-related risk exposure, adapted from (21, e9, e10).
| Exposure/risk factor | Infections/ pathogens to consider |
|
Insect bites: – Mosquitoes |
Malaria, arboviral diseases (e.g. dengue fever, West Nile fever), filariasis, leishmaniasis |
| – Flies | African trypanosomiasis (tsetse fly, painful) |
| – Deer flies (Central/West Africa) | Loiasis (painful bites of the genus Chrysops) |
| – Ticks | Rickettsioses, tick-borne relapsing fever, Crimean-Congo HF, tularemia, Q fever, tick-borne encephalitis (TBE), babesiosis, Lyme disease |
| – Mites (South/Southeast Asia, Pacific) | Tsutsugamushi fever (Orientsia tsutsugamushi) |
| – Flees | Murine spotted fever (Rickettsia typhi), flea-borne spotted fever (Rickettsia felis), plague (Yersinia pestis) |
| – Triatomine bugs (South/Central America) | American trypanosomiasis (Chagas disease) |
| – Lice | Spotted fever (R. prowazekii), louse-borne relapsing fever (Borrelia recurrentis), Wolhynia (trench) fever (Bartonella quintana) |
| Skin contact with soil/sand | Cutaneous larva migrans, ancylostomiasis (hook worms), strongyloidiasis (Strongyloides stercoralis), melioidosis (Burkholderia pseudomallei) |
| Tattoos, intravenous drug abuse, transfusions | HIV infection, hepatitis B/C, CMV infection, WNV infection, malaria, babesiosis, leishmaniasis |
| Unprotected sexual intercourse | Acute HIV, HSV, EBV, CMV infection, Zika fever, hepatitis A/B/C, syphilis, gonorrhea, chlamydial infection, lymphogranuloma venereum |
| Freshwater contact(e.g., rafting, swimming) | Schistosomiasis, leptospirosis, infection with Aeromonas spp., melioidosis, atypical mycobacteriosis, legionellosis (inhalation), amebic infections |
| Saltwater contact | Infection with Vibrio vulnificus, Aeromonas spp., Mycobacterium marinum (swimming pool granuloma), Erysipelothrix rhusiopathiae (erysipeloid) |
| Animal bites | Rabies, cat scratch disease, rat-bite fever, sporotrichosis |
| Close animal contact | Q fever, anthrax, psittacosis, tularemia, avian influenza, MERS, Lassa HF, Nipah virus encephalitis, echinococcosis, brucellosis, other viral HF |
| Visiting friends and relatives (VFR) | Malaria, typhoid fever, tuberculosis, hepatitis A, meningococcal meningitis, Ebola HF |
| Cave visits (especially in the Americas) | Histoplasmosis, rabies (bat bite/scratch) |
| Safari in East/South Africa | African tick bite fever, African trypanosomiasis (sleeping sickness) |
|
Consumption of – Unclean water |
Lambliasis/giardiasis, cryptosporidiosis, amebiasis, hepatitis A/E, typhoid fever |
| – Raw food (unwashed) | Helminthiases (Ascaris, Trichuris, Echinococcus, Fasciola), typhoid/paratyphoid fever, hepatitis A/E |
| – Raw milk/raw milk products | Listeriosis, brucellosis, bovine tuberculosis, yersiniosis, salmonellosis |
| – Raw meat | Salmonellosis, campylobacteriosis, yersiniosis, Clostridium perfringens infection, hemolytic uremic syndrome (due to EHEC), hepatitis A/E, sarcocystosis, toxoplasmosis, trichinellosis, taeniasis |
| – Raw fish/seafood | Gnathostomiasis, infections with Vibrio vulnificus, Aeromonas spp. and Shewanella spp., hepatitis A/E, norovirus enteritis, opisthorchiasis/clonorchiasis (liver flukes), intoxication (e.g. ciguatera) |
| – Snails/crabs (Southeast Asia, pacific) | Eosinophilic meningitis (Angiostrongylus cantonensis), paragonimiasis |
CMV, cytomegalovirus; EBV, Epstein-Barr virus; EHEC, enterohemorrhagic Escherichia coli; TBE, tick-borne encephalitis; HF, hemorrhagic fever;
HIV, human immunodeficiency virus; HSV, herpes simplex virus; MERS, Middle East Respiratory Syndrome; VFR, visiting friends and relatives; WNV, West Nile virus
eFigure 2.
Frequency of occurrence of tropical diseases in the febrile returning traveler by travel region, adapted from (13)
Targeted questions should be asked to identify disease-specific risk factors related to activities, food and contact with animals or people abroad (table 3). Freshwater exposure may be indicative of schistosomiasis (note endemic areas) or leptospirosis (12), sexual contacts or percutaneous exposure (tattoos, piercings, intravenous application of drugs/medications) of acute HIV or hepatitis B virus infections (12, 21, 29). If a patient denies exposure and reports having taken preventive measures, this should not lead to immediate exclusion of certain diagnoses: for example, some travelers may not have noticed insect bites; likewise, travel vaccinations and malaria chemoprophylaxis are not 100% effective. The protective efficacy of parenteral typhoid Vi capsular polysaccharide vaccines, for example, is only 69% in the first year (systematic review, three studies, n = 99 979) (32) and the efficacy of malaria chemoprophylaxis with atovaquone/proguanil is 95.8% (meta-analysis, ten RCTs, n = 4539) (33). Besides travel-related information, information about present and past illnesses, long-term medication and PRN medication as well as the possibility of pregnancy should be obtained. Special attention should be paid to (iatrogenic) immunodeficiency, as it may be associated with an increased risk of infection/complications of infection (21, 26, 34).
Note cosmopolitan diseases
In the traveler returning from the tropics/subtropics, non-tropical cosmopolitan diseases are sometimes overlooked, a phenomenon which is explained to some extent by so-called anchoring bias: The diagnostic assessment is “anchored” to certain pieces of information, such as the recent travel, and misses other/new facts (35). The share of febrile cosmopolitan infectious diseases after stay in the tropics/subtropics is actually about 30% (13). Therefore, autochthonous infectious diseases with “typical“ spectrum of pathogens should also be taken into account: bacterial pneumonia (e.g., Streptococcus pneumoniae, Legionella, Mycoplasma), urinary tract infections (e.g., Enterobacterales), meningitis (e.g., Neisseria meningitidis, Streptococcus pneumoniae, Listeria), endocarditis (e.g., Staphylococcus, Streptococcus, Enterococcus), systemic viral infections (e.g., cytomegalovirus, Epstein-Barr virus [EBV], human herpes virus-6), and bacterial or viral gastroenteritis (e.g., adenovirus, norovirus, nontyphoidal Salmonella, Campylobacter, Clostridioides difficile) (21, 28, 29, 36).
Finally, globally distributed infectious diseases with a high transmission rate (e.g., COVID-19, measles, varicella, influenza, tuberculosis) should always be considered, as the incidence rates of some of these pathogens are significantly higher in non-European countries (21). In addition, among returning travelers non-infectious causes of fever are also relevant: Fever of unknown origin is the initial presentation in 2% to 25% of all cancers (e.g., lymphoma) as well as 5% to 32% of all autoimmune diseases (e.g., rheumatoid arthritis, giant-cell arteritis) and hereditary febrile syndromes (e.g. familial Mediterranean fever) (36). Likewise, febrile episodes in the general population are related to medicines in 3% to 7% of cases (drug-induced fever) (36). In patients with drug rash with eosinophilia and systemic symptoms (DRESS), eosinophilia and rash can misdirect the diagnostic evaluation, especially in returning travelers (for example, putative parasitic disease or exanthematous flavivirus infection). Furthermore, travel-related venous thrombosis/thromboembolism (incidence rate for flights >4 hours: <0.01%; >8 hours: 0.5%) is also a potential cause of fever (37).
International travel is associated with an increased risk of temporary colonization with multidrug-resistant bacteria (MDRB) (38– 40, e1). A German study (38) and a Finnish study (e1) on intestinal colonization with extended-spectrum beta-lactamase (ESBL)-producing Enterobacterales after overseas travel found colonization rates of about 30%, with the risk of colonization depending on the region of travel (highest for India, 73%) and showing a positive correlation with traveler‘s diarrhea and antibiotic treatment (38, e1). Likewise, hospitalization abroad is a major risk factor for MDRB colonization (e2). Consequently, the MDRB risk should be taken into account in the evaluation of the febrile returning traveler, both when selecting an empirical antibiotic treatment and with regard to the diagnostic workup and necessary hospital hygiene procedures (39, 40, e1-e4).
Paraclinical diagnosis
As a general rule, a definite diagnosis or a well-founded tentative diagnosis should precede the initiation of a specific treatment. Only in a few cases, it is possible to make a visual diagnosis (e.g., African tick bite fever: eschar and fever after a trip to South Africa, memory of a tick bite). Therefore, additional investigations are usually required, and should have a favorable cost-benefit ratio.
Basic testing should at least include differential blood count, serum electrolytes, renal function tests (creatinine, urea), liver function tests (aspartate aminotransferase [ASAT], alanine aminotransferase [ALAT]; gamma-glutamyl transferase [GGT]), coagulation status and markers of inflammation (e.g., C-reactive protein). Depending on the clinical presentation and the suspected diagnosis, this standard laboratory workup is expanded by adding, for example, hemolysis tests (suspected malaria), muscle enzyme tests (suspected trichinellosis) or further special analyzes. Eosinophilia may indicate invasive parasitosis (e.g., strongyloidiasis, filariasis); a low eosinophil count may be indicative of typhoid fever, if the clinical presentation is suggestive of the disease.
Many infectious disease can only be detected once a pathogen-specific incubation period or prepatent period (period between parasitic infection and appearance of larvae/eggs) has ended or after production of antibodies. Thus, performing specific diagnostic tests too early (or too late) may lead to false negative results. It may be necessary to repeat the testing (e.g., in patients with suspected malaria every 24 hours; if required, repetitively over a period of several days). The disadvantage of testing for antibodies is that pathogens can only be detected indirectly and false-positive results may occur (e.g., due to polyclonal B-cell stimulation triggered by EBV infection or due to cross-reaction of related pathogens). Antibody specificity can be increased by follow-up testing or measuring avidity (the strength of the interactions between the antigen and antibody—increases over the course of the immune response). Serology results should always be interpreted in context.
In general, direct testing (e.g., antigen tests, polymerase chain reaction [PCR], culture, microscopy) has a higher specificity. Serological tests are utterly unsuitable for the acute diagnosis of diseases such as malaria and typhoid fever: The gold standard of malaria diagnosis remains the direct microscopic visualization of Plasmodium parasites in the stained “thick drop“/blood smear (efigure) with determination of the percentage parasite load, complemented by rapid diagnostic testing [RDT]); Salmonella Typhi bacteria can be cultured initially by blood culture and from the second week onwards from urine and stool samples. In the case of culture-based testing with resistance analysis, it must be borne in mind that it takes several days before results become available and indicated treatments (e.g., empirical administration of antibiotics in patients with suspected typhoid fever) should not be delayed. Sampling for blood culture and malaria diagnosis can be performed whether or not the patient has fever. The possibility of double infections must also be taken into account so that relevant coinfections can simultaneously be ruled out (e.g., if dengue fever is suspected, additional malaria testing should be performed in travelers returning from endemic areas).
Further diagnostic workup and management
In about 20% of febrile returning travelers, the cause of the fever cannot be identified despite comprehensive diagnostic evaluation (13). The reasons are many: Examples include low sensitivity of the diagnostic test, post-infectious condition and misinterpretation of available findings. In some cases, it may be helpful to repeat examinations or choose an alternative diagnostic test (e.g., PCR testing in malaria patients with low-level parasitemia). In most cases, the condition is self-limiting and benign, requiring only pragmatic management (e.g., regular follow-up visits and repetition of the basic diagnostic evaluation until all symptoms have resolved). If in doubt, a specialized center should promptly be contacted in case symptoms persist, the patient‘s condition deteriorates or new symptoms appear (ebox 2).
eBOX 1. Special considerations in children, pregnant women and immunocompromised individuals (selection).
-
Children
In children, P. falciparum malaria is more commonly associated with severe anemia and seizures, while in adults renal failure and respiratory failure are more frequently observed complications.
Pediatric typhoid and paratyphoid cases more often present with diarrhea and vomiting; in addition, infants may have concomitant meningitis
Higher risk of insect bites
Risk of animal bites increased in children
Sexually transmitted diseases significantly less common compared to adults
Limitations to use of malaria chemoprophylaxis
-
Pregnant women
Increased risk of severe disease in P. falciparum malaria
Zika virus infection during pregnancy can result in significant damage to the brain and eyes of the developing fetus (congenital Zika syndrome with microcephaly, retinopathy, neurological damages).
Risk of transplacental transmission of pathogens to the unborn child (e.g., toxoplasmosis, listeriosis, Chagas disease) with infection-related miscarriage or preterm birth
Limitations to use of malaria chemoprophylaxis
-
Immunocompromised individuals
Increased risk of severe and clinically atypical courses in a variety of diseases (e.g. herpes virus infections, strongyloidiasis, visceral leishmaniasis)
Limited immune response after vaccinations
Limited sensitivity of infectious serology testing
Questions on the article in issue 22–23/2023:
Fever in the Returning Traveler
The submission deadline is 6 June 2023. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
What is the most common cause of fever in travelers returning from South East Asia who present at centers for infectious diseases and tropical medicine?
Zika fever
Paratyphoid fever
Leptospirosis
Visceral leishmaniasis
Dengue fever
Question 2
Among travelers returning from which region, P. falciparum malaria is the predominant cause of presentation for fever at centers for infectious diseases and tropical medicine?
Sub-Saharan Africa
Southeast Asia
South America
Spain
Australia
Question 3
Approximately how high is the overall lethality in febrile returning travelers and migrants according to a systematic review?
0.11%
0.22%
3.33%
10.1%
15.5%
Question 4
What diseases can usually be ruled out if symptoms begin at least 21 days after the presumed last exposure?
Malaria and amoebic liver abscess
HIV and brucellosis
Hepatitis B and malaria
Viral hemorrhagic fevers and dengue fever
Visceral leishmaniasis and HIV
Question 5
With what type of travel is P. falciparum malaria most common?
Professional travel
Migration
Visiting relatives in the country of origin
Tourist travel
Visiting friends in the country of origin
Question 6
What is the efficacy of malaria chemoprophylaxis with atovaquone/proguanil according to a meta-analysis?
approx. 47%
approx. 55%
approx. 68%
approx. 81%
approx. 96%
Question 7
For which infection is the consumption of raw milk and/or raw milk products a special risk factor?
Rabies
Amebic infection
Histoplasmosis
Hepatitis A/E
Listeriosis
Question 8
What is the meaning of the term “prepatent period“?
Period between parasitic infection and onset of symptoms
Period between bacterial infection and start of the formation of specific antibodies
Period between parasitic infection and appearance of larvae/eggs
Period between symptom onset after an infection and formation of first specific antibodies
Period between parasitic infection and complete recovery
Question 9
In what percentage of returning travelers with fever can no cause be found despite extensive diagnostic workup?
approx. 5%
approx. 10%
approx. 20%
approx. 40%
approx. 80%
Question 10
What is the term for the type of bias in which a diagnosis is heavily based on certain information, such as a recent travel, and other/new facts are overlooked?
Reporting bias
Tunnel bias
Focusing bias
Anchoring bias
Attrition bias
Acknowledgments
Translated from the original German by Ralf Thoene, MD.
Footnotes
Conflict of interest statement The authors declare that no conflict of interest exists.
References
- 1.United Nations World Tourism Organization (UNWTO) Statistics. www.unwto.org/statistics (last accessed on 28 March 2022) [Google Scholar]
- 2.Wendt S, Beier D, Paquet D, et al. Medical advice for travelers. Dtsch Arztebl Int. 2021;118:349–356. doi: 10.3238/arztebl.m2021.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelo KM, Kozarsky PE, Ryan ET, et al. What proportion of international travellers acquire a travel-related illness? A review of the literature. J Travel Med. 2017;24 doi: 10.1093/jtm/tax046. tax046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fink D, Wani RS, Johnston V. Fever in the returning traveller. BMJ. 2018;360 doi: 10.1136/bmj.j5773. j5773. [DOI] [PubMed] [Google Scholar]
- 5.Grobusch MP, Weld L, Goorhuis A, et al. Travel-related infections presenting in Europe: a 20-year analysis of EuroTravNet surveillance data. Lancet Reg Health Eur. 2020;1 doi: 10.1016/j.lanepe.2020.100001. 100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osman A, Preet R. Dengue, chikungunya and zika in GeoSentinel surveillance of international travellers: a literature review from 1995 to 2020. J Travel Med. 27 doi: 10.1093/jtm/taaa222. taaa222. [DOI] [PubMed] [Google Scholar]
- 7.Robert Koch-Institut (RKI) Infektionsepidemiologisches Jahrbuch meldepflichtiger Krankheiten für 2017. www.rki.de/DE/Content/Infekt/Jahrbuch/Jahrbuch_2017.html (last accessed on 28 March 2022) [Google Scholar]
- 8.Robert Koch-Institut (RKI) Infektionsepidemiologisches Jahrbuch meldepflichtiger Krankheiten für 2018. www.rki.de/DE/Content/Infekt/Jahrbuch/Jahrbuch_2018.html (last accessed on 28 March 2022) [Google Scholar]
- 9.Robert Koch-Institut (RKI) Infektionsepidemiologisches Jahrbuch meldepflichtiger Krankheiten für 2019. www.rki.de/DE/Content/Infekt/Jahrbuch/Jahrbuch_2019.html (last accessed on 28 March 2022) [Google Scholar]
- 10.Falkenhorst G, Enkelmann J, Frank C, Stark K. Zur Situation bei wichtigen Infektionskrankheiten - Reiseassoziierte Krankheiten 2020. Epid Bull. 2021;48:42–43. [Google Scholar]
- 11.Norman FF, Chamorro-Tojeiro S, Crespillo-Andújar C, et al. Travel-related fever in the time of COVID-19 travel restrictions. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa104. taaa104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Acremont V, Ambresin AE, Burnand B, Genton B. Practice guidelines for evaluation of fever in returning travelers and migrants. J Travel Med. 2003;10(Suppl 2):S25–S52. doi: 10.2310/7060.2003.35132. [DOI] [PubMed] [Google Scholar]
- 13.Buss I, Genton B, D’Acremont V. Aetiology of fever in returning travellers and migrants: a systematic review and meta-analysis. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa207. taaa207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensenius M, Han PV, Schlagenhauf P, et al. Acute and potentially life-threatening tropical diseases in western travelers–a GeoSentinel multicenter study, 1996-2011. Am J Trop Med Hyg. 2013;88:397–404. doi: 10.4269/ajtmh.12-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semenza JC, Paz S. Climate change and infectious disease in Europe: impact, projection and adaptation. Lancet Reg Health Eur. 2021;9 doi: 10.1016/j.lanepe.2021.100230. 100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robert Koch-Institut (RKI) Infektionsepidemiologisches Jahrbuch meldepflichtiger Krankheiten für 2020. www.rki.de/DE/Content/Infekt/Jahrbuch/Jahrbuch_2020.html (last accessed on 28 March 2022) [Google Scholar]
- 17.Burchard GD. Import seltener, aber lebensbedrohlicher und hochansteckender Erreger: Heutige Situation und Ausblick. Internist. 2015;56:1149–1161. doi: 10.1007/s00108-015-3776-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robert Koch-Institut. RKI-Ratgeber Lassafieber. www.rki.de/DE/Content/Infekt/EpidBull/Merkblaetter/Ratgeber_Lassa-Fieber.html (last accessed on 28 March 2022) [Google Scholar]
- 19.Wolf T, Ellwanger R, Goetsch U, et al. Fifty years of imported Lassa fever: a systematic review of primary and secondary cases. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa035. taaa035. [DOI] [PubMed] [Google Scholar]
- 20.Ehlkes L, Kreuels B, Schwarz NG, May J. Epidemiologie des Ebolafiebers und anderer, in Deutschland seltener hochkontagiöser, lebensbedrohlicher Erkrankungen. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2015;58:705–713. doi: 10.1007/s00103-015-2165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thwaites GE, Day NPJ. Approach to fever in the returning traveler. N Engl J Med. 2017;376:548–560. doi: 10.1056/NEJMra1508435. [DOI] [PubMed] [Google Scholar]
- 22.Robert Koch-Institut. Rahmenkonzept Ebolafieber: Vorbereitungen auf Maßnahmen in Deutschland. www.rki.de/DE/Content/InfAZ/E/Ebola/Rahmenkonzept_Ebola.pdf (last accessed on 28 March 2022) [Google Scholar]
- 23.Robert Koch-Institut. Informationen des RKI zum MERS-Coronavirus. www.rki.de/DE/Content/InfAZ/M/MERS_Coronavirus/MERS-CoV.html (last accessed on 28 March 2022) [Google Scholar]
- 24.Robert Koch-Institut. RKI Ratgeber Pest. https://www.rki.de/DE/Content/Infekt/EpidBull/Merkblaetter/Ratgeber_Pest.html (last accessed on 28 March 2022) [Google Scholar]
- 25.Brunkhorst FM, Weigand MA, Pletz MW, et al. S3-Leitlinie Sepsis - Prävention, Diagnose, Therapie und Nachsorge. Med Klin Intensivmed Notfmed. 2020;115:37–109. doi: 10.1007/s00063-020-00685-0. [DOI] [PubMed] [Google Scholar]
- 26.Wu HM. Evaluation of the sick returned traveler. Semin Diagn Pathol. 2019;36:197–202. doi: 10.1053/j.semdp.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz MD. Fever in the returning traveler, part one: a methodological approach to initial evaluation. Wilderness Environ Med. 2003;14:24–32. doi: 10.1580/1080-6032(2003)014[0024:fitrtp]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Burchard G. Fieber bei Reiserückkehrern. Internist. 2014;55:274–279. doi: 10.1007/s00108-013-3366-9. [DOI] [PubMed] [Google Scholar]
- 29.Kotlyar S, Rice BT. Fever in the returning traveler. Emerg Med Clin North Am. 2013;31:927–944. doi: 10.1016/j.emc.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deutsche Gesellschaft für Tropenmedizin, Reisemedizin und Globale Gesundheit (DTG) Diagnostik und Therapie der Malaria: Registernummer 042-001. www.awmf.org/uploads/tx_szleitlinien/042-001l_S1_Diagnostik-Therapie-Malaria_2021-08.pdf (last accessed on 28 March 2022) [Google Scholar]
- 31.Bottieau E, Clerinx J, Schrooten W, et al. Etiology and outcome of fever after a stay in the tropics. Arch Intern Med. 2006;166:1642–1648. doi: 10.1001/archinte.166.15.1642. [DOI] [PubMed] [Google Scholar]
- 32.Milligan R, Paul M, Richardson M, Neuberger A. Vaccines for preventing typhoid fever. Cochrane Database Syst Rev. 2018;5 doi: 10.1002/14651858.CD001261.pub4. CD001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakato H, Vivancos R, Hunter PR. A systematic review and meta-analysis of the effectiveness and safety of atovaquone proguanil (Malarone) for chemoprophylaxis against malaria. J Antimicrob Chemother. 2007;60:929–936. doi: 10.1093/jac/dkm337. [DOI] [PubMed] [Google Scholar]
- 34.Rello J, Manuel O, Eggimann P, et al. Management of infections in critically ill returning travellers in the intensive care unit-II: clinical syndromes and special considerations in immunocompromised patients. Int J Infect Dis. 2016;48:104–112. doi: 10.1016/j.ijid.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gäbler M. Denkfehler bei diagnostischen Entscheidungen. Wien Med Wochenschr. 2017;167:333–342. doi: 10.1007/s10354-017-0570-6. [DOI] [PubMed] [Google Scholar]
- 36.Haidar G, Singh N. Fever of unknown origin. N Engl J Med. 2022;386:463–477. doi: 10.1056/NEJMra2111003. [DOI] [PubMed] [Google Scholar]
- 37.Watson HG, Baglin TP. Guidelines on travel-related venous thrombosis. Br J Haematol. 2011;152:31–34. doi: 10.1111/j.1365-2141.2010.08408.x. [DOI] [PubMed] [Google Scholar]
- 38.Lübbert C, Straube L, Stein C, et al. Colonization with extended-spectrum beta-lactamase-producing and carbapenemase-producing enterobacteriaceae in international travelers returning to Germany. Int J Med Microbiol. 2015;305:148–156. doi: 10.1016/j.ijmm.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Leblebicioglu H, Rodriguez-Morales AJ, Rossolini GM, et al. Management of infections in critically ill returning travellers in the intensive care unit-I: considerations on infection control and transmission of resistance. Int J Infect Dis. 2016;48:113–117. doi: 10.1016/j.ijid.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz KL, Morris SK. Travel and the spread of drug-resistant bacteria. Curr Infect Dis Rep. 2018;20 doi: 10.1007/s11908-018-0634-9. [DOI] [PubMed] [Google Scholar]
- E1.Kantele A, Lääveri T, Mero S, et al. Antimicrobials increase travelers’ risk of colonization by extended-spectrum betalactamase-producing enterobacteriaceae. Clin Infect Dis. 2015;60:837–846. doi: 10.1093/cid/ciu957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Khawaja T, Kirveskari J, Johansson S, et al. Patients hospitalized abroad as importers of multiresistant bacteria - a cross-sectional study. Clin Microbiol Infect. 2017;23 doi: 10.1016/j.cmi.2017.02.003. 673.e1-673.e8. [DOI] [PubMed] [Google Scholar]
- E3.Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) Hygienemaßnahmen bei Infektionen oder Besiedlung mit multiresistenten gramnegativen Stäbchen: Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) beim Robert Koch-Institut (RKI) Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55:1311–1354. doi: 10.1007/s00103-012-1549-5. [DOI] [PubMed] [Google Scholar]
- E4.Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) Infektionsprävention im Rahmen der Pflege und Behandlung von Patienten mit übertragbaren Krankheiten: Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) beim Robert Koch-Institut. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2015;58:1151–1170. doi: 10.1007/s00103-015-2234-2. [DOI] [PubMed] [Google Scholar]
- E5.Centers for Disease Control and Prevention (CDC) Yellow Book 2020: Health information for international travel. Oxford: Oxford University Press. 2020 [Google Scholar]
- E6.Bennett JE, Dolin R, Blaser MJ (eds.) Elsevier. 9. Amsterdam: 2020. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. [Google Scholar]
- E7.Farrar J, Hotez PJ, Junghanss T, et al. Saunders Elsevier. 23. London: 2013. Manson’s tropical diseases. [Google Scholar]
- E8.Johnston V, Stockley M, Dockrell D, et al. Fever in returned travellers presenting in the United Kingdom: recommendations for investigation and initial management. J Infect. 2009;59:1–18. doi: 10.1016/j.jinf.2009.05.005. [DOI] [PubMed] [Google Scholar]
- E9.Jiménez-Morillas F, Gil-Mosquera M, et al. Fever in travellers returning from the tropics. Med Clin (Barc) 2019;153:205–212. doi: 10.1016/j.medcle.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E10.Scaggs Huang FA, Schlaudecker E. Fever in the returning traveler. Infect Dis Clin North Am. 2018;32:163–188. doi: 10.1016/j.idc.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E11.Robert Koch-Institut (RKI) Maßnahmen bei Verdacht auf Ebolafieber: Orientierungshilfe für Ärztinnen und Ärzte. www.rki.de/DE/Content/InfAZ/E/Ebola/Massnahmen_Verdachtsfall_Infografik_Tab.html (last accessed on 28 March 2022) [Google Scholar]
- E12.Advisory Committee on Dangerous Pathogens. Management of Hazard Group 4 viral haemorrhagic fevers and similar human infectious diseases of high consequence. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/534002/Management_of_VHF_A.pdf (last accessed on 28 March 2022) [Google Scholar]
- E13.European Network for Diagnostics of „imported“ viral diseases case definition viral hemorrhagic fever (general) www.enivd.de/FS/fs_encdiseases.htm (last accessed on 28 March 2022) [Google Scholar]