Abstract
In this study, Yatağan lignite (YL) and peach kernel shells (PKS) were originally taken separately and in a 1: 1 ratio by weight. Experiments were carried out in a 3-zone heated cylindrical furnace in a steel reactor. Structural characterization of all the solid products obtained was made by FTIR, XRD, and SEM analysis. When the FTIR and XRD spectra of the raw samples are examined, it is seen that they are rich in functional groups. It is seen that the PKS has aliphatic and aromatic structures and cellulosic structure –OH stresses (3500 cm−1). The sharp peak around 2918 cm−1 in Yatağan lignite belongs to the aliphatic C–H stretch. In the XRD spectrum, it is seen that both structures are largely amorphous. The raw PKS contains 3 different amorphous macromolecular structures. Yatagan lignite, on the other hand, contains crystalline peaks of clay and inorganic structures, depending on the ash content, as well as the amorphous structure. As the temperature increases depending on the carbonization temperature, as seen in the FTIR spectrum, the peaks of the functional groups decrease and disappear with the disruption of small macromolecular structures. As a result of the structural adjustment with the temperature increase, M-O-M peaks around 1000 cm−1 remain due to the aromatic C–H stretching and ash content. The paper centers around test assurance of operating temperatures in the consuming layer during co-carbonization. It is obtained that 800 °C is the best temperature condition for the co-carbonization process. It has been concluded that the chars obtained as a result of pyrolysis will be used as a solid fuel in both environmental (the lowest sulfur content) and economic (400 °C) sense. However, the fact that it has a very low sulfur content with the increase in the liquid and gas efficiency obtained at high temperatures again proves the production of an environmentally friendly liquid fuel.
Keywords: Lignite, Biomass, Peach kernel shell, Pyrolysis, Pyrolysis products, Yield, Characterization
Lignite, Biomass, Peach kernel shell, Pyrolysis, Pyrolysis products, Yield, Characterization.
1. Introduction
Waste collected by municipalities is increasing and bringing important environmental problems. For example, it is estimated that the annual waste amount in the USA is around 200 million tons (Chen et al., 2021). This figure is equivalent to approximately one ton of waste per person. Even if these figures are not reached in Turkey, there is significant waste production. In the national contribution intent statement submitted to the UN Secretariat on September 30, 2015, Turkey stated that it would reduce the increase in greenhouse gas emissions by 18%–21% by 2030. According to the data of large cities, it will reach 1.5 kg of municipal waste per capita daily production in Turkey. The problems caused by garbage dumps and the fact that some of the wastes are in the status of hazardous waste gradually bring the methods of incineration to the fore. Although it depends on the type and season of the wastes, it can be easily calculated that the energy level obtained from the wastes is between 11.5 and 14 MJ/kg. Considering that the replacement of costly concrete with pyrolyzed biowaste is well known, particularly in emerging nations that can't bear to give adequate consideration to natural administrative instruments, speculation was fabricated, regardless of whether the current circumstance can be improved by planning an undemanding shower cooler of the pyrolytic gas that would be fused to the pyrolysis unit to lessen the spillage of a particulate matter (PM) that spreads the risky poisons to the environmental elements (Maroušek et al., 2020). Numerous physio-substance primary and compositional elements hamper the absorbability of cellulose present in lignocellulosic biomass. Hindrances in pretreatment processes depend on the connective capacity of lignin, which resembles the weight cover of cellulose and hemicelluloses. Research is centered around crumbling phytomass into its constituents in a market serious and earth maintainable manner in the significance of negligible utilization of energy and synthetic compounds (Maroušek, 2012). Municipal solid waste can be turned into a flammable gas by pyrolysis. As is known, the pyrolysis process is the thermal decomposition of the material in the absence of oxygen. The product obtained can be defined as an intermediate energy gas and can be used in electricity generation or various processes that require thermal energy. The ratio of the calorific value of the gas product to the calorific value of the waste material is about 0.77 or less. Domestic and commercial wastes and their mixtures can also be used as additional fuel in heat boilers. Burners are modified to suit this situation and to ensure that the wastes are burned simultaneously with conventional fuels. Experiments have shown that in this co-burning process, only a small percentage may be of waste origin, given the nominal capacity of the boiler. For example, this ratio should remain between 10–20% in pulverized coal boilers. Also, it should not be forgotten that some problems will occur during the incineration of waste in conventional boilers. At the beginning of these problems is the inhomogeneity of the firewood fuel, its high moisture content and it is being more corrosive than coal and oil. Also, separate facilities will be required for the storage of waste origin fuels. Despite all these disadvantages, solid waste for fuels can be used economically in some power plants. Gases produced from solid waste can also be burned directly or converted into useful gases such as methanol and ammonia. With today's technological possibilities, the amount of methanol obtained from approximately one ton of solid waste is 185 kg and the amount of ammonia is around 225 kg. It does not seem very economical as a result of the positive environmental experiences related to the recovery of solid wastes before. Almost all of the various facilities established are far from operating economically. There are major problems in the marketing of recycled resources and sales are not realized at the desired level. One of the technical problems is the solid waste shredding operation. Huge explosions occurred at various crushing plants. Also, some solid wastes cannot be broken down into small pieces and such wastes have to be sent directly to landfills and buried. Another big problem is that almost no power plant wants to use this fuel due to the problems created by waste-based fuels in the combustion process (Farsi, 2021; Gerasimov et al., 2021; Hamidpour et al., 2021; Moldoveanu, 2021; Moravvej et al., 2021; Nie et al., 2021; Piroozmand et al., 2021; Quereshi et al., 2021; Tursi and Olivito, 2021; Zhou et al., 2021). The historical development of the pyrolysis process is shown in Table 1. Table 2 shows the difference between pyrolysis technology from other technologies. Pecha et alshow the behavior of a wood particle during pyrolysis. In this study in the horizontal direction, there is a rapid pyrolysis stage, on the vertical axis direction there is a relatively slow pyrolysis stage (Pecha and Garcia-Perez, 2020).
Table 1.
Historical background of pyrolysis technology [11].
| Year | Technology |
|---|---|
| 28000 BC | Cave art with biochar |
| 1658 | Pyroligneous acid was found to contain acetic acid |
| 1792 | England commercialized illuminating gas from wood and coal |
| 1835 | Methyl alcohol is isolated from crude wood-spirit |
| 1850 | Wood distilaltion industyr expands rapidly |
| 1920s | Rise of petroleum industry |
| 1920–1960 | Slow pyrolysis for biochar for metallurgy and cooking |
| 1960 | Fundamentals research on biomass pyrolysis reactions |
| 1970 | Oil crisis |
| 1980s | Fast pyrolysis is studied and commercialized |
| 1989 | Ensyn commercializes food flavors from pyrolysis |
| 1990s | New bio-oil upgrading strategies and products to replace petroleum |
| 2000s | Oil prices and global warming create demand for biofuel |
Table 2.
Differences in biomass pyrolysis among other thermal conversion Technologies [11].
| Process | Temperature range (°C) | Products | Description |
|---|---|---|---|
| Evaporation | 100–200 | Solid: Roasted wood | Endothermic; Evaporation; External heat penetrates particle |
| Vapor: Water | |||
| Torrefaction | 225–200 | Solid: Roasted wood | Endothermic; Hemicellulose and amorphous cellulose decomposition, Light extractives evaporation, Intermolecular dehydration reactions; Mass density decreases; Volatile organics can combust |
| Vapor: Water, volatile organics | |||
| Pyrolysis | 300–650 | Solid: Charcoal | Endothermic for fast pyrolysis, exothermic for slow pyrolysis; solid, liquid, and vapor reactions; cellulose decomposition; lignin decomposition; mass density decreases; Volatile organics can combust |
| Vapor: Light organics, heavy organics | |||
| Gasification | 700–850 | Solid: ash | Endothermic if water is oxidizing agent, exothermic if oxygen is oxidizing agent; Volatilization of carbon, hydrogen, and oxygen in char; gasification of volatile pyrolysis oil; Syngas can combust |
| Vapor: Syngas (CO, CO2, H2, CH4, H2O) | |||
| Combustion of vapors | 450–2000 | CO2, CO, H2O | Exothermic; consumption of oxygen; requires ignition at high temperatures and/or pressures |
Once the primary volatiles is formed in the Biomass matrix during pyrolysis, their fate is governed by competing for recombination, cracking, and devolatilization processes. The change in the pore structure during pyrolysis is due to the net effect of these competing processes. As pore diameter changes, pore blinding and connecting with other new pores is not uncommon. Three steps are involved in the transport of the primary volatile: i transferring the volatile to the exterior of the biomass particles, (ii) removing the volatile from the exterior of the biomass and transferring it to the bulk gas phase, and (iii) moving the volatile in the gas phase away from the hot zone and into the cooler regions of the reactor. Through the main devolatilization processes, biomass is thermally transformed into gas, liquid, and solid products in pyrolysis. Any of the phases listed below can cause secondary reactions to occur with thermally unstable primary volatile products such as acetol, Lovoglucosan, Glycoaldehyde, Levoglucosenone, Cellobiosan, Furfural, 1,4:3,6-Dianhydro-α-D-glucopyranose, 1,6-Anhydro-β-D-glucofuronase, Hydrox-ymethylfurfural. Such reactions result in the creation of char or the shattering of larger molecules of tar into smaller ones. The first and second stages of the transport of the volatiles are affected by the particle size of the biomass, the external pressure, and possibly the gas flow velocity past the particles (Aliyu et al., 2021).
The geometry of the reactor and the local hydrodynamic conditions significantly control the third step.. Reactor geometry and reaction circumstances affect secondary reactions.. Secondary reactions are the least severe in the wire-mesh reactors. In this type of reactor, biomass is dispersed between two layers of the wire-mesh screen; a monolayer and volatiles are removed from the biomass as they formed within the sweep gas. In other types of reactors, severe secondary reactions of initial pyrolysis products occur. In a deep fixed-bed hot-rod reactor, the volatiles flow through the hotbed of pyrolyzing biomass particles, where volatilization from the biomass particles occurs. Secondary reactions due to the contact of the volatiles with hot reactor walls are also possible. Gas-phase cracking techniques are known to favor longer residence times in the hot zone within the fixed bed, which increases gas output. Longer stays in the hot zone may further enhance the possibility of depolymerization reactions (caused by close contact between hot solid surfaces and tar vapors), which might result in the development of some secondary char and light gases. It is also probable that comparable processes occur within tar mist globules moving through the hot zone of the reactor, most likely by radical recombination, even though there is no clear proof of this. Reaction parameters such as the temperature of pyrolysis, pressure in the reactor, the particle size of the biomass, gas residence time, solid residence time, heating rate, and sweep gas space velocity determine the total carbon conversion and the transport of volatiles and thereby the product distribution (Akinyemi and Adesina, 2020; Aldana et al., 2015; Bonelli et al., 2001; Chen et al., 2016; Chiappero et al., 2020; Davies et al., 2021; Durak, 2015; Farsi, 2021; Gedik et al., 2020; Gemechu & Kumar, 2021; Gerasimov et al., 2021; Guran, 2018; Hamidpour et al., 2021; Ifa et al., 2020; İlhan Küçük, 2021; Işıtan et al., 2016; Jana et al., 2017; Jung et al., 2021; Kir et al., 2021; Loya-González et al., 2019; Mailaram et al., 2021; Manurung et al., 2009; Martí-Rosselló et al., 2018; Moldoveanu, 2021; Moravvej et al., 2021; Nie et al., 2021; Ortiz et al., 2020; Özsin & Pütün, 2017; Pecha and Garcia-Perez, 2020; Pereira Lopes and Astruc, 2021; Piroozmand et al., 2021; Puig-Gamero et al., 2021; Quereshi et al., 2021; Tacettin Geçkil, 2020; Tacettin GEÇKİL, 2021; Tursi and Olivito, 2021; Zaafouri et al., 2016; Zhou et al., 2021).
Solid benefits in cost and as a pathway of restoring squander into a worth-added item. Contrasted and ignition, pyrolysis enjoys the benefits of less optional contamination, item selectivity, and cycle adaptability. These days, low-temperature carbonization innovation has been industrialized in numerous nations like China and Japan (Cheng et al., 2016). Its advantages include the following: after all MS components have been destroyed, merely a mechanical method can remove 75% of the intracellular water, and its energy consumption is half that of standard drying technology. While high-temperature carbonization can directly utilize the incomplete hotness worth of MS, low-temperature carbonization creates carbides with significant warming worth.. In this manner, the last option is worth a further turn of events (Jiang et al., 2021).
To diminish the expense of initiated coke creation, research has additionally been centered around promptly accessible inexhaustible forerunners, like minimal expense woods and agrarian deposits, similar to corn bodies and corn straw, grain straw, bagasse, and rice husk, espresso endocarp, oak, seeds, date pits. Notwithstanding, a portion of these materials are found in little amounts or are difficult to gather, and, along these lines, they are unrealistic for modern applications. Despite what might be expected, poplar bark, which is a strong waste from the lumber creation industry, is being produced in enormous amounts. China is the biggest poplar establishing nation and a lot of poplar bark is normally piled up in plants. This occupies a room as well as, likewise represents the danger of fire. Plus, as a result of its low calorific worth, direct burning of bark is wasteful and delivers a ton of air poisons. Earlier examination on biomass-based actuated coke showed that it can have great execution and it can assist with resolving natural issues, like diminishing the aggregation of farming strong squander and catching emanations of risky poisons. Hence, the planning of actuated coke from biomass is a suitable technique to understand the compelling usage of bad-quality regular assets (Cao et al., 2019; Li et al., 2021; Lin et al., 2021; Mochizuki and Tsubouchi, 2019; Wang et al., 2020; Wilk et al., 2021). To more conclusively think about the effect powers have on the climate, extra examinations are required (Matzen and Demirel, 2016). Unlike previous studies, in this study, a technological solution was determined to evaluate the commonly used lignite and biomass together, to reduce the negative environmental impact of lignite in the Yatağan region. The chars generated by pyrolysis have been determined to be suitable for use as a solid fuel in terms of both environmental (low sulfur content) and economic (400 °C) considerations. However, the fact that it has a very low sulfur content, along with an improvement in liquid and gas efficiency at high temperatures, indicates that it is possible to produce an ecologically benign liquid fuel.
2. Experimental
2.1. Materials
Lignite used in pyrolysis experiments was supplied by TKI (Turkish Coal Enterprises) from the Muğla Yatağan basin in the Aegean Region of Turkey. The PKS used in the experiment was obtained from the Tabya Factory in Aydın Söke.
Muğla Yatağan lignite was utilized with an absolute sulfur content of 2.69% and natural sulfur was liable for a huge extent of 2.10% of this sulfur. Muğla Yatağan lignite is still up in the air to have launderability of respectably troublesome degree (65% recuperation, 20% debris expulsion, and no sulfur evacuation or undeniably challenging degree (18.84% debris expulsion and no sulfur expulsion (Ünal Sansar, 2018).
2.2. Methods
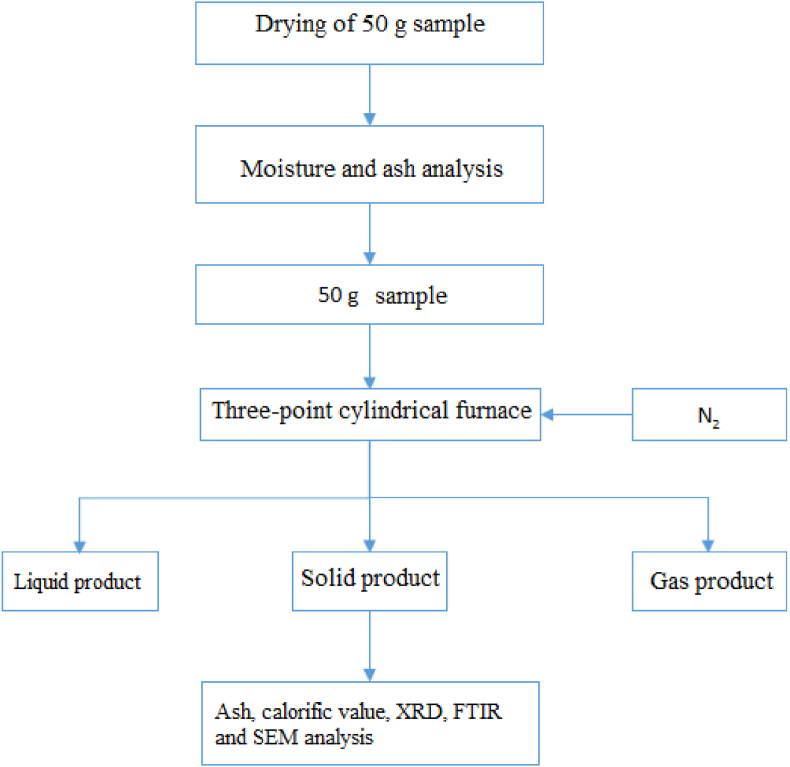
The experiments started with the supply of raw materials. 50 g of bedding coal bedding), peach seed pods, and mixture (1:1, weight) were dried. After their short analysis, their weight was taken. In a horizontal cylindrical pyrolysis furnace (used for carbonization, see Figure 1) at 400, 500, 600, 700, and 800 °C temperatures, it was subjected to a carbonization process in a nitrogen environment at 100 mL/min gas flow rate for 1 h. After the experiment, carbonized solid product liquid product was weighed and a yield calculation was made. The gas products obtained were released into the atmosphere (Figure 2).
Figure 1.
Pyrolysis furnace.
Figure 2.
Experiment flow chart.
2.2.1. Experiment no 1: mix (yatağan lignite + peach seed shell)
The experiments started with the supply of raw materials. 50 g of ground lignite (yatağan) and 50 g of PKS were dried. After a brief analysis, the weights were taken. It was subjected to a carbonization process in a horizontal cylindrical pyrolysis furnace (used for carbonization) at 400, 500, 600, 700, and 800 °C, at a gas flow rate of 100 mL/min, for 1 h, in a Nitrogen environment. After the experiment, carbonized solid product liquid product was weighed and a yield calculation was made. The resulting gaseous products were released into the atmosphere.
2.2.2. Experiment no 2: peach seed shell
50 g of PKS were dried. After a brief analysis, the weights were taken. It was taken into a steel tub. It was subjected to a carbonization process in a horizontal cylindrical pyrolysis furnace (used for carbonization) at 400, 500, 600, 700, and 800 °C temperatures, 100 mL/min gas flow rate, 1 h, in a Nitrogen environment. After the experiment, carbonized solid product liquid product was weighed and a yield calculation was made. The resulting gaseous products were released into the atmosphere.
2.2.3. Experiment no 3: yatağan lignite
50 g of Yatan lignite was dried. After a brief analysis, the weight was taken. It was taken into a steel tub. It was subjected to a carbonization process in a horizontal cylindrical pyrolysis furnace (used for carbonization) at 400, 500, 600, 700, and 800 °C, at a gas flow rate of 100 mL/min, for 1 h, in a nitrogen environment. After the experiment, carbonized solid product liquid product was weighed and a yield calculation was made. The resulting gaseous products were released into the atmosphere.
2.3. The analyses
SEM analyzes were performed with the “LEO-EVO 40/Cambridge-England” brand device in the central research laboratory of İnönü University. Electron acceleration and generation mechanisms in the analyzes were carried out with the traditional method. XRD analyzes are Rigaku RadB-Dmax II and Rigaku RINT-2000 X-ray diffractometers available at the Inonu University scientific research center (IBTAM). There is also a Jade 6 + crystal analysis program and library integrated into these systems. In the analysis, the electromagnetic beam of a certain wavelength was reflected by hitting the material surface and the reflected beam was interpreted according to Bragg's Law. FTIR analyses of the samples were performed with PerkinElmer Spectrum One device in the Central Research Laboratory of İnönü University in the range of 500–4000 cm-1.
3. Results and discussion
3.1. Proximate and elemental analysis results
The proximate analysis results of the raw samples (before pyrolysis) and proximate analysis after pyrolysis products are shown together in Table 3. The elemental analysis of the raw samples (before pyrolysis) and the elemental analysis after pyrolysis products are shown in Table 4.
Table 3.
Proximate analysis of the raw samples and pyrolysis products.
| Sample name | Ash (%) | Moisture (%) | Volatile matter (%) | Fixed carbon (%)∗ |
|---|---|---|---|---|
| Yatağan coal (raw) | 12,00 | 14,27 | 30,50 | 43,23 |
| PKS (Raw) | 0,29 | 5,40 | 78,69 | 15,62 |
| Exp. 1 (400 °C) | 12,61 | -∗∗ | -∗∗ | 87,39 |
| Exp. 2 (500 °C) | 15,10 | -∗∗ | -∗∗ | 84,90 |
| Exp. 3 (600 °C) | 13,02 | -∗∗ | -∗∗ | 86,98 |
| Exp. 4 (700 °C) | 17,82 | -∗∗ | -∗∗ | 82,18 |
| Exp. 5 (800 °C) | 15,00 | -∗∗ | -∗∗ | 85,00 |
| Exp. 6 (PKS 400 °C) | 0,73 | -∗∗ | -∗∗ | 99,27 |
| Exp. 6 (PKS 500 °C) | 0,98 | -∗∗ | -∗∗ | 99,02 |
| Exp. 6 (PKS 600 °C) | 0,99 | -∗∗ | -∗∗ | 99,01 |
| Exp. 6 (PKS 700 °C) | 1,98 | -∗∗ | -∗∗ | 98,02 |
| Exp. 6 (PKS 800 °C) | 0,93 | -∗∗ | -∗∗ | 99,07 |
| Exp. 7 (YL 400 °C) | 17,31 | -∗∗ | -∗∗ | 82,69 |
| Exp. 7 (YL 500 °C) | 19,00 | -∗∗ | -∗∗ | 81,00 |
| Exp. 7 (YL 600 °C) | 22,00 | -∗∗ | -∗∗ | 88,00 |
| Exp. 7 (YL 700 °C) | 24,75 | -∗∗ | -∗∗ | 75,25 |
| Exp. 7 (YL 800 °C) | 24,24 | -∗∗ | -∗∗ | 75,76 |
Calculated by difference.
Undefined.
Table 4.
Elemental analysis of the raw samples and pyrolysis products.
| Sample name | Composition |
(daf, %) |
|||
|---|---|---|---|---|---|
| C | H | N | S | O∗ | |
| Yatağan coal (raw) | 56,42 | 5,40 | 1,35 | 1,10 | 35,73 |
| PKS (Raw) | 67,31 | 0,91 | 0,17 | 0,36 | 31,25 |
| Exp. 1 (400 °C) | 60,08 | 3,89 | 1,16 | 0,62 | 34,25 |
| Exp. 2 (500 °C) | 53,26 | 3,18 | 1,07 | 0,45 | 42,04 |
| Exp. 3 (600 °C) | 60,34 | 2,46 | 0,86 | 0,53 | 35,81 |
| Exp. 4 (700 °C) | 50,99 | 1,71 | 0,71 | 0,58 | 46,01 |
| Exp. 5 (800 °C) | 88,71 | 1,11 | 1,13 | 1,07 | 7,98 |
| Exp. 6 (PKS 800 °C) | 68,08 | 1,22 | 1,27 | 1,26 | 28,17 |
| Exp. 7 (YL 800 °C) | 49,94 | 6,67 | 0,11 | 0,04 | 43,24 |
Calculated by difference.
High volatile matter content also positively affects pyrolysis. Agarwal and Lattimer found that biomass with 80% volatile matter content showed a pyrolysis reaction (Agarwal and Lattimer, 2014). This high volatile matter content is because the biomass structure contains 30–60% cellulose, 20–35% hemicellulose, and 15–30% lignin [30]. As can be seen from Table 5, hydrogen and volatile matter with high hydrogen content accelerate the formation of secondary reactions as they will show different heat transfer characteristics in coal biomass mixtures (Agarwal and Lattimer, 2014). The resulting liquid and gas yields are generally high (Guo and Bi, 2015). Guo and Bi increased the temperature from 480 °C to 650 °C by adding 70% biomass to the coal in their pyrolysis study. According to the results they obtained, the liquid and gas yield increased and the solid yield decreased [30]. As can be seen from Table 3, the volatile matter content of PKS is very high. Thus, when mixed, the gasification and liquefaction state of the volatile matter increased and the solid yield decreased. Moisture is a parameter that negatively affects the pyrolysis process [30]. Mixing high humidity YL with PKS had a positive effect on the pyrolysis efficiency as it would reduce the total moisture content of the mixture.
Table 5.
Calorific value results.
| Sample name | Calorific value (kJ/kg) |
|---|---|
| Yatağan coal (raw) | 18166.93 |
| PKS (Raw) | 20815.40 |
| Exp. 1 Mix (400 °C) | 27289.68 |
| Exp. 2 Mix (500 °C) | 27956.78 |
| Exp. 3 Mix (600 °C) | 26166.02 |
| Exp. 4 Mix (700 °C) | 19728.48 |
| Exp. 5 Mix (800 °C) | 27701.72 |
| Exp. 6 PKS (400 °C) | 37072.79 |
| Exp. 7 PKS (500 °C) | 36060.89 |
| Exp. 8 PKS (600 °C) | 35185.98 |
| Exp. 9 PKS (700 °C) | 33443.13 |
| Exp. 10 PKS (800 °C) | 28781.90 |
| Exp. 11 YL (400 °C) | 19543.21 |
| Exp 12 YL (500 °C) | 23840.06 |
| Exp 13 YL (600 °C) | 26138.66 |
| Exp 14 YL (700 °C) | 26263.01 |
| Exp 15 YL (800 °C) | 27120.65 |
Martinez et al. concluded that the calorific value increases as the oxygen content decreases [30]. As can be seen from Table 4, as the temperature increased, the oxygen value decreased (from 34.25% to 7.98%) and as it can be seen from Table 5, the calorific value increased proportionally (from 27289.68 to 27701.72 kJ/kg). As can be seen from Table 4, the carbon content in the ash is close to the average of crude YL and PKS up to 800 °C with the increase in temperature in the pyrolysis of the mixture. However, it increased significantly at 800 °C (from 60.08% to 88.71%). At the same temperatures separately, carbon contents decreased in YL (from 56.42% to 49.94%) and increased in PKS (from 67.31% to 68.31%). This situation strengthens the situation where hydrogen transfer to coal occurs due to the high hydrogen content of biomass and high volatile matter content. It is known that more oxygenated groups decompose at low temperatures [30]. According to this information, as can be seen from Table 4, the oxygen content of the mixture increased regularly with the increase of the pyrolysis temperature compared to the average of both raw samples, but according to the results obtained separately at 800 °C, the PKS decreased (from 31.25% to 28.17%) while YL has increased (from 35.73% to 43.24%). The low nitrogen and sulfur content in the biomass is a positive factor for the economics of the process [30]. As can be seen from Table 4, nitrogen and sulfur values are lower in the mixture than in YL alone (from 1.35% and 1.10%–1.13% and 1.07% at 800 °C). It should be noted here that sulfur creates the effect of FeS and/or FeS2 catalyst with iron in coal. Sulfur ratios in liquid and gaseous products (products from which liquid fuel is obtained) obtained in the pyrolysis process where YL and PKS are used at 800 °C are close to zero. This makes it possible to produce the liquid fuels obtained independently from sulfur.
3.2. Calorific value results
The calorimetry analysis results are shown in Table 5. In the pyrolysis of Yatağan lignite, the calorific value of the pyrolysis product obtained by increasing the temperature from 400 °C to 800 °C increased regularly (from 19543.21 to 27120.65 kJ/kg). Here, as a result of the removal of volatile matter and moisture with the increase in temperature, the enrichment of the sample with fixed carbon was effective. However, the calorific value of the products obtained as a result of the increase in temperature from 400 °C to 800 °C in the pyrolysis of the PKS significantly (from 37072.79 to 28781.90 kJ/kg). Here, the structure deteriorates with the increase in temperature. In particular, lignin, cellulose, and hemicellulose structures have deteriorated. This situation was also observed in FT-IR analyzes. In the pyrolysis trials performed as a result of mixing YL and PKS in equal proportions, it was investigated whether the mixture had a synergistic effect with each other as a result of increasing the temperature from 400 °C to 800 °C. According to this result, it has been observed that coal and biomass have a synergistic effect on the thermal values of the product obtained as a result of the pyrolysis process together. For example, while PKS is 28781.90 kJ/kg and YL is 27120.65 kJ/kg at 800 °C, the product heating value of the mixture is 27701.72 kJ/kg at the same temperature. As the pyrolysis temperature of YL increases, the calorific value of the char obtained increases by approximately 9000 kJ/kg compared to the raw YL. As the pyrolysis temperature of PKS increases, it is seen that it reaches the highest calorific value at 400 °C. The increase in raw biomass is approximately 16500 kJ/kg. In the mixed state, the calorific value of the char obtained when the temperature increased was higher than both the YL and the raw form of PKS.
3.3. Liquid, solid, and gas yields of PKS, YL, and mix
The product yield results of PKS pyrolysis are shown in Table 6. While the liquid yields obtained as a result of the pyrolysis of PKS from 400 °C to 800 °C increased up to 700 °C (from 2.00% to 13.56%), it turned into the gas at 800 °C due to the deterioration of the structure, increasing the gas yield (from 60.72% to 67.96%). Solid yield decreased overall (from 33.44% to 25.44%). The rate of conversion of liquid and solid products to gas has increased. In YL, on the other hand, by increasing the temperature from 400 °C to 800 °C, the gas yield generally increased (from 41.64% to 52.76%). Solid yield decreased and some conversion to liquid occurred at 800 °C (from 0 to 6.60%). By increasing the pyrolysis temperature from 400 °C to 800 °C with the addition of biomass to the coal, the liquid yield was found to be very high compared to the liquid yield of both PKS and YL products (6,60%, 6,60%, and 23,87%, respectively). However, both gas and solid yields decreased indirectly (67.96%, 52.76, and 41.31, respectively, and 25.44, 43.24, and 34.82, respectively). This indicates that when coal and biomass are mixed, more liquid products are released. It is important to obtain high efficiency without using catalysts for products that can be liquid fuels due to the synergistic effect. It is very important for liquid fuel production that this value is higher at low temperatures (For example, at 400 °C, the liquid product yield is 2 in PKS and 0 in YL, while this value is 52.3 in the mixture.
Table 6.
PKS product yield results.
| Exp. no. | Liquid yield (%) | Gas yield (%) | Solid yield (%) |
|---|---|---|---|
| Exp. 1 PKS (400 °C) | 2,00 | 64,56 | 33,44 |
| Exp. 2 PKS (500 °C) | 2,00 | 68,58 | 29,42 |
| Exp. 3 PKS (600 °C) | 12,40 | 60,54 | 27,06 |
| Exp. 4 PKS (700 °C) | 13,56 | 60,72 | 25,72 |
| Exp. 5 PKS (800 °C) | 6,60 | 67,96 | 25,44 |
| Exp. 1 YL (400 °C) | - | 41,64 | 58,36 |
| Exp. 2 YL (500 °C) | - | 47,72 | 52,28 |
| Exp. 3 YL (600 °C) | - | 52,68 | 47,32 |
| Exp. 4 YL (700 °C) | - | 55,40 | 44,60 |
| Exp. 5 YL (800 °C) | 6,60 | 52,76 | 43,24 |
| Exp. 1 (400 °C) | 52,30 | 1,60 | 46,10 |
| Exp. 2 (500 °C) | 43,39 | 15,83 | 40,78 |
| Exp. 3 (600 °C) | 30,47 | 31,06 | 38,47 |
| Exp. 4 (700 °C) | 26,96 | 37,21 | 35,83 |
| Exp. 5 (800 °C) | 23,87 | 41,31 | 34,82 |
Li et al. obtained high oil yield in low-temperatures pyrolysis between Xilinhot and rice husk. Excessive degradation of cross-linked oxygenated groups at low temperatures is the most important factor in this situation. In the study, they emphasized that biomass with high hydrogen content added to coal produced more liquid and gaseous products than coal pyrolysis alone (Li et al., 2019). Byambajav et al. studied the co-pyrolysis of lignin and low-rank coal. It was determined that the gas efficiency increased by increasing the temperature from 450 °C to 650 °C (Byambajav et al., 2018). Guo and Bi also investigated the pyrolysis of coal and biomass mixtures at various ratios. According to the results they obtained, with the increase in the pyrolysis temperature (from 480 °C to 650 °C), the liquid and oil yield of the mixture from the pyrolysis products increased, while the solid yield decreased (Guo and Bi, 2015). As can be seen in Table 6, liquid and gas yields increased, while solid yields decreased with an increase in temperature from 400 °C to 800 °C. Taro et al. investigated the synergistic effect in the combined pyrolysis of Thai lignite and corncob. The high liquid yield was obtained when the temperature was between 300–400 °C and 350–500 °C. Here they concluded that corncob with high volatile matter content undergoes temperature-related reactions on lignite. They thought that this situation was also caused by hydrogen transfer from biomass to coal (Sonobe et al., 2008).
3.4. Characterization of pyrolysis products
3.4.1. FT-IR analysis
The product yield results of different experimental pyrolysis trials are shown in Figure 3, the crude lignite sample contains –OH structures bound to water in 3000–3750 cm−1 wideband and –OH structures bound to clay and organic structure of lignite. The two peaks around 3900 cm−1 belong to aliphatic stresses. The peak at 1024 cm−1 essentially belongs to the inorganic M-O-M (metal-oxygen-metal) structure. It belongs to a broadband aromatic ring around 1500 cm−1. The width of the 3000-3750 cm−1 band has prevented the aromatic C–H stretch to be seen. At the thinness of the chief check, the wideband cellulosic-OH at 3400 cm−1 peak min, the aliphatic C–H stretch of 2900 cm−1 peak, the C–O stress in the 1024 cm−1 peak cellulose and lignin belong to the C = O stress in hemicellulose around 1700.
Figure 3.
FT-IR analysis results.
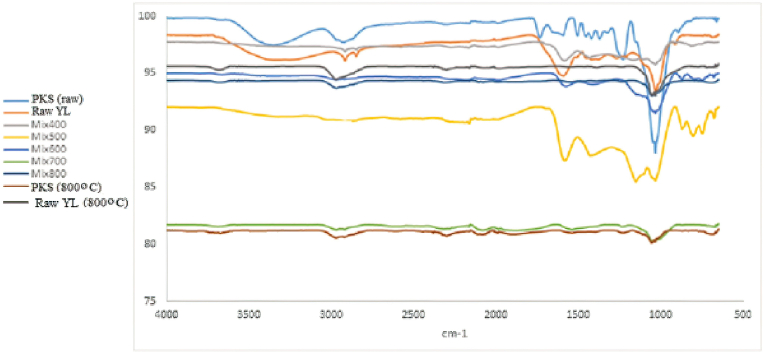
The product yield results of raw PKS and raw YL pyrolysis trials are shown in Figure 4. In Figure 4, it was revealed that the oxygenated groups that were decomposed in the low-temperature pyrolysis of the crude samples did not appear both in the mixtures and in the PKS and YL pyrolysis at 800 °C, as seen in Figure 3. This confirms the degradation of oxygenated groups in low-temperature pyrolysis, as we mentioned in the previous section. Byambajav et al. studied the combined pyrolysis of lignin and low-rank coal. According to the FT-IR analysis of the obtained chars, they determined that the amount of oxygen functional group in the mixture decreased compared to pyrolysis one by one (Byambajav et al., 2018). The functional group distributions of the raw samples in Figure 4 are almost the same as the pyrolysis results of the mixtures performed at 400 °C and 500 °C. In this case, it can be thought that pyrolysis at 800 °C gives the best results.
Figure 4.
FT-IR results for raw samples.
Raw PKS and YL, which have not been heating treated and therefore contain especially volatile compounds, are the richest in functional groups compared to other solids. The slightly broad peak seen at 3396 cm-1 is due to hydroxyl (single bond-OH) groups in the sample, as well as water (Figure 3(a, b)) (Önal and Ceylan, 1995).
3.4.2. XRD analysis
In Figure 5, the comparison of the XRD analyses of the raw PKS and YL samples, the mixture, and the chars obtained as a result of the pyrolysis of PKS and YL at 800 °C is given. According to the XRD results, the optimum temperature at 800 °C determined according to the FT-IR results is also significantly evident in the raw and pyrolysis of PKS. This situation shows the positive effect of PKS addition on pyrolysis conversion when looking at the pyrolysis result of crude YL. Wu et al. studied the combined pyrolysis of coal and lignocellulosic biomass. According to the XRD analysis of the obtained chars, the addition of wheat straw to anthracite coal and bituminous coal showed a positive effect on the pyrolysis conversion made together (Wu et al., 2019).
Figure 5.
XRD analysis results.
As can be seen in Figure 5, 2 peaks were observed at the 40–50 (2Ɵ (°)) diffraction angles. The presence of aromatic compounds, which constitute an important part of the pyrolysis products, also supports the FT-IR results. Wu et al. investigated the effect of cellulose in coals of various ranks in the characterization of chars obtained as a result of pyrolysis. As a result of the test, it was determined that the lignocellulose added to the coal formed two peaks in the region where the aromatic rings in the 40–50 (2Ɵ (°)) diffraction angles are dense (Wu et al., 2017).
3.4.3. SEM analysis
SEM analyzes are shown in Figure 6. According to the structural analysis, SEM analyses of the chars obtained as a result of pyrolysis at varying temperatures as a mixture were compared. Accordingly, it is seen that the porosity deepened with the temperature increase to 80 °C. Wu et al. performed SEM analysis of the co-pyrolysis of microalgae and low-quality coals. According to the results of SEM analysis, it was observed that the porosity increased with the temperature applied to the mixture from 600 °C to 850 °C (Wu et al., 2018). This result is consistent with the best results of PKS and YL at 800 °C due to the synergistic effect of biomass on coal in the best pyrolysis condition as indicated in both the FT-IR result and the XRD result.
Figure 6.
SEM analysis of a) Mix (600 °C), b) Mix (400 °C), c) Mix (500 °C), d) Mix (700 °C), e) Mix (800 °C).
The combined pyrolysis of these two fuels produced a structure that reaches smaller particles. In the mixed state, additional sulfur capture creates more active sites for the chemical reaction to fix the sulfur in the structure (Haykiri-Acma and Yaman, 2007). A large number of accumulated small particles can be seen on the surface of the Mix400 and the pores gradually begin to grow. However, Mix 800 appears to have a more diverse surface morphology, such as a looser structure and more irregular particles; this can be explained by the destruction of the original dense structure of PKS due to the large number of volatile substances released by YL (Yuan et al., 2019). In addition, structures in PKS can catalyze lignin decomposition at higher temperatures. Therefore, it can accelerate the formation of porous structures even more (Lin et al., 2019; Liu et al., 2021; Yuan et al., 2019).
4. Conclusions
From the short and elemental analyzes of the chars obtained as a result of the pyrolysis performed by mixing PKS and YL separately and together from 400 °C to 800 °C, it is stated that the condition at 800 °C had positive effects on the pyrolysis conversion of the mixture. Structural analysis also confirms this situation. In particular, structural deterioration at low temperatures due to oxidation revealed the positive effect of biomass addition. It was tested if the combination had a synergistic impact with each other as a consequence of raising the temperature from 400 °C to 800 °C in the pyrolysis experiments done as a result of combining YL and PKS in equal amounts. According to this finding, coal and biomass have a synergistic influence on the thermal values of the product produced by the pyrolysis process when used together. In XRD analysis, on the other hand, aromatic structures that are important as a result of pyrolysis are an important result of pyrolysis. The increase in the porosity of the chars when the temperature increases has revealed that the necessary conditions for the reaction have a positive effect on the total conversion by adding biomass. In the case of increasing the pyrolysis temperatures of YL and PKS, the calorific values of the chars obtained in general were higher than the raw samples. According to this result, the chars obtained can be considered a new fuel with reduced sulfur. According to the calorific value results, it is possible to economically use the chars obtained as a solid fuel, since the calorific values of the chars increase when the temperature increases due to the synergetic effect of the mixture. According to the results of the elemental analysis, the solid product obtained in the mixture is both an economical and environmentally friendly product due to the lowest sulfur percentage obtained at the lowest pyrolysis temperatures (400 °C). According to the results of elemental analysis, sulfur was almost completely removed in the liquid and gas products obtained at 800 °C as a result of co-pyrolysis. In parallel with this, the yield of liquid product obtained at increasing pyrolysis temperatures showed a very serious increase. Gas product yield also increased. On the other hand, the solid product yield decreased.
Declarations
Author contribution statement
Figen Gündüz: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Yeliz Akbulut: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data.
Cemil Koyunoğlu: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Yunus Önal: Conceived and designed the experiments; Analyzed and interpreted the data.
Hüseyin Karaca: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Agarwal G., Lattimer B. Physicochemical, kinetic and energetic investigation of coal–biomass mixture pyrolysis. Fuel Process. Technol. 2014;124:174–187. [Google Scholar]
- Akinyemi B.A., Adesina A. Recent advancements in the use of biochar for cementitious applications: a review. J. Build. Eng. 2020;32 [Google Scholar]
- Aldana H., Lozano F.J., Acevedo J., Mendoza A. Thermogravimetric characterization and gasification of pecan nut shells. Bioresour. Technol. 2015;198:634–641. doi: 10.1016/j.biortech.2015.09.069. [DOI] [PubMed] [Google Scholar]
- Aliyu A., Lee J.G.M., Harvey A.P. Microalgae for biofuels: a review of thermochemical conversion processes and associated opportunities and challenges. Biores. Technol. Rep. 2021;15 [Google Scholar]
- Bonelli P.R., Della Rocca P.A., Cerrella E.G., Cukierman A.L. Effect of pyrolysis temperature on composition, surface properties and thermal degradation rates of Brazil Nut shells. Bioresour. Technol. 2001;76(1):15–22. doi: 10.1016/s0960-8524(00)00085-7. [DOI] [PubMed] [Google Scholar]
- Byambajav E., Paysepar H., Nazari L., Xu C. Co-pyrolysis of lignin and low rank coal for the production of aromatic oils. Fuel Process. Technol. 2018;181:1–7. [Google Scholar]
- Cao S., Yang J., Li J., Shi K., Li X. Preparation of oxygen-rich hierarchical porous carbon for supercapacitors through the co-carbonization of pitch and biomass. Diam. Relat. Mater. 2019;96:118–125. [Google Scholar]
- Chen D., Chen X., Sun J., Zheng Z., Fu K. Pyrolysis polygeneration of pine nut shell: quality of pyrolysis products and study on the preparation of activated carbon from biochar. Bioresour. Technol. 2016;216:629–636. doi: 10.1016/j.biortech.2016.05.107. [DOI] [PubMed] [Google Scholar]
- Chen J., Li X., Huang K., Eckelman M.J., Chertow M.R., Jiang D. Non-hazardous industrial waste in the United States: 100 Million tonnes of recoverable resources. Resour. Conserv. Recycl. 2021;167 [Google Scholar]
- Chiappero M., Norouzi O., Hu M., Demichelis F., Berruti F., Di Maria F., Mašek O., Fiore S. Review of biochar role as additive in anaerobic digestion processes. Renew. Sustain. Energy Rev. 2020;131 [Google Scholar]
- Davies G., El Sheikh A., Collett C., Yakub I., McGregor J. In: Emerging Carbon Materials for Catalysis. Sadjadi S., editor. 2021. Chapter 5 - catalytic carbon materials from biomass; pp. 161–195. Elsevier. [Google Scholar]
- Durak H. Thermochemical conversion of Phellinus pomaceus via supercritical fluid extraction and pyrolysis processes. Energy Convers. Manag. 2015;99:282–298. [Google Scholar]
- Farsi M. In: Advances in Bioenergy and Microfluidic Applications. Rahimpour M.R., Kamali R., Amin Makarem M., Manshadi M.K.D., editors. 2021. 9 - biomass conversion to biomethanol; pp. 231–252. Elsevier. [Google Scholar]
- Gedik B.K., Önal Y., Başar C.A., Akbulut Y. Chemical vapor deposition synthesis of carbon nanotube using pyrolysis gas products of apricot kernel shell. Adv. Sci. Eng. Med. 2020;12(4):556–563. [Google Scholar]
- Gemechu E.D., Kumar A. In: Renewable-Energy-Driven Future. Ren J., editor. 2021. Chapter 12 - the environmental performance of hydrogen production pathways based on renewable sources; pp. 375–406. Academic Press. [Google Scholar]
- Gerasimov G., Khaskhachikh V., Larina O., Sytchev G., Zaichenko V. In: Handbook of Advanced Approaches towards Pollution Prevention and Control. Rahman R.O.A., Hussain C.M., editors. 2021. 7 - pyrolytic methods of converting municipal solid waste into biofuel; pp. 137–156. Elsevier. [Google Scholar]
- Guo M., Bi J.-C. Characteristics and application of co-pyrolysis of coal/biomass blends with solid heat carrier. Fuel Process. Technol. 2015;138:743–749. [Google Scholar]
- Guran S. In: Sustainable Food Waste-To-Energy Systems. Trabold T.A., Babbitt C.W., editors. 2018. Chapter 8 - sustainable waste-to-energy technologies: gasification and pyrolysis; pp. 141–158. Academic Press. [Google Scholar]
- Hamidpour S., Fouladi N., Sedghamiz M.A., Rahimpour M.R. In: Advances in Bioenergy and Microfluidic Applications. Rahimpour M.R., Kamali R., Amin Makarem M., Manshadi M.K.D., editors. 2021. 18 - biomass technologies industrialization and environmental challenges; pp. 431–453. Elsevier. [Google Scholar]
- Haykiri-Acma H., Yaman S. Synergy in devolatilization characteristics of lignite and hazelnut shell during co-pyrolysis. Fuel. 2007;86(3):373–380. [Google Scholar]
- Ifa L., Yani S., Nurjannah N., Darnengsih D., Rusnaenah A., Mel M., Mahfud M., Kusuma H.S. Techno-economic analysis of bio-briquette from cashew nut shell waste. Heliyon. 2020;6(9) doi: 10.1016/j.heliyon.2020.e05009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- İlhan Küçük Y.Ö., Başar Canan. The activated carbon from walnut shell using CO2 and methylene blue removal. Dicle Üniversitesi Mühendislik Fakültesi Mühendislik Dergisi. 2021;12(2):297–308. [Google Scholar]
- Işıtan S., Ceylan S., Topcu Y., Hintz C., Tefft J., Chellappa T., Guo J., Goldfarb J.L. Product quality optimization in an integrated biorefinery: conversion of pistachio nutshell biomass to biofuels and activated biochars via pyrolysis. Energy Convers. Manag. 2016;127:576–588. [Google Scholar]
- Jana K., Ray A., Majoumerd M.M., Assadi M., De S. Polygeneration as a future sustainable energy solution – a comprehensive review. Appl. Energy. 2017;202:88–111. [Google Scholar]
- Jiang G., Xu D., Hao B., Liu L., Wang S., Wu Z. Thermochemical methods for the treatment of municipal sludge. J. Clean. Prod. 2021;311 [Google Scholar]
- Jung S., Park Y.-K., Kwon E.E. In: Biomass, Biofuels, Biochemicals. Bhaskar T., Pandey A., editors. 2021. 7 - Catalytic Hydrodeoxygenation for Upgrading of Lignin-Derived Bio-Oils; pp. 129–145. Elsevier. [Google Scholar]
- Kir Ş., Dehri İ., Önal Y., Esen R. Graphene quantum dots prepared from dried lemon leaves and microcrystalline mosaic structure. Luminescence. 2021;36(6):1365–1376. doi: 10.1002/bio.4060. [DOI] [PubMed] [Google Scholar]
- Li Y., Huang S., Wang Q., Li H., Zhang Q., Wang H., Wu Y., Wu S., Gao J. Hydrogen transfer route and interaction mechanism during co-pyrolysis of Xilinhot lignite and rice husk. Fuel Process. Technol. 2019;192:13–20. [Google Scholar]
- Li Y., Lu L., Lyu S., Xu H., Ren X., Levendis Y.A. Activated coke preparation by physical activation of coal and biomass co-carbonized chars. J. Anal. Appl. Pyrol. 2021;156 [Google Scholar]
- Lin X., Kong L., Cai H., Zhang Q., Bi D., Yi W. Effects of alkali and alkaline earth metals on the co-pyrolysis of cellulose and high density polyethylene using TGA and Py-GC/MS. Fuel Process. Technol. 2019;191:71–78. [Google Scholar]
- Lin X., Sheng Z., He J., He X., Wang C., Gu X., Wang Y. Preparation of isotropic spinnable pitch with high-spinnability by co-carbonization of coal tar pitch and bio-asphalt. Fuel. 2021;295 [Google Scholar]
- Liu J., Yang X., Liu H., Jia X., Bao Y. Mixed biochar obtained by the co-pyrolysis of shrimp shell with corn straw: Co-pyrolysis characteristics and its adsorption capability. Chemosphere. 2021;282 doi: 10.1016/j.chemosphere.2021.131116. [DOI] [PubMed] [Google Scholar]
- Loya-González D., Loredo-Cancino M., Soto-Regalado E., Rivas-García P., Cerino-Córdova F.d.J., García-Reyes R.B., Bustos-Martínez D., Estrada-Baltazar A. Optimal activated carbon production from corn pericarp: a life cycle assessment approach. J. Clean. Prod. 2019;219:316–325. [Google Scholar]
- Mailaram S., Kumar P., Kunamalla A., Saklecha P., Maity S.K. In: Sustainable Fuel Technologies Handbook. Dutta S., Mustansar Hussain C., editors. 2021. 3 - biomass, biorefinery, and biofuels; pp. 51–87. Academic Press. [Google Scholar]
- Manurung R., Wever D.A.Z., Wildschut J., Venderbosch R.H., Hidayat H., van Dam J.E.G., Leijenhorst E.J., Broekhuis A.A., Heeres H.J. Valorisation of Jatropha curcas L. plant parts: nut shell conversion to fast pyrolysis oil. Food Bioprod. Process. 2009;87(3):187–196. [Google Scholar]
- Maroušek J. Vol. 35. Revista Técnica de la Facultad de Ingeniería Universidad del Zulia; 2012. Finding the Optimal Parameters for the Steam Explosion Process of hay; pp. 170–178. [Google Scholar]
- Maroušek J., Maroušková A., Kůs T. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects. 2020. Shower cooler reduces pollutants release in production of competitive cement substitute at low cost; pp. 1–10. [Google Scholar]
- Martí-Rosselló T., Li J., Lue L. Quantitatively modelling kinetics through a visual analysis of the derivative thermogravimetric curves: application to biomass pyrolysis. Energy Convers. Manag. 2018;172:296–305. [Google Scholar]
- Matzen M., Demirel Y. Methanol and dimethyl ether from renewable hydrogen and carbon dioxide: alternative fuels production and life-cycle assessment. J. Clean. Prod. 2016;139:1068–1077. [Google Scholar]
- Mochizuki Y., Tsubouchi N. Preparation of pelletized coke by co-carbonization of caking coal and pyrolyzed char modified with tar produced during pyrolysis of woody biomass. Fuel Process. Technol. 2019;193:328–337. [Google Scholar]
- Moldoveanu S.C. In: Analytical Pyrolysis of Natural Organic Polymers. second ed. Moldoveanu S.C., editor. Vol. 20. 2021. 15 - applications of analytical pyrolysis in bio-oil production; pp. 471–475. Elsevier. [Google Scholar]
- Moravvej Z., Soroush E., Makarem M.A., Rahimpour M.R. In: Advances in Bioenergy and Microfluidic Applications. Rahimpour M.R., Kamali R., Amin Makarem M., Manshadi M.K.D., editors. 2021. 7 - thermochemical routes for hydrogen production from biomass; pp. 193–208. Elsevier. [Google Scholar]
- Nie J., Zhi D., Zhou Y. In: Sorbents Materials for Controlling Environmental Pollution. Núñez-Delgado A., editor. 2021. Chapter 8 - magnetic biochar-based composites for removal of recalcitrant pollutants in water; pp. 163–187. Elsevier. [Google Scholar]
- Ortiz L.R., Torres E., Zalazar D., Zhang H., Rodriguez R., Mazza G. Influence of pyrolysis temperature and bio-waste composition on biochar characteristics. Renew. Energy. 2020;155:837–847. [Google Scholar]
- Önal Y., Ceylan K. Effects of treatments on the mineral matter and acidic functional group contents of Turkish lignites. Fuel. 1995;74(7):972–977. [Google Scholar]
- Özsin G., Pütün A.E. Insights into pyrolysis and co-pyrolysis of biomass and polystyrene: thermochemical behaviors, kinetics and evolved gas analysis. Energy Convers. Manag. 2017;149:675–685. [Google Scholar]
- Pecha M.B., Garcia-Perez M. In: Bioenergy. second ed. Dahiya A., editor. 2020. Chapter 29 - pyrolysis of lignocellulosic biomass: oil, char, and gas; pp. 581–619. Academic Press. [Google Scholar]
- Pereira Lopes R., Astruc D. Biochar as a support for nanocatalysts and other reagents: recent advances and applications. Coord. Chem. Rev. 2021;426 [Google Scholar]
- Piroozmand M., Balegh Y., Hafizi A., Esfandyari M. In: Advances in Bioenergy and Microfluidic Applications. Rahimpour M.R., Kamali R., Amin Makarem M., Manshadi M.K.D., editors. 2021. 4 - chemical looping conversion of biomass and biomass-derived feedstocks; pp. 87–136. Elsevier. [Google Scholar]
- Puig-Gamero M., Pio D.T., Tarelho L.A.C., Sánchez P., Sanchez-Silva L. Simulation of biomass gasification in bubbling fluidized bed reactor using aspen plus. Energy Convers. Manag. 2021;235 [Google Scholar]
- Quereshi S., Jadhao P.R., Pandey A., Ahmad E., Pant K.K. In: Sustainable Fuel Technologies Handbook. Dutta S., Mustansar Hussain C., editors. 2021. 1 - overview of sustainable fuel and energy technologies; pp. 3–25. Academic Press. [Google Scholar]
- Sonobe T., Worasuwannarak N., Pipatmanomai S. Synergies in co-pyrolysis of Thai lignite and corncob. Fuel Process. Technol. 2008;89(12):1371–1378. [Google Scholar]
- Tacettin Geçkil Y.O., Beyza ince Ceren. Atık polietilen tereftalat (PET) ile modifiye edilmiş saf bitümün fiziksel, morfolojik ve isıl özellikleri. Fırat Üniversitesi Mühendislik Bilimleri Dergisi. 2020;32(1):157–166. [Google Scholar]
- Tacettin Gecki̇l Y.O., Ceren Beyza I.N.C.E. Moisture resistance of bituminous hot mixtures modified with waste PET. J. Polytech. 2021;24(2):461–471. [Google Scholar]
- Tursi A., Olivito F. In: Advances in Bioenergy and Microfluidic Applications. Rahimpour M.R., Kamali R., Amin Makarem M., Manshadi M.K.D., editors. 2021. 1 - biomass conversion: general information, chemistry, and processes; pp. 3–39. Elsevier. [Google Scholar]
- Ünal Sansar İ. Optimization of some parameters on desulfurization process of Muğla Yatağan Bağyaka lignite by ultrasonic waves. Bull. Mineral Res. Exploration. 2018;157:10–20. [Google Scholar]
- Wang A.-Y., Sun K., Wu L., Wu P., Zeng W., Tian Z., Huang Q.-X. Co-carbonization of biomass and oily sludge to prepare sulfamethoxazole super-adsorbent materials. Sci. Total Environ. 2020;698 doi: 10.1016/j.scitotenv.2019.134238. [DOI] [PubMed] [Google Scholar]
- Wilk M., Śliz M., Lubieniecki B. Hydrothermal co-carbonization of sewage sludge and fuel additives: combustion performance of hydrochar. Renew. Energy. 2021;178:1046–1056. [Google Scholar]
- Wu Z., Ma C., Jiang Z., Luo Z. Structure evolution and gasification characteristic analysis on co-pyrolysis char from lignocellulosic biomass and two ranks of coal: effect of wheat straw. Fuel. 2019;239:180–190. [Google Scholar]
- Wu Z., Wang S., Luo Z., Chen L., Meng H., Zhao J. Physico-chemical properties and gasification reactivity of co-pyrolysis char from different rank of coal blended with lignocellulosic biomass: effects of the cellulose. Bioresour. Technol. 2017;235:256–264. doi: 10.1016/j.biortech.2017.03.121. [DOI] [PubMed] [Google Scholar]
- Wu Z., Yang W., Li Y., Yang B. Co-pyrolysis behavior of microalgae biomass and low-quality coal: products distributions, char-surface morphology, and synergistic effects. Bioresour. Technol. 2018;255:238–245. doi: 10.1016/j.biortech.2018.01.141. [DOI] [PubMed] [Google Scholar]
- Yuan R., Yu S., Shen Y. Pyrolysis and combustion kinetics of lignocellulosic biomass pellets with calcium-rich wastes from agro-forestry residues. Waste Manag. 2019;87:86–96. doi: 10.1016/j.wasman.2019.02.009. [DOI] [PubMed] [Google Scholar]
- Zaafouri K., Ben Hassen Trabelsi A., Krichah S., Ouerghi A., Aydi A., Claumann C.A., André Wüst Z., Naoui S., Bergaoui L., Hamdi M. Enhancement of biofuels production by means of co-pyrolysis of Posidonia oceanica (L.) and frying oil wastes: experimental study and process modeling. Bioresour. Technol. 2016;207:387–398. doi: 10.1016/j.biortech.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Zhou J., Wang G., Wang M., Zhu P., Yan B., Luo L., Wong J. In: Current Developments in Biotechnology and Bioengineering. Wong J., Kaur G., Taherzadeh M., Pandey A., Lasaridi K., editors. 2021. Chapter twelve - pyrolysis and gasification of food waste; pp. 325–344. Elsevier. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.