Abstract
The gram-negative bacterium Caulobacter crescentus has a life cycle that includes two distinct and separable developmental stages, a motile swarmer phase and a sessile stalked phase. The cell cycle-controlled biogenesis of the single polar flagellum of the swarmer cell is the best-studied aspect of this developmental program. The flagellar regulon is arranged into a rigid trans-acting hierarchy of gene expression in which successful expression of early genes is required for the expression of genes that are later in the hierarchy and in which the order of gene expression mirrors the order of assembly of gene products into the completed flagellum. The flgBC-fliE genes were identified as a result of the C. crescentus genome sequencing project and encode the homologues of two flagellar proximal rod proteins, FlgB and FlgC, and one conserved protein, FliE, that is of unknown function. Footprint assays on a DNA fragment containing the operon promoter as well as in vivo mutant suppressor analysis of promoter mutations indicate that this operon is controlled by the cell cycle response regulator CtrA, which with ς70 is responsible for regulating transcription of other early flagellar genes in C. crescentus. Promoter analysis, timing of expression, and epistasis experiments place these genes outside of the flagellar regulatory hierarchy; they are expressed in class II mutants, and flgB deletions do not prevent class III gene expression. This operon is also unusual in that it is expressed from a promoter that is divergent from the class II operon containing fliP, which encodes a member of the flagellum-specific protein export apparatus.
A fundamental aspect of biology is the mechanism by which cells are able to control their progression from one stage of the cell cycle to the next in a manner that ensures that all necessary cellular functions are successfully completed. Study of the cell cycle in bacteria has proceeded slowly, primarily due to the difficulty of obtaining pure populations of cells at a particular point in the cell cycle. The gram-negative aquatic bacterium Caulobacter crescentus undergoes a life cycle which, upon division, results in two dissimilar daughter cells: a sessile stalked cell and a motile swarmer cell that possesses a single polar flagellum. The swarmer cell stage lasts for approximately one-third of the life cycle, after which the swarmer differentiates into a stalked cell by ejecting the flagellum and growing a stalk at the pole of the cell that previously harbored the flagellum. Initiation of chromosomal replication depends on this differentiation event, so that swarmer cells are unable to initiate replication. The two cell types are easily separable by differential density gradient centrifugation because stalked cells are less dense than swarmer cells, so a pure population of swarmer cells can be obtained which can be used to study cell cycle-dependent phenomena.
The aspect of the C. crescentus cell cycle that has been most thoroughly studied is the biogenesis of the single polar flagellum (reviewed in references 5, 15, 16, and 66). The flagellar regulon in C. crescentus is arranged in a complex, trans-acting hierarchy composed of approximately 50 genes (11, 12, 31) organized into four levels of gene expression, in which a complete complement of gene products from the early levels is required for proper expression of genes later in the hierarchy (6, 38, 50, 52, 69). In addition, the order of expression of the levels of the hierarchy reflects their order of assembly from the cytoplasm of the cell outward. Cell-proximal components such as inner basal body proteins are expressed before more distal components such as the flagellar hook and filament (6, 7, 17, 19, 34, 41, 46–48). The first level of the hierarchy is occupied by the cell cycle response regulator CtrA, which in response to cell cycle cues activates the transcription of early flagellar genes (55, 59, 68). Class II genes encode proteins that make up the basal body of the flagellum, including the MS ring (encoded by fliF) (29, 52), which anchors the flagellum in the inner membrane of the cell and is the first component to be assembled; the flagellum-specific protein export apparatus (including fliO, fliP, fliQ, fliR, and flhA) (20, 58, 69, 63, 71); the switch components of the flagellum (fliG, fliM, and fliN) (52, 70); and transcription factors flbD (4, 49, 52, 57, 64, 67) and rpoN (2, 5), which are required for expression of class III and IV genes. All the promoters for class II genes share a distinctive motif (20, 52, 60, 63, 71) that includes the binding site for CtrA (55, 59, 68), which along with RNA polymerase holoenzyme containing ς70 (68) is thought to be responsible for transcription of genes in this level of the hierarchy. All of the class II components must be properly expressed before transcription of the class III level of the hierarchy can commence.
The master regulator of the flagellar regulon, CtrA, apparently has multiple roles in the C. crescentus cell cycle. In response to a cell cycle cue, CtrA is thought to bind to 9-mer sequences (TTAA-N7-TTAA) at the origin and at many promoters (28, 40, 41). Binding to the origin has the effect of preventing replication initiation, while binding to the promoters of flagellar genes activates transcription. CtrA is proteolytically degraded in stalked cells following formation of the septum between two incipient daughter cells, so that following division replication can immediately commence in that cell type (10). The presence of CtrA in the swarmer cell ensures that replication is not initiated inappropriately in swarmer cells until the swarmer-to-stalked cell transition has occurred, at which time the CtrA that has remained in that cell type is degraded, thus relieving repression of DNA replication. In addition to its role in suppressing reinitiation of chromosomal replication and in flagellar gene regulation, CtrA also regulates transcription of the critical cell division gene ftsZ (33). Thus, CtrA has an important role in uniting three distinct morphological-developmental processes in C. crescentus cells.
Class III genes encode proteins which compose both the outer components of the basal body and the flagellar hook, which is a protein structure that serves as a flexible universal joint connecting the rod to the flagellar filament. In gram-negative bacteria, the rod spans the periplasm and is composed of five proteins: FlgB, FlgC, FlgF, FlgG, and FliE (1, 22, 32, 45). The distal rod, which spans most of the distance between the inner and outer membranes, is composed of FlgF and FlgG. The proximal rod connects the distal rod to the flagellar motor and consists of FlgB and FlgC. FliE is required for assembly of the proximal rod, although it is itself a protein of unknown function; it may serve as an adapter between the radially symmetric components below the rod and the helically symmetric rod components of the flagellum (43, 45).
The C. crescentus genome sequencing project was used to identify and clone the three components of the proximal rod. Here we report the timing and level of expression of flgB. Additionally, we have determined the start site of transcription of flgB and found that it is close (53 bases) to the start site of a divergent operon that contains the class II component fliP. Epistasis experiments demonstrate that the transcription of these genes does not require complete class II gene expression and that neither does class III gene expression depend on successful expression of the proximal rod genes, indicating that they lie outside the flagellar regulatory hierarchy. In addition, we show that both flgB and fliP are dependent on the transcriptional regulator CtrA for proper levels of expression.
MATERIALS AND METHODS
Bacterial strains, growth media, and plasmid constructions.
Table 1 describes the bacterial strains and plasmids used in this study. C. crescentus strains were grown at 31°C in either peptone yeast extract or M2 minimal medium containing glucose as the carbon source (30). Transcription fusions were to a promoterless Escherichia coli lacZ gene or an E. coli nptII gene, in low-copy-number plasmid placZ/290 or pLacZ-nptII/290. Plasmid subclones and reporter fusions were introduced into C. crescentus cells by mating with E. coli strain S17-1. β-Galactosidase assays were done as described previously (42), and all were conducted on strains grown at 31°C, including the temperature-sensitive ctrA401 strain. The reported values were measured in triplicate from extracts of three different cultures, grown on different days. All routine molecular biology procedures were performed as described by Ausubel et al. (3). Site-directed mutagenesis was performed as described by Kunkel and Roberts (37).
TABLE 1.
Strains and plasmids
| Strain or plasmid | Characteristics | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| S17-1 | Rp4-2; Tc::Mu Km::Tn | 62 |
| ER2566 | F−fhuA2 [lon] ompT lacZ::T7 gene1 gal sulA11 Δ(mcrC-mrr)114:IS10 R(mcr-73::mini-Tn10-TetS)2 R(zgb-210::Tn10) (Tets) endA1[dcm] | New England Biolabs |
| C. crescentus | ||
| NA1000 | syn-1000 (synchronizable derivative of CB15) | 13 |
| LS2195 | syn-1000 ctrA401 | 55 |
| SC508 | flaS153 (ΔfliQR) | 31 |
| SC1032 | flbD198::Tn5 | 57 |
| SC1055 | rpoN::Tn5 proA str-140 | 11 |
| SC1131 | fliM::Tn5 str-152 | 53 |
| SC1132 | flhA::Tn5 str-152 | 53 |
| JG3108 | ΔflgBC-fliE syn-1000 | This work |
| JG3110 | ΔflgBC-fliE flhA::Tn5 syn-1000 | This work and reference 53 |
| JG3111 | ΔflgBC-fliE fliM::Tn5 syn-1000 | This work and reference 53 |
| Plasmids | ||
| J13 | pLAFR5-derived cosmid containing flgBC-fliE operon | 20 |
| placZ290 | lacZ reporter vector, Tetr | 17 |
| placZ-nptII | Bidirectional lacZ nptII reporter vector | This work |
| flbG/placZ290 | flbG-lacZ transcriptional reporter fusion | 17 |
| flgB/placZ290 | HindIII-AflII fragment containing the dual flgBC-fliE–fliOP promoter cloned into placZ290 in the flgBC-fliE orientation | This work |
| fljL/placZ290 | fljL-lacZ transcriptional reporter fusion | 38 |
| pNPTS129 | sacB counterselection vector, Kanr | Dickon Alley |
| pMR4 | Broad-host-range cloning vector, Tetr | C. Mohr and R. Roberts |
| pJS14 | Broad-host-range cloning vector, Camr | 35 |
| pCB4.2 | pJS14 containing the 4.2-kb BamHI fragment with fliO-fliP and flgBC-fliE | This work |
| pKJH5 | envZ C terminus in pMAL-c2 (New England Biolabs) | 23 |
| ctrA/pET15b | ctrA coding sequence in pET15b (Novagen) | This work |
| pCB321 | 321-bp flgBC-fliE, fliO-fliP promoter fragment in pIC20R | 20 |
In order to clone flgBC-fliE DNA, a cosmid (J13) known to contain the DNA adjacent to the flgBC-fliE genes (20) was digested with restriction enzymes NcoI, NarI, and BssHII (Promega) in order to isolate fragments that contained flgB, flgC, and fliE, respectively. flgB resides on a 602-bp NcoI fragment, flgC resides on a 640-bp NarI fragment, and fliE resides on a 590-bp BssHII fragment. Each fragment was verified to contain the gene of interest by dideoxy chain termination sequencing. The promoter for flgB resides on a 321-bp HindIII-AflII fragment that has been previously described (20). All three genes as well as the fliO-fliP operon reside on a 4.2-kb BamHI fragment isolated from the same cosmid.
Mapping the 5′ end of the flgB transcript.
The 5′ end of the flgB transcript was mapped by an S1 nuclease protection assay. RNA was obtained from strain NA1000 grown to an optical density at 600 nm of 1.0 in M2 minimal medium. Cells were pelleted and resuspended in 20 mM sodium acetate with 1 mM EDTA (pH 5.4). Sodium dodecyl sulfate (10%) was added to make the suspension 0.4%, and then an equal volume of phenol equilibrated with 20 mM sodium acetate and 1 mM EDTA (pH 5.4) was added. The mixture was vortexed and incubated at 65°C for 10 min. This extraction was repeated twice, after which the aqueous phase was ethanol precipitated, washed twice with 70% ethanol, dried, and then dissolved in water. The 321-bp HindIII-AflII fragment containing the predicted promoter region was labeled at the HindIII site with [α-32P]ATP and the Klenow fragment of DNA polymerase. The labeled probe was gel purified and hybridized (6.0 × 104 cpm) to total cellular RNA (30 or 60 mg) for 1 h at 45°C after boiling. Following digestion with S1 nuclease (Promega), the protected fragment was phenol extracted, ethanol precipitated, and electrophoresed on an 8% denaturing polyacrylamide gel. The mobility of the protected fragment was compared to the mobility of the products of a dideoxy chain termination sequencing reaction that used a single-stranded DNA fM13 template and a primer that precisely matched the sequence of the 5′-labeled end of the S1 nuclease probe.
Construction of mutants.
Gene knockouts were accomplished by selection against an integrated mutant present on the vector pNPTS129. This vector contains a sacB gene which confers sensitivity to sucrose in the growth medium unless it is excised by homologous recombination (61). The entire flgBC-fliE region was deleted by cloning approximately 1 kb of the 5′-flanking region and 1 kb of the 3′-flanking region together into pNPTS129, creating a chimeric fragment that is missing the flgBC-fliE coding sequence. The primers used to amplify the 5′-flanking region were 5′-GGTACCCTTCAGTTCCGAGATCATGA and 3′-GGATCCCATGCCGAAAAGAGGGATGT. The primers used to amplify the 3′-flanking region were 5′-GGATCCTCCATCTTGTGATTTCTCCC and 3′-GAATTCTCGACTACGGCAACAACATC. When amplified by PCR, the 5′-flanking sequence has a KpnI site at the 5′ end and a BamHI site at the 3′ end. The 3′-flanking sequence has a BamHI site at the 5′ end and an EcoRI site at the 3′ end. The chimeric deletion was then constructed by sequentially cloning the two flanking regions into pNPTS129, using the introduced BamHI sites to connect them. Following introduction of this deletion into strain NA1000, cultures were grown overnight in peptone yeast extract and then plated onto peptone yeast extract agar containing 2% sucrose. Survivors that were nonmotile, sucrose resistant, and kanamycin sensitive were selected and verified by Southern blotting to have the entire flgBC-fliE region deleted. Additionally, the resultant knockout strain was successfully complemented by the 4.2-kb BamHI fragment on plasmid pJS14, indicating that this operon and not an independent locus is the cause of the lack of motility. The knockouts of flgB and fliE were accomplished by introduction via pNPTS129 of frameshifted mutants. The 602-bp NcoI fragment that harbors flgB contains a unique NdeI site at position 356 (codon 48 of 114). This NdeI site was cut, filled in with Klenow fragment, and religated with T4 DNA ligase (Promega) to create a frameshift mutation. The resultant polypeptide is out of frame from codon 48 and terminates 60 codons later. The 590-bp fliE fragment contains a unique NcoI site at position 175 (codon 42 of 103). This site was cut, filled in with the Klenow fragment of DNA polymerase (Promega), and religated to create a +2 frameshift mutation; the resultant polypeptide is out of frame from codon 42 and terminates 43 codons later. Both the flgB and fliE knockouts were mediated by sucrose antiselection and verified by Southern blotting and complementation of the respective fragments.
Assay of temporal expression.
The temporal expression of flgB was determined by assaying the cell cycle expression of both flgB-lacZ and flgB-nptII transcriptional fusions. To accomplish the assay, the 321-bp HindIII-AflII fragment containing the promoters for both the flgB and the fliO-fliP operon was cloned in both orientations into the bidirectional reporter placZ-npt/290, which contains a promoterless lacZ reporter in one direction and a divergent promoterless nptII gene. Cultures of NA1000 harboring either of the resulting constructs were synchronized by isolating pure swarmer cells via density gradient centrifugation in Percoll (Sigma) as described previously (13). The swarmer cells were resuspended in minimal medium and incubated with shaking at 31°C. The cells were allowed to progress through the cell cycle, and aliquots were pulse-labeled at intervals with 5 mCi of [35S]methionine translabel (ICN) per ml for 10 min. The label was chased with cold methionine, following which the cells were pelleted; washed with buffer containing 0.45 M NaCl, 50 mM Tris (pH 8.3), and 0.5% Triton X-100; resuspended in the same buffer; and then lysed and subjected to immunoprecipitation with both anti-β-galactosidase and anti-NptII monoclonal antibodies as previously described (21). The immunoprecipitated proteins were then separated on a 10% polyacrylamide biphasic Laemmli sodium dodecyl sulfate-polyacrylamide gel and visualized on a PhosphorImager 445 Si (Molecular Dynamics).
CtrA footprinting assays.
Soluble CtrA-His6 was purified as described from vector pET15b (Novagen) in E. coli strain ER2566 (New England Biolabs). PCR primers used to amplify ctrA for cloning into pET15b were 5′-ATGCAGCATATGCGCGTACTGTTGATCGAG and 3′-ATGCAGCGCGAGTCAGGCGGCGTTAACCTGCT. Products were amplified with Pfu polymerase (Stratagene) according to the manufacturer's directions, cloned directly into pET15b, and verified by dye termination sequencing. EnvZ′-MalE fusion protein from pKJH5 (23, 26) was purified as described in the pMAL protein fusion manual (New England Biolabs). CtrA-His6 (4.8 mM) and EnvZ′-MalE (0.5 mM) were added to phosphorylation buffer (50 mM Tris [pH 7.5], 50 mM KCl, 20 mM MgCl2, 1 mM dithiothreitol, 0.5 mM ATP) and incubated at 37°C for 1 h (59). Various amounts of the phosphorylated protein were mixed with 1.5 × 105 cpm of end-labeled probe (the 321-bp HindIII-AflII fragment already described) in buffer A (200 mM Tris [pH 8.0], 100 mM KCl, 6 mM MgCl2, 1 mM CaCl2, 2 mM dithiothreitol, and 4 mg of salmon sperm DNA per ml). The mix (volume of 190 μl) was allowed to bind for 20 min at room temperature, and then 0.05 U of DNase I (Boehringer Mannheim) was added and allowed to digest for 3 min. At that time, 100 μl of DNase stop buffer (0.6 M ammonium acetate, 150 mM EDTA, 20 mg of calf thymus DNA per ml) was added to stop the reactions, and the reaction mixtures were then extracted once with phenol-chloroform-isoamyl alcohol (25:24:1; equilibrated with Tris-EDTA, pH 8.0), precipitated with 3 volumes of ethanol, washed with 70% ethanol, dried, and resuspended in 12 μl of formamide loading buffer. One microliter from each sample was counted in a scintillation counter, and 1.5 × 104 cpm per lane was loaded and run on an 8% denaturing polyacrylamide sequencing gel. The gel was dried, and the bands were visualized on a PhosphorImager 445 Si (Molecular Dynamics).
RESULTS
The C. crescentus flgB, flgC, and fliE genes encode homologues of basal body rod components.
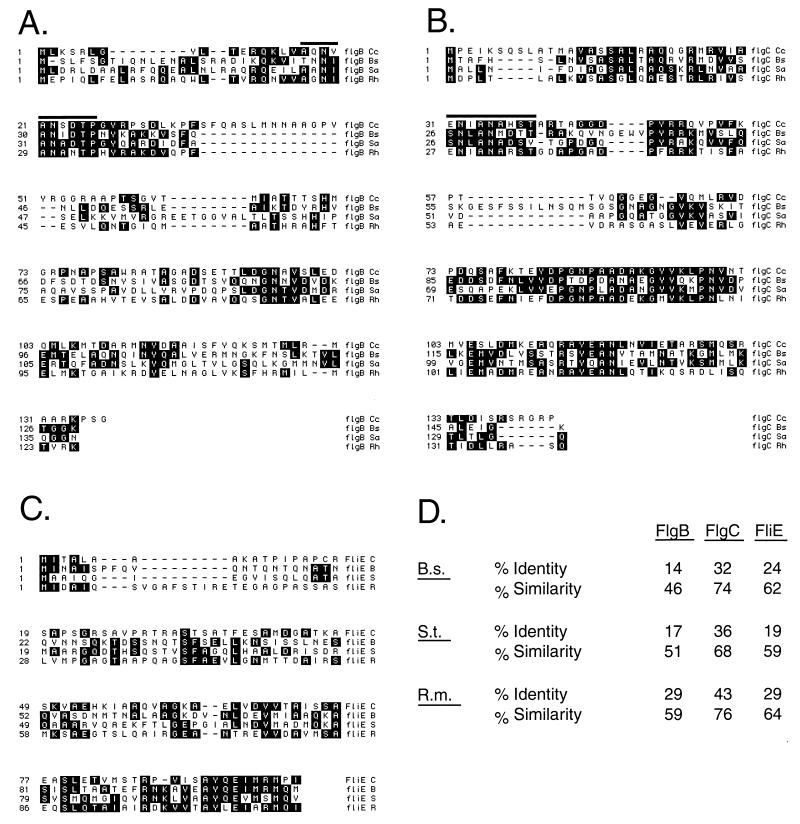
The sequences encoding the C. crescentus homologues of the flagellar rod proteins FlgB, FlgC, and FliE were identified by comparison of enteric flagellar gene sequences to contigs of the C. crescentus genome sequencing project. The results of a sequence alignment program (BLAST) indicated that the C. crescentus homologue of FlgB is 29% identical and 59% similar to the Rhizobium meliloti FlgB; the C. crescentus homologue of FlgC is 43% identical and 76% similar to that of R. meliloti; and for FliE, the C. crescentus homologue is 29% identical and 64% similar to that of R. meliloti (Fig. 1). In addition, the characteristic rod motif that is present in all flagellar rod proteins, including both distal (FlgF and FlgG) and proximal (FlgB and FlgC) rod proteins, is present (18). FliE is known to interact with FlgB (43) and is required for rod assembly in Salmonella enterica serovar Typhimurium (31). It lacks the rod motif, but the high degree of similarity between the C. crescentus homologue of FliE and that of other diverse bacterial species, including serovar Typhimurium and Bacillus subtilis, indicates that the gene encodes the C. crescentus homologue of the bacterial flagellar FliE protein (Fig. 1).
FIG. 1.
The C. crescentus FlgB, FlgC, and FliE gene products. (A to C) Alignments of the three predicted gene products encoded by flgBC-fliE. Conserved residues are shaded. FlgB and FlgC from C. crescentus share a rod motif common to all gram-negative bacterial flagellar rod proteins, the so-called ANNLAN motif indicated by a bar above the sequences in panels A and B. The presence of the ANNLAN motif is diagnostic of rod proteins and shows that FlgB and FlgC are indeed proximal rod structural proteins. FliE lacks the ANNLAN motif, so it is thought that FliE might serve as an adapter between the helical symmetry of the rod and the radial symmetry of the MS ring (43, 45). (D) Degree of similarity to homologues from the three bacterial species used in the alignments in panels A to C. B.s., B. subtilis; S.t., S. enterica serovar Typhimurium, R.m., R. meliloti. Of the three species listed, R. meliloti is most closely related to C. crescentus (14).
The flgBC-fliE genes are not part of the flagellar regulatory hierarchy.
Class II genes of the flagellar hierarchy in C. crescentus are so called because their expression is required for the transcription of genes later in the hierarchy. To test whether the flgBC-fliE genes lie within the C. crescentus flagellar hierarchy, we created frameshift mutations of flgB and fliE by filling in and religating restriction sites within the coding regions of the genes and then assayed the effect on the regulation of flagellar gene transcription using reporter fusions to lacZ. We also deleted the entire operon by splicing together the two flanking regions by PCR and assayed transcription using the same reporter fusions. In all cases, the mutant versions were cloned into vector pNPTS129, which allows integration into the chromosome via homologous recombination and counterselection against the plasmid by virtue of a sacB cassette when grown on media containing sucrose (47). The resulting kanamycin-sensitive, sucrose-resistant strains were then screened for motility on semisolid agar. Nonmotile colonies were verified by Southern blotting to contain the desired mutation (data not shown). The reporters used were the class II genes fliP, fliL, and fliF, all fused to lacZ to quantitate their respective transcription levels. All were expressed at levels that were approximately 1.6 times greater than wild-type levels (data not shown); this slightly elevated expression is well documented for class II genes in class II mutant backgrounds, indicating that the flgBC-fliE genes behave as early flagellar genes. In order to test the idea that flgBC-fliE are class II flagellar genes, we assayed the expression of a flgB-lacZ reporter fusion in several class II flagellar mutants. This fusion was expressed in all class II null strains tested, indicating that it is expressed at the class II level of the hierarchy (Table 2). This result was somewhat surprising; based on their similarity to genes for other rod proteins, we expected that flgBC-fliE would be class III genes, because all other known rod components in C. crescentus are class III (8). rpoN and flbD (the class II genes mutated in SC1055 and SC1032, respectively) encode essential transcription factors for class III and IV gene expression (2, 4, 5, 49, 52, 57, 64, 67), so the fact that the flgBC-fliE genes are transcribed in strains lacking those proteins provides additional strong evidence that they are not expressed at the class III level.
TABLE 2.
Epistatic analysis of the flgBC-fliE operon
| Strain | β-Galactosidase activity (Miller units) for gene fusion:
|
||
|---|---|---|---|
| flgB-lacZ | flbG-lacZ | fljL-lacZ | |
| NA1000 | 5,510 | 2,770 | 5,370 |
| JG3108 | 5,740 | 2,620 | 10,400 |
| SC508 | 5,430 | 394 | 249 |
| SC1032 | 5,370 | 427 | 179 |
| SC1055 | 5,810 | 299 | 200 |
| NA1000/pCB4.2 | 5,450 | 3,410 | 4,590 |
| JG3110 | 127 | ||
| JG3111 | 106 | ||
We next examined the effect of deletions of the flgBC-fliE genes on the expression levels of class III and IV flagellar genes. If these genes are located at the class II level of the hierarchy, then deleting the operon should eliminate expression of class III and IV flagellar genes. In order to accomplish this, we assayed the expression of the class III (flbG::lacZ) and class IV (fljL::lacZ) reporter fusions in strain JG3108, which has the entire flgBC-fliE region deleted. Surprisingly, both reporters were expressed in strain JG3108, indicating that the flgBC-fliE gene products are not necessary for completion of the class II level of the hierarchy (Table 2). In fact, fljL::lacZ expression was elevated, showing approximately a twofold increase in β-galactosidase levels over those of the wild type. These results indicate that the flgBC-fliE genes are not components of the C. crescentus regulatory hierarchy.
The placement of the flgBC-fliE genes outside the regulatory hierarchy is intriguing, given that all other components of the basal body that are located in the periplasmic space are class III genes. Perhaps these gene products serve as a signal to the cell that the basal body is competent for export of the distal rod, hook, and other components that must be exported in order to be incorporated into the flagellum. It is possible that an increase in cytoplasmic pools of the flgBC-fliE gene products might inhibit class III transcription, as would occur in the absence of a functional flagellar export apparatus. The elevated expression of the fljL::lacZ fusion in strain JG3108 would tend to support this possibility. In order to test this hypothesis, we combined the flgBC-fliE deletion (JG3108) with a well-characterized class II mutant, either SC1131 (fliM::Tn5) or SC1132 (flhA::Tn5), and assayed expression of fljL::lacZ in the resultant double-mutant background. We reasoned that if any one of the flgBC-fliE gene products serves as a negative regulator in the absence of export, then eliminating them in either of these two export-deficient strains should restore normal expression to a fljL::lacZ transcriptional fusion. No such restoration in expression was evident (Table 2), indicating that the flgBC-fliE gene products do not serve as a negative regulator of late flagellar gene expression in the absence of a functional export assembly.
Determination of the flgB transcription start site and the identification of a CtrA binding site.
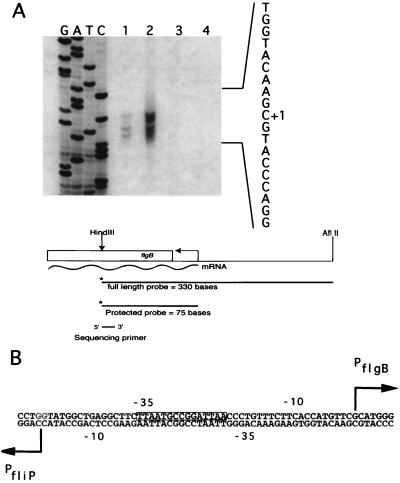
Initial examination of the sequence between the first residues encoding FlgB and the start site of fliO-fliP transcription showed that there can be only a very short distance between the two promoters. In order to determine the extent of the intergenic region between the fliO-fliP operon and flgB, S1 nuclease protection assays were used to identify the start site of transcription for flgB. Together with previously published data on the start site of transcription for the fliO-fliP operon (20), this permitted the precise determination of the size of the intergenic region that contains these divergent promoters. The region of protection from S1 nuclease digestion indicated that the major start site of flgB transcription lies 53 bp upstream of the fliO-fliP transcription start sequence (Fig. 2A). Therefore, the expression of flgB and the fliO-fliP operon is regulated by an unusually compact arrangement of divergent promoters with overlapping −10 and −35 regions of each promoter (Fig. 2B).
FIG. 2.
Identification of the transcription start site for flgB. (A) In order to map the start site of transcription for flgB, an S1 nuclease protection assay was carried out using a 321-bp HindIII-AflII fragment labeled at the HindIII terminus as the probe. The sequencing primer used to generate the dideoxy chain-termination sequencing ladder has its 5′ end corresponding to the HindIII site in the probe. Lane 1, 30 mg of total cellular RNA; lane 2, 60 mg of total cellular RNA; lane 3, 50 mg of yeast tRNA; lane 4, probe alone. The start site of transcription indicated corresponds to the top band in lanes 1 and 2. (B) The resulting bands on the gel correspond to a transcriptional start site for flgB that is only 53 bases upstream of the start site of transcription for the fliO-fliP operon. The 9-mer sequence corresponding to the CtrA recognition site (40) is indicated by a box around the sequence.
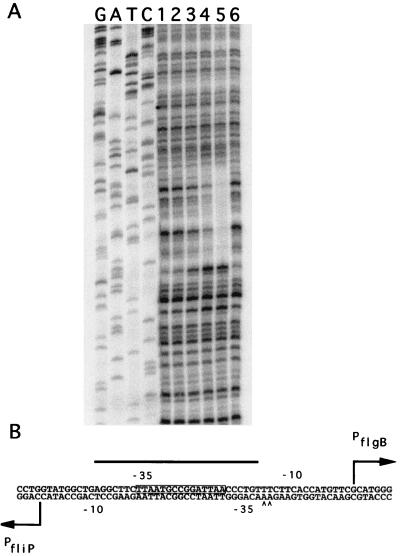
The transcription factor CtrA has been shown to regulate the transcription of several class II flagellar genes. Examination of the sequence between the two transcription start sites of these divergent promoters revealed the presence of a consensus core CtrA binding sequence (TTAA-N7-TTAA) (Fig. 2), indicating the possibility that CtrA regulates the transcription of either the fliO-fliP operon or that of flgB. If this is indeed the case, the idea of two promoters both controlled by the same trans-acting factor (CtrA) in close proximity to one another raises the question of which sequences within the promoter are bound by the transcription apparatus. Is there enough room in the 53-bp divergent promoter region to have separate and distinct CtrA binding sites, are there separate but overlapping binding sites, or does one binding site serve the same function for both promoters? To determine the area of the divergent promoter that interacts with CtrA to direct transcription, DNase I protection assays were utilized. The CtrA footprint extends from 14 bp on the map shown in Fig. 3 to 41 bp, with hypersensitive bands appearing at positions 42 and 43 bp (Fig. 3). This 28-bp region is of approximately the same extent as most other published CtrA footprints (33, 56, 59). Thus, it is clearly shown that there is only one segment of the dual promoter bound by CtrA; how this single binding site serves to direct transcription from both promoters is not clear.
FIG. 3.
DNase I protection assay of purified, phosphorylated CtrA on the 321-bp flgB–fliO-fliP divergent promoter fragment. (A) GATC represents dideoxy chain termination sequencing reactions. Lanes 1 and 6, probe alone (no CtrA added); lane 2, 0.12 mM CtrA; lane 3, 0.25 mM CtrA; lane 4, 0.5 mM CtrA; lane 5, 1.0 mM CtrA. DNase I protection assays were performed as described in Materials and Methods. (B) The CtrA footprint extends 28 bases, from position 14 to position 41, indicated by the bar over the sequence. This footprint completely covers the putative 9-mer sequence (CtrA binding site) indicated by the box around the sequence. Arrowheads indicate DNase I-hypersensitive sites. The positions of the transcriptional start sites for flgB and the fliO-fliP operon are indicated by large arrows and are at positions 57 and 4, respectively.
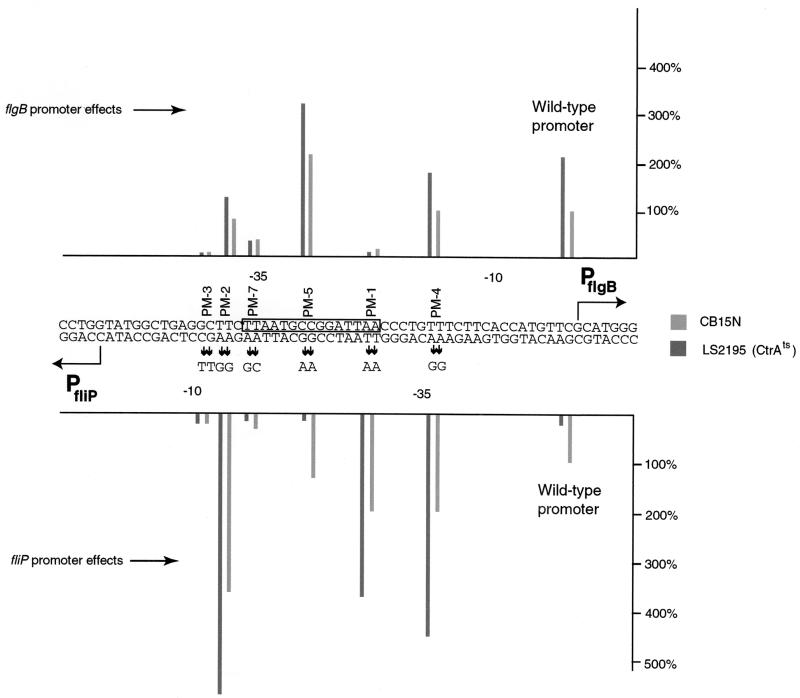
DNA binding assays indicated that CtrA may regulate the expression of both flgB and the fliO-fliP operon. To test this idea, the expression of flgB::lacZ and fliO-fliP::lacZ transcriptional reporter fusions was assayed in either wild-type (NA1000) or CtrA temperature-sensitive (LS2195, ctrA401) (56) strains (Fig. 4). Assays were conducted at the permissive temperature (31°C) for the ctrA401 allele, conditions which have been demonstrated previously to affect class II gene expression (56). Comparison of the transcription levels of the wild-type promoter sequence in strain LS2195 indicated that CtrA acts as a repressor of flgB expression and an activator of fliO-fliP expression. flgB transcriptional activity approximately doubled in the ctrA401 strain at the permissive temperature, while fliO-fliP transcription was reduced to about 10% of levels in a wild-type background (Fig. 4). To define the sequences that are important for transcriptional regulation of either flgB or the fliO-fliP operon, site-directed mutagenesis was used to create mutants in the promoter region. The site-directed mutants were cloned in both orientations into the transcriptional reporter plasmid placZ290, which contains a promoterless lacZ gene, in order to assay the effect of the mutations on transcription levels for each promoter. β-Galactosidase activity generated from the mutant promoters was assayed in either wild-type (NA1000) or CtrA temperature-sensitive (LS2195) strains (Fig. 4). We created mutations within the consensus core CtrA binding sequence (mutants PM-1, PM-5, and PM-7), as well as mutations that lie outside this region (mutants PM-2, PM-3, and PM-4). The results of this analysis were complex, indicating that, in the case of fliO-fliP expression, some mutations within this promoter region can shift the role of CtrA from a repressor to an activator. For example, mutations PM-1, PM-2, and PM-4 resulted in an increase in fliO-fliP-generated β-galactosidase expression in wild-type cells, ranging from a 2- to a 3.5-fold increase over the level for the wild-type promoter. These mutant promoters were expressed at even higher levels in the ctrA401 strain, an effect opposite from that of the wild-type fliO-fliP promoter. We speculate that these mutations may alter the position of CtrA bound to the promoter. One mutation, PM-7, clearly had a deleterious effect on both flgB and fliO-fliP transcription (Fig. 4). The bases mutated in PM-7 are located within the TTAA-N7-TTAA core CtrA binding sequence, as well as in the −35 region of the flgB promoter. The reduction in transcription in the fliO-fliP direction in this case may be mediated by failure of CtrA to activate transcription. The reduction in transcription in the flgB direction is probably a consequence of the mutation interfering with the binding of RNA polymerase. The same may be true of the PM-3 mutation in the case of flgB transcription. In all cases, however, when transcription of the flgB promoter was active, CtrA still apparently had a repressive effect. These data taken as a whole indicate that CtrA has a differential effect on the activity of these divergent promoters.
FIG. 4.
Mutational analysis of the divergent flgB and fliO-fliP promoters. Site-directed mutants of the flgB and fliO-fliP promoter region were created using the method of Kunkel and Roberts (37). The site-directed mutants were then cloned in both orientations into the reporter plasmid placZ290, which contains a promoterless lacZ gene, in order to assay the effect of the mutations on transcription levels for each promoter. The transcriptional activity was measured by mating the various reporter constructs into either wild-type (NA1000) or CtrA temperature-sensitive (LS2195, ctrA401) strains (54) and then performing β-galactosidase assays. Cells were grown and assays were conducted at the permissive temperature (31°C) for the ctrA401 allele (54). β-Galactosidase activity levels of the wild-type promoters are nearest the vertical axes; 100% activity corresponds in each case to the activity of the wild-type promoter in strain NA1000.
Cell cycle expression of the flgB and fliO-fliP promoters.
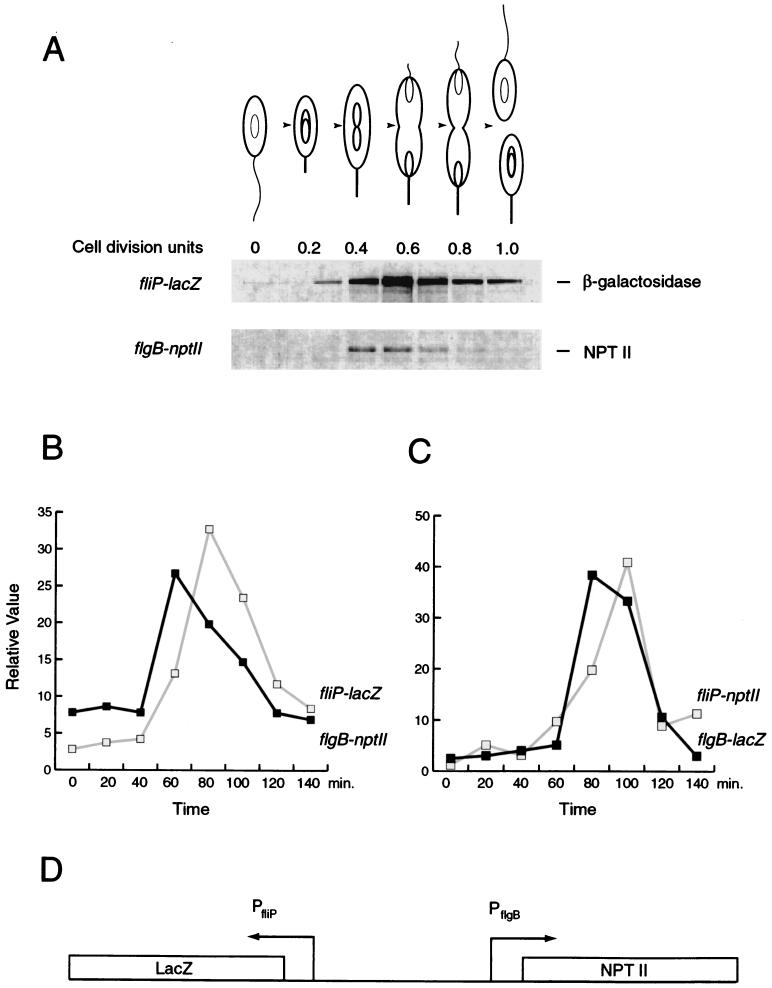
The results of the epistatic analysis indicate that the position occupied by the flgBC-fliE genes is unique in the C. crescentus flagellar hierarchy. No other flagellar structural genes are known that are expressed as class II genes and yet have no effect on class III gene expression when mutated. The very short segment of DNA between the two transcription start sites would seem to leave little room for two complete promoters that are both able to specify not only correct levels of expression but also correct timing of expression within the cell cycle. Furthermore, the apparent differential effects of CtrA on the transcription of these divergent promoters would suggest that their timing of expression during the C. crescentus cell cycle may be different. To test this idea, we assayed the cell cycle expression of both promoters simultaneously (Fig. 5). In order to accomplish this, the 321-bp HindIII-AflII fragment that was used to determine the start site of transcription was cloned into a dual-reporter plasmid. This reporter plasmid (placZ-nptII) contains a dual fusion of the 321-bp HindIII-AflII fragment with lacZ in one direction and nptII (encoding neomycin phosphotransferase) in the other (Fig. 5D). Labeled cell extracts from synchronized cultures were subjected to immunoprecipitation with both anti-β-galactosidase and anti-NptII antibodies. The results indicated that both the fliO-fliP and the flgB promoters are present in wholly functional form, including the ability to specify proper timing of expression in the cell cycle (Fig. 5A). Since both immunoprecipitations are from the same labeled cell extract, a direct comparison of the timing of expression is possible. In all dual synchrony experiments conducted, the peak in expression of flgB preceded that of the fliO-fliP operon by approximately 5 to 10% of the cell cycle (Fig. 5B and C). This was confirmed by repeating the experiment with the promoter fragment reversed in orientation (Fig. 5C).
FIG. 5.
Cell cycle pattern of expression of flgB and the fliO-fliP operon. (A) A dual synchrony experiment utilizing flgB fused to nptII and fliO-fliP fused to lacZ. The peak in transcriptional activity for both promoters occurs near the middle of the cell cycle, at approximately 0.5 cell division units for flgB and approximately 0.6 cell division units for fliO-fliP. This temporal pattern of expression is approximately the same as that for other class II genes that have been assayed. In all cases tested, expression of flgB slightly preceded expression of the fliO-fliP operon. To accomplish the assay, a 321-bp HindIII-AflII fragment containing the promoters for both flgB and the fliO-fliP operons was cloned into the bidirectional reporter placZ-npt290, which contains a promoterless lacZ reporter in one direction and a divergent promoterless nptII gene. The constructions were then assayed for cell cycle gene expression by synchronizing a culture of NA1000 harboring the construct being tested. (B) Graphic representation of the expression levels in panel A. The relative value of expression at each time point is expressed as a percentage of the sum of expression from all time points in the cell cycle for each reporter. (C) Graphic representation of a cell cycle experiment using NA1000 containing flgB::lacZ and fliP::nptII on placZ-npt290. (D) A diagram of the bidirectional reporter used in the cell cycle expression assay described for panels A and B.
DISCUSSION
The study of flagellar biogenesis in C. crescentus has proven to be very fruitful over the last several years in elucidating many aspects of the bacterial cell cycle. Several connections between essential cell cycle processes such as the initiation of DNA replication and cell division and flagellar biogenesis have emerged, illustrating the high degree of interwining of motility and development in this organism. Most of the connections that have been made are in the form of cell cycle checkpoints. For instance, the swarmer-to-stalked cell transition, while marked by ejection of the flagellum and growth of a stalk, is accompanied by changes in the protein complement of the nucleoid (13); also, flagellar biogenesis does not occur in the absence of replication initiation (9, 54), and cell division is altered in mutants that lack proper expression of genes that are early in the flagellar hierarchy (20, 70, 71). Determining the identity and function of the proteins that mediate these checkpoints has become the primary focus of study in the field of C. crescentus flagellar biogenesis, so that the examination of motility provides a window for understanding how a bacterial cell coordinates and synchronizes the many varied aspects of the cell cycle to successfully complete the reproductive process.
In this work, we have characterized the genes that encode three components of the proximal rod, FlgB, FlgC, and FliE. We show that these genes lie outside the C. crescentus flagellar hierarchy because, while the operon is expressed in class II mutant backgrounds, class III genes are still expressed in flgBC-fliE null strains. We show that flgB is expressed in a temporal pattern that is similar to that of other class II genes. We have mapped the start site of transcription and used this information to construct site-directed mutations in the promoter region to examine the promoter architecture, in regard to both flgB and fliO-fliP transcription. By analyzing expression of wild-type and mutant promoters in a CtrA mutant strain, we have provided genetic evidence that CtrA is responsible for regulating expression of both sets of genes, and we have provided direct biochemical evidence that CtrA interacts with the promoter sequences on the DNA itself.
A question in the study of C. crescentus flagellar biogenesis is how the cell determines that assembly of the class II elements of the flagellum is complete. Expression of all the class II elements is required before transcription of class III promoters can begin, so there must be some mechanism for sensing their presence, their assembly, or their function, but no such mechanism has been identified. It is difficult to imagine a sensing protein or apparatus that can detect assembly of the class II components, because such a sensing protein would be required to simultaneously sample many points on the surface of a rather large, multisubunit structure. A much simpler checkpoint control would be to test the function of the class II structure by assaying export of a component of the next part of the flagellum to be assembled, a role for which the proximal rod proteins, FlgBC and FliE, would seem to be suited. There are two possibilities in this scenario: either a sensor protein in the periplasmic space sends a signal to begin class III gene expression once it detects the presence of the proximal rod, or the buildup of unexported signal proteins (proximal rod or otherwise) in the cytoplasm as a result of a nonfunctional flagellar export apparatus sends a negative signal that inhibits commencement of class III gene expression. In either case, the signal protein(s) must be expressed even in the absence of complete class II gene product assembly. The results of the epistasis analysis provide evidence that the flgBC-fliE gene products are not this signal. None of the proteins encoded acts either positively by activating transcription when exported or negatively by inhibiting transcription if unexported (Table 2). There may be an as-yet-undiscovered signal protein that is also expressed outside the hierarchy, or there may in fact be a sensor that detects assembly of the class II components directly. Determination of how the cell acts to monitor the assembly of such a large, complex structure would be of general interest to the study of other such structures, for example, photosynthesis reaction centers and other secretion machinery.
Recent work on both general cell cycle regulation and the flagellar hierarchy of C. crescentus has shown that CtrA is responsible for regulating both chromosomal replication initiation and transcription of early flagellar genes, thus uniting these two regulatory aspects of the cell cycle of this organism (reviewed in references 27 and 66). However, the mode of action of CtrA at class II promoters has been largely uncharacterized. It is known that CtrA cooperates with ς70 holoenzyme to regulate class II transcription, but the nature of the relationship between the two is not clear. CtrA seems to play a dual role in the cell, sometimes exerting its effect by virtue of its ability to repress transcription, as at the origin of replication and some flagellar genes (56), and sometimes by activating transcription (55). In the case of the flgB and fliO-fliP divergent promoters, CtrA appears to function as a repressor in the former case and as an activator in the latter. This is an apparent consequence of the compact nature of the divergent flgB–fliO-fliP promoter, and we speculate that it has an effect on their relative timing of expression. We have shown here that the flgB promoter, which is repressed by CtrA, is expressed earlier in the cell cycle than is the fliO-fliP promoter, which is activated by CtrA. We hypothesize that the temporal phosphorylation and increase in CtrA levels are responsible for the cessation of flgB transcription and the subsequent increase in the transcription of the divergent fliO-fliP promoter.
The differential regulation and sequence organization of the flgB and fliO-fliP divergent promoters by a single transcription factor, CtrA, contrast with those of other C. crescentus divergent flagellar promoters. An interesting aspect of flagellar gene regulation in C. crescentus is the apparent coordinated expression within the hierarchy of components for substructures within the basal body. In particular, there are two divergent flagellar promoters in C. crescentus that regulate the expression of basal body genes, the flgI-fliX (39, 44) and the fliL-flgF (41, 70) divergent promoters. The flgI-fliX and fliL-flgF pairs are arranged as divergent class II-class III promoters. They include a CtrA binding site proximal to the class II member of the class II-class III pair, several sites that are used for class III transcription including ftr sequences (binding sites for the transcription factor FlbD), integration host factor binding sites, and consensus ς54 promoter elements proximal to the class III member of the pair. The flgB–fliO-fliP divergent promoter sequence is similar to neither of these examples; it is the only divergent promoter that consists of two class II-type promoters, and it is much more compact than the class II-class III divergent promoters, which are approximately 160 bases in extent versus only 53 bases for the flgB–fliO-fliP divergent promoter. The class II-class III divergent promoters do share an interesting trait with the flgB–fliO-fliP promoter, however: with the work presented here characterizing the expression of the proximal rod proteins FlgB, FlgC, and FliE, it is now known that all of the components of the flagellum that are located between the MS ring, which is embedded in the inner membrane, and the outer membrane (i.e., the outer basal body components) are divergently transcribed from class II operons. The reasons for this arrangement of all periplasmic flagellar components being divergently transcribed may be related to the requirement for sequential expression of the different elements within each promoter. For example, binding of the transcriptional activator FlbD to the class III member of a divergently transcribed flagellar promoter may act to shut off transcription from the class II member of the pair as well as to activate the class III member. It will be of interest to test this hypothesis by determining if FlbD and CtrA are mutually incompatible at divergent promoters, or if FlbD can bind while CtrA remains bound, or if phosphorylated FlbD can displace CtrA or otherwise inhibit transcription. Active, phosphorylated FlbD is already known to inhibit transcription from the class II promoter that governs its own transcription, the fliF promoter (65), but competitive binding assays with CtrA have not been undertaken, either at that promoter or at any other class II promoter.
ACKNOWLEDGMENTS
We thank Ann Reisenauer and Ellen Quardokus for advice on CtrA purification and footprinting, Michelle Igo for permission to use the EnvZ′-MalE fusion, and Dane Mohl and members of the Gober lab for assistance with the manuscript.
This work was funded by grant GM48417 from the National Institutes of Health to J.W.G.
REFERENCES
- 1.Aizawa S-I. Flagellar assembly in Salmonella typhimurium. Mol Microbiol. 1996;19:1–5. doi: 10.1046/j.1365-2958.1996.344874.x. [DOI] [PubMed] [Google Scholar]
- 2.Anderson D K, Ohta N, Wu J, Newton A. Regulation of the Caulobacter crescentus rpoN gene and function of the purified sigma 54 in flagellar gene transcription. Mol Gen Genet. 1995;246:697–706. doi: 10.1007/BF00290715. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons, Inc.; 1989. [Google Scholar]
- 4.Benson A K, Wu J, Newton A. The role of FlbD in regulation of flagellar gene transcription in Caulobacter crescentus. Res Microbiol. 1994;145:420–430. doi: 10.1016/0923-2508(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 5.Brun Y V, Marczynski G, Shapiro L. The expression of asymmetry during Caulobacter cell differentiation. Annu Rev Biochem. 1994;63:419–450. doi: 10.1146/annurev.bi.63.070194.002223. [DOI] [PubMed] [Google Scholar]
- 6.Champer R, Dingwall A, Shapiro L. Cascade regulation of Caulobacter flagellar and chemotaxis genes. J Mol Biol. 1987;194:71–80. doi: 10.1016/0022-2836(87)90716-9. [DOI] [PubMed] [Google Scholar]
- 7.Chen L-S, Mullin D, Newton A. Identification, nucleotide sequence and control of developmentally regulated promoters in the hook operon region of Caulobacter crescentus. Proc Natl Acad Sci USA. 1986;83:2860–2864. doi: 10.1073/pnas.83.9.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dingwall A, Garman J, Shapiro L. Organization and ordered expression of Caulobacter genes encoding flagellar basal body rod and ring proteins. J Mol Biol. 1990;228:1147–1162. doi: 10.1016/0022-2836(92)90322-b. [DOI] [PubMed] [Google Scholar]
- 9.Dingwall A, Zhuang W, Quon K, Shapiro L. Expression of an early gene in the flagellar regulatory hierarchy is sensitive to an interruption in DNA replication. J Bacteriol. 1992;174:1760–1768. doi: 10.1128/jb.174.6.1760-1768.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domian I, Quon K, Shapiro L. Cell type-specific phosphorylation and proteolysis of a transcriptional regulator controls the G1-to-S transition in a bacterial cell cycle. Cell. 1997;90:415–424. doi: 10.1016/s0092-8674(00)80502-4. [DOI] [PubMed] [Google Scholar]
- 11.Ely B, Ely T W. Use of pulsed field gel electrophoresis and transposon mutagenesis to estimate the minimal number of genes required for motility in Caulobacter crescentus. Genetics. 1989;123:649–654. doi: 10.1093/genetics/123.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ely B, Croft R H, Gerardot C J. Genetic mapping of genes required for motility in Caulobacter crescentus. Genetics. 1984;108:523–532. doi: 10.1093/genetics/108.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evinger M, Agabian N. Envelope-associated nucleoid from Caulobacter crescentus stalked and swarmer cells. J Bacteriol. 1977;132:294–301. doi: 10.1128/jb.132.1.294-301.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox G E, Stackebrandt E, Hespell R B, Gibson J, Maniloff J, Dyer T A, Wolfe R S, Balch W E, Tanner R S, Magrum L J, Zablen L B, Blakemore R, Gupta R, Bonen L, Lewis B J, Stahl D A, Luehrsen K R, Chen K N, Woese C R. The phylogeny of prokaryotes. Science. 1980;209:457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- 15.Gober J W, England J C. Regulation of flagellum biosynthesis and motility in Caulobacter. In: Brun Y V, Shimkets L J, editors. Prokaryotic development. Washington, D.C.: ASM Press; 2000. pp. 319–339. [Google Scholar]
- 16.Gober J W, Marques M. Regulation of cellular differentiation in Caulobacter crescentus. Microbiol Rev. 1995;59:31–47. doi: 10.1128/mr.59.1.31-47.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gober J W, Shapiro L. A developmentally regulated Caulobacter flagellar promoter is activated by 3′ enhancer and IHF binding elements. Mol Biol Cell. 1992;3:913–926. doi: 10.1091/mbc.3.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gober J W, Champer R, Reuter S, Shapiro L. Positional information during Caulobacter cell differentiation. Curr Opin Genet Dev. 1991;1:324–329. doi: 10.1016/s0959-437x(05)80295-3. [DOI] [PubMed] [Google Scholar]
- 19.Gober J W, Xu H, Dingwall A, Shapiro L. Identification of cis and trans elements involved in the timed control of a Caulobacter flagellar gene. J Mol Biol. 1991;217:247–257. doi: 10.1016/0022-2836(91)90539-i. [DOI] [PubMed] [Google Scholar]
- 20.Gober J W, Boyd C, Jarvis M, Mangan E, Rizzo M, Wingrove J. Temporal and spatial regulation of fliP, an early flagellar gene of Caulobacter crescentus that is required for motility and cell division. J Bacteriol. 1995;177:3656–3667. doi: 10.1128/jb.177.13.3656-3667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomes S L, Shapiro L. Differential expression and positioning of chemotaxis methylation proteins in Caulobacter. J Mol Biol. 1992;178:551–568. doi: 10.1016/0022-2836(84)90238-9. [DOI] [PubMed] [Google Scholar]
- 22.Homma M, Kutsukake K, Hasebe M, Iino T, MacNab R. FlgB, FlgC, FlgF, and FlgG: a family of structurally related proteins in the flagellar basal body of Salmonella typhimurium. J Mol Biol. 1990;211:465–477. doi: 10.1016/0022-2836(90)90365-S. [DOI] [PubMed] [Google Scholar]
- 23.Huang K-J, Igo M M. Identification of the bases in the ompF regulatory region which interact with the transcription factor OmpR. J Mol Biol. 1996;262:615–628. doi: 10.1006/jmbi.1996.0540. [DOI] [PubMed] [Google Scholar]
- 24.Hughes K T, Gillen K L, Semon M J, Karlinsey J E. Sensing structural intermediates in bacterial flagellar assembly by export of a negative regulator. Science. 1993;262:1277–1280. doi: 10.1126/science.8235660. [DOI] [PubMed] [Google Scholar]
- 25.Hung D, McAdams H, Shapiro L. Regulation of the Caulobacter cell cycle. In: Brun Y V, Shimkets L J, editors. Prokaryotic development. Washington, D.C.: ASM Press; 2000. pp. 361–378. [Google Scholar]
- 26.Igo M M, Ninfa A J, Stock J B, Silhavy T J. Phosphorylation and dephosphorylation of a bacterial transcriptional activator by a transmembrane receptor. Genes Dev. 1989;3:1725–1734. doi: 10.1101/gad.3.11.1725. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs C, Shapiro L. Microbial asymmetric cell division: localization of cell fate determinants. Curr Opin Genet Dev. 1998;8:386–391. doi: 10.1016/s0959-437x(98)80107-x. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs C, Domian I, Maddock J, Shapiro L. Cell cycle-dependent polar localization of an essential bacterial histidine kinase that controls DNA replication and cell division. Cell. 1999;97:111–120. doi: 10.1016/s0092-8674(00)80719-9. [DOI] [PubMed] [Google Scholar]
- 29.Jenal U, Shapiro L. Cell cycle-controlled proteolysis of a flagellar motor protein that is asymmetrically distributed in the Caulobacter predivisional cell. EMBO J. 1996;15:2393–2406. [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson R C, Ely B. Isolation of spontaneously derived mutants of C. crescentus. Genetics. 1977;86:25–32. doi: 10.1093/genetics/86.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson R C, Ely B. Analysis of nonmotile mutants of the dimorphic bacterium Caulobacter crescentus. J Bacteriol. 1979;137:627–634. doi: 10.1128/jb.137.1.627-634.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones C J, Macnab R M, Okino H, Aizawa S-I. Stoichiometric analysis of the flagellar hook-(basal-body) complex of Salmonella typhimurium. J Mol Biol. 1990;212:377–387. doi: 10.1016/0022-2836(90)90132-6. [DOI] [PubMed] [Google Scholar]
- 33.Kelly A J, Sackett M J, Din N, Quardokus E, Brun Y V. Cell cycle-dependent transcriptional and proteolytic regulation of FtsZ in Caulobacter. Genes Dev. 1998;12:880–893. doi: 10.1101/gad.12.6.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khambaty F M, Ely B. Molecular genetics of the flgI region and its role in flagellum biosynthesis in Caulobacter crescentus. J Bacteriol. 1992;174:4101–4109. doi: 10.1128/jb.174.12.4101-4109.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovach M E, Phillips R W, Elzer P H, Roop R M, 2nd, Peterson K M. pBBRIMCS: a broad host range cloning vector. BioTechniques. 1994;16:800–802. [PubMed] [Google Scholar]
- 36.Kubori T, Shimamoto N, Yamaguchi S, Namba K, Aizawa S-I. Morphological pathway of flagellar assembly in Salmonella typhimurium. J Mol Biol. 1992;226:433–446. doi: 10.1016/0022-2836(92)90958-m. [DOI] [PubMed] [Google Scholar]
- 37.Kunkel T A, Roberts J D. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 38.Mangan E K, Bartamian M, Gober J W. A mutation that uncouples flagellum assembly from transcription alters the temporal pattern of flagellar gene expression in Caulobacter crescentus. J Bacteriol. 1995;177:3176–3184. doi: 10.1128/jb.177.11.3176-3184.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marczynski G, Shapiro L. Cell-cycle control of a cloned chromosomal origin of replication from Caulobacter crescentus. J Mol Biol. 1992;226:959–977. doi: 10.1016/0022-2836(92)91045-q. [DOI] [PubMed] [Google Scholar]
- 40.Marczynski G, Lentine K, Shapiro L. A developmentally regulated chromosomal origin of replication uses essential transcription elements. Genes Dev. 1995;9:1543–1557. doi: 10.1101/gad.9.12.1543. [DOI] [PubMed] [Google Scholar]
- 41.Marques M V, Gober J. Activation of a temporally regulated Caulobacter promoter by upstream and downstream sequence elements. Mol Microbiol. 1995;16:279–289. doi: 10.1111/j.1365-2958.1995.tb02300.x. [DOI] [PubMed] [Google Scholar]
- 42.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. pp. 352–355. [Google Scholar]
- 43.Minamino T, Yamaguchi S, Macnab R M. Interaction between FliE and FlgB, a proximal rod component of the flagellar basal body of Salmonella. J Bacteriol. 2000;182:3029–3036. doi: 10.1128/jb.182.11.3029-3036.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohr C D, Mackichan J, Shapiro L. A membrane-associated protein, FliX, is required for an early step in Caulobacter flagellar assembly. J Bacteriol. 1998;180:2175–2185. doi: 10.1128/jb.180.8.2175-2185.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muller V, Jones C, Kawagishi I, Aizawa S, MacNab R. Characterization of the fliE genes of Escherichia coli and Salmonella typhimurium and identification of the FliE protein as a component of the flagellar hook-basal body complex. J Bacteriol. 1992;174:2298–2304. doi: 10.1128/jb.174.7.2298-2304.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mullin D, Minnich S, Chen L S, Newton A. A set of positively regulated flagellar gene promoters in Caulobacter crescentus with sequence homology to the nif gene promoters of Klebsiella pneumoniae. J Mol Biol. 1987;195:939–943. doi: 10.1016/0022-2836(87)90497-9. [DOI] [PubMed] [Google Scholar]
- 47.Mullin D A, Newton A. Ntr-like promoters and upstream regulatory sequence ftr are required for transcription of a developmentally regulated Caulobacter crescentus flagellar gene. J Bacteriol. 1989;171:3218–3227. doi: 10.1128/jb.171.6.3218-3227.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mullin D A, Newton A. A ς54 promoter and downstream sequence elements ftr2 and ftr3 are required for regulated expression of divergent transcription units flaN and flbG in Caulobacter crescentus. J Bacteriol. 1993;175:2067–2076. doi: 10.1128/jb.175.7.2067-2076.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mullin D A, Van Way S M, Blankenship C A, Mullin A H. FlbD has a DNA-binding activity near its carboxy terminus that recognizes ftr sequences involved in positive and negative regulation of flagellar gene transcription in Caulobacter crescentus. J Bacteriol. 1994;176:5971–5981. doi: 10.1128/jb.176.19.5971-5981.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newton A, Ohta N, Ramakrishnan G, Mullin D, Raymond G. Genetic switching in the flagellar gene hierarchy of Caulobacter requires negative as well as positive regulation of transcription. Proc Natl Acad Sci USA. 1989;86:6651–6655. doi: 10.1073/pnas.86.17.6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohta N, Chen L-S, Swenson E, Newton A. Transcriptional regulation of a periodically controlled flagellar gene operon in Caulobacter crescentus. J Mol Biol. 1985;186:107–115. doi: 10.1016/0022-2836(85)90261-x. [DOI] [PubMed] [Google Scholar]
- 52.Ohta N, Chen L-S, Mullin D A, Newton A. Timing of flagellar gene expression in the Caulobacter cell cycle is determined by a transcriptional cascade of positive regulatory genes. J Bacteriol. 1991;173:1514–1522. doi: 10.1128/jb.173.4.1514-1522.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohta N, Swanson E, Ely B, Newton A. Physical mapping and complementation analysis of transposon Tn5 mutations in Caulobacter crescentus: organization of transcriptional units in the hook gene cluster. J Bacteriol. 1984;158:897–904. doi: 10.1128/jb.158.3.897-904.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Osley M A, Sheffery M, Newton A. Regulation of flagellin synthesis in the cell cycle of Caulobacter: dependence on DNA replication. Cell. 1977;12:393–400. doi: 10.1016/0092-8674(77)90115-5. [DOI] [PubMed] [Google Scholar]
- 55.Quon K C, Marczynski G, Shapiro L. Cell cycle control by an essential bacterial two component signal transduction proteins. Cell. 1996;84:83–93. doi: 10.1016/s0092-8674(00)80995-2. [DOI] [PubMed] [Google Scholar]
- 56.Quon K C, Yang B, Domian I J, Shapiro L, Marczynski G. Negative control of bacterial DNA replication by a cell cycle regulatory protein that binds at the chromosome origin. Proc Natl Acad Sci USA. 1998;95:120–125. doi: 10.1073/pnas.95.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramakrishnan G, Newton A. FlbD of Caulobacter crescentus is a homologue of the NtrC (NRI) protein and activates sigma 54-dependent flagellar gene promoters. Proc Natl Acad Sci USA. 1990;87:2369–2373. doi: 10.1073/pnas.87.6.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramakrishnan G, Zhao J-L, Newton A. The cell cycle-regulated flagellar gene flbF of Caulobacter crescentus is homologous to a virulence locus (lcrD) of Yersinia pestis. J Bacteriol. 1991;173:7283–7292. doi: 10.1128/jb.173.22.7283-7292.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reisenauer A, Quon K, Shapiro L. The CtrA response regulator mediates temporal control of gene expression during the Caulobacter cell cycle. J Bacteriol. 1999;181:2430–2439. doi: 10.1128/jb.181.8.2430-2439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanders L A, Van Way S, Mullin D A. Characterization of the Caulobacter crescentus flbF promoter and identification of the inferred flbF product as a homolog of the LcrD protein from a Yersinia enterocolitica virulence plasmid. J Bacteriol. 1992;174:857–866. doi: 10.1128/jb.174.3.857-866.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schweizer H P. Allelic exchange in Pseudomonas aeruginosa using novel ColE1-type vectors and a family of cassettes containing a portable oriT and the counter-selectable Bacillus subtilis sacB marker. Mol Microbiol. 1992;6:1195–1204. doi: 10.1111/j.1365-2958.1992.tb01558.x. [DOI] [PubMed] [Google Scholar]
- 62.Simons R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–790. [Google Scholar]
- 63.Stephens C M, Shapiro L. An unusual promoter controls cell-cycle regulation and dependence on DNA replication of the Caulobacter fliLM early flagellar operon. Mol Microbiol. 1993;9:1169–1179. doi: 10.1111/j.1365-2958.1993.tb01246.x. [DOI] [PubMed] [Google Scholar]
- 64.Wingrove J A, Mangan E K, Gober J W. Spatial and temporal phosphorylation of a transcriptional activator regulates pole-specific gene expression in Caulobacter. Genes Dev. 1993;7:1979–1992. doi: 10.1101/gad.7.10.1979. [DOI] [PubMed] [Google Scholar]
- 65.Wingrove J A, Gober J W. A ς54 transcriptional activator also functions as a pole-specific repressor in Caulobacter. Genes Dev. 1994;8:1839–1852. doi: 10.1101/gad.8.15.1839. [DOI] [PubMed] [Google Scholar]
- 66.Wu J, Newton A. Regulation of the Caulobacter flagellar gene hierarchy: not just for motility. Mol Microbiol. 1997;24:233–239. doi: 10.1046/j.1365-2958.1997.3281691.x. [DOI] [PubMed] [Google Scholar]
- 67.Wu J, Benson A K, Newton A. Global regulation of a ς54-dependent flagellar gene family in Caulobacter crescentus by the transcriptional activator FlbD. J Bacteriol. 1995;177:3241–3450. doi: 10.1128/jb.177.11.3241-3250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu, Ohta J N, Newton A. An essential, multicomponent signal transduction pathway required for cell cycle regulation in Caulobacter. Proc Natl Acad Sci USA. 1998;95:1443–1448. doi: 10.1073/pnas.95.4.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu H, Dingwall A, Shapiro L. Negative transcriptional regulation in the Caulobacter flagellar hierarchy. Proc Natl Acad Sci USA. 1989;86:6656–6660. doi: 10.1073/pnas.86.17.6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu J, Shapiro L. Early Caulobacter crescentus genes fliL and fliM are required for flagellar gene expression and normal cell division. J Bacteriol. 1992;174:3327–3338. doi: 10.1128/jb.174.10.3327-3338.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhuang W Y, Shapiro L. Caulobacter FliQ and FliR membrane proteins, required for flagellar biogenesis and cell division, belong to a family of virulence factor export proteins. J Bacteriol. 1995;177:343–356. doi: 10.1128/jb.177.2.343-356.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]