Abstract
Background
Vitamin D plays an invaluable role in reproductive health, but vitamin D insufficiency and deficiency are generally common among couples of childbearing age and pregnant women. This study aimed to evaluate the evolution, development trend, and research hotspot of publications on vitamin D and reproductive health.
Methods
The literature on vitamin D and reproductive health between 2012 and 2021 was retrieved from the Web of Science Core Collection (WoSCC). We used VOSviewer and CiteSpace to analyze publication years, countries, institutions, journals, highly cited authors and publications, and co-occurrence and citation bursts of keywords.
Results
A total of 1,828 articles and reviews on vitamin D and reproductive health published between 2012 and 2021 were identified. The annual publication outputs showed steady growth, with the most publications (272) and citations (7,097) in 2021. The United States contributed the most publications (458) and had the highest h-index (58). In terms of the number of publications and h-index, the journal named Nutrients ranked first. Nutrition dietetics, obstetrics gynecology, and endocrinology metabolism were three well-represented disciplines in research on vitamin D and reproductive health. Hollis BW, Wagner CL, and Litonjua AA were the top three most productive authors in this field during the last decade. Apart from vitamin D, the five keywords with the most frequent occurrence were vitamin D deficiency, pregnancy, risk, vitamin D supplementation, and 25-hydroxyvitamin D. Keyword citation burst analysis revealed that low birth weight, adipose tissue, marker, and embryo had a citation burst lasting until 2021.
Conclusion
In conclusion, vitamin D has received continuous attention in the field of reproductive health, and there appears to have a higher level of research in North America. Multidisciplinary intersection contributed to the in-depth exploration in this field. And the effect of maternal vitamin D levels on fetal lipid metabolism and the prediction of fertility by vitamin D-related markers might be hotspots for the research.
Keywords: vitamin D, vitamin D deficiency, pregnancy, bibliometric analysis, reproductive health, marker
Introduction
Vitamin D is an essential fat-soluble vitamin that serves a vital role in physiological processes such as calcium and phosphorus metabolism, immune regulation, cell growth, differentiation, and apoptosis in the body (1). Vitamin D is a steroid derivative, which can be divided into ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3) according to the chemical constitution (2). Vitamin D2 is sourced from the UV irradiation of ergosterol in some plants or fungi, while vitamin D3 is mostly synthesized from 7-dehydrocholesterol in the skin following the UV irradiation (3). It is estimated that about 80% of the body’s vitamin D comes from the endogenous synthesis in the skin (ambient UV exposure), and only 20% comes from exogenous intake, including vitamin D3–exogenous (egg yolks and oily fish), vitamin D2 fortified foods (margarine and breakfast cereals) and vitamin supplements (3, 4). After binding to the multifunctional vitamin D-binding protein (VDBP), vitamin D is transported to the liver, where it is transformed by the action of 25-hydroxylation into 25-hydroxy vitamin D3 [25-(OH)D3], which is its main form of circulation and storage in the human body. Then, 25-(OH)D3 needs to be metabolized to 1,25-(OH)2D3, the main active form of vitamin D, by the action of 1α-hydroxylase, which is expressed by many cell types (i.e., skin, immune cells, bone cells, placenta), but the highest concentration is found in the kidney proximal tubule cells (5, 6).
1,25-(OH)2D3 exerts hormone-like effects by binding to the vitamin D receptor (VDR). VDR belongs to the steroid receptor family, widely present in various tissues of the body, including the intestines, renal tubules, skin, pancreas, skeleton, immune system, and germ tissues (7). The binding of 1,25-(OH)2D3 to the ligand-binding domain of VDR promotes the phosphorylation of VDR and the heterodimerization of retinoid X receptor (RXR) to form a 1,25-(OH)2D3-VDR/RXR complex which interacts with the vitamin D responsive elements (VDRE) in the promotor region of target genes to exert the genomic effects (6, 8). In the non-genomic pathway, the 1,25-(OH)2D3-VDR-caveolin-1 complex induces modifications in cellular signaling pathways, thereby affecting cellular function (9). The VDR gene, located on chromosome 12, has single nucleotide polymorphism, which can have biological functions (8). VDR polymorphisms are closely related to renal diseases, bone biology, cancer, diabetes, and other diseases, which are expected to be a diagnostic tool for the diseases, but further large-scale population studies are needed (10).
The high prevalence of vitamin D deficiency is a worldwide problem, especially in pregnant and reproductive-age women (11, 12). Recent studies have shown that vitamin D played an important role in the regulation of female reproductive health and was involved in the oocyte development, and production of anti-mullerian (AMH), ovarian steroidogenesis, and endometrial receptivity (13). Vitamin D deficiency can lead to adverse pregnancy outcomes and cause a variety of reproductive disorders. Some meta-analyses have reported that vitamin D deficiency could increase the risks of preeclampsia, gestational diabetes mellitus (GDM), maternal infections, intrauterine growth restriction, and preterm birth (14–16). Vitamin D deficiency is associated with a high risk of hypertensive disorders in pregnancy (17). Matias et al. found that vitamin D can downregulate the activation of the inflammasome and the TLR4-MyD88-NF-κB pathway in preeclampsia (18). Evidence suggested that vitamin D could improve insulin sensitivity by enhancing insulin responsiveness to glucose transport (19) and thus prevent the occurrence of GDM. A prospective study demonstrated follicular fluid vitamin D level was positively correlated with clinical pregnancy and implantation rates and was an independent predictor of the success of in vitro fertilization treatment (20). Moreover, vitamin D was also involved in the development of reproductive disorders including polycystic ovary syndrome (PCOS) (21), endometriosis (22), and uterine fibroids (23).
Bibliometric analysis is a world-accepted statistical evaluation of published articles and has grown in popularity (24), which was first proposed by American bibliographers in 1969. Through qualitative and quantitative analysis of publications, it could use literature metrology characteristics to provide investigators with crucial messages and discover frontiers and evaluate the distribution of countries/regions, authors, and journals in a certain specific field (25). In line with the growing interest in the role of vitamin D in reproductive health, there is a growing body of literature, including numerous systematic reviews and meta-analyses, but few bibliometric analyses explored the hot spots and frontiers of research in this field. In this study, we aimed to provide a general description of quantitative and visual information in the global literature on the associations between vitamin D and reproductive health, identifying its emerging trends and potential hot spots from various aspects through integrative analysis of relevant information from manuscripts published worldwide from 2012 to 2021. We presented a brief discussion of vitamin D-reproductive research and predicted possible trends in this field over the next few years, laying a foundation for the direction and development of future research.
Materials and methods
Data collection and search strategy
All relevant articles published between 2012 and 2021 were retrieved from the Web of Science (WoS) Core Collection (WoSCC), one of the most widely used literature search databases. The main merits of the WoS database are a wide range of applications, few utilization restrictions, convenience to support horizontal comparison, and robust tools that assist in conducting advanced assessments over gathered information. The search strategies were as follows: Topic = (“reproducti*” OR “*fertil*” OR “steril*” OR “pregnan*”) AND Topic = (“*Vitamin D*”) AND Language = (English). The timespan was set from January 1, 2012, to December 31, 2021. For document types, only original articles and reviews were included in the study, with a total of 4,187 publications. Similarly, we searched for studies with the Topic = (“Vitamin D*”) following the same search strategies. A total of 48,493 relevant literature were retrieved. To avoid possible bias produced by continuous database updating, the retrieval and export of documents were created within 1 day (May 1, 2022). As well, to ensure the accuracy of retrieval, two independent authors (Xudong Zhang and Yimeng Lu) performed the search process and confirmed the search query and results. After excluding those on unrelated search topics (n = 2,359), the remaining 1,828 publications were included in the subsequent analyses.
Data extraction and analysis
The retrieved data were downloaded and exported into different formats for further analysis. The “Analyze Results” and “Citation Report” functions of WoS were utilized for basic information statistics, including annual production, WoS categories, and quality of publications by country, institution, author, and journal. The quality of publications in this study mainly referred to the number of publications, the sum of the times cited, average citations per item (ACI), and h-index. Impact factors and category quartiles of journals for individual publications were collected from the 2021 Journal Citation Reports (JCR) (Clarivate Analytics, Philadelphia, United States). The country distribution maps were created using the online mapchart.1
VOSviewer and CiteSpace, two common software for bibliometric analysis, were employed to further analyze the underlying connections and key themes. We used VOSviewer (1.6.17, Leiden University, the Netherlands) to visualize co-authorship among countries/institutions/authors, co-citation of references, and co-occurrence of keywords. The specific steps included creating a map based on the bibliographic data, reading data from bibliographic database files, selecting the appropriate type and unit of analysis, and setting the counting method to full counting. In the figures obtained, the node represented country/institution/author/keyword, the size of the node indicated the number of publications or frequency of occurrence, the thickness of the curve showed the strength of the link, and the color reflected the cluster. CiteSpace is a Java-based software program and one of its features is citation burst analysis (26). CiteSpace (5.8. R3) was utilized to view citation bursts and a timeline of keywords. The parameters of CiteSpace were set as follows: link retaining factor (LRF = 3), look back years (LBY = 5), time slicing (from 2012 to 2021), years per slice (1), links (strength = cosine; scope = within slices), selection criteria (g-index, k = 25), and minimum duration (MD = 2). In the network graph, nodes represented various keywords, whereas the size of nodes reflected the frequency, and the connections between nodes represented the link.
Results
Temporal distribution map of the literature
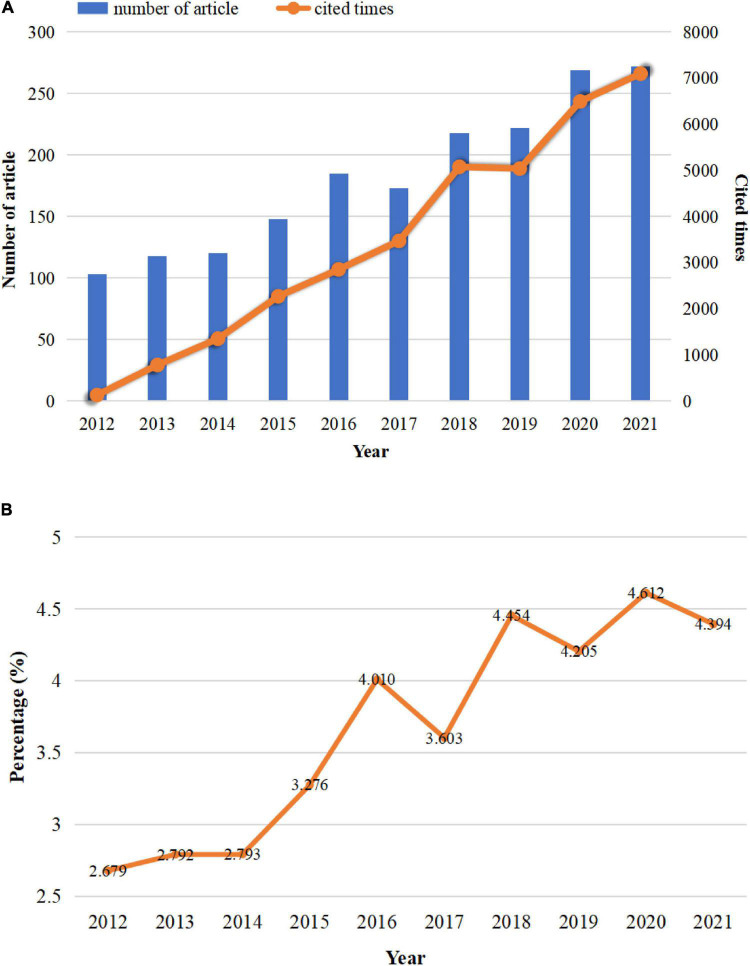
A total of 1,828 pieces of literature from 2012 to 2021 related to Vitamin D and reproductive health were retrieved from WoS. There were 1,534 articles (83.92%) and 294 reviews (16.08%), respectively. As shown in Figure 1A, the number of publications on vitamin D and reproductive health generally showed an upward trend. From 2012 to 2016, the number of publications increased steadily, with a slight decline in 2017, and then continued to increase until reaching its peak (272 publications) in 2021. Similarly, the percentage of research on vitamin D and reproductive health in all vitamin D-related publications gradually increased from 2012 to 2016, declined in 2017, and remained volatile at approximately 4.40% from 2018 to 2021 (Figure 1B). Cited times of publications increased year by year, except for a slight drop in 2019, and the most citations (7,097 times) were achieved in 2021 (Figure 1A).
FIGURE 1.
Trends in the growth of publications from 2012 to 2021. (A) The number of publications and citations per year on vitamin D and reproductive health. (B) Trends in the proportion of research on vitamin D and reproductive health in vitamin D-related research.
Distribution of countries and institutions
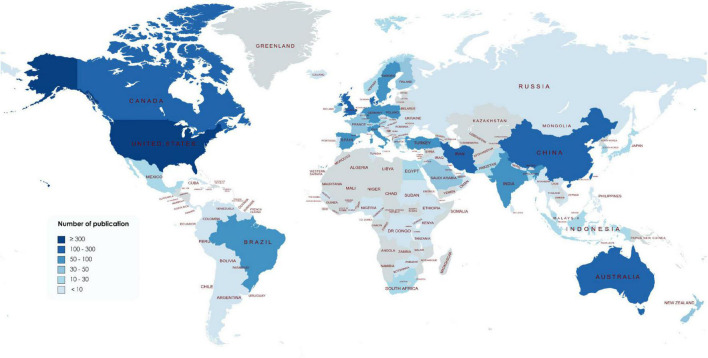
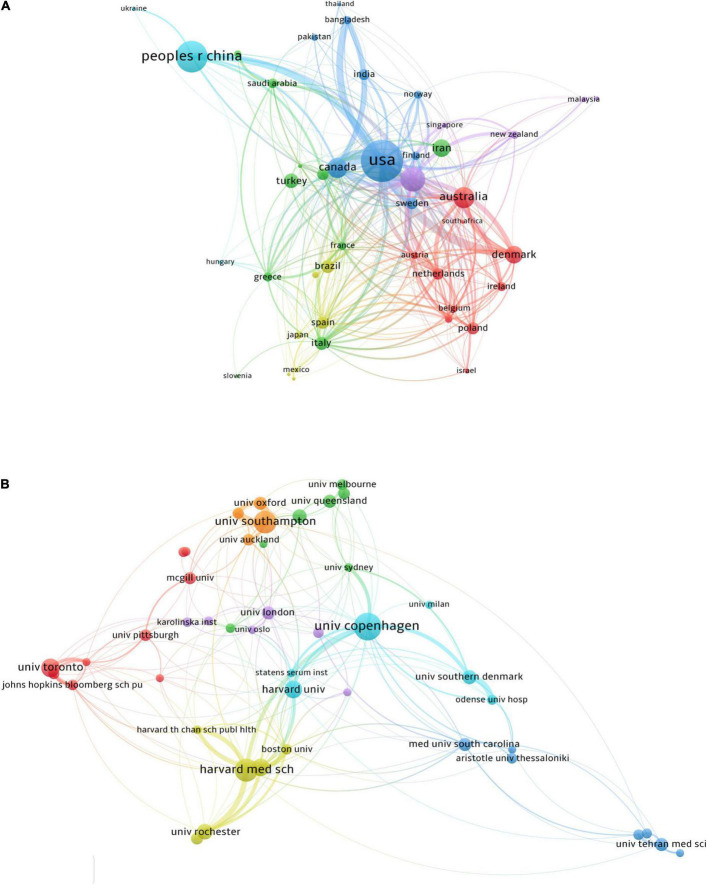
A total of 96 countries contributed to publications in this field (Figure 2). As shown in Table 1, the United States contributed the greatest number of publications (458, 25.06% of all), followed by China (283, 15.48%), England (187, 10.23%), Australia (147, 8.04%), and Canada (139, 7.60%). The top five countries in terms of ACI values were Canada (29.00), the United States (28.91), England (27.78), Denmark (24.29), and Australia (21.61). Regarding the h-index, the United States (58), England (38), and Canada (35) ranked in the top three. A total of 44 countries with more than five publications in the field were analyzed in the co-authorship analysis (Figure 3A). The five countries with the highest total link strength were the United States (total link strength = 359 times), England (264), Canada (168), Australia (135), and Denmark (124) (Table 1).
FIGURE 2.
World map of countries contributing to vitamin D and reproductive health.
TABLE 1.
Top 10 productive countries regarding the research on vitamin D and reproductive health.
| Rank | Country | Region | Quantity | Percentage | ACI | h | Total link strength |
| 1 | United States | North America | 458 | 25.06% | 28.91 | 58 | 359 |
| 2 | China | Eastern Asia | 283 | 15.48% | 13.21 | 31 | 65 |
| 3 | England | Western Europe | 187 | 10.23% | 27.78 | 38 | 264 |
| 4 | Australia | Oceania | 147 | 8.04% | 21.61 | 32 | 135 |
| 5 | Canada | North America | 139 | 7.60% | 29.00 | 35 | 168 |
| 6 | Iran | Western Asia | 107 | 5.85% | 17.71 | 28 | 25 |
| 7 | Denmark | Northern Europe | 106 | 5.80% | 24.29 | 30 | 124 |
| 8 | Turkey | Western Asia | 82 | 4.49% | 8.76 | 14 | 20 |
| 9 | Italy | Southern Europe | 68 | 3.72% | 19.87 | 23 | 109 |
| 10 | Brazil | South America | 66 | 3.61% | 9.15 | 15 | 53 |
ACI, Average Citations per Item; h, h-index.
FIGURE 3.
Co-authorship analysis of countries and institutions. (A) Network map of co-authorship between countries with more than five publications. (B) Network map of co-authorship between institutions with more than 15 publications.
As shown in Table 2, the institution with the highest number of research publications in this field is Harvard University with a quantity of 96, followed by the University of Copenhagen (68) and Harvard Medical School (47). The research institution with the highest ACI value in this field was the Medical University of South Carolina, which had an ACI value of 65.62, followed by the University of London (31.72) and Brigham and Women’s Hospital (31.40). We analyzed the co-authorship of 46 institutions with more than 15 publications. The exclusion of one item that was not connected revealed the collaborations of 45 institutions (Figure 3B). The three institutions with the highest total link strength were the University of Copenhagen (total link strength = 149 times), Harvard Medical School (145), and Brigham and Women’s Hospital (127) (Table 2).
TABLE 2.
Top 10 institutions in the studies of vitamin D and reproductive health.
| Rank | Institution | Country | Quantity | STC | ACI | Total link strength |
| 1 | Harvard University | United States | 96 | 2,884 | 30.04 | 95 |
| 2 | University of Copenhagen | Denmark | 68 | 1,601 | 23.54 | 149 |
| 3 | Harvard Medical School | United States | 47 | 1,031 | 21.94 | 145 |
| 4 | Harvard T.H. Chan School of Public Health | United States | 45 | 1,316 | 29.24 | 61 |
| 5 | University of Southampton | England | 45 | 1,351 | 30.02 | 126 |
| 6 | University of Toronto | Canada | 44 | 1,066 | 24.23 | 82 |
| 7 | Brigham and Women’s Hospital | United States | 43 | 1,350 | 31.40 | 127 |
| 8 | University of London | England | 43 | 1,364 | 31.72 | 63 |
| 9 | Medical University of South Carolina | United States | 42 | 2,756 | 65.62 | 43 |
| 10 | National Institutes of Health | United States | 41 | 792 | 19.32 | 55 |
STC, sum of the times cited; ACI, average citations per item.
Analysis of journals and distribution of disciplines
A total of 1,828 articles were published in 518 journals. As shown in Table 3, the journal with the highest number of articles in this field was Nutrients (108), followed by PLoS One (68), Journal of Steroid Biochemistry and Molecular Biology (49), Journal of Maternal Fetal Neonatal Medicine (43), and BMC Pregnancy and Childbirth (42). The journal with the highest ACI value was Journal of Clinical Endocrinology Metabolism (41.69), followed by American Journal of Clinical Nutrition (34.78), Fertility and Sterility (34.68), PLoS One (22.46), and European Journal of Clinical Nutrition (21.95). The top five journals in terms of h-index were Nutrients (25), PLoS One (24), Journal of Clinical Endocrinology Metabolism (24), BMC Pregnancy and Childbirth (17), and American Journal of Clinical Nutrition (17).
TABLE 3.
Top 15 journals in the studies of vitamin D and reproductive health.
| Rank | Journal title | Quantity | ACI | IF | Q | h |
| 1 | Nutrients | 108 | 18.64 | 6.706 | Q1 | 25 |
| 2 | PLoS One | 68 | 22.46 | 3.752 | Q2 | 24 |
| 3 | Journal of Steroid Biochemistry and Molecular Biology | 49 | 17.76 | 5.011 | Q2 | 16 |
| 4 | Journal of Maternal Fetal Neonatal Medicine | 43 | 14.67 | 2.323 | Q3 | 10 |
| 5 | BMC Pregnancy and Childbirth | 42 | 17.79 | 3.105 | Q2 | 17 |
| 6 | Journal of Clinical Endocrinology Metabolism | 35 | 41.69 | 6.134 | Q1 | 24 |
| 7 | British Journal of Nutrition | 34 | 19.59 | 4.125 | Q3 | 15 |
| 8 | American Journal of Clinical Nutrition | 27 | 34.78 | 8.162 | Q1 | 17 |
| 9 | Gynecological Endocrinology | 27 | 8.33 | 2.277 | Q3 | 9 |
| 10 | Clinical Nutrition | 23 | 13.39 | 7.643 | Q1 | 10 |
| 11 | Fertility and Sterility | 22 | 34.68 | 7.49 | Q1 | 14 |
| 12 | Scientific Reports | 22 | 11.68 | 4.996 | Q2 | 9 |
| 13 | European Journal of Clinical Nutrition | 21 | 21.95 | 4.884 | Q2 | 9 |
| 14 | Journal of Nutrition | 21 | 13.76 | 4.687 | Q2 | 8 |
| 15 | American Journal of Reproductive Immunology | 17 | 10.59 | 3.777 | Q2 | 9 |
ACI, average citations per item; IF, impact factors; Q, quartile in the category; h, h-index.
As shown in Table 4, the top three disciplines according to the number of publications were nutrition dietetics (393, 21.50%), obstetrics gynecology (384, 21.01%), and endocrinology metabolism (337, 18.44%). Additional disciplines represented in the literature were reproductive biology (142, 7.77%), pediatrics (119, 6.51%), biochemistry molecular biology (110, 6.02%), medicine general internal (105, 5.74%), public environmental occupational health (97, 5.31%), immunology (96, 5.25%), multidisciplinary sciences (94, 5.14%), and other disciplines.
TABLE 4.
The top 20 subject categories in the studies of vitamin D and reproductive health.
| Rank | WOS categories | Quantity | Percentage |
| 1 | Nutrition dietetics | 393 | 21.50% |
| 2 | Obstetrics gynecology | 384 | 21.01% |
| 3 | Endocrinology metabolism | 337 | 18.44% |
| 4 | Reproductive biology | 142 | 7.77% |
| 5 | Pediatrics | 119 | 6.51% |
| 6 | Biochemistry molecular biology | 110 | 6.02% |
| 7 | Medicine general internal | 105 | 5.74% |
| 8 | Public environmental occupational health | 97 | 5.31% |
| 9 | Immunology | 96 | 5.25% |
| 10 | Multidisciplinary sciences | 94 | 5.14% |
| 11 | Medicine research experimental | 71 | 3.88% |
| 12 | Allergy | 46 | 2.52% |
| 13 | Pharmacology pharmacy | 39 | 2.13% |
| 14 | Physiology | 33 | 1.81% |
| 15 | Cell biology | 32 | 1.75% |
| 16 | Agriculture dairy animal science | 27 | 1.48% |
| 17 | Psychiatry | 25 | 1.37% |
| 18 | Environmental sciences | 23 | 1.26% |
| 19 | Food science technology | 22 | 1.20% |
| 20 | Andrology | 20 | 1.09% |
Analysis of authors
As shown in Table 5, Hollis BW from the Medical University of South Carolina has the highest number of published articles (33), followed by Wagner CL from the Medical University of South Carolina (30) and Litonjua AA from the University of Rochester (26). The top three authors in terms of ACI values were Hollis BW (71.00), Wagner CL (64.93), and Camargo CA (49.88) from Massachusetts General Hospital. Five of the top 10 authors are from the United States, four are from England, and one is from Canada.
TABLE 5.
Top 10 authors in the studies of vitamin D and reproductive health.
| Rank | Author | Country | Institute | TP | P | ACI | h |
| 1 | Hollis BW | United States | Medical University of South Carolina | 33 | 1.81% | 71.00 | 23 |
| 2 | Wagner CL | United States | Medical University of South Carolina | 30 | 1.64% | 64.93 | 20 |
| 3 | Litonjua AA | United States | University of Rochester | 26 | 1.42% | 40.58 | 14 |
| 4 | Cooper C | England | University of Southampton | 25 | 1.37% | 38.28 | 16 |
| 5 | Harvey NC | England | University of Southampton | 24 | 1.31% | 40.29 | 16 |
| 6 | Godfrey KM | England | University of Southampton | 23 | 1.26% | 35.48 | 16 |
| 7 | Weiss ST | United States | Brigham and Women’s Hospital | 23 | 1.26% | 34.09 | 11 |
| 8 | Roth DE | Canada | University of Toronto | 22 | 1.20% | 26.55 | 12 |
| 9 | Camargo CA | United States | Massachusetts General Hospital | 16 | 0.88% | 49.88 | 13 |
| 10 | Hewison M | England | University of Birmingham | 16 | 0.88% | 42.50 | 10 |
TP, total publications; P, percentage; ACI, average citations per item; h, h-index.
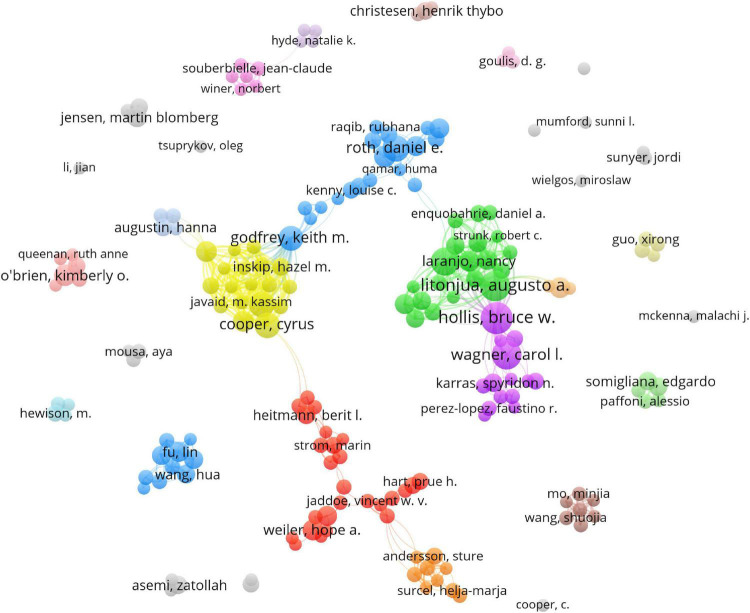
We analyzed a total of 204 authors that were co-authored in more than four publications (Figure 4). The five authors with the highest total link strength were Weiss ST (total link strength = 142 times), Litonjua AA (138), Harvey NC (130), Cooper C (129), and Godfrey KM (119). Three of the five are from the same institution called the University of Southampton.
FIGURE 4.
Network map of co-authorship between authors with more than four publications.
Citation and co-citation analyses
Table 6 lists the top 10 documents with the highest citations. There were 501 citations for “Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-A review of recent evidence,” (27) followed by “Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies,” (15) with 335 citations. The third-ranked article for the largest number of citations was “Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis,” (14) with 290 citations.
TABLE 6.
Top 10 citation analysis of documents on vitamin D and reproductive health.
| Rank | Title | Journal | Type | Authors | Y | C | IN | CN |
| 1 | Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence | Autoimmunity Reviews | Review | Pludowski et al. (27) | 2013 | 501 | 3 | 1 |
| 2 | Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies | British Medical Journal | Review | Aghajafari et al. (15) | 2013 | 335 | 1 | 1 |
| 3 | Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis | Journal of Maternal-Fetal & Neonatal Medicine | Review | Wei et al. (14) | 2013 | 290 | 2 | 2 |
| 4 | Vitamin D supplementation for women during pregnancy | Cochrane Database of Systematic Reviews | Review | De-Regil et al. (104) | 2012 | 238 | 3 | 2 |
| 5 | Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years the VDAART randomized clinical trial | Journal of the American Medical Association | Article | Litonjua et al. (73) | 2016 | 237 | 11 | 2 |
| 6 | Micronutrient deficiencies in pregnancy worldwide: health effects and prevention | Nature Reviews Endocrinology | Review | Gernand et al. (105) | 2016 | 205 | 3 | 1 |
| 7 | Vitamin D supplementation for women during pregnancy | Cochrane Database of Systematic Reviews | Review | De-Regil et al. (106) | 2016 | 196 | 4 | 3 |
| 8 | Vitamin D and fertility: a systematic review | European Journal of Endocrinology | Review | Lerchbaum et al. (107) | 2012 | 192 | 1 | 1 |
| 9 | Vitamin D supplementation in pregnancy: a systematic review | Health Technology Assessment | Review | Harvey et al. (108) | 2014 | 189 | 4 | 1 |
| 10 | Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis | Pediatric and perinatal epidemiology | Review | Thorne-Lyman et al. (109) | 2012 | 188 | 1 | 1 |
Y, publication year; C, citations; IN, institute number; CN, country number.
Table 7 lists the top 10 references with the highest co-citations. The five references with the largest number of citations were by Aghajafari et al. (15), Holick (28), Holick et al. (29), Hollis et al. (30), and Bodnar et al. (31).
TABLE 7.
Top 10 co-citation analysis of cited reference on vitamin D and reproductive health.
| Rank | Title | Journal | Type | Authors | Y | C | IN | CN |
| 1 | Vitamin D deficiency | The New England Journal of Medicine | Review | Holick (28) | 2007 | 381 | 1 | 1 |
| 2 | Evaluation, treatment, and prevention of vitamin D Deficiency: an endocrine society clinical practice guideline | Journal of Clinical Endocrinology & Metabolism | Article | Holick et al. (29) | 2011 | 369 | 8 | 3 |
| 3 | Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness | Journal of Bone and Mineral Research | Article | Hollis et al. (30) | 2011 | 280 | 1 | 1 |
| 4 | Maternal vitamin D deficiency increases the risk of preeclampsia | Journal of Clinical Endocrinology & Metabolism | Article | Bodnar et al. (31) | 2007 | 249 | 3 | 1 |
| 5 | Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies | British Medical Journal | Review | Aghajafari et al. (15) | 2013 | 201 | 1 | 1 |
| 6 | Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study | Lancet | Article | Javaid et al. (110) | 2006 | 183 | 2 | 1 |
| 7 | Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis | Journal of Maternal-Fetal & Neonatal Medicine | Review | Wei et al. (14) | 2013 | 180 | 2 | 2 |
| 8 | Maternal vitamin D status during pregnancy and child outcomes | European Journal of Clinical Nutrition | Article | Gale et al. (111) | 2008 | 172 | 1 | 1 |
| 9 | High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates | Journal of Nutrition | Article | Bodnar et al. (31) | 2007 | 157 | 2 | 1 |
| 10 | Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: results of the multi-ethnic Amsterdam born children and their development cohort | British Journal of Nutrition | Article | Leffelaar et al. (112) | 2010 | 139 | 3 | 1 |
Y, publication year; C, citations; IN, institute number; CN, country number.
Research hotspots and frontier analysis
Keywords are highly summarized and focused on descriptions of the subject of the article, which means that keywords with high frequency can reflect the research hotspots and trends of major issues in related fields. As shown in Table 8, in addition to vitamin D, keywords with a high frequency of occurrence were vitamin D deficiency (890), pregnancy (858), risk (493), vitamin D supplementation (396), 25-hydroxyvitamin D (378), and women (377).
TABLE 8.
The top 20 keywords in the studies of vitamin D and reproductive health.
| Rank | Keywords | Occurrences | Total link strength | Rank | Keywords | Occurrences | Total link strength |
| 1 | Vitamin D | 912 | 5,584 | 11 | Outcomes | 204 | 1,501 |
| 2 | Vitamin D deficiency | 890 | 5,724 | 12 | Preeclampsia | 196 | 1,369 |
| 3 | Pregnancy | 858 | 5,369 | 13 | Insulin resistance | 172 | 1,122 |
| 4 | Risk | 493 | 3,317 | 14 | Expression | 166 | 881 |
| 5 | Vitamin D supplementation | 396 | 2,805 | 15 | Vitamin D receptor | 162 | 876 |
| 6 | 25-hydroxyvitamin D | 378 | 2,613 | 16 | Children | 161 | 999 |
| 7 | Women | 377 | 2,436 | 17 | Prevalence | 153 | 1,005 |
| 8 | Association | 288 | 1,929 | 18 | Supplementation | 146 | 1,012 |
| 9 | Health | 213 | 1,438 | 19 | Pregnant women | 144 | 986 |
| 10 | Calcium | 205 | 1,261 | 20 | Meta-analysis | 135 | 991 |
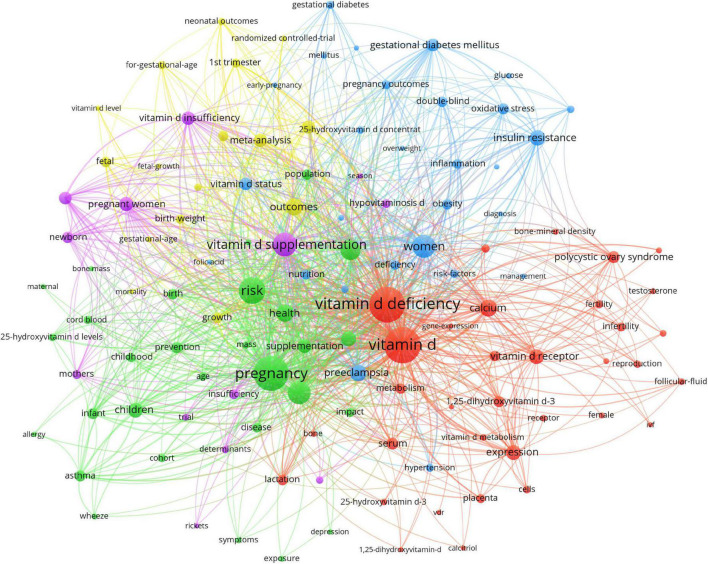
We analyzed a total of 120 keywords that were identified as occurring more than 20 times (Figure 5). The size of the node represents the number of times the keyword appears, and the thickness of the curve between the nodes represents the frequency of the two keywords appearing together. The keywords formed five clusters, which represented the five major research directions in the field.
FIGURE 5.
Network visualization of keywords in studies on vitamin D and reproductive health.
The red cluster was dominated by female, infertility, semen quality, PCOS, and VDR. Vitamin D could affect the synthesis of human hormones (32), the development of oocytes in women (13), the quality of sperm in men (33), and the implantation of the embryo (34). PCOS is one of the critical causes of infertility in childbearing women and its incidence was closely linked to vitamin D deficiency. VDR polymorphisms played an important role in the development of PCOS and associated hormonal and metabolic abnormalities (35).
The yellow cluster focused on randomized controlled trial (RCT), meta-analysis, and neonatal outcomes. RCTs and meta-analyses were the more common types of studies investigating the association between vitamin D and reproductive health. Most RCTs evaluated the efficacy of vitamin D supplementation on IVF outcomes (36), infant acute respiratory infections (37), psychological distress (38), maternal postnatal bone indices (39), and so on. The meta-analysis was a secondary analysis primarily based on RCTs. Neonatal outcomes involved in exploring the effects of maternal vitamin D deficiency on offspring, including small-for-gestational-age (40), neonatal bone mass (41), infant glucose metabolism (42), infant atopic dermatitis (43), infant acute respiratory infections (37), infant birth weight (44), offspring sex ratio (45), infant gut microbiota (46), offspring socioemotional development (47), infant neurodevelopment (48, 49).
The blue cluster was mainly composed of diet, nutrition, and pregnancy outcomes. Vitamin D is an exogenous nutrient that can be influenced by dietary patterns (50), making diet and nutrition a significant subject in this field. Pregnancy outcomes are primarily concerned with adverse maternal effects of abnormal vitamin D status (insufficiency or deficiency), including GDM, hypertension, and preeclampsia (14).
The main research topics of the green cluster were allergy, asthma, wheeze, population, and depression. The first three parts were mainly to study the relationship between preconception and pregnancy serum vitamin D concentration and childhood atopic diseases (51–53). Population referred to discussing the relevant social factors affecting vitamin D levels (54). Depression discussed the role of poor vitamin D in perinatal depression (55).
The high-frequency ones in the purple cluster were vitamin D supplementation, season, and determinants. This clustering mainly addressed relevant determinants of vitamin D levels, such as vitamin D supplementation and season.
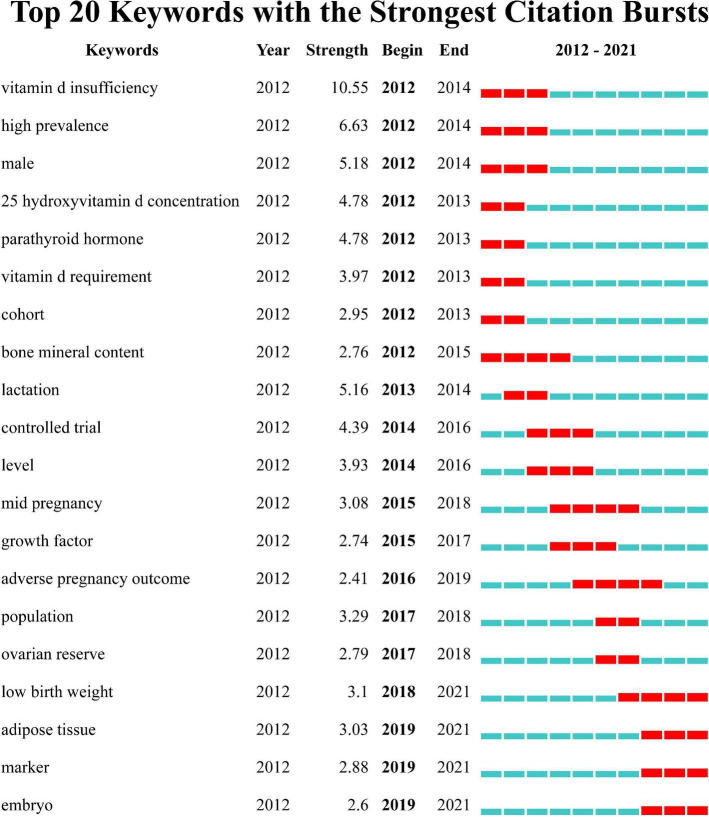
Burst patterns of keywords can reveal the frontiers and priorities of research between vitamin D and reproductive health. As shown in Figure 6, the timeline is depicted as a year-sliced blue line, where the red section is the detected burst, indicating the start and end years and duration of the burst. Vitamin D insufficiency ranked first with the highest burst strength (10.55), followed by high prevalence (6.63), male (5.18), lactation (5.16), 25 hydroxyvitamin D concentration (4.78), and parathyroid hormone (4.78). The burst times of the terms bone mineral content, adverse pregnancy outcome, and low birth weight lasted for 4 years. Low birth weight, adipose tissue, marker, and embryo are currently within the burst period and might become new research foci in this field.
FIGURE 6.
Top 20 keywords with the strongest citation bursts based on CiteSpace.
Discussion
In this paper, we conducted a bibliometric analysis of the literature on vitamin D and reproductive health published from 2012 to 2021 based on the visualization software CiteSpace and VOSviewer. The spatial and temporal distribution, author and journal contribution, core literature, research hotspots, and research frontier analysis were assessed. The timeline of keywords revealed the evolution of the research theme and the burst analysis suggested current research hotspots.
The research on vitamin D in reproductive health showed a steady upward trend. Research on vitamin D in the field of reproductive health can still arouse great interest so far. On one hand, it is inextricably linked to the benefits of vitamin D. Vitamin D has a physiological role in ovarian follicular development and luteinization, including alteration of AMH signaling, FSH sensitivity, and progesterone production and release (56). Vitamin D also performed vital functions in male reproduction. Serum 25-(OH)D3 level was positively associated with sperm motility and VDR in human spermatozoa could be regarded as a positive predictor of sperm quality (57). On the other hand, some of the effects of vitamin D on reproductive health are controversial and have not yet been elucidated. For example, there were differing views on whether vitamin D was associated with IVF outcomes or whether vitamin D supplementation could improve IVF pregnancy outcomes. A retrospective study that analyzed the association between serum 25-(OH)D3 level 7 days prior to embryo transfer and pregnancy revealed that vitamin D deficiency impaired clinical pregnancy rate in a single blastocyst transfer cycle (58). Conversely, a RCT by Somigliana et al. showed that a single oral dose of 600,000 IU of vitamin D3 did not improve IVF clinical pregnancy rates in women with normal weight (36).
The study of vitamin D in the reproductive health field showed a pattern of extensive coverage, but uneven development. As shown in Figure 1, approximately more than half of the countries in the world, covering 5 continents, have been involved in this area of research in the last decade. This was probably due to the high prevalence of vitamin D abnormalities (vitamin D insufficiency and deficiency) among the reproductive-aged and pregnant women, which was a worldwide phenomenon. Even in abundant sunshine’s tropical countries like India, the prevalence of vitamin D deficiency among reproductive-aged women is as high as 80%. Numerous studies indicated that vitamin D insufficiency or deficiency varied with geography, economic situation, social status, national policy (food fortification), and individual awareness (59–62). From raising population self-awareness, national intervention to the actual improvement of vitamin D levels, each country needs to further explore its own solutions.
Overall, the America, Europe, Asia, and Oceania were relatively active in this field, while Africa might be in a low active state due to limited economic development. Of these, North America, especially the United States and Canada, probably hold a stronger basis and more mature research system in the field, as 7 and 6 of the top 10 productive institutions and authors come from these two countries, respectively. The Medical University of South Carolina in the United States had the highest ACI, and the top two productive authors are from this institution (Tables 1, 2). The research team mainly focused on prenatal vitamin D screening (63), and the effects of vitamin D supplementation on early life (pregnancy, lactation, and childhood), including glycemic, lipidemic, oxidative stress biomarkers (64), inflammatory biomarkers (65), growth factors, immune-mediators (66), bone mineral density (67), the vaginal microbiome (68), preeclampsia (69) during pregnancy, preterm birth (70), offspring epigenetic clock (71), asthma, allergies and recurrent wheeze (72–75). The conclusions of most original studies were derived from RCTs, with high-level evidence and instructive significance. Furthermore, they also dabbled in basic research, using model animals to explore the effects of a low vitamin D diet on maternal hypertension as well as placental and fetal development (76). Researchers of the University of Southampton focused more on the association between vitamin D and bone-related outcomes such as maternal osteoporosis (39, 77), offspring bone structure (78), bone formation (79), bone mass (80), bone health (81–83) and fractures in late childhood (84). Concurrently, genetic variation in gene expression also received greater interest, with studies showing that detection of genetic susceptibility-related variant genes for vitamin D deficiency may guide vitamin D supplementation (85–87). Europe and Asia are also undergoing rapid development and are represented by the University of Southampton and Tehran University of Medical Sciences, respectively. Tehran University of Medical Sciences paid more attention to the role of vitamin D in male reproduction (88–90) and the mechanism by which vitamin D improved female reproductive disorders including endometriosis (91, 92), PCOS (93, 94), repeated implantation failure (51, 95), and endometritis (96).
It formed a development landscape of multidisciplinary intersection with nutrition, obstetrics and gynecology, and endocrinology metabolism as the core in this field. From Tables 3, 4, we can note that nutrition, gynecology and obstetrics, and endocrinology metabolism are the most dominant WoS categories and the scope of the top 15 journals. These were reflected in the literature on topics such as diet and vitamin D supplementation, the effects of vitamin D on the maternal and offspring, and the expression, role, and variation of vitamin D-related receptors and enzymes. Meanwhile, we should also take note of public environmental, environmental sciences, immunology, multidisciplinary sciences, biochemistry molecular biology, medicine research experimental, pharmacology, cell biology, and so on (Table 4). Multidisciplinary intersection contributed to a more comprehensive understanding of the role of vitamin D in reproductive health to guide the prevention, treatment, and even reversal of the consequences of vitamin D deficiency. The participation of basic disciplines is to split the whole into the tissue, cellular, and molecular levels, to gain an in-depth understanding of the mechanism.
Risk factors and adverse impacts of vitamin D deficiency were the focus of current research. The effect of maternal vitamin D levels on fetal lipid metabolism and the prediction of fertility by vitamin D-related markers might be hot spots and frontier areas in the field. Adipose tissue serves as a reservoir for vitamin D and affects its activity (97). Maternal vitamin D deficiency may affect fetal weight by influencing lipid metabolism, but the current findings are controversial. A birth cohort-based study in China suggested that maternal serum 25-(OH)D3 was positively associated with fetal birth weight and that vitamin D deficiency during pregnancy increased the risk of low birth weight infants (98). A study performed by Morales et al. indicated that maternal deficit of 25(OH)D3 was associated with an increased risk of fetal overweight and overweight in offspring at age 1 year (99). This might be due to maternal vitamin D deficiency resulting in polarization in the adipose depots (100). Moreover, vitamin D-related indexes are expected to be new markers for fertility assessment. Li et al. reported urine VDBP levels were significantly positively correlated with ovarian reserve and were expected to be a biomarker for predicting ovarian reserve (101). In assisted reproductive technology, vitamin D level in the follicular fluid could be used as a marker of oocyte quality, and vitamin D level in serum could be used as a marker of in vitro fertilization outcome (102). For men of childbearing age, the vitamin D metabolizing enzyme CYP24A1 was positively correlated with total sperm count, concentration, motility, and morphology, and the expression of CYP24A1 at the annulus of human spermatozoa might serve as a novel marker of semen quality (103). Therefore, further research is needed to clarify the above-mentioned effects of vitamin D, and RCT with a higher level of evidence might be a more meaningful research approach.
To our knowledge, this study is the first bibliometric analysis to explore the research on vitamin D in reproductive health. However, it had a few limitations. First, we only selected the WoS database and were limited by visual analysis software to include only the WoSCC. Second, we only analyzed the literature over the last decade, but this field has developed for such a considerable period of time that some previous views might be ignored by us. Finally, some of the more recently published articles may have been overlooked by the hotspot analysis because they did not have sufficient citations.
Conclusion
Vitamin D holds great research significance and the clinical application potential in the field of reproductive health. Based on visual analysis software, we evaluated the evolution process, contribution distribution, development trend, research hotspots, and frontiers of vitamin D research in reproductive health over the past decade. The field has received great attention globally, and the multidisciplinary intersection is the development trend. Furthermore, the effect of maternal vitamin D levels on fetal lipid metabolism and the prediction of fertility by vitamin D-related markers might be the focus of the research, and the exploration of population heterogeneity in the diagnostic criteria of vitamin D deficiency and the specific mechanisms of vitamin D effects on reproductive health should also be paid attention to.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
XZ and YL were responsible for experiment conception and design, collection and assembly of data, data analysis and interpretation, and manuscript writing. SW and SZ contributed acquisition data and revised the manuscript. JT designed the work, provided technical guidance, and finally approved the manuscript. All authors read and approved the final manuscript.
Footnotes
Funding
This work was supported by the National Key Research and Development Program (2018YFC1002105), the National Natural Science Foundation of China (82071601), the Major Special Construction Plan for Discipline Construction Project of China Medical University (3110118033), and the Shengjing Freelance Researcher Plan of Shengjing Hospital of China Medical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Zmijewski MA. Vitamin D and human health. Int J Mol Sci. (2019) 20:145. 10.3390/ijms20010145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson LR, Tripkovic L, Hart KH, Lanham-New SA. Vitamin D deficiency as a public health issue: using vitamin D2 or vitamin D3 in future fortification strategies. Proc Nutr Soc. (2017) 76:392–9. 10.1017/S0029665117000349 [DOI] [PubMed] [Google Scholar]
- 3.Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. (2012) 95:1357–64. 10.3945/ajcn.111.031070.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young AR, Morgan KA, Harrison GI, Lawrence KP, Petersen B, Wulf HC, et al. A revised action spectrum for vitamin D synthesis by suberythemal UV radiation exposure in humans in vivo. Proc Natl Acad Sci USA. (2021) 118:1–8. 10.1073/pnas.2015867118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikle D, Christakos S. New aspects of vitamin D metabolism and action — addressing the skin as source and target. Nat Rev Endocrinol. (2020) 16:234–52. 10.1038/s41574-019-0312-5 [DOI] [PubMed] [Google Scholar]
- 6.Martens P, Gysemans C, Verstuyf A, Mathieu C. Vitamin D ’s effect on immune function. Nutrients. (2020) 12:1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys. (2012) 523:123–33. 10.1016/j.abb.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 8.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. (2015) 96:365–408. 10.1152/physrev.00014.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hii CS, Ferrante A. The non-genomic actions of vitamin D. Nutrients. (2016) 8:1–14. 10.3390/nu8030135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valdivielso JM, Fernandez E. Vitamin D receptor polymorphisms and diseases. Clin Chim Acta. (2006) 371:1–12. 10.1016/j.cca.2006.02.016 [DOI] [PubMed] [Google Scholar]
- 11.Ginde AA, Sullivan AF, Mansbach JM, Jr., Camargo CA. Vitamin D insufficiency in pregnant and nonpregnant women of childbearing age in the United States. Am J Obstet Gynecol. (2010) 202:.e1–436. 10.1016/j.ajog.2009.11.036.Vitamin [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peterlik M, Boonen S, Cross HS, Lamberg-Allardt C. Vitamin D and calcium insufficiency-related chronic diseases: an emerging world-wide public health problem. Int J Environ Res Public Health. (2009) 6:2585–607. 10.3390/ijerph6102585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahrokhi SZ, Ghaffari F, Kazerouni F. Role of vitamin D in female reproduction. Clin Chim Acta. (2016) 455:33–8. 10.1016/j.cca.2015.12.040 [DOI] [PubMed] [Google Scholar]
- 14.Wei SQ, Qi HP, Luo ZC, Fraser WD. Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis. J Matern Neonatal Med. (2013) 26:889–99. 10.3109/14767058.2013.765849 [DOI] [PubMed] [Google Scholar]
- 15.Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O’Beirne M, Rabi DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational Studies. BMJ. (2013) 346:1–14. 10.1136/bmj.f1169 [DOI] [PubMed] [Google Scholar]
- 16.Nassar N, Halligan GH, Roberts CL, Morris JM, Ashton AW. Systematic review of first-trimester vitamin D normative levels and outcomes of pregnancy. Am J Obstet Gynecol. (2011) 205:.e1–208. 10.1016/j.ajog.2011.03.058 [DOI] [PubMed] [Google Scholar]
- 17.Magnus MC, Miliku K, Bauer A, Engel SM, Felix JF, Jaddoe VWV, et al. Vitamin D and risk of pregnancy related hypertensive disorders: Mendelian randomisation study. BMJ. (2018) 361:k2167. 10.1136/bmj.k2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matias ML, Romao-Veiga M, Ribeiro VR, Nunes PR, Gomes VJ, Devides AC, et al. Progesterone and vitamin D downregulate the activation of the NLRP1/NLRP3 inflammasomes and TLR4-MyD88-NF-κB pathway in monocytes from pregnant women with preeclampsia. J Reprod Immunol. (2021) 144:103286. 10.1016/j.jri.2021.103286 [DOI] [PubMed] [Google Scholar]
- 19.Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest. (2005) 115:485–91. 10.1172/JCI200524531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C, et al. Replete vitamin D stores predict reproductive success following IVF. Fertil Steril. (2010) 94:1314–9. 10.1016/j.fertnstert.2009.05.019.Replete [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He C, Lin Z, Robb SW, Ezeamama AE. Serum vitamin d levels and polycystic ovary syndrome: a systematic review and meta-analysis. Nutrients. (2015) 7:4555–77. 10.3390/nu7064555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sayegh L, Fuleihan GEH, Nassar AH. Vitamin D in endometriosis: a causative or confounding factor? Metabolism. (2014) 63:32–41. 10.1016/j.metabol.2013.09.012 [DOI] [PubMed] [Google Scholar]
- 23.Vergara D, Catherino WH, Trojano G, Tinelli A. Vitamin D: mechanism of action and biological effects in uterine fibroids. Nutrients. (2021) 13:1–11. 10.3390/nu13020597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Lu Y, Wu S, Zhang S, Li S, Tan J. An overview of current research on mesenchymal stem cell-derived extracellular vesicles: a bibliometric analysis from 2009 to 2021. Front Bioeng Biotechnol. (2022) 10:910812. 10.3389/fbioe.2022.910812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukherjee D, Lim WM, Kumar S, Donthu N. Guidelines for advancing theory and practice through bibliometric research. J Bus Res. (2022) 148:101–15. 10.1016/j.jbusres.2022.04.042 [DOI] [Google Scholar]
- 26.Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci USA. (2004) 101:5303–10. 10.1073/pnas.0307513100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pludowski P, Holick MF, Pilz S, Wagner CL, Hollis BW, Grant WB, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun Rev. (2013) 12:976–89. 10.1016/j.autrev.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 28.Holick MF. Vitamin D deficiency. N Engl J Med. (2007) 357:266–81. 10.1136/bmj.318.7193.1284a [DOI] [PubMed] [Google Scholar]
- 29.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2011) 96:1911–30. 10.1210/jc.2011-0385 [DOI] [PubMed] [Google Scholar]
- 30.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. (2011) 26:2341–57. 10.1002/jbmr.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. (2007) 92:3517–22. 10.1210/jc.2007-0718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan T, Sun H, Mao Z, Zhang L, Chen X, Shi Y, et al. Vitamin D deficiency inhibits microRNA-196b-5p which regulates ovarian granulosa cell hormone synthesis, proliferation, and apoptosis by targeting RDX and LRRC17. Ann Transl Med. (2021) 9:1775–1775. 10.21037/atm-21-6081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Angelis C, Galdiero M, Pivonello C, Garifalos F, Menafra D, Cariati F, et al. The role of vitamin D in male fertility: a focus on the testis. Rev Endocr Metab Disord. (2017) 18:285–305. 10.1007/s11154-017-9425-0 [DOI] [PubMed] [Google Scholar]
- 34.Cai S, Li J, Zeng S, Hu L, Peng Y, Tang S, et al. Impact of vitamin D on human embryo implantation—a prospective cohort study in women undergoing fresh embryo transfer. Fertil Steril. (2021) 115:655–64. 10.1016/j.fertnstert.2020.09.005 [DOI] [PubMed] [Google Scholar]
- 35.Wehr E, Trummer O, Giuliani A, Gruber HJ, Pieber TR, Obermayer-Pietsch B. Vitamin D-associated polymorphisms are related to insulin resistance and vitamin D deficiency in polycystic ovary syndrome. Eur J Endocrinol. (2011) 164:741–9. 10.1530/EJE-11-0134 [DOI] [PubMed] [Google Scholar]
- 36.Somigliana E, Sarais V, Reschini M, Ferrari S, Makieva S, Cermisoni GC, et al. Single oral dose of vitamin D3 supplementation prior to in vitro fertilization and embryo transfer in normal weight women: the SUNDRO randomized controlled trial. Am J Obstet Gynecol. (2021) 225:.e1–283. 10.1016/j.ajog.2021.04.234 [DOI] [PubMed] [Google Scholar]
- 37.Morris SK, Pell LG, Rahman MZ, Al Mahmud A, Shi J, Ahmed T, et al. Effects of maternal vitamin D supplementation during pregnancy and lactation on infant acute respiratory infections: follow-up of a randomized trial in Bangladesh. J Pediatric Infect Dis Soc. (2021) 10:901–9. 10.1093/jpids/piab032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajabi-Naeeni M, Dolatian M, Qorbani M, Vaezi AA. Effect of omega-3 and vitamin D co-supplementation on psychological distress in reproductive-aged women with pre-diabetes and hypovitaminosis D: a randomized controlled trial. Brain Behav. (2021) 11:1–10. 10.1002/brb3.2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curtis EM, Parsons C, Maslin K, D’Angelo S, Moon RJ, Crozier SR, et al. Bone turnover in pregnancy, measured by urinary CTX, is influenced by vitamin D supplementation and is associated with maternal bone health: findings from the maternal vitamin D osteoporosis study (MAVIDOS) trial. Am J Clin Nutr. (2021) 114:1600–11. 10.1093/ajcn/nqab264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen B, Chen Y, Xu Y. Vitamin D deficiency in pregnant women: influenced by multiple risk factors and increase the risks of spontaneous abortion and small-for-gestational age. Medicine (Baltimore). (2021) 100:e27505. 10.1097/MD.0000000000027505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gharibeh N, Razaghi M, Vanstone CA, Wei S, McNally D, Rauch F, et al. Maternal vitamin d status and gestational weight gain as correlates of neonatal bone mass in healthy term breastfed young infants from Montreal, Canada. Nutrients. (2021) 13:4189. 10.3390/nu13124189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palacios C, Trak-Fellermeier MA, Melendez M, Campos M, Pomeroy J, Guo K, et al. Associations between vitamin d levels and glucose metabolism markers among pregnant women and their infants in Puerto Rico. Nutr Hosp. (2021) 38:1224–31. 10.20960/nh.03600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian Y, Ye Y, Zhang Y, Dou L, Dou Y, Zhao P, et al. Maternal serum 25-hydroxyvitamin D levels and infant atopic dermatitis: a prospective cohort study. Pediatr Allergy Immunol. (2021) 32:1637–45. 10.1111/pai.13582 [DOI] [PubMed] [Google Scholar]
- 44.Yang W, Jiao M, Xi L, Han N, Luo S, Xu X, et al. The association between maternal fat-soluble vitamin concentrations during pregnancy and infant birth weight in China. Br J Nutr. (2021) 125:1058–66. 10.1017/S0007114520003347 [DOI] [PubMed] [Google Scholar]
- 45.Purdue-Smithe AC, Kim K, Nobles C, Schisterman EF, Schliep KC, Perkins NJ, et al. The role of maternal preconception vitamin D status in human offspring sex ratio. Nat Commun. (2021) 12:1–9. 10.1038/s41467-021-23083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaidi AZ, Moore SE, Okala SG. Impact of maternal nutritional supplementation during pregnancy and lactation on the infant gut or breastmilk microbiota: a systematic review. Nutrients. (2021) 13:1137. 10.3390/nu13041137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Francis EC, Charron E, Li M, Chen L, Mayo R, Butler LS, et al. Third trimester maternal vitamin D and early childhood socioemotional development. Paediatr Perinat Epidemiol. (2021) 35:350–8. 10.1111/ppe.12736 [DOI] [PubMed] [Google Scholar]
- 48.Sass L, Vinding RK, Stokholm J, Bjarnadóttir E, Noergaard S, Thorsen J, et al. High-dose vitamin D supplementation in pregnancy and neurodevelopment in childhood: a prespecified secondary analysis of a randomized clinical trial. JAMA Netw open. (2020) 3:e2026018. 10.1001/jamanetworkopen.2020.26018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Voltas N, Canals J, Hernández-Martínez C, Serrat N, Basora J, Arija V. Effect of vitamin D status during pregnancy on infant neurodevelopment: the eclipses study. Nutrients. (2020) 12:1–20. 10.3390/nu12103196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bolesławska I, Kowalówka M, Dobrzyńska M, Karaźniewicz-łada M, Przysławski J. Differences in the concentration of vitamin D metabolites in plasma due to the low-carbohydrate-high-fat diet and the Eastern European diet—a pilot study. Nutrients. (2021) 13:2774. 10.3390/nu13082774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu M, Litonjua AA, O’Connor GT, Zeiger RS, Bacharier L, Schatz M, et al. Effect of early and late prenatal vitamin D and maternal asthma status on offspring asthma or recurrent wheeze. J Allergy Clin Immunol. (2021) 147:1234–1241.e3. 10.1016/j.jaci.2020.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knihtilä HM, Stubbs BJ, Carey VJ, Laranjo N, Chu SH, Kelly RS, et al. Low gestational vitamin D level and childhood asthma are related to impaired lung function in high-risk children. J Allergy Clin Immunol. (2021) 148:110–9. 10.1016/j.jaci.2020.12.647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Briceno Noriega D, Savelkoul HFJ. Vitamin D and allergy susceptibility during gestation and early life. Nutrients. (2021) 13:1–19. 10.3390/nu13031015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weiler HA, Vanstone CA, Razaghi M, Gharibeh N, Patel S, Wei SQ, et al. Disparities in vitamin D status of Newborn infants from a diverse sociodemographic population in Montreal, Canada. J Nutr. (2022) 152:255–68. 10.1093/jn/nxab344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan Q, Liu S, Chen D. Poor vitamin D status and the risk of maternal depression: a dose-response meta-analysis of observational studies. Public Health Nutr. (2021) 24:2161–70. 10.1017/S1368980019004919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Irani M, Merhi Z. Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertil Steril. (2014) 102:460–8. 10.1016/j.fertnstert.2014.04.046 [DOI] [PubMed] [Google Scholar]
- 57.Jensen MB. Vitamin D and male reproduction. Nat Rev Endocrinol. (2014) 10:175–86. 10.1038/nrendo.2013.262 [DOI] [PubMed] [Google Scholar]
- 58.Polyzos NP, Anckaert E, Guzman L, Schiettecatte J, Van Landuyt L, Camus M, et al. Vitamin D deficiency and pregnancy rates in women undergoing single embryo, blastocyst stage, transfer (SET) for IVF/ICSI. Hum Reprod. (2014) 29:2032–40. 10.1093/humrep/deu156 [DOI] [PubMed] [Google Scholar]
- 59.Miller KM, De Klerk NH, Davis EA, Lucas RM, Hart PH, Haynes A. Demographic and clinical predictors of vitamin D status in pregnant women tested for deficiency in Western Australia. Aust N Z J Public Health. (2021) 45:474–81. 10.1111/1753-6405.13150 [DOI] [PubMed] [Google Scholar]
- 60.Chang CJ, Barr DB, Zhang Q, Dunlop AL, Smarr MM, Kannan K, et al. Associations of single and multiple per- and polyfluoroalkyl substance (PFAS) exposure with vitamin D biomarkers in African American women during pregnancy. Environ Res. (2021) 202:111713. 10.1016/j.envres.2021.111713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moos C, Duus KS, Frederiksen P, Heitmann BL, Andersen V. Exposure to the danish mandatory vitamin D fortification policy in prenatal life and the risk of developing coeliac disease—the importance of season: a semi ecological study. Nutrients. (2020) 12:1–11. 10.3390/nu12051243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han T, Dong J, Zhang J, Zhang C, Wang Y, Zhang Z, et al. Nutrient supplementation among pregnant women in China: an observational study. Public Health Nutr. (2021) 25:1537–42. 10.1017/S1368980021001269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rostami M, Tehrani FR, Simbar M, Yarandi RB, Minooee S, Hollis BW, et al. Effectiveness of prenatal Vitamin D deficiency screening and treatment program: a stratified randomized field trial. J Clin Endocrinol Metab. (2018) 103:2936–48. 10.1210/jc.2018-00109 [DOI] [PubMed] [Google Scholar]
- 64.Motamed S, Nikooyeh B, Kashanian M, Chamani M, Hollis BW, Neyestani TR. Evaluation of the efficacy of two doses of vitamin D supplementation on glycemic, lipidemic and oxidative stress biomarkers during pregnancy: a randomized clinical trial. BMC Pregnancy Childbirth. (2020) 20:619. 10.1186/s12884-020-03311-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Motamed S, Nikooyeh B, Kashanian M, Hollis BW, Neyestani TR. Efficacy of two different doses of oral vitamin D supplementation on inflammatory biomarkers and maternal and neonatal outcomes. Matern Child Nutr. (2019) 15:1–10. 10.1111/mcn.12867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khatiwada A, Wolf BJ, Mulligan JK, Shary JR, Hewison M, Baatz JE, et al. Effects of vitamin D supplementation on circulating concentrations of growth factors and immune-mediators in healthy women during pregnancy. Pediatr Res. (2021) 89:554–62. 10.1038/s41390-020-0885-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei W, Shary JR, Garrett-Mayer E, Anderson B, Forestieri NE, Hollis BW, et al. Bone mineral density during pregnancy in women participating in a randomized controlled trial of Vitamin D supplementation. Am J Clin Nutr. (2017) 106:1422–30. 10.3945/ajcn.116.140459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jefferson KK, Parikh HI, Garcia EM, Edwards DJ, Serrano MG, Hewison M, et al. Relationship between Vitamin D status and the vaginal microbiome during pregnancy. J Perinatol. (2019) 39:824–36. 10.1038/s41372-019-0343-8.Relationship [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mirzakhani H, Carey VJ, McElrath TF, Laranjo N, O’Connor G, Iverson RE, et al. The association of maternal asthma and early pregnancy vitamin D with risk of preeclampsia: an observation from vitamin D antenatal asthma reduction trial (VDAART). J Allergy Clin Immunol Pract. (2018) 6:600.e–8.e. 10.1016/j.jaip.2017.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McDonnell SL, Baggerly KA, Baggerly CA, Aliano JL, French CB, Baggerly LL, et al. Maternal 25(OH)D concentrations =40 ng/mL associated with 60% lower preterm birth risk among general obstetrical patients at an urban medical center. PLoS One. (2017) 12:e0180483. 10.1371/journal.pone.0180483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen L, Wagner CL, Dong Y, Wang X, Shary JR, Huang Y, et al. Effects of maternal vitamin D3 supplementation on offspring epigenetic clock of gestational age at birth: a post-hoc analysis of a randomized controlled trial. Epigenetics. (2020) 15:830–40. 10.1080/15592294.2020.1734148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Litonjua AA, Lange NE, Carey VJ, Brown S, Laranjo N, Harshfield BJ, et al. The vitamin D antenatal asthma reduction trial (VDAART): rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemp Clin Trials. (2014) 38:37–50. 10.1016/j.cct.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O’Connor GT, et al. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years: the VDAART randomized clinical trial. JAMA. (2016) 315:362–70. 10.1001/jama.2015.18589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Litonjua AA, Carey VJ, Laranjo N, Stubbs BJ, Mirzakhani H, O’Connor GT, et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med. (2020) 382:525–33. 10.1056/nejmoa1906137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolsk HM, Harshfield BJ, Laranjo N, Carey VJ, O’Connor G, Sandel M, et al. Vitamin D supplementation in pregnancy, prenatal 25(OH)D levels, race, and subsequent asthma or recurrent wheeze in offspring: secondary analyses from the vitamin D antenatal asthma reduction trial. J Allergy Clin Immunol. (2017) 140:1423–9. 10.1016/j.jaci.2017.01.013 [DOI] [PubMed] [Google Scholar]
- 76.Liu NQ, Ouyang Y, Bulut Y, Lagishetty V, Chan SY, Hollis BW, et al. Dietary vitamin D restriction in pregnant female mice is associated with maternal hypertension and altered placental and fetal development. Endocrinology. (2013) 154:2270–80. 10.1210/en.2012-2270 [DOI] [PubMed] [Google Scholar]
- 77.Harvey NC, Javaid K, Bishop N, Kennedy S, Papageorghiou AT, Fraser R, et al. MAVIDOS maternal vitamin D osteoporosis study: study protocol for a randomized controlled trial. Trials. (2012) 13:1–9. 10.1186/1745-6215-13-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lanham SA, Roberts C, Habgood AK, Alexander S, Burne THJ, Eyles DW, et al. Effect of vitamin D deficiency during pregnancy on offspring bone structure, composition and quality in later life. J Dev Orig Health Dis. (2013) 4:49–55. 10.1017/S2040174412000542 [DOI] [PubMed] [Google Scholar]
- 79.Gopal-Kothandapani JS, Rigby AS, Harrison R, Eastell R, Moon RJ, Curtis EM, et al. Maternal pregnancy vitamin D supplementation increases offspring bone formation in response to mechanical loading: findings from a MAVIDOS trial sub-study. J Musculoskelet Neuronal Interact. (2020) 20:4–11. [PMC free article] [PubMed] [Google Scholar]
- 80.Cooper C, Harvey NC, Bishop NJ, Kennedy S, Papageorghiou AT, Schoenmakers I, et al. Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): a multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol. (2016) 4:393–402. 10.1016/S2213-8587(16)00044-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moon RJ, Curtis EM, Woolford SJ, Ashai S, Cooper C, Harvey NC. The importance of maternal pregnancy vitamin D for offspring bone health: learnings from the MAVIDOS trial. Ther Adv Musculoskelet Dis. (2021) 13:1–14. 10.1177/1759720X211006979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ioannou C, Javaid MK, Mahon P, Yaqub MK, Harvey NC, Godfrey KM, et al. The effect of maternal vitamin D concentration on fetal bone. J Clin Endocrinol Metab. (2012) 97:2070–7. 10.1210/jc.2012-2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Curtis EM, Moon RJ, Dennison EM, Harvey NC. Prenatal calcium and vitamin D intake, and bone mass in later life. Curr Osteoporos Rep. (2014) 12:194–204. 10.1007/s11914-014-0210-7 [DOI] [PubMed] [Google Scholar]
- 84.Händel MN, Frederiksen P, Osmond C, Cooper C, Abrahamsen B, Heitmann BL. Prenatal exposure to vitamin D from fortified margarine and risk of fractures in late childhood: period and cohort results from 222 000 subjects in the D-tect observational study. Br J Nutr. (2017) 117:872–81. 10.1017/S000711451700071X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moon RJ, Harvey NC, Cooper C, D’Angelo S, Curtis EM, Crozier SR, et al. Response to antenatal cholecalciferol supplementation is associated with common vitamin D-related genetic variants. J Clin Endocrinol Metab. (2017) 102:2941–9. 10.1210/jc.2017-00682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simner CL, Ashley B, Cooper C, Harvey NC, Lewis RM, Cleal JK. Investigating a suitable model for the study of vitamin D mediated regulation of human placental gene expression. J Steroid Biochem Mol Biol. (2020) 199:105576. 10.1016/j.jsbmb.2019.105576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sampathkumar A, Tan KM, Chen L, Chong MFF, Yap F, Godfrey KM, et al. Genetic link determining the maternal-fetal circulation of vitamin D. Front Genet. (2021) 12:721488. 10.3389/fgene.2021.721488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mahmoudi AR, Zarnani AH, Jeddi-Tehrani M, Katouzian L, Tavakoli M, Soltanghoraei H, et al. Distribution of vitamin D receptor and 1α-hydroxylase in male mouse reproductive tract. Reprod Sci. (2013) 20:426–36. 10.1177/1933719112459235 [DOI] [PubMed] [Google Scholar]
- 89.Abbasihormozi S, Kouhkan A, Alizadeh AR, Shahverdi AH, Nasr-Esfahani MH, Sadighi Gilani MA, et al. Association of vitamin D status with semen quality and reproductive hormones in Iranian subfertile men. Andrology. (2017) 5:113–8. 10.1111/andr.12280 [DOI] [PubMed] [Google Scholar]
- 90.Hajianfar H, Karimi E, Mollaghasemi N, Rezaei S, Arab A. Is there a relationship between serum vitamin D and semen parameters? A cross-sectional sample of the Iranian infertile men. Basic Clin Androl. (2021) 31:1–8. 10.1186/s12610-021-00147-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Almassinokiani F, Khodaverdi S, Solaymani-Dodaran M, Akbari P, Pazouki A. Effects of vitamin D on endometriosis-related pain: a double-blind clinical trial. Med Sci Monit. (2016) 22:4960–6. 10.12659/MSM.901838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pazhohan A, Danaei-Mehrabad S, Mohamad-Rezaeii Z, Amidi F, Khodarahmian M, Shabani Nashtaei M, et al. The modulating effects of vitamin D on the activity of β-catenin in the endometrium of women with endometriosis: a randomized exploratory trial. Gynecol Endocrinol. (2021) 37:278–82. 10.1080/09513590.2020.1858780 [DOI] [PubMed] [Google Scholar]
- 93.Bakhshalizadeh S, Amidi F, Shirazi R, Shabani Nashtaei M. Vitamin D3 regulates steroidogenesis in granulosa cells through AMP-activated protein kinase (AMPK) activation in a mouse model of polycystic ovary syndrome. Cell Biochem Funct. (2018) 36:183–93. 10.1002/cbf.3330 [DOI] [PubMed] [Google Scholar]
- 94.Kyei G, Sobhani A, Nekonam S, Shabani M, Ebrahimi F, Qasemi M, et al. Assessing the effect of MitoQ10 and vitamin D3 on ovarian oxidative stress, steroidogenesis and histomorphology in DHEA induced PCOS mouse model. Heliyon. (2020) 6:e04279. 10.1016/j.heliyon.2020.e04279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hosseinirad H, Novin MG, Hosseini S, Nazarian H, Amidi F, Paktinat S, et al. Effect of 1,25(OH)2-vitamin D3 on expression and phosphorylation of progesterone receptor in cultured endometrial stromal cells of patients with repeated implantation failure. Acta Histochem. (2020) 122:151489. 10.1016/j.acthis.2019.151489 [DOI] [PubMed] [Google Scholar]
- 96.Ghanavatinejad A, Rashidi N, Mirahmadian M, Rezania S, Mosalaei M, Ghasemi J, et al. Vitamin D3 controls TLR4- and TLR2-mediated inflammatory responses of endometrial cells. Gynecol Obstet Invest. (2021) 86:139–48. 10.1159/000513590 [DOI] [PubMed] [Google Scholar]
- 97.Carrelli A, Bucovsky M, Horst R, Cremers S, Zhang C, Bessler M, et al. Vitamin D storage in adipose tissue of obese and normal weight women. J Bone Miner Res. (2017) 32:237–42. 10.1002/jbmr.2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen YH, Fu L, Hao JH, Yu Z, Zhu P, Wang H, et al. Maternal vitamin D deficiency during pregnancy elevates the risks of small for gestational age and low birth weight infants in Chinese population. J Clin Endocrinol Metab. (2015) 100:1912–9. 10.1210/jc.2014-4407 [DOI] [PubMed] [Google Scholar]
- 99.Morales E, Rodriguez A, Valvi D, Iñiguez C, Esplugues A, Vioque J, et al. Deficit of vitamin D in pregnancy and growth and overweight in the offspring. Int J Obes. (2015) 39:61–8. 10.1038/ijo.2014.165 [DOI] [PubMed] [Google Scholar]
- 100.Li P, Li P, Liu Y, Liu W, Zha L, Chen X, et al. Maternal vitamin D deficiency increases the risk of obesity in male offspring mice by affecting the immune response. Nutrition. (2021) 87–88:111191. 10.1016/j.nut.2021.111191 [DOI] [PubMed] [Google Scholar]
- 101.Li S, Hu L, Zhang C. Urinary vitamin D-binding protein as a marker of ovarian reserve. Reprod Biol Endocrinol. (2021) 19:80. 10.1186/s12958-021-00762-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dul AJ, Kowaleczko E, Che K, Kurzawa R. Vitamin D as a follicular marker of human oocyte quality and a serum marker of in vitro fertilization outcome. J Assist Reprod Genet. (2018) 25:1265–76. 10.1007/s10815-018-1179-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blomberg Jensen M, Jørgensen A, Nielsen JE, Bjerrum PJ, Skalkam M, Petersen JH, et al. Expression of the vitamin D metabolizing enzyme CYP24A1 at the annulus of human spermatozoa may serve as a novel marker of semen quality. Int J Androl. (2012) 35:499–510. 10.1111/j.1365-2605.2012.01256.x [DOI] [PubMed] [Google Scholar]
- 104.De-Regil LM, Palacios C, Ansary A, Regina K, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. (2012) 2:CD008873. 10.1002/14651858.CD008873.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gernand AD, Schulze KJ, Stewart CP, West KP, Christian P. Micronutrient deficiencies in pregnancy worldwide: health effects and prevention. Nat Rev Endocrinol. (2016) 12:274–89. 10.1038/nrendo.2016.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.De-Regil LM, Palacios C, Lombardo LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. (2016) 134:274–5. 10.1590/1516-3180.20161343T2 [DOI] [PubMed] [Google Scholar]
- 107.Lerchbaum E, Obermayer-Pietsch B. Vitamin D and fertility: a systematic review. Eur J Endocrinol. (2012) 166:765–78. 10.1530/EJE-11-0984 [DOI] [PubMed] [Google Scholar]
- 108.Harvey NC, Holroyd C, Ntani G, Javaid K, Cooper P, Moon R, et al. Vitamin D supplementation in pregnancy: a systematic review. Health Technol Assess (Rockv). (2014) 18:1–190. 10.3310/hta18450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thorne-Lyman A, Fawzi WW. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis Andrew. Paediatr Perinat Epidemiol. (2012) 26 Suppl 1:75–90. 10.1111/j.1365-3016.2012.01283.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Crozier MK, Sr., Harvey NC, Gale CR, Dennison EM, Boucher BJ, Arden NK, et al. Princess Anne Hospital Study Group. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. (2006) 367:36–43. 10.1016/S0140-6736(06)67922-1 [DOI] [PubMed] [Google Scholar]
- 111.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, et al. Europe PMC funders group maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. (2008) 62:68–77. 10.1038/sj.ejcn.1602680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leffelaar ER, Vrijkotte TGM, Van Eijsden M. Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: results of the multi-ethnic amsterdam born children and their development cohort. Br J Nutr. (2010) 104:108–17. 10.1017/S000711451000022X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.