Abstract
Purpose of Review
Both traumatic and acquired brain injury can result in diffuse multifocal injury affecting both the pyramidal and extrapyramidal tracts. Thus, these patients may exhibit signs of both upper motor neuron syndrome and movement disorder simultaneously which can further complicate diagnosis and management. We will be discussing movement disorders following acquired and traumatic brain injury.
Recent Findings
Multiple functions including speech, swallowing, posture, mobility, and activities of daily living can all be affected. Medical treatment and rehabilitation-based therapy can be especially challenging due to accompanying cognitive deficits and severity of the disorder which can involve multiple limbs in addition to muscles of the face and axial skeleton. Tremor and dystonia are the most reported movement disorders following traumatic brain injury. Dystonia and myoclonus are well documented following hypoxic ischemic brain injuries. Electrophysiological studies such as dynamic surface poly-electromyography can assist with identifying phenomenology, especially differentiating between jerk-like phenomenon and help guide further work up and management. Management with medications remains challenging due to potential adverse effects. Surgical interventions including stereotactic surgery, deep brain stimulation, and intrathecal baclofen pumps have been reported, but most of the evidence supporting them has been limited to primarily case reports except for post-traumatic tremor.
Summary
Brain injury can lead to motor disorders, movement disorders, visual (processing) deficits, and vestibular deficits which often coexist with cognitive deficits making it challenging to treat and rehabilitate these patients. Unfortunately, the evidence regarding the medical management and rehabilitation of brain injury patients with movement disorders is sparse and leaves much to be desired.
Keywords: Tremor, Dystonia, Myoclonus, Ballism, Rigidity, Bradykinesia
Introduction
Disorders of movement are frequently encountered following acquired and traumatic brain injury as well as other central nervous system disorders including stroke, multiple sclerosis, and cerebral palsy. Disorders of movement encompass the upper motor neuron syndrome which includes paresis, hyperreflexia, and spasticity due to lesions of the corticospinal and corticobulbar tract; movement disorders due to dysfunction of the thalamus, basal ganglia, and/or associated circuitry; and ataxias due to injury to the cerebellum and associated pathways. Both traumatic and acquired brain injury can result in diffuse multifocal injury affecting both the pyramidal and extrapyramidal tracts. Thus, these patients may exhibit signs of both upper motor neuron syndrome and movement disorder simultaneously which can further impede diagnosis and management. Clinical presentation can be further complicated by co-existing visual, vestibular, sensory, and proprioceptive deficits which can be central and/or peripheral in origin. Medical treatment and rehabilitation-based therapy can be especially challenging due to accompanying cognitive deficits and severity of the disorder. This can involve multiple limbs in addition to the facial muscles and axial skeleton affecting multiple functions including speech, swallowing, posture, mobility, and activities of daily living. Unfortunately, reviews of movement disorders following brain injury have concentrated more on phenomenological aspects and treatment and less on neurorehabilitation and neurosurgical options [1–3]. For the purposes of this review, we will be primarily discussing movement disorders following acquired and traumatic brain injury.
Classification
The term “extrapyramidal disorders” used to describe movement disorders has fallen out of favor as the corticospinal and corticobulbar pathways, i.e., pyramidal tracts and basal ganglia pathways, are more integrated than originally thought. In addition, certain movement disorders are not directly associated with basal ganglia pathology. Therefore, the classification system of movement disorders, both as a group of disorders and as individual conditions, is now based on phenomenology as opposed to anatomic localization [4–6]. Movement disorders can be separated into two major classifications based on the presence of either excessive, abnormal involuntary movements (hyperkinetic movement disorders) or involuntary slowness and lack of movement (hypokinetic movement disorders) [4, 5]. The primary characteristics of the major hyperkinetic and hypokinetic movement disorders are summarized in Table 1.
Table 1.
Hyperkinetic and hypokinetic movement disorders
| Tremors | Involuntary, alternating movements involving one or more joints occurring at a regular frequency resulting in “rhythmic oscillations” |
| Dystonia | Involuntary, slow, sustained contractions of agonist and sometimes also antagonist muscles producing twisting movements and/or abnormal posturing |
| Chorea | Involuntary, non-rhythmic, abrupt movements resulting from continuous flow of muscle contractions from one muscle group to another resulting in jerky or dance like movements |
| Athetosis | Involuntary, slow, non-rhythmic, writhing movements with alternating postures in the limbs |
| Ballism | Involuntary, rapid, non-rhythmic, non-suppressible movements of the proximal joints producing wild, flinging, high-amplitude movements |
| Myoclonus | Involuntary, sudden, brief muscle contractions (positive myoclonus) or inhibition of muscle contractions (negative myoclonus) leading to shock like movements |
| Tics | Simple or complex, repetitive, abnormal movements or sounds usually preceded by an uncomfortable feeling or sensory urge that is relieved by carrying out the behavior. Tics can often be easily mimicked and suppressed by short efforts of will |
| Stereotypy | Simple or complex, repetitive, coordinated, ritualistic movement, posture or utterance that is continuous and purposeless |
| Bradykinesia | Involuntary slowness or poverty of movement |
| Rigidity | Involuntary increase in resistance to slow passive movement which is not velocity dependent |
Hyperkinetic movement disorders, also known as dyskinesias, can be further divided based on the presence of simple jerky movements (tremors and myoclonus) versus complex movements or postures (dystonia, athetosis, chorea, ballism and tics). Tremor is defined as an involuntary, rhythmic, oscillatory movement of a body part. Tremor can be further characterized by its presence at rest or with action (kinetic) such as volitionally holding the limb against gravity (postural tremor) or during a specific task (task-specific) [7••]. Myoclonus is defined as sudden involuntary, nonrhythmic movements caused by muscle contractions (positive myoclonus) or muscle tone lapses (negative myoclonus) which occur spontaneously at rest, during movement (action myoclonus), or provoked by external tactile or acoustic stimuli (reflex myoclonus) [8, 9]. Hyperekplexia is characterized by a heightened startle response to sudden unexpected stimuli which can result in falls [10, 11].
Dystonia is characterized by sustained or intermittent muscle contractions causing abnormal, often repetitive movements, postures, or both [12]. Dystonia may be present at rest but is usually brought on by voluntary activity (action dystonia) and sometimes accompanied by a tremor. The term athetosis is often used to describe slow, non-rhythmic, writhing movements with alternating postures in the limbs of patients with generalized secondary dystonia, usually seen in cerebral palsy [13]. Chorea and ballism represent a spectrum of disorders that overlap in phenomenology, pathophysiology, and etiology [13, 14]. Patients with chorea demonstrate non-rhythmic, jerky, non-suppressible movements of the distal limbs and face. Ballism is a unilateral, rapid, nonrhythmic, non-suppressible movement of the proximal limb. Because of its proximal involvement, usually at the shoulder and elbow, it produces wild, flinging, high-amplitude movements that can not only be psychologically distressing to the patient but also increase the risk of self-injury and falls due to loss of balance.
Tics are rapid, involuntary jerk-like movements or produced vocalizations (sounds or words) occurring out of a background of typical activity. They are differentiated from other hyperkinetic movement disorders as they may be preceded by premonitory feelings or sensations, variability, temporary suppressibility, and distractibility [5]. Stereotypy is an involuntary, repetitive, coordinated, ritualistic movement, posture, or utterance that is continuous and purposeless [5]. Tics and stereotypy may not be easy to distinguish, especially when irregular; however, stereotypy typically occurs in patients with intellectual disabilities. Both can be either simple or complex in nature. Although both tics and stereotypy are usually harmless, they can become especially problematic when manifesting as self-injurious behavior [15].
Bradykinesia, or the involuntary slowness or poverty of movement, is the clinical hallmark of hypokinetic movement disorder [5, 16]. It is sometimes used interchangeably with akinesia and hypokinesia. However, akinesia refers specifically to the poverty of spontaneous movement or associated movement, e.g., lack of facial expression, freezing of gait, or reduced arm swing during ambulation [16]. Hypokinesia describes movement that is both slow and smaller than desired, i.e., micrographia [16]. Rigidity is demonstrated by an increase in resistance to slow passive movement which is not velocity dependent [5]. Constant resistance to passive range of motion is referred to as lead pipe rigidity [5]. “Cogwheel rigidity” is due to a superimposed tremor which causes a “ratcheting” effect during passive range of motion [5]. Parkinsonism is characterized by bradykinesia, postural instability, rigidity, and/or resting tremor [6, 17].
Paratonia is a type of rigidity demonstrated by the inability to relax the muscles during passive range of motion. It may be confused with spasticity on clinical examination. It can be oppositional (gegenhalten) with increased involuntary resistance to passive movement or facilitatory (mitgehen) when the patient involuntarily assists with passive movements [18, 19]. Gegenhalten is velocity-dependent like spasticity; however, hypertonia felt with spasticity should subsequently reduce with repeated stretching. Paratonia has been associated with frontal lobe dysfunction, particularly with dementia [20]. Paratonia may be underappreciated in the brain injury population and warrants further study.
Epidemiology
Few epidemiological studies have investigated the incidence of movement disorders following brain injury. Most have looked at incidence following traumatic brain injury with wide variability in their reporting from 13 to 66% [21]. The delayed onset of movement disorders following brain injury likely further complicates data collection. One extreme case even reported the onset of tremors over 20 years after sustaining brain stem trauma [22]. This delay suggests that restorative neuroplastic processes including aberrant sprouting, ephaptic transmission, and alterations of neurotransmitter sensitivity may be contributing to the development of the movement disorder themselves [23].
Krauss and coworkers studied 398 patients admitted with severe traumatic brain injury and found that among survivors, 22.6% developed a movement disorder [24]. Tremor was the most common movement disorder reported, followed by dystonia [24]. These movement disorders were transient in 10.4% and persistent in 12.2% of patients but only 5.4% reported experiencing significant disability as a result [24]. The most disabling posttraumatic tremors were reportedly high-amplitude postural and kinetic tremors of the upper extremity. In terms of mild to moderate traumatic brain injury, Krauss and coworkers also conducted a similar study looking at 158 patients and found that 10.1% had developed movement disorders; however, only 2.6% experienced persistent movement disorders [25]. The most common movement disorder in this group was the postural/intention tremor [25]. The higher incidence of tremors seen in severe closed head injury, most commonly in motor vehicle accidents or in pedestrians who were struck by vehicles, suggests that diffuse axonal injury plays a role and is supported by imaging data [26–28].
Secondary dystonia is usually due to injury of the basal ganglia and/or thalamus (rarely globus pallidus) either from trauma, stroke, or anoxia and can often have associated hemiparesis or quadriparesis [29–32]. Hemidystonia affecting both the upper and lower extremity was the most common presentation following traumatic brain injury [33–39]. Dystonia is more likely in younger patients and there is often a delay in its appearance from the time of injury. Dystonia has also been reported with brain injury during their stay in the ICU; however, these are mainly cervical or oromandibular dystonia, and fortunately, most seem to improve over time with recovery [40].
Myoclonus has been reported following traumatic brain injury, involving the limbs as well as palate (palatal myoclonus) or oculomotor muscles (opsoclonus) but may often go undiagnosed and thus be underreported [41–45]. Ballism, chorea, and athetosis are rare following brain injury but have been reported in the literature. The presence of ballism does not necessarily guarantee structural abnormalities in the subthalamic nucleus [46–49]. Subdural and epidural hematomas may present with contralateral, ipsilateral, or bilateral chorea and/or choreoathetosis [50–55].
Adult-onset motor and vocal tics secondary to a known cause are referred to as “tourettism” to avoid confusion with the more common idiopathic Gilles de la Tourette’s syndrome [56]. Tourettism occurring after traumatic brain injury and stroke have been reported, but because tics are relatively common in the general population, coincidental occurrence should be also considered [56–60]. However, tourettism has been reported to have an older age of onset in contrast to Tourette’s syndrome [57].
Parkinsonism has been known to occur after repeated subclinical head trauma in professional sports, most commonly boxing. However [61, 62], the epidemiologic relationship between parkinsonism and repetitive subclinical traumatic brain injury has been difficult to elucidate as only rare cases have been reported with other sports. Parkinsonism associated with boxing has been termed “Pugilistic” parkinsonism, and presentation is delayed by at least several years following the end of a boxing career [63–65]. Imaging and neuropathological studies have also demonstrated the involvement of the substantia nigra [66, 67].
The relationship between Parkinson’s disease and traumatic brain injury has been even more difficult to establish. Although several studies have shown a higher incidence of head injury in patients with Parkinson’s disease, the causal effect is difficult to solidify as the head trauma occurred up to 20 to 30 years prior to the initial onset of symptoms [68–75]. Direct lesions to the substantia nigra because of traumatic brain injury from weapons and projectiles as well as strokes have been reported in the literature, usually presenting as contralateral hemiparkinsonism [76–79].
Hypoxic-ischemic brain injury (HIBI) can occur in patients who suffer sudden cardiac arrest, drug overdose, shock, and head trauma. Of those who survive to discharge, many are left with permanent neurological impairments including movement disorders. The most frequently encountered movement disorder in subacute to chronic HIBI is myoclonus and tremors [80, 81]. However, they may likely be confused with one another or even coexist. In 1963, Lance and Adams described “myoclonus in patients after severe hypoxic episodes” [82–84]. Since then, many such cases of Lance-Adams syndrome have been described, and it is characterized by multifocal action myoclonus that appears after resolution of coma [85, 86]. This is also referred to as chronic post hypoxic myoclonus and can be significantly disabling. It is important that this be distinguished from the epileptic myoclonus that occurs almost immediately after cardiac arrest/resuscitation, which is termed acute post hypoxic myoclonus or myoclonic status epilepticus [86]. Dystonia, parkinsonism, and akinetic rigidity involving the face and limbs can also occur following HIBI [80, 87]. Choreiform disorders are rarely reported with HIBI, but have been associated with lesions of the globus pallidus, caudate, and putamen [88].
Diagnosis
The clinical identification of movement disorders is primarily based on history and physical examination. Based on initial evaluation, the examiner should be able to answer the following questions:
Is there an excess or paucity of movement?
If excess movements, are the movements rhythmic, irregular, or arrhythmic?
Are the muscle contractions or postures sustained or not sustained?
Are the movements paroxysmal, continual (repeatedly), or continuous (without stopping)?
Do the movements only occur when the patient is awake, or do they persist during sleep?
With further examination, one should determine if the movement is present at rest, with action, or both. It must also be determined if the movement is patterned, nonpatterned, or if it is a combination of a variety of movements. If there is abnormal posturing, physical examination should reveal if the movement is powerful, easy to overcome as well as velocity dependent. Finally, determine if the movement is suppressible or not suppressible [5].
If there is a paucity of movement, then a hypokinetic movement disorder such as bradykinesia and/or rigidity is most likely. Rigidity may be accentuated by a voluntary movement in the contralateral limb (Froment’s maneuver) or reduced depending on the static posture of the body [5]. The presence of rigidity, in particular cogwheel rigidity, with bradykinesia would suggest underlying Parkinson’s disease, especially if there is also a resting tremor.
Excess rhythmic movements in an oscillatory fashion are consistent with a tremor. Resting tremor occurs when the extremity is relaxed. Action tremor occurs during attempts to sustain posture (postural tremor) or with movement (kinetic, intention, and task-specific tremors). A midbrain (Holmes) tremor usually consists of a combination of a rest tremor with worse action and a postural tremor. A dystonic tremor can be rhythmic or irregular and usually manifests as the patient is attempting to suppress a dystonic posture. The frequency of the tremor may not be helpful in diagnosis as most are 4–8 Hz with some exceptions [7••]. The intention and Holmes tremor frequencies are typically slow, usually less than 5 Hz [7••]. An essential tremor is generally between 4 and 11 Hz. Physiologic tremor is typically between 8 and 12 Hz [7••]. A primary orthostatic tremor is usually greater than 12 Hz [7••]. Tremor in Parkinson’s disease occurs at a frequency of 4 to 6 Hz and is classically described as a “pill rolling” resting tremor [89, 90]. Essential tremor and tremor in Parkinson’s disease can also affect the head, trunk, jaw, and lips [89, 90]. Considering that essential tremor and Parkinson’s disease are the most common movement disorders in the general population, it would not be unreasonable to suspect that a patient with traumatic brain injury due to fall might have had either condition as they both can affect gait and predispose a person to falls [91–94].
Sustained muscle contractions and postures are usually due to rigidity or dystonia though stereotypy is also a possibility. Stereotypy should be suppressible and easily overcome with passive range of movement. Dystonic postures may require a little more force to correct. Patients with dystonia may have a sensory trick (geste antagoniste) which is a physical gesture that temporarily suppresses the movement disorder. However, a limb with rigidity, especially lead pipe and oppositional paratonia (gegenhalten), can be very difficult to range even if attempted with great force and velocity by the examiner; hence, it can also demonstrate velocity-dependent resistance like spasticity [5, 12].
Athetosis is like dystonia as it often produces sustained nonrhythmic, continuous contractions with abnormal posturing, but the speed of these movements is faster than dystonia, and the direction of the movement changes randomly and in a somewhat flowing pattern. Chorea is similarly irregular, nonrhythmic movements that flow from one body part to another, but they are more rapid, unsustained, and are unpredictable in timing, direction, and distribution [13].
Myoclonus consists of sudden, brief shock-like involuntary movements or jerks that are either caused by muscle contractions (positive myoclonus) or inhibition of muscle contraction (negative myoclonus). They are usually arrhythmic except for segmental, ocular, and palatal myoclonus which are rhythmic. Ocular myoclonus should not be confused with opsoclonus which is arrhythmic. Segmental myoclonus suggests lesion in the brain stem or spinal cord. Rhythmic myoclonus can be differentiated from tremors as they tend to persist during sleep. Examination should further determine if the myoclonus is present at rest, with activity, and/or triggered by sudden stimuli such as sound, light, visual threat, or movement (reflex myoclonus). Action myoclonus along with negative myoclonus is often encountered with HIBI (Lance-Adams syndrome) which manifests as a “bouncing gait” when the patient attempts to stand or walk [8, 9, 95].
The correct categorization of the movement disorder is a critical first step toward diagnostic workup and management. Unfortunately, there are many challenges to identifying the correct movement disorder with history and clinical examination alone. A patient presenting with “jerk-like” movements may have tic disorder, myoclonus, dystonia, ballism, chorea, tremor, functional movement disorder, or even spasms and clonus. In addition, an individual may have more than one type of movement disorder or co-existing upper motor neuron syndrome producing these movements.
Recent imaging studies when available should also be reviewed to correlate phenomenology to lesion and decide if further workup for metabolic, infectious, or other causes including side effects of medications is warranted. This is especially true in ballism, which is a very rare post-traumatic movement disorder that can be confused with large-amplitude tremors or irregular myoclonic jerks, but it corresponds with lesion to the subthalamic nucleus [46–48, 96]. Posttraumatic dystonia is most frequently correlated with basal ganglia or thalamic lesions. Rare cases of chorea and athetosis have been reported with epidural and subdural hematomas [51–55]. Upper extremity bradykinesia was the most common movement disorder reported with hydrocephalus [97]. Lead pipe rigidity can be caused by a number of central nervous system lesions to the corpus striatum, cortical-basal, mid-brain (decorticate rigidity), medulla (decerebrate rigidity), or spinal cord (tetanus) [5]. However, correlating lesion with imaging can be limited in diffuse axonal injury and HIBI where there is more widespread injury, and some patients may exhibit a specific movement disorder without correlating imaging findings.
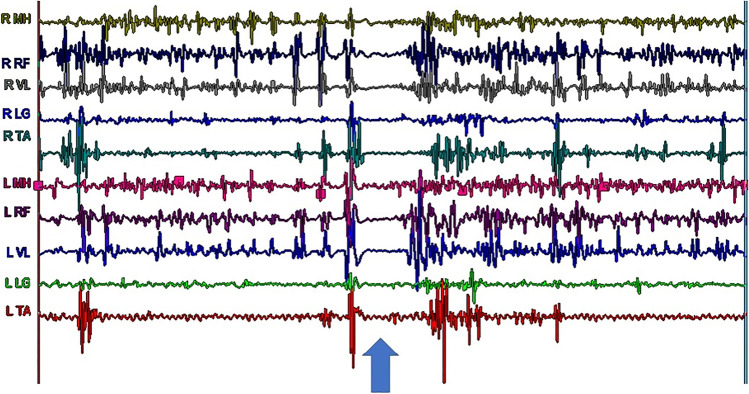
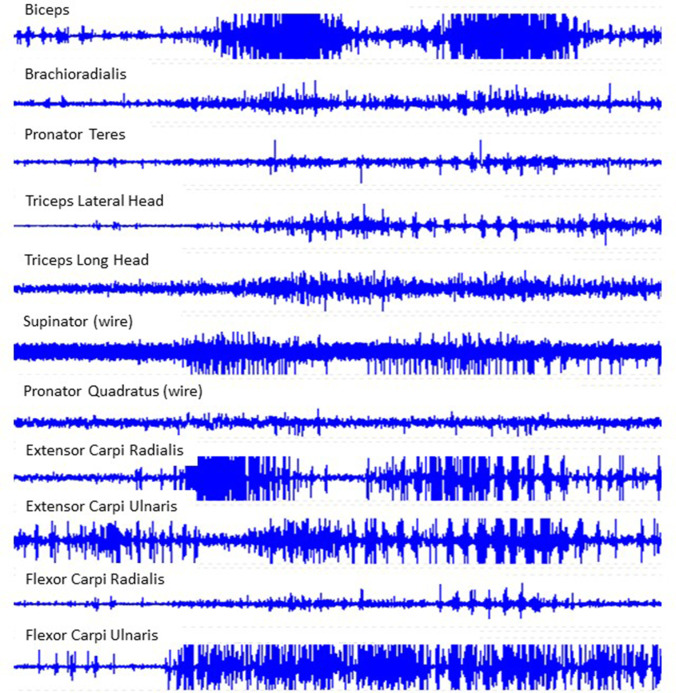
Although they may appear similar, hyperkinetic movement disorders have distinct electrophysiological characteristics that can be elucidated using electrophysiological testing. The use of surface poly-electromyography (EMG) recordings, electroencephalogram (EEG) studies with or without EMG, sensory-evoked potentials (SEP) and cortical reflexes, and startle studies have all been reported to help with the characterization of myoclonic movements [98••]. Poly-EMG recordings of a patient with Lance-Adams syndrome while standing clearly shows alternating positive and negative myoclonus which causes the characteristic “bouncy” appearance as well as a prolonged period of negative myoclonus leading to a sudden “drop attack” and near fall (Fig. 1). The use of poly-EMG recordings has been described to evaluate tremors and muscle overactivity associated with upper motor neuron syndrome [7••, 99, 100]. Surface poly-EMG recordings can be utilized to document the presence of tremor, measure frequency, and evaluate EMG burst morphology and rhythmicity which is helpful in differentiating tremor from myoclonus (Fig. 2) [7••]. To make matters more complicated, bradykinesia can occur with hyperkinesias such as Huntington’s disease and dystonia [16]. Poly-EMG recording during attempted rapid arm movements can help elucidate if bradykinesia is due to co-contraction of antagonistic muscles as shown in patients with Huntington’s disease and dystonia or impaired recruitment without co-contraction seen in parkinsonism [101].
Fig. 1.
Dynamic poly-electromyographic recordings of a patient with Lance-Adams syndrome while standing shows alternating positive and negative myoclonus leading to “bouncing” as well as a prolonged period of negative myoclonus (noted by the arrow) leading to a “drop attack.” Right (R), left (L), medial hamstrings (MH), rectus femoris (RF), vastus lateralis (VL), lateral gastrocnemius (LG), and tibialis anterior (TA). Data collected in the MossRehab Sheer Gait and Motion Analysis Laboratory
Fig. 2.
Dynamic poly-electromyographic recordings of select muscles in a patient’s right arm while they are bringing a spoon to their mouth (2 cycles) demonstrate a posttraumatic kinetic tremor. This patient demonstrated clinical improvement with levodopa-carbidopa. Data collected in the MossRehab Motor Control Analysis Laboratory
Finally, other causes of the movement disorder (or its exacerbation) including drug or toxin-related, metabolic (endocrine), autoimmune, and infectious etiologies should be considered and ruled out. Dopaminergic agents, anti-epileptics, stimulants, serotoninergic agents, and even muscle relaxants such as baclofen and benzodiazepines have been reported to cause or exacerbate pre-existing movement disorders. Antipsychotics are well known for causing or worsening movement disorders in psychiatric patients, as well as Parkinson’s and brain injury patients [50]. Illicit drug use and drug withdrawal are also known to cause movement disorders [102, 103]. Asterixis is negative myoclonus elicited when the patient attempts to extend wrists against gravity which is a clinical sign often found in patients with liver failure. Chorea and myoclonus have been reported in association with glycemic disturbances as well as hyperthyroidism [104–111]. With the current ongoing COVID-19 pandemic, there has been a recent flood of literature reporting on the neurological side effects of coronavirus infection including movement disorders [112•, 113, 114]. Thus, it would be logical to include COVID-19 testing in the workup though a positive result would not necessarily confirm causality considering how common the infection is now in the world. However, more studies are warranted investigating COVID-19-associated movement disorders for further guidance.
Management
Treatment of patients with movement disorders due to brain injury must include addressing both the neurological and psychosocial dysfunction resulting from the brain injury. These patients can be severely disabled from associated neurological deficits including the movement disorder resulting from the brain injury. The rehabilitative multidisciplinary team approach involving physiatrists, neurologists, occupational and physical therapists, speech language pathologists, and psychologists would be ideal. However, the patient can already be discharged from the acute rehabilitation hospital prior to onset of the movement disorder making it more challenging to coordinate care in the outpatient, subacute rehabilitation, or skilled nursing home setting.
Posttraumatic tremor, especially those with persistent “violent” shaking movements, can be very difficult to treat. Some medications reported to help include beta blockers such as propranolol, benzodiazepines, primidone, levodopa/carbidopa, carbamazepine, anticholinergics, and even isoniazid [115–117]. Botulinum toxin injections may be helpful temporarily but a high total dosage with medication administered to multiple muscles in the proximal and distal arm and any positive effects wearing off before treatment can be repeated limit its use [118].
Chronic post-anoxic myoclonus can also be very disabling and psychologically devastating to the patient. Not only do they have to deal with positive myoclonic jerks which sometimes persist when they sleep but also negative myoclonus leading to sudden loss of muscle tone and falls. Levetiracetam, clonazepam, and valproic acid have been shown to decrease myoclonus but are not well tolerated due to side effects [85, 95, 119–121]. Piracetam, baclofen, and ethanol have also shown some success [119]. Regardless, post-anoxic myoclonus remains very challenging to manage especially due to the presence of positive myoclonic jerks followed by lapses in muscle activity.
Treatment of dystonia following brain injury may include anticholinergics and muscle relaxants such as benzodiazepines, tizanidine, baclofen, and tizanidine. Occasionally, there is mild symptom relief as these patients may also feel discomfort, but oral/enteral medicine is usually ineffective. However, there has been some improvement following botulinum toxin injections; therefore, it has become the treatment of first choice in patients with brain injury–associated focal dystonias [118, 122, 123]. Haloperidol, risperidone, diazepam, and cyclobenzaprine have been reportedly used with some success in post-anoxic and posttraumatic chorea and athetosis [50, 88]. In chorea and athetosis following brain injury, treatment should also involve addressing the underlying cause including cessation of suspected medication and surgical drainage of subdural or epidural hematoma if present [13, 51–55, 88, 104, 105]. Ballism can be treated conservatively with tetrabenazine.
Patients with posttraumatic parkinsonism due to lesions of the substantia nigra may improve with amantadine and levodopa therapy; however, efficacy may be limited by adverse effects, especially nausea and vomiting [78, 79, 124]. Identification and management of other potential causes of rigidity and bradykinesia include hydrocephalus and antipsychotics.
Stereotactic surgery/radio-frequency lesioning in the ventrolateral thalamus and subthalamic region has been relatively well documented in the treatment of post-traumatic tremor [28, 125–127]. One study found striking improvements in the postural and kinetic tremor, but there was an increased risk of adverse effects, primarily exacerbation of preoperative symptoms such as dysarthria or gait disturbance [28]. Stereotactic surgery targeting the ventrolateral thalamus, subthalamic lesion, the pulvinar, and the globus pallidus has been performed in patients with severe hemidystonia, segmental dystonia, and hemiballism [37, 128]. However, it is still unclear if thalamic or pallidal targets should be preferred in secondary dystonia, and it is generally found to be less effective in patients with extensive structural cerebral lesions [129].
Due to the increased risk of adverse effects, there has been increasing use of thalamic deep brain stimulation (DBS) for treatment of posttraumatic tremor. However, it has been found to be less effective in comparison to the treatment of parkinsonian or essential tremor, and some even claim it was ineffective [130–134]. DBS to the thalamus or globus pallidus has also been used more frequently recently in attempts to treat patients with hemidystonia following traumatic brain injury, but response has been mixed [135–137]. Though infrequently utilized, there has been some success reported with the utilization of DBS to the bilateral globus pallidus and thalamus in the treatment of post-anoxic myoclonus and dystonia respectively [138–140]. Combined DBS to the subthalamic nucleus and nucleus ventralis intermedius was also reportedly successful in the treatment of a patient with posttraumatic parkinsonism with kinetic tremor [141]. Intrathecal baclofen pumps usually used for patients with spasticity have also been used in the management of post-traumatic generalized dystonia and hemiballismus with some success reported [142–145]. There have also been several reports of their use in patients with Lance-Adams syndrome [146, 147].
Role of rehabilitation
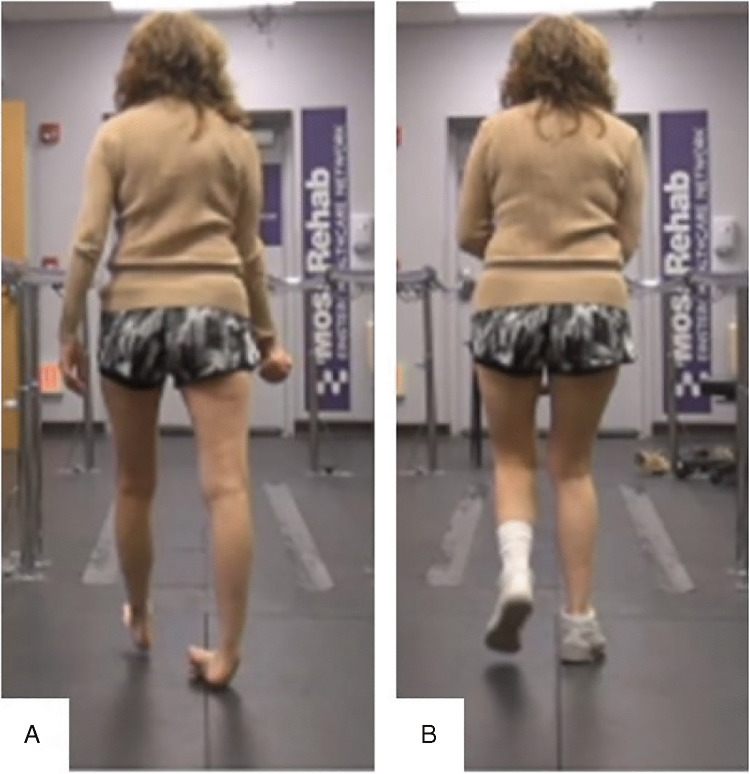
The potential benefits of exercise in movement disorders are well documented not only in improving their strength and endurance but also in improving their psychological wellbeing [148, 149]. However, no definitive rehabilitation guidelines are available for addressing movement disorders following brain injury though these patients are commonly seen by therapists during their inpatient rehabilitation as well as once they are discharged. Clearly, exercises to improve strength and endurance, strategies (i.e., use of sensory tricks, relaxation techniques, limiting degrees of freedom), and adaptive equipment (i.e., orthotics, weighted utensils and garments, assistive walking devices) are beneficial and should be explored with every patient. Regular stretching and splinting may prevent the worsening of contractures which could be especially detrimental to their mobility. The use of orthoses/orthotics, assistive devices, and wheelchairs is indicated to enhance mobility and safety. The author has had some success with use of orthoses and orthotics in helping to control and stabilize lower limb in movement disorders (Fig. 3). Further investigations into the potential for electrical nerve stimulation and robotics in symptom suppression and facilitation of movement have been reported, but there is an obvious scarcity of literature in general with regards to this topic [150, 151]. Most of the rehabilitation research with regard to movement disorders has been dedicated to Parkinson’s disease.
Fig. 3.
A Patient with history of traumatic brain injury as a child demonstrates dystonic right ankle varus posturing with weight bearing. B With implementation of a UCBL orthotic in her right shoe, her varus posturing is significantly improved. University of California Berkeley Laboratory (UCBL)
Conclusion
Brain injury can affect movement in a variety of ways. Motor disorders, movement disorders, and visual and vestibular deficits can often coexist with cognitive deficits which interfere with motor learning. Accurately identifying the various phenomena utilizing clinical history and examination, imaging studies, and electrophysiological studies provides an important framework for evaluation and treatment of the functional deficits that result from them. Although movement disorders are typically delayed in onset from initial brain injury, further workup should be done to rule out any other potential causes or exacerbators including underlying metabolic/endocrine issues, adverse effects of medications, infections, toxins, illicit drugs, and even undiagnosed pre-existing movement disorder. Brain injury patients with movement disorders should be managed by a multidisciplinary team providing psychological support, medical management including trials of medications and injections when appropriate, assistive devices for mobility and safety, rehabilitation services to optimize strength, endurance, range of motion, and functional independence and possibly neurosurgical interventions. However, the evidence regarding the medical management and rehabilitation of brain injury patients with movement disorders is sparse warranting further investigation.
Declarations
Conflict of Interest
The author has served on an advisory board for Ipsen Pharmaceuticals.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Footnotes
This article is part of the Topical Collection on Brain Injury Medicine and Rehabilitation
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Koller WC, Wong GF, Lang A. Posttraumatic movement disorders: a review. Mov Disord. 1989;4(1). 10.1002/mds.870040106. [DOI] [PubMed]
- 2.Goetz CG, Pappert EJ. Trauma and movement disorders. Neurol Clin. 1992;10(4). 10.1016/s0733-8619(18)30187-7. [PubMed]
- 3.Jankovic J. Post-traumatic movement disorders: central and peripheral mechanisms. Neurology. 1994;44(11). 10.1212/wnl.44.11.2006. [DOI] [PubMed]
- 4.Jankovic J. International classification of diseases, tenth revison: Neurological adaptation (ICD‐10 NA): extrapyramidal and movement disorders. Mov Disord. 1995;10(5). 10.1002/mds.870100502. [DOI] [PubMed]
- 5.Jankovic J, Hallett M, Okun MS, Comella C, Fahn S, Goldman J. Clinical overview and phenomenology of movement disorders. In: Principles and Practice of Movement Disorders; 2021. 10.1016/b978-0-323-31071-0.00001-9.
- 6.Fahn S. Classification of movement disorders. Mov Disord. 2011;26(6):947–957. Accessed April 30, 2022. 10.1002/mds.26445. [DOI] [PubMed]
- 7.••.Bhatia KP, Bain P, Bajaj N, et al. Consensus statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord. 2018;33(1). 10.1002/mds.27121. This review highlights the criteria for diagnosis and classification of tremor. [DOI] [PMC free article] [PubMed]
- 8.Fahn S, Marsden CD, van Woert MH. Definition and classification of myoclonus. Adv Neurol. 1986;43. [PubMed]
- 9.Fahn S. Overview, history, and classification of myoclonus. Adv Neurol. 2002;89. [PubMed]
- 10.Zhan F xia, Wang SG, Cao L. Advances in hyperekplexia and other startle syndromes. Neurol Sci. 2021;42(10). 10.1007/s10072-021-05493-8. [DOI] [PubMed]
- 11.Dreissen YEM, Bakker MJ, Koelman JHTM, Tijssen MAJ. Exaggerated startle reactions. Clin Neurophysiol. 2012;123(1). 10.1016/j.clinph.2011.09.022. [DOI] [PubMed]
- 12.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28(7). 10.1002/mds.25475. [DOI] [PMC free article] [PubMed]
- 13.Biglan KM. Chorea, athetosis, and ballism. In: Hyperkinetic Movement Disorders; 2015. 10.1093/med/9780199925643.003.0005.
- 14.Dewey RB, Jankovic J. Hemiballism-hemichorea: clinical and pharmacologic findings in 21 patients. Arch Neurol. 1989;46(8). 10.1001/archneur.1989.00520440044020. [DOI] [PubMed]
- 15.Muehlmann AM, Lewis MH. Abnormal repetitive behaviors: shared phenomenology and pathophysiology. J Intellect Disabil Res. 2012;56(5). 10.1111/j.1365-2788.2011.01519.x. [DOI] [PubMed]
- 16.Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in parkinson’s disease. Brain. 2001;124(11). 10.1093/brain/124.11.2131. [DOI] [PubMed]
- 17.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30(12). 10.1002/mds.26424. [DOI] [PubMed]
- 18.Beversdorf DQ, Heilman KM. Facilitory paratonia and frontal lobe functioning. Neurology. 1998;51(4). 10.1212/WNL.51.4.968. [DOI] [PubMed]
- 19.Hobbelen JSM, Koopmans RTCM, Verhey FRJ, van Peppen RPS, de Bie RA. Paratonia: A delphi procedure for consensus definition. J Geriatr Phys Ther. 2006;29(2). 10.1519/00139143-200608000-00002.. [PubMed]
- 20.Drenth H, Zuidema S, Bautmans I, et al. Paratonia in dementia: a systematic review. J Alzheimer’s Dis. 2020;78(4). 10.3233/JAD-200691. [DOI] [PMC free article] [PubMed]
- 21.Krauss JK, Polemikos M, Jankovic J. Movement disorders after traumatic brain injury. In: Brain Injury Medicine.; 2021. 10.1891/9780826143051.0039.
- 22.Krack P, Deuschl G, Kaps M, Warnke P, Schneider S, Traupe H. Delayed onset of “rubral tremor” 23 years after brainstem trauma. Mov Disord. 1994;9(2). 10.1002/mds.870090225. [DOI] [PubMed]
- 23.Scott BL, Jankovic J. Delayed-onset progressive movement disorders after static brain lesions. Neurology. 1996;46(1). 10.1212/WNL.46.1.68. [DOI] [PubMed]
- 24.Krauss JK, Tränkle R, Kopp KH. Post-traumatic movement disorders in survivors of severe head injury. Neurology. 1996;47(6). 10.1212/WNL.47.6.1488. [DOI] [PubMed]
- 25.Krauss JK, Tränkle R, Kopp KH. Posttraumatic movement disorders after moderate or mild head injury. Mov Disord. 1997;12(3). 10.1002/mds.870120326. [DOI] [PubMed]
- 26.Krauss JK. Movement disorders secondary to craniocerebral trauma. In: Handbook of Clinical Neurology. Vol 128.; 2015. 10.1016/B978-0-444-63521-1.00030-3. [DOI] [PubMed]
- 27.Yaguchi H, Niida H, Yaguchi M, Tachikawa H, Sekihara Y. A case of postural and kinetic tremor caused by diffuse axonal injury. Clin Neurol. 2003;43(1–2). [PubMed]
- 28.Krauss JK, Wakhloo AK, Nobbe F, Trankle R, Mundinger F, Seeger W. Lesions of dentatothalamic pathways in severe post-traumatic tremor. Neurol Res. 1995;17(6). 10.1080/01616412.1995.11740353. [PubMed]
- 29.Lee MS, Marsden CD. Movement disorders following lesions of the thalamus or subthalamic region. Mov Disord. 1994;9(5). 10.1002/mds.870090502. [DOI] [PubMed]
- 30.Oztekin MF, Oztekin N. Anatomic localization of secondary dystonias: analysis of 21 patients. J Neurol Sci. 2013;333. 10.1016/j.jns.2013.07.631.
- 31.A. M, D. M, T. C, N.P. Q, C.D. M, K.P. B. Unilateral lesions of the globus pallidus: Report of four patients presenting with focal or segmental dystonia. J Neurol Neurosurg Psychiatry. 2000;69(4). [DOI] [PMC free article] [PubMed]
- 32.Marsden CD, Obeso JA, Zarranz JJ, Lang AE. The anatomical basis of symptomatic hemidystonia. Brain. 1985;108(2). 10.1093/brain/108.2.463. [DOI] [PubMed]
- 33.Lee MS, Rinne JO, Ceballos-Baumann A, Thompson PD, Marsden CD. Dystonia after head trauma. Neurology. 1994;44(8). 10.1212/wnl.44.8.1374. [DOI] [PubMed]
- 34.Nadaf SN, Chakor RT, Kothari KV, Bharote H. Progressive delayed hemidystonia following clinically mild traumatic brain injury. BMJ Case Rep. 2017;2017. 10.1136/bcr-2017-220334. [DOI] [PMC free article] [PubMed]
- 35.Gowda V, Manjeri V, Srinivasan V, Sajjan S, Benakappa A. Mineralizing angiopathy with basal ganglia stroke after minor trauma: case series including two familial cases. J Pediatr Neurosci. 2018;13(4). 10.4103/JPN.JPN_89_17. [DOI] [PMC free article] [PubMed]
- 36.Secondary hemidystonia following head trauma. Curr Clin Neurol. 2012;36. 10.1007/978-1-60327-426-5_45.
- 37.Krauss JK, Mohadjer M, Braus DF, Wakhloo AK, Nobbe F, Mundinger F. Dystonia following head trauma: a report of nine patients and review of the literature. Mov Disord. 1992;7(3). 10.1002/mds.870070313. [DOI] [PubMed]
- 38.Lingappa L, Varma RD, Siddaiahgari S, Konanki R. Mineralizing angiopathy with infantile basal ganglia stroke after minor trauma. Dev Med Child Neurol. 2014;56(1). 10.1111/dmcn.12275. [DOI] [PubMed]
- 39.Chuang C, Fahn S, Frucht SJ. The natural history and treatment of acquired hemidystonia: Report of 33 cases and review of the literature. J Neurol Neurosurg Psychiatry. 2002;72(1). 10.1136/jnnp.72.1.59. [DOI] [PMC free article] [PubMed]
- 40.Lo SE, Rosengart AJ, Novakovic RL, et al. Identification and treatment of cervical and oromandibular dystonia in acutely brain-injured patients. Neurocrit Care. 2005;3(2). 10.1385/NCC:3:2:139. [DOI] [PubMed]
- 41.Turazzi S, Alexandre A, Bricolo A, Rizzuto N. Opsoclonus and palatal myoclonus during prolonged post-traumatic coma: a clinico-pathologic study. Eur Neurol. 1977;15(5). 10.1159/000114811. [DOI] [PubMed]
- 42.Jacob PC, Pratap Chand R. A posttraumatic thalamic lesion associated with contralateral action myoclonus. Mov Disord. 1999;14(3). 10.1002/1531-8257(199905)14:3<512::AID-MDS1022>3.0.CO;2-7. [DOI] [PubMed]
- 43.Mayer SA, Fink ME, Galetta SL, Silver AJ, Hilal SK. Posttraumatic Oculopalatal Myoclonus. Neurorehabil Neural Repair. 1993;7(1). 10.1177/136140969300700105.
- 44.Starosta-Rubinstein S, Bjork RJ, Snyder BD, Tulloch JW. Posttraumatic intention myoclonus. Surg Neurol. 1983;20(2). 10.1016/0090-3019(83)90463-9. [DOI] [PubMed]
- 45.Keane JR. Galloping tongue: post-traumatic, episodic, rhythmic movements. Neurology. 1984;34(2). 10.1212/wnl.34.2.251. [DOI] [PubMed]
- 46.Hopewell CA. Hemichorea-hemiballismus as conversion reaction following head trauma. Clin Neuropsychol. 1983;5(1).
- 47.King RB, Fuller C, Collins GH. Delayed onset of hemidystonia and hemiballismus following head injury: a clinicopathological correlation. Case report. J Neurosurg. 2001;94(2). 10.3171/jns.2001.94.2.0309. [DOI] [PubMed]
- 48.Kant R, Zeiler D. Hemiballismus following closed head injury. Brain Injury. 1996;10(2). 10.1080/026990596124665. [DOI] [PubMed]
- 49.Lévesque MF, Markham CH. Ventral intermediate thalamotomy for posttraumatic hemiballismus. Stereotactic Funct Neurosurg. 1992;58(1–4). 10.1159/000098967. [DOI] [PubMed]
- 50.Krasna D, Montgomery E, Koffer J, Segal M. Bilateral chorea following severe traumatic brain injury treated with risperidone. BMJ Case Rep. 2021;14(5). 10.1136/bcr-2021-241929. [DOI] [PMC free article] [PubMed]
- 51.Shiraishi T, Sengoku R, Takanashi S, Shibukawa M, Kanemaru K, Murayama S. Chorea due to chronic subdural hematoma. Clin Neurol. 2018;58(6). 10.5692/clinicalneurol.cn-001102. [DOI] [PubMed]
- 52.Bae SH, Vates TS, Kenton EJ. Generalized chorea associated with chronic subdural hematomas. Ann Neurol. 1980;8(4). 10.1002/ana.410080422. [DOI] [PubMed]
- 53.Sung YF, Ma HI, Hsu YD. Generalized chorea associated with bilateral chronic subdural hematoma. Eur Neurol. 2004;51(4). 10.1159/000078546. [DOI] [PubMed]
- 54.Gilmore PC, Brenner RP. Chorea: a late complication of a subdural hematoma. Neurology. 1979;29(7). 10.1212/WNL.29.7.1044. [DOI] [PubMed]
- 55.Adler JR, Winston KR. Chorea as a manifestation of epidural hematoma: Case report. J Neurosurg. 1984;60(4). 10.3171/jns.1984.60.4.0856. [DOI] [PubMed]
- 56.Kwak CH, Jankovic J. Tourettism and dystonia after subcortical stroke. Mov Disord. 2002;17(4). 10.1002/mds.10207. [DOI] [PubMed]
- 57.Krauss JK, Jankovic J. Tics secondary to craniocerebral trauma. Mov Disord. 1997;12(5). 10.1002/mds.870120527. [DOI] [PubMed]
- 58.Ranjan N, Nair KPS, Romanoski C, Singh R, Venketswara G. Tics after traumatic brain injury. Brain Injury. 2011;25(6). 10.3109/02699052.2011.572944. [DOI] [PubMed]
- 59.Singer C, Sanchez‐Ramos J, Weiner WJ. A case of post‐traumatic tic disorder. Mov Disord. 1989;4(4). 10.1002/mds.870040409. [DOI] [PubMed]
- 60.Fahn S. A case of post-traumatic tic syndrome. Adv Neurol. 1982;35. [PubMed]
- 61.Mckee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalography in athletes. J Neuropathol Exp Neurol. 2009;68(7). [DOI] [PMC free article] [PubMed]
- 62.Gavett BE, Stern RA, McKee AC. Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin Sports Med. 2011;30(1). 10.1016/j.csm.2010.09.007. [DOI] [PMC free article] [PubMed]
- 63.Guterman A, Smith RW. Neurological sequelae of boxing. Sports Medicine. 1987;4(3). 10.2165/00007256-198704030-00004. [DOI] [PubMed]
- 64.Jordan BD. Chronic traumatic brain injury associated with boxing. Semin Neurol. 2000;20(2). 10.1055/s-2000-9826. [DOI] [PubMed]
- 65.Friedman JH. Progressive parkinsonism in boxers. S Med J. 1989;82(5). 10.1097/00007611-198905000-00002. [DOI] [PubMed]
- 66.Davie CA, Pirtosek Z, Barker GJ, Kingsley DPE, Miller PH, Lees AJ. Magnetic resonance spectroscopic study of parkinsonism related to boxing. J Neurol Neurosurg Psychiatry. 1995;58(6). 10.1136/jnnp.58.6.688. [DOI] [PMC free article] [PubMed]
- 67.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68(7). 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed]
- 68.Morley JF, Duda JE. Head injury and the risk of Parkinson’s disease. Mov Disord. 2012;27(13). 10.1002/mds.25230. [DOI] [PubMed]
- 69.Taylor KM, Saint-Hilaire MH, Sudarsky L, et al. Head injury at early ages is associated with risk of Parkinson’s disease. Parkinsonism Relat Disord. 2016;23. 10.1016/j.parkreldis.2015.12.005. [DOI] [PMC free article] [PubMed]
- 70.Rugbjerg K, Ritz B, Korbo L, Martinussen N, Olsen JH. Risk of Parkinson’s disease after hospital contact for head injury: population based case-control study. BMJ (Online). 2009;338(7685). 10.1136/bmj.a2494. [DOI] [PMC free article] [PubMed]
- 71.Brown E, Goldman S, Meng C, Tanner C. Head injury and Parkinson’s disease (PD) phenotype. Neurology. 2020;94(15 Supplement).
- 72.Spangenberg S, Hannerz H, Tüchsen F, Mikkelsen KL. A nationwide population study of severe head injury and parkinson’s disease. Parkinsonism Relat Disord. 2009;15(1). 10.1016/j.parkreldis.2008.02.004. [DOI] [PubMed]
- 73.Fang F, Chen H, Feldman AL, Kamel F, Ye W, Wirdefeldt K. Head injury and Parkinson’s disease: a population-based study. Mov Disord. 2012;27(13). 10.1002/mds.25143. [DOI] [PubMed]
- 74.Goldman SM, Tanner CM, Oakes D, Bhudhikanok GS, Gupta A, Langston JW. Head injury and Parkinson’s disease risk in twins. Ann Neurol. 2006;60(1). 10.1002/ana.20882. [DOI] [PubMed]
- 75.Harris MA, Shen H, Marion SA, Tsui JKC, Teschke K. Head injuries and Parkinson’s disease in a case-control study. Occup Environ Med. 2013;70(12). 10.1136/oemed-2013-101444. [DOI] [PubMed]
- 76.Bajaj P, Contractor A, Pramanick A. Post-traumatic Parkinsonism in diffuse axonal injury: a case report. J Parkinson’s Dis. 2016;6.
- 77.Harik SI, Al-Hinti JT, Archer RL, Angtuaco EJC. Hemiparkinsonism after unilateral traumatic midbrain hemorrhage in a young woman. Neurology. 2013;3(1). 10.1212/CPJ.0b013e318283fef6. [DOI] [PMC free article] [PubMed]
- 78.Takeda M, Okuda B, Tomino Y, Tachibana H, Sugita M. A case of posttraumatic parkinsonism. Clin Neurol. 1991;31(8). [PubMed]
- 79.Rosero E, Cho S, Watanabe MMTK. Posttraumatic parkinsonism: a case report. PM&R. 2011;3(10).
- 80.Venkatesan A, Frucht S. Movement disorders after resuscitation from cardiac arrest. Neurol Clin. 2006;24(1). 10.1016/j.ncl.2005.11.001. [DOI] [PubMed]
- 81.Scheibe F, Neumann WJ, Lange C, et al. Movement disorders after hypoxic brain injury following cardiac arrest in adults. Eur J Neurol. 2020;27(10). 10.1111/ene.14326. [DOI] [PubMed]
- 82.LANCE JW. The falling attacks of myoclonus. Proc Aust Assoc Neurol. 1963;72. [PubMed]
- 83.Lance JW, Adams RD. Negative myoclonus in posthypoxic patients: historical note. Mov Disord. 2001;16(1). 10.1002/1531-8257(200101)16:1<162::AID-MDS1029>3.0.CO;2-9. [DOI] [PubMed]
- 84.Lance JW, Adams RD. The syndrome of intention or action myoclonus as a sequel to hypoxic encephalopathy. Brain. 1963;86(1). 10.1093/brain/86.1.111. [DOI] [PubMed]
- 85.Frucht S, Fahn S. The clinical spectrum of posthypoxic myoclonus. Mov Disord. 2000;15(S1). 10.1002/mds.870150702. [DOI] [PubMed]
- 86.Gupta H v, Caviness JN. Post-hypoxic myoclonus: current concepts, neurophysiology, and treatment. Tremor and Other Hyperkinetic Movements. 2016;6. 10.5334/tohm.323. [DOI] [PMC free article] [PubMed]
- 87.Bhatt MH, Obeso JA, Marsden CD. Time course of postanoxic akinetic-rigid and dystonic syndromes. Neurology. 1993;43(2). 10.1212/wnl.43.2.314. [DOI] [PubMed]
- 88.Hermann A, Walker RH. Diagnosis and treatment of chorea syndromes. Curr Neurol Neurosci Rep. 2015;15(2). 10.1007/s11910-014-0514-0. [DOI] [PubMed]
- 89.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor Ad Hoc. Scientific Committee. Mov Disord. 1998;13(Suppl):3. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 90.Thenganatt MA, Louis ED. Distinguishing essential tremor from Parkinson’s disease: Bedside tests and laboratory evaluations. Exp Rev Neurother. 2012;12(6). 10.1586/ern.12.49. [DOI] [PMC free article] [PubMed]
- 91.Rao AK, Gilman A, Louis ED. Balance confidence and falls in nondemented essential tremor patients: the role of cognition. Arch Phys Med Rehabil. 2014;95(10). 10.1016/j.apmr.2014.04.001. [DOI] [PMC free article] [PubMed]
- 92.Baudendistel ST, Schmitt AC, Rodriguez A v., McFarland NR, Hass CJ. A turn for the worse: turning performance in Parkinson’s disease and essential tremor. Clin Biomech. 2019;70. 10.1016/j.clinbiomech.2019.09.008. [DOI] [PubMed]
- 93.Arkadir D, Louis ED. The balance and gait disorder of essential tremor: what does this mean for patients? Ther Adv Neurol Disord. 2013;6(4). 10.1177/1756285612471415. [DOI] [PMC free article] [PubMed]
- 94.Camacho-Soto A, Warden MN, Searles Nielsen S, et al. Traumatic brain injury in the prodromal period of Parkinson’s disease: a large epidemiological study using medicare data. Ann Neurol. 2017;82(5). 10.1002/ana.25074. [DOI] [PMC free article] [PubMed]
- 95.Fahn S. Posthypoxic action myoclonus: literature review update. Adv Neurol. 1986;43. [PubMed]
- 96.Krauss JK, Borremans JJ, Nobbe F, Mundinger F. Ballism not related to vascular disease: a report of 16 patients and review of the literature. Parkinsonism Relat Disord. 1996;2(1). 10.1016/1353-8020(95)00018-6. [DOI] [PubMed]
- 97.Krauss JK, Regel JP, Droste DW, Orszagh M, Borremans JJ, Vach W. Movement disorders in adult hydrocephalus. Mov Disord. 1997;12(1). 10.1002/mds.870120110. [DOI] [PubMed]
- 98.••.Merchant SHI, Vial-Undurraga F, Leodori G, van Gerpen JA, Hallett M. Myoclonus: an electrophysiological diagnosis. Mov Disord Clin Pract. 2020;7(5). 10.1002/mdc3.12986This review emphasizes the use of neurophysiological studies to better characterize myoclonus as well as differentiation from other movement disorders which can have important therapeutic and prognostic implications. [DOI] [PMC free article] [PubMed]
- 99.Esquenazi A, Mayer NH. Laboratory analysis and dynamic polyEMG for assessment and treatment of gait and upper limb dysfunction in upper motoneuron syndrome. Europa Medicophysica. 2004;40(2). [PubMed]
- 100.Esquenazi A, Mayer NH. Instrumented assessment of muscle overactivity and spasticity with dynamic polyelectromyographic and motion analysis for treatment planning. In: American Journal of Physical Medicine and Rehabilitation. Vol 83.; 2004. 10.1097/01.PHM.0000141127.63160.3E. [DOI] [PubMed]
- 101.Berardelli A, Hallett M, Rothwell JC, et al. Single-joint rapid arm movements in normal subjects and in patients with motor disorders. Brain. 1996;119(2). 10.1093/brain/119.2.661. [DOI] [PubMed]
- 102.Brust JCM. Substance abuse and movement disorders. Mov Disord. 2010;25(13). 10.1002/mds.22599. [DOI] [PubMed]
- 103.Narula N, Krishnan N, Siddiqui F, Chalhoub M. Cracking the crack dance: a case report on cocane-induced choreoathetosis. Chest. 2017;152(4). 10.1016/j.chest.2017.08.381. [DOI] [PMC free article] [PubMed]
- 104.Ristić AJ, Svetel M, Dragašević N, Žarković M, Koprivšek K, Kostić VS. Bilateral chorea-ballism associated with hyperthyroidism. Mov Disord. 2004;19(8). 10.1002/mds.20119. [DOI] [PubMed]
- 105.Isaacs JD, Rakshi J, Baker R, Brooks DJ, Warrens AN. Chorea associated with thyroxine replacement therapy. Mov Disord. 2005;20(12). 10.1002/mds.20603. [DOI] [PubMed]
- 106.Laureno R. Neurologic manifestations of thyroid disease. Endocrinologist. 1996;6(6). 10.1097/00019616-199611000-00007.
- 107.Neupane P, Satyavolu B, Adhikari A, Seedat Z. Movement disorder as a manifestation of diabetic ketoacidosis. Chest. 2020;158(4). 10.1016/j.chest.2020.08.798.
- 108.Tan EK, Chan LL. Movement disorders associated with hyperthyroidism: expanding the phenotype [1]. Mov Disord. 2006;21(7). 10.1002/mds.20883. [DOI] [PubMed]
- 109.Pitton Rissardo J, Fornari Caprara A. Movement disorders associated with hypoglycemia and hyperglycemia. Ann Mov Disord. 2020;3(2). 10.4103/AOMD.AOMD_18_20.
- 110.Shah VS, Sardana V. Sudden jerky head movement in hypoglycemia. Ann Mov Disord. 2020;3(1). 10.4103/AOMD.AOMD_29_19.
- 111.Shaw C, Haas L, Miller D, Delahunt J. A case report of paroxysmal dystonic choreoathetosis due to hypoglycaemia induced by an insulinoma. J Neurol Neurosurg Psychiatry. 1996;61(2). 10.1136/jnnp.61.2.194. [DOI] [PMC free article] [PubMed]
- 112.•.Brandão PRP, Grippe TC, Pereira DA, Munhoz RP, Cardoso F. New-onset movement disorders associated with covid-19. Tremor and Other Hyperkinetic Movements. 2021;11(1). 10.5334/tohm.595This early review reports that although prevalence of movement disorders is low, myoclonus is the most associated new-onset movement disorder with COVID-19 infection. [DOI] [PMC free article] [PubMed]
- 113.Salari M, Zaker Harofteh B, Etemadifar M, Sedaghat N, Nouri H. Movement disorders associated with COVID-19. Parkinson’s Dis. 2021;2021. 10.1155/2021/3227753. [DOI] [PMC free article] [PubMed]
- 114.Schneider SA, Hennig A, Martino D. Relationship between COVID-19 and movement disorders: a narrative review. Eur J Neurol. 2022;29(4). 10.1111/ene.15217. [DOI] [PubMed]
- 115.Jacob PC, Chand RP. Posttraumatic rubral tremor responsive to clonazepam. Mov Disord. 1998;13(6). 10.1002/mds.870130622. [DOI] [PubMed]
- 116.Harmon RL, Long DF, Shirtz J. Treatment of post-traumatic midbrain resting-kinetic tremor with combined levodopa/carbidopa and carbamazepine. Brain Injury. 1991;5(2). 10.3109/02699059109008092. [DOI] [PubMed]
- 117.Ellison PH. Propranolol for severe post-head injury action tremor. Neurology. 1978;28(2). 10.1212/wnl.28.2.197. [DOI] [PubMed]
- 118.Anandan C, Jankovic J. Botulinum toxin in movement disorders: an update. Toxins (Basel). 2021;13(1). 10.3390/toxins13010042. [DOI] [PMC free article] [PubMed]
- 119.Budhram A, Lipson D, Nesathurai S, Harvey D, Rathbone MP. Postanoxic myoclonus: two case presentations and review of medical management. Arch Phys Med Rehabil. 2014;95(3). 10.1016/j.apmr.2013.09.008. [DOI] [PubMed]
- 120.Fahn S. Posthypoxic action myoclonus: review of the literature and report of two new cases with response to valproate and estrogen. Adv Neurol. 1979;26. [PubMed]
- 121.Frucht SJ, Louis ED, Chuang C, Fahn S. A pilot tolerability and efficacy study of Levetiracetam in patients with chronic myoclonus. Neurology. 2001;57(6). 10.1212/WNL.57.6.1112. [DOI] [PubMed]
- 122.Bellows S, Jankovic J. Treatment of dystonia and tics. Clin Parkinsonism Relat Disord. 2020;2. 10.1016/j.prdoa.2019.11.005. [DOI] [PMC free article] [PubMed]
- 123.Ramirez-Castaneda J, Jankovic J. Long-term efficacy and safety of botulinum toxin injections in dystonia. Toxins (Basel). 2013;5(2). 10.3390/toxins5020249. [DOI] [PMC free article] [PubMed]
- 124.Kivi A, Trottenberg T, Kupsch A, Plotkin M, Felix R, Niehaus L. Levodopa-responsive posttraumatic Parkinsonism is not associated with changes of echogenicity of the substantia nigra [1]. Mov Disord. 2005;20(2). 10.1002/mds.20323. [DOI] [PubMed]
- 125.Bullard DE, Nashold BS. Stereotaxic thalamotomy for treatment of posttraumatic movement disorders. J Neurosurg. 1984;61(2). 10.3171/jns.1984.61.2.0316. [DOI] [PubMed]
- 126.Fox JL, Kurtzke JF. Trauma-induced intention tremor relieved by stereotaxic thalamotomy. Arch Neurol. 1966;15(3). 10.1001/archneur.1966.00470150025005. [DOI] [PubMed]
- 127.Andrew J, Fowler CJ, Harrison MJG. Tremor after head injury and its treatment by stereotaxic surgery. J Neurol Neurosurg Psychiatry. 1982;45(9). 10.1136/jnnp.45.9.815. [DOI] [PMC free article] [PubMed]
- 128.Cardoso F, Jankovic J, Grossman RG, Hamilton WJ. Outcome after stereotactic thalamotomy for dystonia and hemiballismus. Neurosurgery. 1995;36(3). 10.1227/00006123-199503000-00009. [DOI] [PubMed]
- 129.Alkhani A, Khan F, Lang AE, Hutchison WD, Dostrovsky J. 716 The response to pallidal surgery for dystonia is dependent on the etiology. Neurosurgery. 2000;47(2). 10.1097/00006123-200008000-00064.
- 130.Sitsapesan HA, Holland P, Oliphant Z, et al. Deep brain stimulation for tremor resulting from acquired brain injury. J Neurol Neurosurg Psychiatry. 2014;85(7). 10.1136/jnnp-2013-305340. [DOI] [PubMed]
- 131.Rojas-Medina LM, Esteban-Fernández L, Rodríguez-Berrocal V, del Álamo De Pedro M, Ley Urzaiz L, Bailly-Baillere IR. Deep brain stimulation in posttraumatic tremor: a series of cases and literature review. Stereotactic Funct Neurosurg. 2017;94(6). 10.1159/000448078. [DOI] [PubMed]
- 132.Umemura A, Samadani U, Jaggi JL, Hurtig HI, Baltuch GH. Thalamic deep brain stimulation for posttraumatic action tremor. Clin Neurol Neurosurg. 2004;106(4). 10.1016/j.clineuro.2003.12.004. [DOI] [PubMed]
- 133.Benabid AL, Pollak P, Gao D, et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996;84(2). 10.3171/jns.1996.84.2.0203. [DOI] [PubMed]
- 134.Broggi G, Brock S, Franzini A, Geminiani G. A case of posttraumatic tremor treated by chronic stimulation of the thalamus. Mov Disord. 1993;8(2). 10.1002/mds.870080217. [DOI] [PubMed]
- 135.Loher TJ, Capelle HH, Kaelin-Lang A, et al. Deep brain stimulation for dystonia: outcome at long-term follow-up. J Neurol. 2008;255(6). 10.1007/s00415-008-0798-6. [DOI] [PubMed]
- 136.Sellal F, Hirsch E, Barth P, Blond S, Marescaux C. A case of symptomatic hemidystonia improved by ventroposterolateral thalamic electrostimulation. Mov Disord. 1993;8(4). 10.1002/mds.870080418. [DOI] [PubMed]
- 137.Loher TJ, Hasdemir MG, Burgunder JM, Krauss JK. Long-term follow-up study of chronic globus pallidus internus stimulation for posttraumatic hemidystonia: Case report. J Neurosurg. 2000;92(3). 10.3171/jns.2000.92.3.0457. [DOI] [PubMed]
- 138.Ghika J, Villemure JG, Miklossy J, et al. Postanoxic generalized dystonia improved by bilateral Voa thalamic deep brain stimulation. Neurology. 2002;58(2). 10.1212/WNL.58.2.311. [DOI] [PubMed]
- 139.Asahi T, Kashiwazaki D, Dougu N, et al. Alleviation of myoclonus after bilateral pallidal deep brain stimulation for Lance–Adams syndrome. J Neurol. 2015;262(6). 10.1007/s00415-015-7748-x. [DOI] [PubMed]
- 140.Yamada K, Sakurama T, Soyama N, Kuratsu JI. GPi Pallidal stimulation for Lance-Adams syndrome. Neurology. 2011;76(14). 10.1212/WNL.0b013e31821482f4. [DOI] [PubMed]
- 141.Reese R, Herzog J, Falk D, et al. Successful deep brain stimulation in a case of posttraumatic tremor and hemiparkinsonism. Mov Disord. 2011;26(10). 10.1002/mds.23686. [DOI] [PubMed]
- 142.Francisco GE. Successful treatment of posttraumatic hemiballismus with intrathecal baclofen therapy. Am J Phys Med Rehabil. 2006;85(9). 10.1097/01.phm.0000233173.32432.6f. [DOI] [PubMed]
- 143.Meythaler JM, Guin-Renfroe S, Grabb P, Hadley MN. Long-term continuously infused intrathecal baclofen for spastic-dystonic hypertonia in traumatic brain injury: 1-year experience. Arch Phys Med Rehabil. 1999;80(1). 10.1016/S0003-9993(99)90301-5. [DOI] [PubMed]
- 144.Walker RH, Danisi FO, Swope DM, Goodman RR, Germano IM, Brin MF. Intrathecal baclofen for dystonia: benefits and complications during six years of experience. Mov Disord. 2000;15(6). 10.1002/1531-8257(200011)15:6<1242::AID-MDS1028>3.0.CO;2-Z. [DOI] [PubMed]
- 145.Meythaler JM, DeVivo MJ, Hadley M. Prospective study on the use of bolus intrathecal baclofen for spastic hypertonia due to acquired brain injury. Arch Phys Med Rehabil. 1996;77(5). 10.1016/S0003-9993(96)90034-9. [DOI] [PubMed]
- 146.Birthi P, Walters C, Vargas OO, Karandikar N. The use of intrathecal baclofen therapy for myoclonus in a patient with Lance Adams syndrome. PM&R. 2011;3(7). 10.1016/j.pmrj.2010.12.023. [DOI] [PubMed]
- 147.Whitlock JA, Dumigan RW. Treatment of postanoxic action myoclonus with intrathecal baclofen: a case report. PM&R. 2018;10(8). 10.1016/j.pmrj.2017.12.010. [DOI] [PubMed]
- 148.Bilney B, ME M, Denisenko S. Physiotherapy for people with movement disorders arising from basal ganglia dysfunction. N Z J Physiother. 2003;31(2).
- 149.Rochester L, Lord S, Morris ME. The role of physiotherapy in the rehabilitation of people with movement disorders. Rehabil Mov Disord Published online. 2011 doi: 10.1017/CBO9781139012942.007. [DOI] [Google Scholar]
- 150.Gregoric M, Stefanovska A, Vodovnik L, Rebersek S, Gros N. Rigidity in parkinsonism: characteristics and influences of passive exercise and electrical nerve stimulation. Funct Neurol. 1988;3(1). [PubMed]
- 151.Rocon E, Belda-Lois JM, Ruiz AF, Manto M, Moreno JC, Pons JL. Design and validation of a rehabilitation robotic exoskeleton for tremor assessment and suppression. IEEE Trans Neural Syst Rehabil Eng. 2007;15(3). 10.1109/TNSRE.2007.903917. [DOI] [PubMed]