Abstract
Genetic diversity and genetic relationships among 42 Pseudomonas stutzeri strains belonging to several genomovars and isolated from different sources were investigated in an examination of 20 metabolic enzymes by multilocus enzyme electrophoresis analysis. Forty-two distinct allele profiles were identified, indicating that all multilocus genotypes were represented by a single strain. All 20 loci were exceptionally polymorphic, with an average of 15.9 alleles per locus. To the best of our knowledge, this P. stutzeri sample exhibited the highest mean genetic diversity (H = 0.876) found to date in all bacterial species studied by multilocus enzyme electrophoresis. A high frequency of occurrence of null alleles was identified. The index of association (IA) for the P. stutzeri strains analyzed was 1.10. The IA values were always significantly different from zero for all subgroups studied, including clinical and environmental isolates and strains classified as genomovar 1. These results suggest that the population structure of P. stutzeri is strongly clonal, indicating that there is no significant level of assortative recombination that might destroy linkage disequilibrium.
Pseudomonas stutzeri was first isolated by Burri and Stutzer (6) as Bacillus denitrificans II and named P. stutzeri by Van Niel and Allen (47). It has an unusual colony shape and consistency when directly isolated, being described as wrinkly, dry, and tenaciously coherent. P. stutzeri, a gram-negative rod-shaped bacterium that is mobile by means of a single polar flagellum, is a nonpigmented denitrifier that liberates nitrogen gas from nitrate, is amylase positive and gelatinase negative, and is able to grow on maltose and starch (4, 43). P. stutzeri has a wide environmental distribution but is found mainly in soil and water. Many strains have been isolated from clinical specimens (20). The members of the species share physiological characteristics that make P. stutzeri of special interest in ecological studies. This species shows high metabolic versatility (35) including the degradation of environmental pollutants (1, 37) and high-molecular-weight polyethylene glycols (30). P. stutzeri serves as a model for the study of the biochemistry and genetics of denitrification and natural transformation processes.
Pseudomonas species are grouped on the basis of rRNA-DNA hybridization studies (31). P. stutzeri is a nonfluorescent denitrifying species of the genus Pseudomonas included in the rRNA group I. P. stutzeri forms a homogeneous group within the genus Pseudomonas, with phenotypic traits that permit description to the species level. However, P. stutzeri is a heterogeneous species with respect to many phenotypic characteristics and DNA composition. Several studies have demonstrated that P. stutzeri consists of a complex collection of strains that might be distributed in more than one species (2, 25, 31, 35). DNA-DNA hybridization studies (35, 43) have shown the existence of at least eight genomic groups, called genomovars. Confirmation of this system of internal subdivisions was reported following other approaches to bacterial phylogeny which included 16S rRNA gene sequencing (2), chemotaxonomic total fatty acid analysis, total protein pattern analysis (36), and macrorestriction fragment analysis of genomic DNA (16). The difference in 16S rRNA sequence of one of these genomic groups, together with differential phenotypic traits, was sufficient for genomovar 6 to be renamed the new species Pseudomonas balearica (2). Genetic relationships among most of the P. stutzeri strains used in the present study, based on molecular typing methods (repetitive extragenic palindromic PCR, enterobacteria repetitive inverted consensus PCR, and internally transcribed spacer [ITS] fingerprinting), have been published previously (4, 19).
Recently 16 P. stutzeri strains (including 9 of the 42 reported here) have been analyzed by PCR-based genomic fingerprinting procedures and multilocus enzyme electrophoresis (MLEE). A distinct genotype of each strain, indicating great genotypic diversity, was found within P. stutzeri (43). However, calculation of genetic diversity and linkage disequilibrium from MLEE results were not provided by the authors, and the nature of genetic population structure within P. stutzeri remains unclear.
In the present study, MLEE was used to examine genetic relationships among 43 strains identified phenotypically as P. stutzeri. One of the strains phenotypically classified as P. stutzeri (SD29577), however, was not identified as P. stutzeri by previously described molecular methods (3). Two strains of P. balearica and three reference strains of closely related Pseudomonas species, included in rRNA group I (31), were also examined by MLEE. All strains of P. stutzeri had been classified previously in genomovars by DNA-DNA hybridizations or 16S-23S ITS1 restriction fragment length polymorphism analysis (19), except CLN100, A122/76, and A147/68, which could not be assigned to any known genomovar. The results of our analysis indicate that the P. stutzeri population exhibits very high genetic diversity and significant linkage disequilibrium, which implies a basic clonal population structure.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study were originally obtained from culture collections or were isolated as indicated in Table 1. Some of these strains have already been characterized physiologically and genomically (2, 4, 16, 19, 32, 35, 36, 38, 43). All strains were cultured by plating on Trypticase soy agar (BBL, Cockeysville, Md.) at 30°C for 48 h.
TABLE 1.
Pseudomonas strains used in this study
| ET | Species | Straina | Genomovar | Source (place and date of isolation) |
|---|---|---|---|---|
| 1 | P. stutzeri | ATCC 17594 | 1 | Clinical (Copenhagen, Denmark, before 1966) |
| 2 | P. stutzeri | CCUG 11256T (ATCC 17588) | 1 | Clinical (Copenhagen, Denmark, before 1966) |
| 3 | P. stutzeri | ATCC 17589 | 1 | Clinical (Copenhagen, Denmark, before 1966) |
| 4 | P. stutzeri | ATCC 17591 | 2 | Clinical (Copenhagen, Denmark, before 1966) |
| 5 | P. stutzeri | ATCC 17587 | 2 | Clinical (Copenhagen, Denmark, before 1966) |
| 6 | P. stutzeri | ZoBell (ATCC 14405) | 2 | Marine (Pacific Ocean, California, before 1944) |
| 7 | P. stutzeri | DSM 50227 (ATCC 11607) | 3 | Clinical (before 1952) |
| 8 | P. stutzeri | AN10 | 3 | Marine (Barcelona, Spain, 1983) |
| 9 | P. stutzeri | AN11 | 3 | Marine (Barcelona, Spain, 1983) |
| 10 | P. stutzeri | 19SMN4 (DSM 6084) | 4 | Marine (Barcelona, Spain, 1988) |
| 11 | P. stutzeri | ST27MN3 | 4 | Marine (Barcelona, Spain, 1988) |
| 12 | P. stutzeri | DNSP21 (DSM 6082) | 5 | Wastewater treatment plant (Mallorca, Spain, 1988) |
| 13 | P. balearica | SP1402 (DSM 6083T) | 6 | Wastewater treatment plant (Mallorca, Spain, 1988) |
| 14 | P. balearica | LS401 | 6 | Marine (Barcelona, Spain, 1988) |
| 15 | P. stutzeri | DSM 50238 (ATCC 17832) | 7 | Soil (Berkeley, Calif., before 1966) |
| 16 | P. stutzeri | JM300 (DSM 10701) | 8 | Soil (California, before 1980) |
| 17 | P. stutzeri | CLN100 | NDb | Industrial waste (Germany, 1992) |
| 18 | P. stutzeri | ATCC 27951 | 1 | Clinical |
| 19 | P. stutzeri | Aer 2.5 | 1 | Aircraft oil-contaminated soil (Mallorca, Spain, 1995) |
| 20 | P. stutzeri | Aer 2.7 | 7 | Aircraft oil-contaminated soil (Mallorca, Spain, 1995) |
| 21 | P. stutzeri | Aer 2.8 | 1 | Aircraft oil-contaminated soil (Mallorca, Spain, 1995) |
| 22 | P. stutzeri | Aer 5.1 | 3 | Aircraft oil-contaminated soil (Mallorca, Spain, 1995) |
| 23 | P. stutzeri | SADN19 | 1 | Brackish water sediment (Mallorca, Spain, 1993) |
| 24 | P. stutzeri | SADN27 | 1 | Brackish water sediment (Mallorca, Spain, 1993) |
| 25 | P. stutzeri | SD20240 | 1 | Clinical (Mallorca, Spain, 1995) |
| 26 | P. stutzeri | SD55473 | 1 | Clinical (Mallorca, Spain, 1994) |
| 27 | P. stutzeri | SD93936 | 1 | Clinical (Mallorca, Spain, 1993) |
| 28 | Pseudomonas sp. | SD29577 | Clinical (Mallorca, Spain, 1996) | |
| 29 | P. stutzeri | SD17204 | 1 | Clinical (Mallorca, Spain, 1995) |
| 30 | P. stutzeri | JD4 | 5 | Soil (Mallorca, Spain, 1995) |
| 31 | P. stutzeri | Pll1 | 3 | Wastewater treatment plant (Mallorca, 1996) |
| 32 | P. stutzeri | NF13 | 3 | Marine (Galápagos Rift, 1979) |
| 33 | P. stutzeri | A122/76 | ND | Clinical (Great Britain, between 1965 and 1984) |
| 34 | P. stutzeri | A63/70 | 1 | Clinical (Great Britain, between 1965 and 1984) |
| 35 | P. stutzeri | A95/69 | 1 | Clinical (Great Britain, between 1965 and 1984) |
| 36 | P. stutzeri | A60/68 | 2 | Clinical (Great Britain, between 1965 and 1984) |
| 37 | P. stutzeri | A75/66 | 1 | Clinical (Great Britain, between 1965 and 1984) |
| 38 | P. stutzeri | A85a/66 | 2 | Clinical (Great Britain, between 1965 and 1984) |
| 39 | P. stutzeri | A48/66 | 1 | Clinical (Great Britain, between 1965 and 1984) |
| 40 | P. stutzeri | A60/72 | 2 | Clinical (Great Britain, between 1965 and 1984) |
| 41 | P. stutzeri | A42/69 | 1 | Clinical (Great Britain, between 1965 and 1984) |
| 42 | P. stutzeri | A31/70 | 2 | Clinical (Great Britain, between 1965 and 1984) |
| 43 | P. stutzeri | A69/69 | 1 | Clinical (Great Britain, between 1965 and 1984) |
| 44 | P. stutzeri | A94/69 | 1 | Clinical (Great Britain, between 1965 and 1984) |
| 45 | P. stutzeri | A147/68 | ND | Clinical (Great Britain, between 1965 and 1984) |
| 46 | P. aeruginosa | CCM 1960T | ||
| 47 | P. pseudoalcaligenes | ATCC 17440T | Clinical | |
| 48 | P. mendocina | ATCC 25411T | Soil (Argentina) |
ATCC, American Type Culture Collection, Manassas, Va.; CCUG, Culture Collection, University of Göteborg, Göteborg, Sweden; DSM, Deutsche Sammlung von Mikroorganismen und Zelkulturen, Braunschweig, Germany; CECT, Colección Española de Cultivos Tipo, Valencia, Spain; LMG, Laboratorium Microbiologie Rijksuniversiteit, Gent, Belgium; CCM, Czechoslovak Collection of Microorgannisms, Brno, Czech Republic.
ND, not determined.
Cell extracts.
Solid cultures were suspended in sterile 50 mM Na2HPO4-NaH2PO4 buffer (pH 7.5). Cell suspensions were disrupted by sonication in an ice-water bath for four 30-s cycles with a Branson Sonifier model 250 (Branson Ultrasonics Co., Danbury, Conn.), using setting 3 and duty cycle 50%. Cell debris was removed by centrifugation for 10 min at 13,500 rpm and room temperature (Sigma centrifuge model 201M). The supernatant fluid was dispensed in sterile Eppendorf tubes and stored immediately at −40°C until used.
Protein was measured by the method of Lowry et al. (24), with bovine serum albumin (Sigma) as a standard.
Electrophoresis and running conditions for MLEE.
Discontinuous nondenaturing vertical polyacrylamide gel electrophoresis was used for all enzymes. The acrylamide concentration in the gels was 10%/8% or 8%/5%, depending on the enzyme studied; 0.4 M Tris-HCl (pH 8.8) resolving buffer and 0.125 M Tris-HCl (pH 6.8) stacking buffer were used in all gels; 0.19 M Tris-glycine (pH 8.3) buffer was used for the electrode compartments. Gels were used within 4 h of preparation and run at 7°C. A constant voltage, depending on the acrylamide concentration in the gel, was applied until the bromophenol blue band reached the bottom of the gel. All strains were run at least twice to confirm their genotype.
The following 20 enzymes were assayed: two glucose-6-phosphate dehydrogenases (G6P-1 and G6P-2), two lactate dehydrogenases (LDH-1 and LDH-2), two ethanol dehydrogenases (EDH-1 and EDH-2), leucine aminopeptidase (LAP), esterases (EST), nucleoside phosphorilase (NSP), leucine dehydrogenase (LED), two alanine dehydrogenases (ALD-1 and ALD-2), threonine dehydrogenase (THD), two lysine dehydrogenases (LYS-1 and LYS-2), glutamate dehydrogenase-NADP (GD2), glutamate dehydrogenase-NAD (GD1), malic enzyme (ME), catechol 2,3 dioxygenase (C23O), and catechol 1,2 dioxygenase (C12O). The electrophoretic mobilities of the enzymes were determined by staining for specific enzyme activity as recommended by Selander et al. (41), except for catechol dioxygenases. Dioxygenase activity was revealed by placing a filter paper soaked with 0.02 M Tris-HCl (pH 7) containing 0.03 M pyrocatechol (Sigma) over the gel for 3 h at 37°C. The stain solution was then poured off, the filter paper was discarded, and the gel slice was kept at room temperature for an additional 3 h.
Catechol dioxygenases appeared on gels as well-defined brown bands. To differentiate between C23O and C12O activities, the following procedure was used. Cell extracts were maintained at 55°C for 10 min and then cooled in ice. The resulting cell extracts were used to reveal C23O activity only (15). The bands which appeared in gels with complete cell extracts but not in gels with heat-treated cell extracts were assigned to C12O activity. For each enzyme, distinct mobility variants were designated as electromorphs and numbered in order of decreasing anodal migration. Electromorphs were taken as products of alleles at the analyzed enzyme loci. The absence of enzyme activity was attributed to a null allele and designated 0.
To verify that the lack of enzymatic activity was due to the true absence of the enzyme, rather than a laboratory artifact associated with the poor quality of the cell extracts, we measured the protein content of each lysate (ranging from 2.5 to 11.4 mg/ml). We prepared concentrated cell lysates from several isolates (with apparent null alleles) being repeatedly grown, in separate preparations, to a high cell density, which never yielded detectable activity for the target enzyme. In addition, among isolates with null alleles, gels stained for other enzymatic activities revealed uniformly high activity. Distinct combinations of alleles over the 20 loci assayed were assigned as electrophoretic types (ETs).
Data treatment.
Genetic diversity (hj) among ETs at an enzyme locus (i.e., the probability that two isolates differ at the j locus) was calculated from allele frequencies using the formula hj = n(1 − ∑ pij2)/(n − 1), j = 1,2, … m, where pij is the frequency of the ith allele at the j locus, n is the number of isolates or ETs, and m is the number of alleles assayed (27, 29). Mean genetic diversity (H) was calculated as the arithmetic mean of the hj values for all 20 loci. Clustering of the values obtained by isoenzyme electrophoresis was performed with a matrix of coefficients of genetic distances by the unweighted pair-group method for arithmetic averages by using the PHYLIP package (12). The genetic distances between pairs of ETs were calculated as the proportions of loci at which dissimilar electromorphs occurred. The cophenetic correlation coefficient was calculated using NTSYS-pc, version 1.80 (34). Multilocus linkage disequilibrium was calculated on the basis of the distribution of allelic mismatches between pairs of ETs among all loci examined as the index of association (IA) developed by Brown et al. (5) and Maynard-Smith et al. (27). The ratio of the observed variance in mismatches (Vo) to that expected at linkage equilibrium [VE = ∑ hj (1 − hj)] provides a measure of multilocus linkage disequilibrium that can be expressed as IA = Vo/VE − 1. For populations at linkage equilibrium, Vo equals VE and IA has an expected value of zero (5, 27). To test if IA differed significantly from its expected value of zero (i.e., the ratio Vo/VE is significantly differently from 1), a Monte Carlo randomization test with 10,000 resamplings was used. Samples of the same size as the original data set were generated by randomly sampling alleles according to their frequencies at each locus (11, 18). For each random sample, Vo and VE were calculated and the minimum (Vmin) and maximum (Vmax) values of Vo for the 10,000 samples were recorded. Significance was estimated as the probability of observing an Vo/VE ratio at least as extreme as that determined for the original data. The null hypothesis of a random association of alleles (i.e., the population is at linkage equilibrium) was rejected if this probability was smaller than the selected significance level. A GST statistics was used to compare the mean genetic diversity of clinical and environmental isolates (41). A Wilcoxon signed-rank test with continuity correction where necessary was used to compare the number of null alleles of samples with respect to the origin of isolates (45). Unless otherwise stated, statistical significance was taken to be indicated by P values of less than 0.05. Computer programs written by T. S. Whittam (41) and J. G. Lorén (14) were used to calculate genetic diversity, Vo and VE, and IA and to perform the Monte Carlo randomizations.
RESULTS
ETs and genetic diversity.
Table 2 summarizes the allelic profiles of the P. stutzeri and other Pseudomonas strains used in this study. All multilocus genotypes were represented by a single strain. In the collection of 48 isolates, all 20 loci were exceptionally polymorphic, ranging from 8 (LAP) to 31 (EST) alleles, with an average of 18.6 alleles per locus. The mean genetic diversity in the sample was 0.885 (Tables 3 and 4). In the P. stutzeri population studied, all 20 loci were also highly polymorphic, ranging from 7 (LAP) to 27 (EST and THD) alleles, with an average of 15.9 alleles per locus. The mean genetic diversity was 0.876 (Tables 3 and 4). Tables 3 and 4 also show the results obtained for several population subsets studied. No significant differences were detected in mean genetic diversity among clinical (H = 0.844, χ2 = 176.08) and environmental (H = 0.890, χ2 = 60.31) isolates (P > 0.9).
TABLE 2.
Allele profiles of ETs of Pseudomonas strains
| ET | Strain | Allele at indicated polymorphic enzyme locus
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G6P-1 | G6P-2 | C12O | C23O | LDH-1 | LDH-2 | EDH-1 | EDH-2 | LAP | EST | NSP | LED | ALD-1 | ALD-2 | THD | LYS-1 | LYS-2 | GD2 | GD1 | ME | ||
| 1 | P. stutzeri ATCC 17594 | 10 | 2 | 0 | 9 | 4 | 7 | 4 | 5 | 2 | 31 | 3 | 14 | 7 | 4 | 7 | 17 | 5 | 5 | 13 | 14 |
| 2 | P. stutzeri CCUG 11256 | 11 | 4 | 0 | 9 | 4 | 7 | 0 | 5 | 2 | 26 | 0 | 22 | 7 | 4 | 7 | 18 | 6 | 6 | 13 | 14 |
| 3 | P. stutzeri ATCC 17589 | 10 | 2 | 4 | 0 | 4 | 12 | 4 | 5 | 4 | 15 | 3 | 4 | 7 | 2 | 20 | 6 | 3 | 5 | 2 | 14 |
| 4 | P. stutzeri ATCC 17591 | 15 | 13 | 6 | 4 | 4 | 8 | 4 | 5 | 2 | 5 | 3 | 21 | 7 | 12 | 32 | 20 | 12 | 3 | 15 | 19 |
| 5 | P. stutzeri ATCC 17587 | 15 | 13 | 6 | 4 | 4 | 8 | 4 | 5 | 2 | 15 | 3 | 21 | 7 | 12 | 32 | 4 | 20 | 3 | 15 | 19 |
| 6 | P. stutzeri ZoBell | 15 | 13 | 0 | 1 | 2 | 15 | 1 | 4 | 4 | 5 | 1 | 30 | 7 | 22 | 13 | 1 | 21 | 3 | 13 | 19 |
| 7 | P. stutzeri DSM 50227 | 14 | 13 | 0 | 1 | 5 | 11 | 6 | 3 | 3 | 16 | 5 | 4 | 11 | 2 | 0 | 13 | 10 | 4 | 7 | 2 |
| 8 | P. stutzeri AN10 | 14 | 13 | 7 | 1 | 5 | 12 | 6 | 3 | 4 | 15 | 13 | 4 | 11 | 2 | 21 | 0 | 1 | 4 | 7 | 14 |
| 9 | P. stutzeri AN11 | 14 | 13 | 0 | 1 | 6 | 11 | 18 | 0 | 4 | 15 | 6 | 29 | 7 | 18 | 21 | 0 | 1 | 4 | 7 | 14 |
| 10 | P. stutzeri 19SMN4 | 15 | 17 | 7 | 7 | 5 | 12 | 5 | 5 | 3 | 34 | 4 | 29 | 8 | 18 | 31 | 20 | 15 | 3 | 14 | 18 |
| 11 | P. stutzeri ST27MN3 | 15 | 17 | 4 | 7 | 5 | 12 | 5 | 5 | 3 | 34 | 4 | 4 | 8 | 8 | 31 | 20 | 15 | 3 | 14 | 18 |
| 12 | P. stutzeri DNSP21 | 9 | 3 | 3 | 2 | 5 | 0 | 4 | 2 | 2 | 1 | 1 | 6 | 7 | 0 | 0 | 5 | 0 | 3 | 2 | 4 |
| 13 | P. balearica SP1402 | 23 | 12 | 0 | 10 | 22 | 13 | 0 | 12 | 4 | 21 | 2 | 3 | 7 | 1 | 5 | 0 | 9 | 4 | 2 | 7 |
| 14 | P. balearica LS401 | 23 | 9 | 7 | 3 | 22 | 13 | 0 | 12 | 0 | 21 | 0 | 4 | 6 | 5 | 23 | 15 | 8 | 5 | 10 | 12 |
| 15 | P. stutzeri DSM 50238 | 15 | 0 | 0 | 1 | 9 | 17 | 11 | 11 | 2 | 25 | 10 | 6 | 2 | 3 | 9 | 2 | 4 | 7 | 6 | 2 |
| 16 | P. stutzeri JM300 | 6 | 6 | 0 | 8 | 21 | 0 | 8 | 0 | 6 | 20 | 2 | 8 | 0 | 0 | 0 | 7 | 0 | 6 | 2 | 16 |
| 17 | P. stutzeri CLN100 | 8 | 8 | 10 | 0 | 23 | 0 | 0 | 1 | 11 | 32 | 1 | 26 | 12 | 2 | 30 | 3 | 3 | 9 | 2 | 9 |
| 18 | P. stutzeri ATCC 27951 | 12 | 6 | 0 | 9 | 10 | 9 | 2 | 5 | 2 | 10 | 3 | 19 | 7 | 13 | 6 | 17 | 5 | 7 | 13 | 15 |
| 19 | P. stutzeri Aer 2.5 | 12 | 6 | 5 | 1 | 0 | 2 | 7 | 4 | 2 | 9 | 4 | 19 | 5 | 4 | 18 | 17 | 4 | 7 | 13 | 15 |
| 20 | P. stutzeri Aer 2.7 | 8 | 6 | 0 | 6 | 1 | 15 | 3 | 18 | 2 | 9 | 1 | 18 | 1 | 10 | 14 | 2 | 4 | 3 | 12 | 6 |
| 21 | P. stutzeri Aer 2.8 | 12 | 6 | 5 | 1 | 7 | 14 | 7 | 4 | 2 | 9 | 4 | 19 | 3 | 0 | 0 | 5 | 5 | 2 | 13 | 15 |
| 22 | P. stutzeri Aer 5.1 | 14 | 13 | 7 | 1 | 19 | 5 | 9 | 4 | 5 | 26 | 4 | 5 | 9 | 2 | 29 | 0 | 1 | 3 | 2 | 14 |
| 23 | P. stutzeri SADN19 | 3 | 14 | 4 | 1 | 12 | 2 | 4 | 3 | 2 | 24 | 3 | 19 | 7 | 17 | 1 | 20 | 0 | 7 | 13 | 15 |
| 24 | P. stutzeri SADN27 | 2 | 6 | 6 | 1 | 6 | 2 | 0 | 15 | 2 | 9 | 3 | 16 | 4 | 10 | 15 | 0 | 1 | 7 | 11 | 20 |
| 25 | P. stutzeri SD20240 | 12 | 6 | 4 | 1 | 11 | 2 | 2 | 6 | 2 | 26 | 3 | 19 | 7 | 4 | 1 | 20 | 0 | 7 | 11 | 17 |
| 26 | P. stutzeri SD55473 | 10 | 1 | 6 | 1 | 13 | 2 | 2 | 5 | 11 | 27 | 3 | 30 | 7 | 6 | 6 | 16 | 11 | 7 | 13 | 17 |
| 27 | P. stutzeri SD93936 | 3 | 15 | 11 | 1 | 13 | 2 | 2 | 5 | 2 | 35 | 3 | 6 | 7 | 4 | 6 | 16 | 11 | 7 | 1 | 14 |
| 28 | Pseudomonas sp. strain SD29577 | 20 | 0 | 0 | 5 | 15 | 2 | 14 | 7 | 7 | 4 | 1 | 25 | 14 | 8 | 16 | 12 | 13 | 2 | 23 | 5 |
| 29 | P. stutzeri SD17204 | 12 | 6 | 4 | 2 | 16 | 2 | 3 | 7 | 2 | 26 | 3 | 7 | 12 | 10 | 24 | 12 | 18 | 8 | 11 | 16 |
| 30 | P. stutzeri JD4 | 13 | 7 | 9 | 0 | 3 | 16 | 7 | 14 | 2 | 11 | 1 | 30 | 7 | 19 | 22 | 3 | 17 | 8 | 13 | 11 |
| 31 | P. stutzeri Pll1 | 14 | 13 | 0 | 1 | 5 | 1 | 12 | 13 | 5 | 28 | 13 | 5 | 12 | 2 | 19 | 17 | 19 | 8 | 3 | 16 |
| 32 | P. stutzeri NF13 | 16 | 0 | 6 | 7 | 18 | 0 | 16 | 10 | 2 | 12 | 3 | 27 | 13 | 20 | 5 | 21 | 0 | 2 | 16 | 2 |
| 33 | P. stutzeri A122/76 | 13 | 6 | 0 | 2 | 0 | 0 | 4 | 0 | 2 | 13 | 0 | 0 | 0 | 0 | 16 | 10 | 14 | 10 | 4 | 16 |
| 34 | P. stutzeri A63/70 | 3 | 16 | 7 | 1 | 0 | 10 | 13 | 7 | 2 | 26 | 1 | 7 | 7 | 10 | 25 | 14 | 18 | 8 | 11 | 14 |
| 35 | P. stutzeri A95/69 | 17 | 0 | 4 | 1 | 0 | 10 | 15 | 7 | 2 | 30 | 3 | 30 | 7 | 20 | 27 | 4 | 4 | 8 | 11 | 14 |
| 36 | P. stutzeri A60/68 | 0 | 10 | 0 | 12 | 0 | 0 | 6 | 0 | 2 | 0 | 2 | 22 | 0 | 0 | 1 | 0 | 0 | 8 | 2 | 5 |
| 37 | P. stutzeri A75/66 | 13 | 6 | 0 | 10 | 0 | 12 | 0 | 7 | 2 | 14 | 0 | 18 | 7 | 10 | 24 | 3 | 4 | 8 | 20 | 14 |
| 38 | P. stutzeri A85a/66 | 19 | 4 | 4 | 1 | 0 | 6 | 0 | 16 | 2 | 13 | 1 | 20 | 5 | 11 | 26 | 4 | 16 | 3 | 14 | 11 |
| 39 | P. stutzeri A48/66 | 11 | 4 | 7 | 1 | 1 | 15 | 3 | 7 | 3 | 36 | 1 | 6 | 10 | 9 | 27 | 12 | 18 | 8 | 11 | 14 |
| 40 | P. stutzeri A60/72 | 16 | 0 | 0 | 11 | 0 | 13 | 0 | 7 | 5 | 5 | 0 | 21 | 5 | 12 | 6 | 4 | 20 | 3 | 14 | 11 |
| 41 | P. stutzeri A42/69 | 11 | 4 | 5 | 0 | 1 | 15 | 7 | 16 | 2 | 27 | 3 | 14 | 7 | 11 | 33 | 4 | 22 | 8 | 5 | 10 |
| 42 | P. stutzeri A31/70 | 4 | 5 | 0 | 1 | 6 | 2 | 8 | 9 | 2 | 23 | 2 | 15 | 5 | 4 | 11 | 8 | 5 | 1 | 17 | 3 |
| 43 | P. stutzeri A69/69 | 11 | 4 | 0 | 10 | 1 | 15 | 2 | 6 | 2 | 8 | 3 | 18 | 5 | 10 | 3 | 11 | 12 | 4 | 1 | 14 |
| 44 | P. stutzeri A94/69 | 12 | 6 | 5 | 0 | 1 | 15 | 2 | 0 | 2 | 3 | 3 | 17 | 7 | 7 | 3 | 3 | 4 | 4 | 6 | 14 |
| 45 | P. stutzeri A147/68 | 0 | 10 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 2 | 8 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 |
| 46 | P. aeruginosa CCM 1960 | 22 | 0 | 0 | 5 | 14 | 4 | 7 | 9 | 2 | 22 | 0 | 13 | 15 | 14 | 10 | 23 | 7 | 3 | 21 | 13 |
| 47 | P. pseudoalcaligenes ATCC 7440 | 14 | 0 | 0 | 2 | 8 | 3 | 10 | 19 | 2 | 17 | 0 | 23 | 17 | 21 | 10 | 4 | 0 | 3 | 9 | 3 |
| 48 | P. mendocina ATCC 25411 | 14 | 0 | 0 | 1 | 4 | 14 | 10 | 17 | 2 | 4 | 9 | 24 | 7 | 16 | 17 | 4 | 2 | 3 | 9 | 13 |
TABLE 3.
Genetic diversity at 20 enzyme loci for all Pseudomonas strains and P. stutzeri
| Enzyme locus | All strains
|
P. stutzeri
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All strains
|
Clinical isolates
|
Environmental isolates
|
Genomovar 1
|
|||||||
| No. of alleles | h | No. of alleles | h | No. of alleles | h | No. of alleles | h | No. of alleles | h | |
| G6P-1 | 19 | 0.937 | 16 | 0.932 | 12 | 0.931 | 10 | 0.908 | 7 | 0.842 |
| G6P-2 | 17 | 0.890 | 15 | 0.881 | 10 | 0.887 | 8 | 0.856 | 8 | 0.795 |
| C12O | 9 | 0.749 | 9 | 0.787 | 6 | 0.746 | 8 | 0.862 | 6 | 0.836 |
| C23O | 13 | 0.804 | 11 | 0.773 | 8 | 0.829 | 6 | 0.679 | 5 | 0.695 |
| LDH-1 | 22 | 0.928 | 18 | 0.912 | 9 | 0.837 | 13 | 0.928 | 10 | 0.906 |
| LDH-2 | 18 | 0.920 | 16 | 0.909 | 11 | 0.916 | 10 | 0.915 | 7 | 0.824 |
| EDH-1 | 18 | 0.925 | 16 | 0.923 | 10 | 0.880 | 13 | 0.960 | 7 | 0.853 |
| EDH-2 | 19 | 0.916 | 16 | 0.900 | 7 | 0.815 | 12 | 0.941 | 8 | 0.842 |
| LAP | 8 | 0.595 | 7 | 0.573 | 6 | 0.442 | 6 | 0.732 | 4 | 0.298 |
| EST | 31 | 0.973 | 27 | 0.967 | 17 | 0.963 | 13 | 0.947 | 13 | 0.941 |
| NSP | 11 | 0.828 | 10 | 0.804 | 6 | 0.681 | 7 | 0.843 | 4 | 0.526 |
| LED | 24 | 0.961 | 19 | 0.953 | 13 | 0.956 | 11 | 0.947 | 10 | 0.912 |
| ALD-1 | 17 | 0.795 | 13 | 0.777 | 6 | 0.637 | 12 | 0.921 | 6 | 0.538 |
| ALD-2 | 22 | 0.940 | 17 | 0.923 | 11 | 0.913 | 11 | 0.928 | 11 | 0.883 |
| THD | 30 | 0.977 | 27 | 0.974 | 15 | 0.956 | 14 | 0.967 | 12 | 0.953 |
| LYS-1 | 21 | 0.937 | 19 | 0.940 | 14 | 0.942 | 9 | 0.915 | 12 | 0.953 |
| LYS-2 | 23 | 0.944 | 18 | 0.935 | 13 | 0.945 | 9 | 0.895 | 10 | 0.918 |
| GD2 | 11 | 0.854 | 11 | 0.858 | 9 | 0.865 | 7 | 0.843 | 6 | 0.777 |
| GD1 | 20 | 0.924 | 16 | 0.909 | 13 | 0.927 | 9 | 0.882 | 7 | 0.783 |
| ME | 19 | 0.907 | 16 | 0.882 | 11 | 0.822 | 11 | 0.941 | 6 | 0.695 |
| Mean (H) | 18.6 | 0.885 | 15.9 | 0.876 | 10.4 | 0.844 | 10 | 0.890 | 8 | 0.788 |
TABLE 4.
Multilocus linkage disequilibrium analysis of Pseudomonas strains used in this study
| Isolate group | No. of ETs | Mean no. of alleles per locus | Mean genetic diversity (H) | VE | 95% confidence limits of VEa | Vo | Monte Carlo randomization
|
Pb | IA ± SDa | |
|---|---|---|---|---|---|---|---|---|---|---|
| Vmin | Vmax | |||||||||
| P. stutzeri isolates | ||||||||||
| Clinical | 24 | 10.4 | 0.844 | 2.29 | 0.95–3.62 | 5.37 | 1.85 | 4.23 | <0.0001 | 1.34 ± 0.29 |
| Environmental | 18 | 10 | 0.890 | 1.84 | 0.56–3.11 | 5.13 | 1.44 | 3.90 | <0.0001 | 1.78 ± 0.35 |
| Genomovar 1 | 19 | 8 | 0.788 | 2.79 | 0.98–4.60 | 4.96 | 1.84 | 5.32 | 0.005 | 0.77 ± 0.32 |
| All | 42 | 15.9 | 0.876 | 2.00 | 1.10–2.90 | 4.22 | 1.77 | 3.19 | <0.0001 | 1.10 ± 0.22 |
| P. stutzeri + P. balearica | 44 | 16.8 | 0.882 | 1.93 | 1.08–2.78 | 4.12 | 1.67 | 2.82 | <0.0001 | 1.13 ± 0.22 |
| All Pseudomonas | 48 | 18.6 | 0.885 | 1.86 | 1.07–2.64 | 3.75 | 1.62 | 2.86 | <0.0001 | 1.01 ± 0.21 |
| All Pseudomonas except ETs: | ||||||||||
| 4, 5 | 46 | 18.4 | 0.887 | 1.84 | 1.05–2.64 | 3.65 | 1.59 | 2.90 | <0.0001 | 0.97 ± 0.21 |
| 4, 5, 10, 11 | 44 | 18.1 | 0.882 | 1.89 | 1.06–2.73 | 3.52 | 1.73 | 2.97 | <0.0001 | 0.85 ± 0.22 |
| 4, 5, 10, 11, 19, 21 | 42 | 17.9 | 0.883 | 1.87 | 1.03–2.71 | 3.44 | 1.58 | 2.89 | <0.0001 | 0.83 ± 0.22 |
| 4, 5, 10, 11, 19, 21, 1, 2 | 40 | 17.7 | 0.886 | 1.84 | 0.99–2.69 | 3.39 | 1.64 | 3.14 | <0.0001 | 0.83 ± 0.23 |
| 4, 5, 10, 11, 19, 21, 1, 2, 7, 8 | 38 | 17.4 | 0.884 | 1.85 | 0.97–2.72 | 3.22 | 1.65 | 2.96 | <0.0001 | 0.74 ± 0.23 |
| 4, 5, 10, 11, 19, 21, 1, 2, 7, 8, 23, 25 | 36 | 17.1 | 0.888 | 1.80 | 0.92–2.68 | 2.94 | 1.62 | 2.96 | 0.0005 | 0.63 ± 0.24 |
| 4, 5, 10, 11, 19, 21, 1, 2, 7, 8, 23, 25, 26, 27 | 34 | 16.6 | 0.888 | 1.79 | 0.89–2.69 | 2.80 | 1.59 | 3.03 | 0.022 | 0.56 ± 0.25 |
Calculated as described by Brown et al. (5).
Probability of observing an Vo/VE ratio as or more extreme as the original data based on 10,000 Monte Carlo randomizations.
High frequency of occurrence of null alleles.
Only 8 (16.7%) of the 48 strains studied (ETs 4, 5, 10, 11, 27, 28, 30, and 40) presented activity for all 20 enzymes, and at least one metabolic enzyme activity could not be detected in the cell lysates prepared from 40 (83.3%) of the organisms. Of these 40 isolates, 16 lacked detectable activity for one enzyme, 9 lacked detectable activity for two enzymes, and three enzyme activities were not found in lysates of 7 isolates. Moreover, cell lysates of ET 45 (P. stutzeri A147/68), ET 36 (P. stutzeri A60/68), ET 33 (P. stutzeri A122/76), and ET 16 (P. stutzeri JM300) showed metabolic activities for only 6, 10, 12, and 13 enzymes, respectively. There was a significant relationship between the presence or absence of detectable enzyme activity and the source of isolates. However, both clinical and environmental isolates exhibited detectable enzyme activities for all 20 enzymes studied.
Relationships among multilocus genotypes.
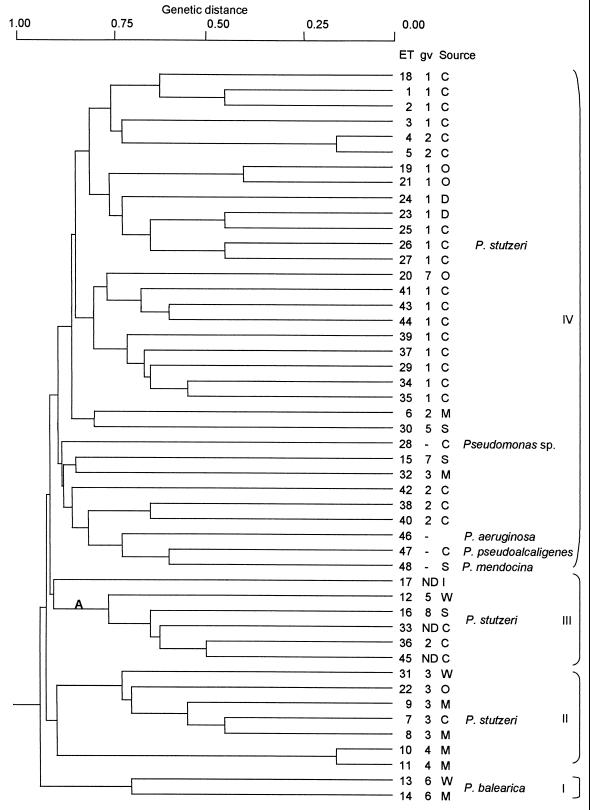
Genetic relationships among the 42 ETs of P. stutzeri and the ETs of the other species of Pseudomonas studied are shown in the dendrogram in Fig. 1. The cophenetic correlation coefficient of the total sample was R = 0.78. ETs differed at least at three loci, ETs 4 and 5 and ETs 10 and 11, which were separated by a genetic distance of 0.15. At a genetic distance of 0.9, which reflects differences between groups at approximately 14 or more loci, there were four cluster groups, numbered I to IV.
FIG. 1.
Dendrogram showing genetic relationships among P. stutzeri and related Pseudomonas strains. Abbreviations: gv, genomovar; C, clinical; D, brackish water sediment; I, industrial waste; M, marine; ND, not determined; O, aircraft oil-contaminated soil; S, soil; W, wastewater treatment plant.
Group I contained both isolates of P. balearica (ETs 13 and 14), and these were separated from all Pseudomonas isolates by a genetic distance of >0.93. The two P. stutzeri strains of genomovar 4 (ETs 10 and 11) were separated from each other by a genetic distance of 0.15. These strains were grouped in cluster II. Five of the six ETs of genomovar 3 were also found in cluster II, forming a homogeneous group; ET 32 (the remaining isolate of genomovar 3) was found in group IV.
Group III clustered ETs 36, 12, 16, 17, 33, and 45 of P. stutzeri (genomovars 2, 5, and 8 and strains CLN100, A122/76, and A147/68, respectively). At a genetic distance of 0.76, there were one subcluster (A) and a single ET (P. stutzeri CLN100). Subcluster A comprised three ETs of genomovars 2, 5, and 8 (ETs 36, 12, and 16, respectively) and two strains of P. stutzeri (ETs 33 and 45).
Group IV contained all ETs of P. stutzeri genomovars 1, 2 (except ET 36), and 7, an isolate identified as Pseudomonas sp. (ET 28), and the ETs of reference strains of P. aeruginosa (ET 46), P. pseudoalcaligenes (ET 47), and P. mendocina (ET 48). These three Pseudomonas species and P. stutzeri were placed in the same DNA homology group within RNA group I (31). The ETs of genomovar 5 (ETs 12 and 30) were found in groups III and IV.
The same cluster pattern was found for the P. stutzeri population alone. There was no clear association in clusters among isolates of P. stutzeri when we considered source and place of isolation. However, when two strains were grouped in the dendrogram at genetic distances below 0.55 (i.e., ETs 1–2, 4–5, 19–21, 26–27, 34–35, 36–45, and 10–11), each pair of strains belonged to the same genomovar (except ET 45, which could not be assigned to any known genomovar) and were isolated from the same source and geographic location. ETs 23 and 25 belonged to genomovar 1 and were both isolated in Mallorca, Spain, but ET 23 is an environmental strain and ET 25 was obtained from a clinical sample. ETs 7 and 8 belonged to genomovar 3. Whereas ET 7 is a clinical strain isolated before 1952, ET 8 is a marine strain isolated in 1983.
Linkage disequilibrium analysis.
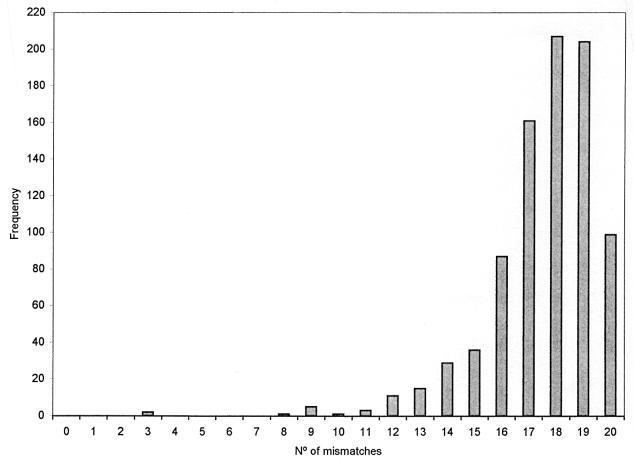
The complete set of isolates and population subsets were analyzed for multilocus linkage disequilibrium (Table 4). Figure 2 shows the allele mismatch distribution among the P. stutzeri strains.
FIG. 2.
Allele mismatch distribution among 42 P. stutzeri strains.
The IA, value which is an estimate of the existence of assortative recombination for the 42 isolates of P. stutzeri studied, was 1.10 ± 0.22, which differs significantly from zero (P < 0.0001). Moreover, calculation of IA for clinical isolates resulted in a value of 1.34 ± 0.29 (P < 0.0001), and it was 1.78 ± 0.35 (P < 0.0001) for environmental isolates. The IA of the genomovar 1 population was 0.77 ± 0.32 (P < 0.005). To verify the robustness of the linkage disequilibrium analysis, we performed repetitive calculations of IA in subsets of the total population analyzed in which the most closely related ET pairs (up to a genetic distance of 0.5 [Fig. 1]) were stepwise eliminated. Table 4 shows the IA values for these subsets along with their significance. In this study, all values of Vo exceeded the values expected in a corresponding population at linkage equilibrium (VE). Values of Vo also exceeded the 95% confidence limits of VE and the Vmax and Vmin values calculated by the Monte Carlo randomizations (Table 4), indicating that the population structure is clonal (27).
DISCUSSION
Despite the exhaustive phenetic and molecular studies that had been conducted on P. stutzeri, little was known about the population genetics of isolates identified as P. stutzeri. Data obtained by DNA-DNA hybridization (34, 42), 16S rRNA gene sequencing (2), internally transcribed 16S-23S rRNA gene spacer regions (19), total fatty acid analysis, total protein pattern (36), and macrorestriction fragment analysis of genomic DNA and DNA fingerprinting (16) have shown that there are substantial levels of variability among natural isolates of this species. For a thorough understanding of the characteristics of a species, a knowledge of the genomic structure of the population is essential, especially in studies of the population dynamics or the colonization of habitats, in order to elucidate genetic exchange in natural populations. MLEE records variation in chromosomal genes and thereby enables the degree of gene transfer within species to be estimated, permits relationships between bacterial isolates to be determined, and allows phylogenetic frameworks to be constructed. Therefore, we used the MLEE method to determine the genotypic diversity and relationships among several strains, representing all genomovars of P. stutzeri described to date with isolates from very different sources.
High mean genetic diversity.
Study of the allelic variation of housekeeping genes by MLEE has generated considerable data about the genetic diversity of bacterial populations (41, 42, 48). The amount of genetic variation in a bacterial species is roughly 10-fold greater than that of higher eukaryotes (28). Our results show that the level of genetic diversity of P. stutzeri (H = 0.876) exceeds that of the other bacteria studied (17, 23). This high degree of genetic diversity is consistent with data obtained by other experimental methods (2, 4, 32, 35, 43). Moreover, Sikorsky et al. (43) determined the allozyme variation of 21 enzymes for 16 P. stutzeri strains. Using Sikorsky's data, we calculated the mean genetic diversity (H = 0.811). Although the latter used a low number of strains and starch gels for the separation of the enzymes, a method which has been reported to be less discriminatory than use of polyacrylamide gels (13, 22), Sikorsky et al. (43) also found that all 21 loci were highly polymorphic and that all multilocus genotypes were represented by a single strain. These results agree with our analysis of a significantly higher number of strains. We completed our analysis by comparing two subgroups, clinical and environmental isolates. The mean levels of genetic diversity of these groups were not significantly different, which indicates that the clinical isolates of P. stutzeri do not represent distinct populations from the environmental isolates. This may have important implications for the microbiology of P. stutzeri infections.
High frequency of occurrence of null alleles.
In addition to the large mean number of alleles per locus, our study also demonstrated an extremely high frequency of occurrence of null alleles. It is of interest that these results are in agreement with those published by Sikorsky et al. (43), who showed that 13 (81.25%) of the 16 P. stutzeri strains analyzed in their study lacked detectable activity for at least one enzyme. Although only nine strains of P. stutzeri and six enzymes were studied by both laboratories, the coincidence of observation of a high frequency of occurrence of null alleles seems to confirm that the absence of enzymatic activity was due to lack of the protein rather than an error in manipulation. A high frequency of occurrence of null alleles has been reported also for Helicobacter pylori, another species with a high genetic diversity (17). Nevertheless, the frequency of occurrence of null alleles was far higher in P. stutzeri (43; this study) than in H. pylori (17). Certain characteristics of P. stutzeri that might explain the high occurrence of null alleles in this species, including the great variation in the length of its genome (10, 16, 32), the large size of its natural populations, the nonconserved organization of its genome, and the chromosome rearrangements that it frequent carries (16, 32, 43). Several other explanations are possible for the high occurrence of null alleles, such as the absence of all or part of the structural gene, downregulation of enzyme production, and occurrence of enzyme-inactivating mutations. Currently, we lack experimental data to differentiate between these hypotheses. Significant differences in the number of null alleles were detected among clinical and environmental isolates. However, there was only moderate evidence against the null hypothesis of no difference between the two populations (0.01 < P < 0.05), and we do not know the biological significance of this observation.
Relationships among multilocus genotypes.
The deep branching pattern of the dendrogram reveals the distant relationships among the P. stutzeri isolates which differed in at least three loci (Fig. 1). The value of the cophenetic correlation obtained (R = 0.78) falls into the range (0.74 to 0.90) of most frequently occurring cophenetic correlations reported by Sneath and Sokal (44). The dendrogram derived in the present study shows no clear association with respect to the source or geographic site of isolation. Both clinical and environmental strains are distributed in clusters II, III, and IV. We found strains from Mallorca in clusters II, III, and IV, and we found strains from Great Britain and from California in both clusters III and IV. Barcelona strains and Denmark isolates were in clusters II and IV, respectively. In contrast, if we consider the distribution in genomovars, we find a good correlation among strains belonging to genomovars 1, 4, and 6. All 19 strains from genomovar 1 were found in cluster IV. The strains from genomovar 4 (ETs 10 and 11) and those from genomovar 6 (ETs 13 and 14, which belonged to P. balearica species) were in clusters II and I, respectively. Only two strains have been described in genomovars 5 and 7, and strain DSM50238 (genomovar 7) has been described genotypically as an atypical isolate. Strain NF13 (genomovar 3, ET 32) is placed in cluster IV, while the rest of strains in genomovar 3 are in cluster II. However, strain NF13 was isolated from a sample taken in the Pacific Ocean (39), and the other five members of genomovar 3 were isolated in the Mediterranean area. Strain NF13 was isolated from the Galápagos Rift hydrothermal vents, located at depths of 2,500 to 2,600 m, and it grows at low temperatures (e.g., 4°C). These characteristics might explain the differences in enzymatic activity between strain NF13 and the other strains classified in genomovar 3 by ITS fingerprinting (4). Of the six strains included in cluster III, three have not been classified in known genomovars and one is the sole representative of a genomovar (JM300, genomovar 8). On the other hand, a very good overall correlation can be found in the clustering of strains obtained by the MLEE method and the DNA fingerprinting methods previously applied. For example, strains 19SMN4 (ET 10) and ST27MN3 (ET 11) have the lowest genetic distance found for the strains studied and have been proposed as very closely related clones in the genomic analyses conducted to date (4, 16, 19); the MLEE results now show this to be the case. The remote relationships previously established between P. stutzeri and P. balearica (2, 3, 4, 16, 43) were confirmed in our analysis. The two P. balearica strains included in this study appeared together in the dendrogram and distantly related (at a genetic distance of 0.93) to the P. stutzeri strains and the other Pseudomonas isolates analyzed.
Linkage disequilibrium analysis.
Some authors proposed the existence of recombinational events to explain some of the diversity found in P. stutzeri (43). Our results are clear in this context and argue strongly against the presence of electrophoretically detectable recombination by means of linkage disequilibrium analysis. The IA value for the P. stutzeri population analyzed was 1.10 ± 0.22 (P < 0.0001). The IA values were always significantly different from zero for all of the subgroups studied (clinical and environmental isolates and strains classified as genomovar 1 [Table 4]). Moreover, the stepwise elimination of the seven most closely related ET pairs (Fig. 1) revealed that the remaining ETs were also in linkage disequilibrium (Table 4). All of these results strongly suggest that the population structure of P. stutzeri sampled isolates is clonal and thus in accordance with the hypothesis that in the P. stutzeri populations analyzed, there is no significant level of assortative recombination (27). Furthermore, the IA value calculated from the data of Sikorsky et al. (43) was 2.93 ± 0.35, which further supports this hypothesis. The difference between the IA values calculated from our results and those of Sikorsky et al. (43) could be due to the different sample sizes of P. stutzeri analyzed by the two laboratories.
Population structure of P. stutzeri.
P. stutzeri is widely distributed in natural environments and shows a great metabolic versatility (1, 30, 35, 37), characteristics consistent with a large effective population size. Our results and data of Sikorsky et al. (43) suggest the existence of very low recombination rates in this bacterial species.
When there is a large population size and no assortative recombination, bacterial clones will diverge freely by accumulating neutral mutations. The occurrence in a particular population of adaptive mutations that confer selective advantages in specific ecological situations leads to the elimination of genetic diversity within the population but will not prevent, in the presence of very low recombination rates, genetic divergence between populations (9). Thus, the exceptionally high genetic diversity of P. stutzeri could be the result of niche-specific selection that occurs during colonization and adaptation to a wide range of microenvironments (40).
The observations that this species is naturally competent (7, 8, 46), with the presence of insertion sequences and reports of mosaic gene structures together with considerable variation in the length of the genome (16), suggest that some different events may contribute to the overall species diversity. However, the IA values obtained indicate that horizontal gene transfer and recombination processes, if they exist, are not sufficient to disrupt the allele associations because there is still a strong linkage disequilibrium among the P. stutzeri isolates.
In conclusion, our results indicate that P. stutzeri exhibits an exceptionally high diversity within a clonal population structure. However, the existence of a strong linkage disequilibrium can be explained in these cases considering that, like many bacterial species, P. stutzeri forms a metapopulation integrated by multiple ecological populations. These populations occupy different ecological niches, and recombination, although possible within populations, is rare or absent between distinct populations. Such a population structure can introduce linkage disequilibrium in a sample of isolates from different populations (26, 33, 48). Although more extensive studies are necessary to assess the population structure of these ecological populations of P. stutzeri, the results reported here are consistent with the conclusion that this bacterial species represents a good example of a phenetically cosmopolitan ecological species sensu Istock (21), i.e., a species form characterized by a circumscribed phenotypic variation, restricted local sets of genetic clones, and no or rare recombination. The clonal sets are genetically diverse, but phenotypic resemblance is sufficient to make phenetic classification and identification possible. This bacterial species represents the highest genetic diversity described to date. MLEE data confirm the results obtained by other techniques to the effect that some clones of P. stutzeri are distinct enough to warrant taxonomic differentiation (2).
ACKNOWLEDGMENTS
We thank B. Holmes for kindly supplying strains. We are also grateful to Maribel Farfán for her valuable contribution. We also thank two anonymous reviewers for their helpful comments and suggestions.
REFERENCES
- 1.Baggi G, Barbieri P, Galli E, Tollari S. Isolation of a Pseudomonas stutzeri strain that degrades o-xylene. Appl Environ Microbiol. 1987;53:2129–2132. doi: 10.1128/aem.53.9.2129-2132.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennasar A, Rosselló-Mora R, Lalucat J, Moore E R B. 16S rRNA gene sequence analyses relative to genomovars of Pseudomonas stutzeri and proposal of Pseudomonas balearica sp. nov. Int J Syst Bacteriol. 1996;46:200–205. doi: 10.1099/00207713-46-1-200. [DOI] [PubMed] [Google Scholar]
- 3.Bennasar A, Guasp C, Lalucat J. Molecular methods for the detection and identification of Pseudomonas stutzeri in pure culture and environmental samples. Microb Ecol. 1998;35:22–33. doi: 10.1007/s002489900057. [DOI] [PubMed] [Google Scholar]
- 4.Bennasar A, Guasp C, Tesar M, Lalucat J. Genetic relationships among Pseudomonas stutzeri strains based on molecular typing methods. J Appl Microbiol. 1998;85:643–656. doi: 10.1111/j.1365-2672.1998.00572.x. [DOI] [PubMed] [Google Scholar]
- 5.Brown A H D, Feldman M W, Nevo E. Multilocus structure of natural populations of Hordeum spontaneum. Genetics. 1980;96:523–536. doi: 10.1093/genetics/96.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burri R, Stutzer A. Über Nitrat Zerstörende Bakterien und den durch dieselben bedingten Stickstoffverlust. Zentrbl Bakteriol Parasitenkd II Abt. 1895;1:257–265. , 350–364, 392–398, 422–432. [Google Scholar]
- 7.Carlson C A, Pierson L S, Rosen J, Ingraham J L. Pseudomonas stutzeri and related species undergo natural transformation. J Bacteriol. 1983;153:93–99. doi: 10.1128/jb.153.1.93-99.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson C A, Steenbergen S M, Ingraham J L. Natural transformation of Pseudomonas stutzeri by plasmids that contain cloned fragments of chromosomal deoxyribonucleic acid. Arch Microbiol. 1984;140:134–138. [Google Scholar]
- 9.Cohan F M. Genetic exchange and evolutionary divergence in prokaryotes. Trends Ecol Evol. 1994;9:175–180. doi: 10.1016/0169-5347(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 10.Döhler K, Huss V A R, Zumft W G. Transfer of Pseudomonas perfectomarina Baumann, Bowditch, Baumann and Beaman 1983 to Pseudomonas stutzeri (Lehmann and Neumann, 1896) Sijderins 1946. Int J Syst Bacteriol. 1987;37:1–3. [Google Scholar]
- 11.Farfán M, Miñana D, Fusté M C, Lorén J G. Genetic relationships between clinical and environmental Vibrio cholerae isolates based on multilocus enzyme electrophoresis. Microbiology. 2000;146:2613–2626. doi: 10.1099/00221287-146-10-2613. [DOI] [PubMed] [Google Scholar]
- 12.Felsenstein J. PHYLIP package, version 3.5. Seattle, Wash: University of Washington; 1993. [Google Scholar]
- 13.Flint S H, Hartley N J, Avery S M, Hudson J A. A comparison between starch and polyacrylamide gels for the analysis of Listeria monocytogenes using multilocus enzyme electrophoresis. Lett Appl Microbiol. 1996;22:16–17. doi: 10.1111/j.1472-765x.1996.tb01098.x. [DOI] [PubMed] [Google Scholar]
- 14.Fusté M C, Pineda M A, Palomar J, Viñas M, Lorén J G. Clonality of multidrug-resistant nontypeable strains of Haemophilus influenzae. J Clin Microbiol. 1996;34:2760–2765. doi: 10.1128/jcm.34.11.2760-2765.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson D T. Assay of enzymes of aromatic metabolism. Methods Microbiol. 1971;6A:463–478. [Google Scholar]
- 16.Ginard M, Lalucat J, Tümmler B, Römling U. Genome organization of Pseudomonas stutzeri and resulting taxonomic and evolutionary considerations. Int J Syst Bacteriol. 1997;47:132–143. doi: 10.1099/00207713-47-1-132. [DOI] [PubMed] [Google Scholar]
- 17.Go M F, Kapur V, Graham D Y, Musser J M. Population genetic analysis of Helicobacter pylori by multilocus enzyme electrophoresis: extensive allelic diversity and recombinational population structure. J Bacteriol. 1996;178:3934–3938. doi: 10.1128/jb.178.13.3934-3938.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon D M. The genetic structure of Escherichia coli populations in feral house mice. Microbiology. 1997;143:2039–2046. doi: 10.1099/00221287-143-6-2039. [DOI] [PubMed] [Google Scholar]
- 19.Guasp C, Moore E R B, Lalucat J, Bennasar A. Utility of internally-transcribed 16-23S rDNA spacer regions for the definition of Pseudomonas stutzeri genomovars and other Pseudomonas species. Int J System Evol Microbiol. 2000;50:1629–1639. doi: 10.1099/00207713-50-4-1629. [DOI] [PubMed] [Google Scholar]
- 20.Holmes B. Identification and distribution of Pseudomonas stutzeri in clinical material. J Appl Bacteriol. 1986;60:401–411. doi: 10.1111/j.1365-2672.1986.tb05085.x. [DOI] [PubMed] [Google Scholar]
- 21.Istock C A, Bell J A, Ferguson N, Istock N L. Bacterial species and evolution: theoretical and practical perspectives. J Ind Microbiol. 1996;17:137–150. [Google Scholar]
- 22.John M A, Hussain Z. Multilocus enzyme electrophoresis using ultrathin polyacrylamide gels. J Microbiol Methods. 1994;19:307–313. [Google Scholar]
- 23.Johnson W M, Tyler S D, Rozee K R. Linkage analysis of geographic and clinical clusters in Pseudomonas cepacia infections by multilocus enzyme electrophoresis and ribotyping. J Clin Microbiol. 1994;32:924–930. doi: 10.1128/jcm.32.4.924-930.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Mandel M. Deoxyribonucleic acid base composition in the genus Pseudomonas. J Genet Microbiol. 1966;43:273–292. doi: 10.1099/00221287-43-2-273. [DOI] [PubMed] [Google Scholar]
- 26.Maynard-Smith J, Dowson C G, Spratt B G. Localized sex in bacteria. Nature. 1991;349:29–31. doi: 10.1038/349029a0. [DOI] [PubMed] [Google Scholar]
- 27.Maynard-Smith J, Smith N H, O'Rourke M, Spratt B G. How clonal are bacteria? Proc Natl Acad Sci USA. 1993;90:4384–4388. doi: 10.1073/pnas.90.10.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neel J V. A revised estimate of the amount of genetic variation in human proteins: implications for the distribution of DNA polymorphism. Am J Hum Genet. 1984;36:1135–1148. [PMC free article] [PubMed] [Google Scholar]
- 29.Nei M. Estimation of average heterozygosity and genetic distance from a small sample of individuals. Genetics. 1978;89:583–590. doi: 10.1093/genetics/89.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obradors N, Aguilar J. Efficient biodegradation of high-molecular-weight polyethylene glycols by pure cultures of Pseudomonas stutzeri. Appl Environ Microbiol. 1991;57:2383–2388. doi: 10.1128/aem.57.8.2383-2388.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palleroni N J, Kunisawa R, Contopoulou R, Doudoroff M. Nucleic acid homologies in the genus Pseudomonas. Int J Syst Bacteriol. 1973;23:333–339. [Google Scholar]
- 32.Rainey P B, Thompson I P, Palleroni N J. Genome and fatty acid analysis of Pseudomonas stutzeri. Int J Syst Bacteriol. 1994;44:54–61. doi: 10.1099/00207713-44-1-54. [DOI] [PubMed] [Google Scholar]
- 33.Reeves P R. Variation in O-antigens, niche specific selection and bacterial populations. FEMS Microbiol Lett. 1992;100:509–516. doi: 10.1111/j.1574-6968.1992.tb14085.x. [DOI] [PubMed] [Google Scholar]
- 34.Rohlf F J. Numerical taxonomy and multivariate analysis system, version 1.80. New York, N.Y: Exeter Software; 1993. [Google Scholar]
- 35.Rosselló R, García-Valdés E, Lalucat J, Ursing J. Genotypic and phenotypic diversity of Pseudomonas stutzeri. Syst Appl Microbiol. 1991;14:150–157. [Google Scholar]
- 36.Rosselló-Mora R, Lalucat J, Dott W, Kämpfer P. Biochemical and chemotaxonomic characterization of Pseudomonas stutzeri genomovars. J Appl Bacteriol. 1994;76:226–233. [Google Scholar]
- 37.Rosselló-Mora R A, Lalucat J, García-Valdés E. Comparative biochemical and genetic analysis of naphthalene degradation among Pseudomonas stutzeri strains. Appl Environ Microbiol. 1994;60:966–972. doi: 10.1128/aem.60.3.966-972.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosselló-Mora R A, Lalucat J, Moore E R B. Strain JM300 represents a new genomovar within Pseudomonas stutzeri. Syst Appl Microbiol. 1996;19:596–599. [Google Scholar]
- 39.Ruby E G, Wirren C D, Jannasch H W. Chemolithotrophic sulfur-oxidizing bacteria from the Galapagos rift hydrothermal vents. Appl Environ Microbiol. 1981;42:317–324. doi: 10.1128/aem.42.2.317-324.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt K D, Tümmler B, Römling U. Comparative genome mapping of Pseudomonas aeruginosa PAO with P. aeruginosa C which belongs to a major clone in cystic fibrosis patients and aquatic habitats. J Bacteriol. 1996;178:85–93. doi: 10.1128/jb.178.1.85-93.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selander R K, Caugant D A, Ochman H, Musser J M, Gilmour M N, Whittam T S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986;51:873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Selander R K, Musser J M, Caugant D A, Gilmour M N, Whittam T S. Population genetics of pathogenic bacteria. Microb Pathog. 1987;3:1–7. doi: 10.1016/0882-4010(87)90032-5. [DOI] [PubMed] [Google Scholar]
- 43.Sikorsky J, Rosselló-Mora R, Lorenz M G. Analysis of genotypic diversity and relationships among Pseudomonas stutzeri strains by PCR-based genomic fingerprinting and multilocus enzyme electrophoresis. Syst Appl Microbiol. 1999;22:393–402. doi: 10.1016/S0723-2020(99)80048-4. [DOI] [PubMed] [Google Scholar]
- 44.Sneath P H A, Sokal R R. Numerical taxonomy. W. H. San Francisco, Calif: Freeman & Co.; 1973. [Google Scholar]
- 45.Sokal R R, Rohlf F J. Biometry: the principles and practice of statistics in biology research. W. H. San Francisco, Calif: Freeman & Co.; 1994. [Google Scholar]
- 46.Stewart G L, Carlson C A, Ingraham J L. Evidence for an active role of donor cells in natural transformation of Pseudomonas stutzeri. J Bacteriol. 1983;156:30–35. doi: 10.1128/jb.156.1.30-35.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Niel C B, Allen M B. A note on Pseudomonas stutzeri. J Bacteriol. 1952;64:413–422. doi: 10.1128/jb.64.3.413-422.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young J P W. The population genetics of bacteria. In: Hopwood D A, Chater K E, editors. Genetics of bacterial diversity. London, England: Academic Press; 1989. pp. 417–438. [Google Scholar]