Abstract
Fatty acid desaturation catalyzed by fatty acid desaturases requires molecular oxygen (O2). Saccharomyces cerevisiae cells derepress expression of OLE1 encoding Δ9 fatty acid desaturase under hypoxic conditions to allow more-efficient use of limited O2. It has been proposed that aerobic conditions lead to repression of OLE1 by well-established O2-responsive repressor Rox1p, since putative binding sequences for Rox1p are present in the promoter of OLE1. However, we revealed in this study that disruption of ROX1 unexpectedly did not affect the O2 repression of OLE1, indicating that a Rox1p-independent novel mechanism operates for this repression. We identified by promoter deletion analysis the 50-bp O2-regulated (O2R) element in the OLE1 promoter approximately 360 bp upstream of the start codon. Site-directed mutagenesis of the O2R element showed that the putative binding motif (5′-GATAA-3′) for the GATA family of transcriptional factors is important for O2 repression. Anaerobic derepression of OLE1 transcription was repressed by unsaturated fatty acids (UFAs), and interestingly the O2R element was responsible for this UFA repression despite not being included within the fatty acid-regulated (FAR) element previously reported. The fact that such a short 50-bp O2R element responds to both O2 and UFA signals implies that O2 and UFA signals merge in the ultimate step of the pathways. We discuss the differential roles of FAR and O2R elements in the transcriptional regulation of OLE1.
The lipid composition of cellular membranes is regulated to maintain membrane fluidity (22). A key enzyme involved in this process is the membrane-bound Δ9 fatty acid desaturase, which catalyzes the introduction of the initial double bond between the 9th and 10th carbons of palmitoyl-coenzyme A (CoA) and stearoyl-CoA (35). The correct ratio of saturated to monounsaturated fatty acids contributes to membrane fluidity. Alterations of this ratio have been implicated in various diseases including cardiovascular diseases, obesity, non-insulin-dependent diabetes mellitus, hypertension, neurological diseases, immune disorders, and cancer in mammals (35). In yeast, this ratio has been suggested to be related to the heat shock response, ethanol tolerance, and mitochondrial movement and inheritance (1, 8, 45). The regulation of the expression of Δ9 fatty acid desaturase is, therefore, of considerable physiological importance.
In Saccharomyces cerevisiae, Δ9 fatty acid desaturase is encoded by OLE1 (46). The steady-state level of OLE1 mRNA is regulated at the level of transcription and by mRNA stability, and both regulatory processes are affected by the presence of unsaturated fatty acids (UFAs) in the growth medium (6, 10, 20). Addition of exogenous UFA represses the transcription of OLE1 and promotes the decay of OLE1 mRNA. A fatty acid-regulated (FAR) element which is essential for UFA repression of OLE1 transcription under aerobic conditions has been identified by promoter deletion analysis (10). Simultaneous disruption of the two long-chain (C14 to C18) fatty acyl-CoA synthetase genes, FAA1 and FAA4, blocks incorporation of long-chain saturated fatty acids and UFAs such as oleic acid into cells and does not cause UFA repression of OLE1 transcription (9, 10). A putative fatty acid transport protein (FATP) with 33% homology to an adipocyte FATP (42) was identified (15). However, more-recent reports indicate that this protein is an acyl-CoA synthetase specific for very-long-chain fatty acids rather than a plasma membrane fatty acid transporter (9, 52). Therefore, a fatty acid transport protein and UFA signal transducers and responding transcriptional regulators have not yet been identified.
Since the desaturation reaction requires molecular oxygen (O2) as an electron acceptor (35), it has been proposed that cells derepress expression of OLE1 under hypoxic conditions to allow more-efficient use of limiting O2 (53). Well-established O2-responsive repressor Rox1p is believed to repress OLE1 transcription under aerobic conditions because putative Rox1p-binding sequences are present in the OLE1 promoter (53). However, there had been no conclusive evidence that OLE1 transcription is indeed repressed by O2 until the recent report by Kwast et al. (26), who showed that OLE1 transcription was induced when cells were shifted to anaerobic conditions.
In this report, we show that O2 repression of OLE1 is mediated by a Rox1p-independent novel mechanism. Furthermore, we newly identified a 50-bp region essential for O2 repression, designated the O2R element, and this element was also responsible for UFA repression of anaerobically derepressed OLE1 transcription despite not being included within the FAR element. Therefore, there appears to be a close connection between O2 and UFA signals that act on the short 50-bp element in the OLE1 promoter.
MATERIALS AND METHODS
Microorganisms and media.
The S. cerevisiae strains used in this work are listed in Table 1. Escherichia coli strains TG1 (40) and DH5α (40) were used as hosts for the propagation and manipulation of plasmid DNA. Yeast cells were grown in nutrient medium (YPDA; i.e., nutrient high-Pi medium) (38) or YPDA supplemented with 1 mM oleic acid (Wako Chemicals, Osaka, Japan) and 1% (vol/vol) Triton X-100 (Wako Chemicals) (16). The Luria-Bertani medium for E. coli was as described previously (40).
TABLE 1.
S. cerevisiae strains used
| Strain | Genotypea | Source |
|---|---|---|
| SH4041 | MATa ura3 trp1 leu2-3,112 his3Δ pho3-1 pho5-1 | Our laboratory |
| SH5128 | MATa ura3 trp1 leu2-3,112 his3Δ pho3-1 pho5-1 rox1::LEU2 | This study |
| SH5420 | MATa ura3::[URA3 OLE1p-PHO5] trp1 leu2-3,112 his3Δ pho3-1 pho5-1 | This study |
| SH5421 | MATa ura3::[URA3 OLE1p-PHO5] trp1 leu2-3,112 his3Δ pho3-1 pho5-1 rox1::LEU2 | This study |
| SH5143 | MATα ura3-52 leu2-3,112 his4-519 trp1 pho3-1 pho5-1 canr | Our laboratory |
| SH5141 | MATα ura3-52::[URA3 OLE1p-PHO5] trp1 leu2-3,112 his4-519 pho3-1 pho5-1 canr | This study |
ura3::[URA3 OLE1p-PHO5] and ura3-52::[URA3 OLE1p-PHO5] represent the integration of the OLE1p-PHO5 fusion reporter gene into the ura3 locus using the plasmid p1166 as described in Materials and Methods. Other symbols are as described previously (32).
Disruption of ROX1.
A 1,109-bp fragment of ROX1 (nucleotides [nt] −2 to +1107) was amplified by PCR using chromosomal DNA of S. cerevisiae strain S288C (33) as a template and oligonucleotides 5′-GCGGATCCCAATGAATCCTAAATCCTCTACAC-3′ and 5′-CGCTCGAGTCATTTCGGAGAAACTAGGC-3′, corresponding to the nt −2 to +22 and +1107 to +1088 of ROX1, as forward and reverse primers, respectively. The PCR product was doubly digested with BamHI and XhoI and cloned into the BamHI-XhoI gap of pRS305 (44) to obtain plasmid p1513. A 1,148-bp fragment of ROX1 (nt −1121 to +27) was amplified by PCR using chromosomal DNA of S288C as a template and oligonucleotides 5′-CCAAGCTTCCATTGAGAAGGACAACATT-3′ and 5′-CTGGATCCTTAGGTGTAGAGGATTTAGG-3′, corresponding to the nt −1121 to −1102 and +27 to +7 of ROX1, as forward and reverse primers, respectively. The PCR product was doubly digested with HindIII and BamHI and cloned into the HindIII-BamHI gap of pUC18 (40) to obtain plasmid p1514. A 2.9-kb BamHI-ScaI fragment from p1513 and a 2.1-kb BamHI-ScaI fragment from p1514 were ligated to obtain plasmid p1515. A 525-bp BamHI-BglII fragment of p1515 containing the ROX1 open reading frame (ORF), corresponding to nt +28 to +523, was replaced with a 1.7-kb BamHI fragment containing LEU2 from YDp-L (4) to obtain plasmid p1516. The rox1::LEU2 disruptant, SH5128, was constructed by transformation of SH4041 with HindIII- and XhoI-digested plasmid p1516. The rox1::LEU2 disruption was verified by Southern analysis.
OLE1p-PHO5 fusions.
Construction of plasmid p1166, which contains a 935-bp fragment upstream of OLE1 fused with the structural region of PHO5 encoding repressible acid phosphatase (rAPase; EC 3.1.3.2), was described previously (16). The activation and repression assay vector pRAV, containing the UASPHO84E-PHO84p-PHO5 reporter gene, was constructed previously (31). The UASPHO84E-PHO84p-PHO5 reporter gene consists of a 272-bp fragment upstream of PHO84 containing Pho4p binding site E fused with the PHO5 ORF. Plasmid p1785 (see Fig. 2), which has the −934 to −586 region of OLE1 (taking base A of the ATG start codon as +1) upstream of the UASPHO84E-PHO84p-PHO5 reporter gene was constructed as follows. A 349-bp region of OLE1 was amplified by PCR using p1166 as a template and oligonucleotides 5′-CTCGAATTCAGCTTTTCGTTTGCAGGTTT-3′ and 5′-CTCAAGCTTAGTTAGTTTTTGGGCCACCG-3′, corresponding to the nt −934 to −915 and −586 to −605 of OLE1, as the forward and reverse primers, respectively. The PCR product was doubly digested with EcoRI and HindIII and cloned into the EcoRI-HindIII gap of pRAV to obtain plasmid p1785. Plasmids p1787, p1781, p1783, p1860, p1862, and p1864, which each contain nt −585 to −456 (the FAR element), −455 to −307, −306 to −159, −455 to −406, −405 to −357, and −356 to −307 of OLE1, respectively, were constructed in a similar way to plasmid p1785 by inserting EcoRI-HindIII fragments containing each region amplified by PCR into the EcoRI-HindIII gap of pRAV. Plasmid p1872, which carries OLE1p lacking the O2R element (OLE1pΔO2R) fused with the structural region of PHO5 was constructed as follows. The −306 to −1 region of OLE1 was amplified by PCR using p1166 as a template and oligonucleotides 5′-CTCAAGCTTTTCTACGAGTCTTGCTCACT-3′ and 5′-GCGGATCCTTTGTTGTAATGTTTTAG-3′, corresponding to the nt −306 to −287 and −1 to −19 of OLE1, as the forward and reverse primers, respectively. The PCR product was doubly digested with HindIII and BamHI and cloned into the HindIII-BamHI gap of pSH39 (34) to obtain plasmid p1870. The −934 to −357 region of OLE1 was amplified by PCR using p1166 as a template and oligonucleotides 5′-CTCGAATTCAGCTTTTCGTTTGCAGGTTT-3′ and 5′-CTCAAGCTTAAAGAAAGCTGCCGACTATG-3′, corresponding to nt −934 to −915 and −357 to −376 of OLE1, as the forward and reverse primers, respectively. The PCR product was doubly digested with EcoRI and HindIII and cloned into the EcoRI-HindIII gap of p1870 to obtain plasmid p1872. Plasmid p1904, in which the 5′-GATAA-3′ sequence in the O2R element is changed to 5′-ACGCC-3′ by site-directed mutagenesis, was constructed as follows. Oligonucleotides 5′-AATTCCGGACGTTGAAACACTCAACAAACCGGCGTTAGTGCCCAACCAGGTGTGCA-3′ and 5′-AGCTT GCACACCTGGTTGGGCACTAACGCCGGTTTGTTGAGTGTTTCAACG TCCGG-3′, corresponding to nt −356 to −307 and −307 to −356 of OLE1, respectively, in which the 5′-GATAA-3′ sequence (−331 to −327) is changed to 5′-ACGCC-3′ (underlined) were annealed and cloned into the EcoRI-HindIII gap of pRAV to obtain plasmid p1904. Plasmid p1858, which contains a 567-bp fragment upstream of ANB1 (27) fused with the PHO5 ORF, was constructed as follows. A 567-bp fragment of ANB1 (nt −567 to −1) was amplified by PCR using chromosomal DNA of S288C as a template and oligonucleotides 5′-CTCAAGCTTCCGGGAATTTTAGATTCAGG-3′ and 5′-CTCGGATCCGTTTTAGTGTGTGAATGAAA-3′, corresponding to nt −567 to −548 and −1 to −20 of ANB1, as the forward and reverse primers, respectively. The PCR product was doubly digested with HindIII and BamHI and cloned into the HindIII-BamHI gap of pSH39 to obtain plasmid p1858. All constructs were analyzed by sequencing the respective promoter regions. The resulting plasmids were digested with StuI and integrated into the URA3 locus of SH5143, SH4041, or SH5128 by transformation. Single-copy integration was confirmed by Southern analysis of genomic DNA digested with HindIII from the respective transformants.
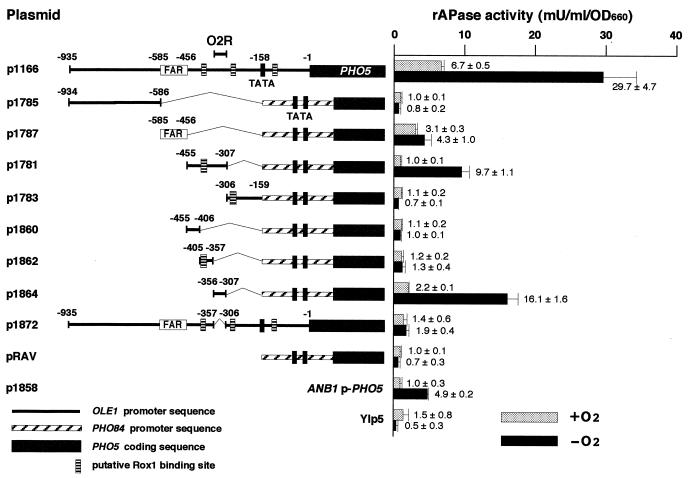
FIG. 2.
The O2R element is present in the −356 to −307 region of the OLE1 promoter. rAPase activity in cells of the wild-type strain (SH5143) harboring the respective reporter genes cultivated aerobically (+O2) or anaerobically (−O2) in YPDA medium was measured as described previously (48). Numbers indicate the position relative to the first nucleotide of the initiation codon (+1) of OLE1. PHO84 promoter sequences (nt −272 to −1) contain binding site E (nt −262 to −257) for the transactivator Pho4p and two putative TATA boxes (nt −122 and −99). Shaded boxes, structural regions of PHO5. The ANB1p-PHO5 reporter gene was used to verify anaerobic conditions. Error bars, standard deviations determined from a minimum of three independent measurements. The actual values of rAPase activity with their standard deviations are indicated. Other symbols are the same as those described for Fig. 1B.
Northern blot analysis.
The preparation of RNA and Northern blot hybridization were performed as described previously (38). Cells were cultivated in 10 ml of YPDA medium to stationary phase at 30°C with vigorous shaking. The stationary-phase culture was inoculated into 30 ml of YPDA or YPDA containing oleic acid in a 100-ml Erlenmeyer flask to give an optical density at 660 nm (OD660) of 0.1. The cultures were shaken at 30°C for aerobic growth. For anaerobic growth, the cultures were sealed with rubber stoppers and bubbled with pure nitrogen gas for 2 min to purge O2 after inoculation or sampling, and shaken at 30°C. Anaerobic conditions were verified based on confirming the transcription of ANB1 (28), a well-known hypoxic gene. Total RNAs were prepared from the cells harvested in the logarithmic growth phase (OD660 = 0.7 to 1.6). DNA fragments containing the OLE1 ORF (nt −9 to +1905 relative to ATG), the PHO5 ORF (nt −18 to +2116), and the ANB1 ORF (nt +1 to +455), amplified by PCR, and the 1.0-kb HindIII-XhoI fragment carrying ACT1 prepared from pYA301 (19) were labeled with 32P as described previously (39) to generate DNA probes.
rAPase assay.
Cells were cultivated in 10 ml of YPDA medium to stationary phase at 30°C with vigorous shaking. The stationary-phase culture (0.2 ml) was inoculated into 10 ml of YPDA medium or YPDA medium containing oleic acid. The cultures were shaken for 5 h at 30°C for aerobic growth or left to stand for 24 h at 30°C in a sealed Anaero Pack (Mitsubishi Gas Chemical Co., Tokyo, Japan) for anaerobic growth, and then rAPase activities were measured as described previously (48). The rAPase activities presented are the averages of at least three independent experiments.
Genetic and biochemical methods.
S. cerevisiae and E. coli cells were transformed as described by Ito et al. (24) and Sambrook et al. (40), respectively. Yeast chromosomal DNA was prepared as described previously (23). Southern blot analysis and other DNA manipulations were performed using standard methods (40). Bacterial plasmid DNA was isolated by the alkaline lysis method (40). Nucleotide sequences were determined by the dideoxy chain termination method (41).
RESULTS AND DISCUSSION
Transcription of OLE1 is repressed by O2 in a Rox1p-independent manner.
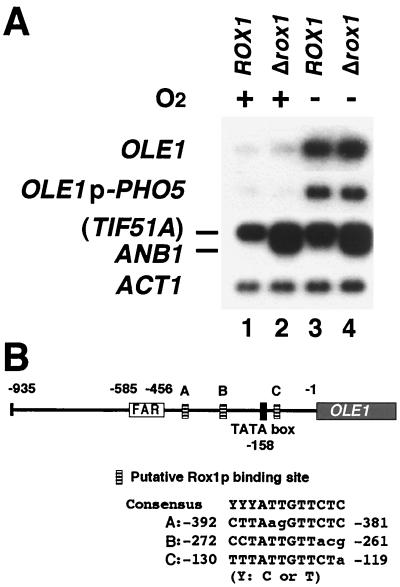
To clarify whether OLE1 transcription is indeed repressed by O2, we investigated OLE1 transcription in cells cultivated aerobically or anaerobically. Northern blot analysis (Fig. 1A) showed that the levels of transcripts of OLE1 and OLE1p-PHO5 fusion gene are significantly increased under anaerobic conditions, compared to the levels under aerobic conditions, in the wild-type strain (Fig. 1A, lanes 1 and 3), indicating that OLE1 transcription is repressed by O2. The same observation that OLE1 transcription is induced when cells are shifted to anaerobic conditions has been reported recently (26). In S. cerevisiae, a Rox1p-dependent mechanism is known to mediate transcriptional repression of various hypoxic genes by O2 (53). Since the biosynthesis of heme requires O2, heme accumulates under aerobic conditions and binds to Hap1p. Hap1p with bound heme acts as a transcriptional activator to activate ROX1 transcription. Rox1p binds to its recognition sites, 5′-YYYATTGTTCTC-3′ (where Y represents pyrimidine) (3), upstream of anaerobically expressed genes such as ANB1 and forms a complex with the general repressors Tup1p and Ssn6p to repress target genes under aerobic conditions (53). As three putative binding sites for Rox1p are present in the OLE1 promoter (Fig. 1B), we disrupted ROX1 to determine whether OLE1 repression requires Rox1p. In the rox1 disruptant, ANB1 transcription was, as reported previously (53), derepressed under aerobic conditions (Fig. 1A, lane 2). However, OLE1 transcription was unexpectedly not derepressed (Fig. 1A, lane 2), indicating that Rox1p is not involved in the O2 repression of OLE1. Although there are many hypoxic genes which have consensus binding sequences for Rox1p in their promoters (53), for some of them the function of these sequences has not been experimentally determined by, for example, deletion or mutation analysis of the consensus sequence or disruption of ROX1. Our results for OLE1 demonstrate that one must not decide on the operating mechanism only by the presence of a consensus sequence. What is the mechanism of O2 repression of OLE1? Recently, another group has shown that the respiratory chain is involved in the anaerobic induction of OLE1 transcription and that cytochrome c oxidase is likely the hemoprotein sensor for O2 (26). However, nothing is known about the signal transduction pathway downstream of the O2 sensor. Identification of O2-responsive transcriptional factors involved in Rox1p-independent O2 repression should make it possible to elucidate the repression mechanism.
FIG. 1.
Transcription of OLE1 is repressed by O2 in a Rox1p-independent manner. (A) Northern analysis of the OLE1, OLE1p-PHO5, and ANB1 transcripts in rox1 disruptants. Total RNA samples were prepared from cells of the wild-type strain (SH5420) and rox1 disruptant (SH5421) cultivated aerobically (lanes 1 and 2) or anaerobically (lanes 3 and 4) in YPDA medium. Equal amounts of RNA (5 μg) were electrophoresed in a 1.5% agarose gel in the presence of formaldehyde, transferred to a nylon filter, blotted, and hybridized with probes consisting of 32P-labeled DNA fragments containing OLE1, ANB1, and PHO5 for detection of the transcripts of OLE1p-PHO5 or ACT1 as an internal control. The ANB1 probe also hybridized to the TIF51A transcript, which is not repressed by Rox1p (53). (B) Putative Rox1p binding sites in the OLE1 promoter. Numbers indicate positions relative to the first nucleotide of the initiation codon (+1). Lowercase letters, nucleotides that differ from the consensus Rox1p-binding sequence (3).
The 50-bp O2R element is sufficient and essential for O2 repression of OLE1 transcription.
To elucidate the Rox1p-independent O2 repression mechanism of OLE1, we first identified the O2 signal-responsive region in the OLE1 promoter. The OLE1 promoter was divided into three subregions, i.e., the region harboring the FAR element and the regions upstream and downstream of this region, and these regions were inserted into the region upstream of the PHO84p-PHO5 reporter gene in the activation and repression assay vector pRAV (31). The transcription of the PHO84p-PHO5 reporter gene is repressed when cells are cultivated under conditions whereby a sufficient amount of Pi is used, such as YPDA medium (31). The levels of expression of these reporter genes were measured as rAPase activities under aerobic and anaerobic conditions (Fig. 2). Control experiments with the ANB1p-PHO5 reporter gene (p1858) validated this reporter assay system to monitor O2-mediated transcriptional repression. The rAPase activity from p1166 was derepressed 4.4-fold under anaerobic conditions compared with aerobic conditions. Although p1787, which contains a FAR element, was not as responsive to anaerobic conditions (rAPase activity was derepressed 1.4-fold), p1781, containing the downstream region of the FAR element, responded significantly (rAPase activity was derepressed 9.7-fold). In order to delineate the O2-regulated element in the 149-bp OLE1p region of p1781, we further divided this region into three subregions and determined which region responds to anaerobic conditions. Only p1864 showed significant derepression of rAPase activity (7.3-fold) under anaerobic conditions, indicating that the 50-bp OLE1p region (nt −356 to −307) is sufficient for anaerobic derepression. Deletion of the 50-bp region from OLE1p (p1872) decreased the level of anaerobic derepression from 4.4- to 1.4-fold, indicating that the 50-bp region is essential for anaerobic derepression of OLE1 transcription. We therefore designated the 50-bp region as an O2-regulated element (O2R). That the O2R element does not contain consensus Rox1p-binding sites is consistent with a Rox1p-independent mechanism for O2 repression of OLE1 transcription (Fig. 2). These results indicate that the O2R element is an anaerobic upstream activation site and further imply that some anaerobically responsive activators acting on the element exist. Thus, the system for O2 repression of OLE1 transcription appears to be similar to the mammalian HIF-1 hypoxia-sensing system, whose regulation is mediated by transcriptional activator HIF-1 (51). On the other hand, the Rox1p-dependent O2 repression system is not parallel to the HIF-1 hypoxia-sensing system because it is mediated by transcriptional repressor Rox1p, which binds to the upstream repression site of O2-repressed genes (53). It is noted that a homologue of HIF-1 is not found in S. cerevisiae (21) and that the O2R element does not contain the consensus core sequence, 5′-RCGTG-3′, of HIF-1 binding sites (43).
5′-GATAA-3′ sequence in the O2R element is important for O2 repression.
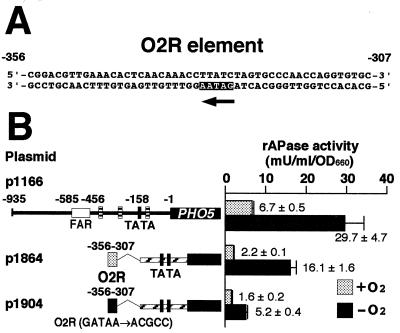
What is the transcriptional activator acting on the O2R element? We searched for binding motifs of already-known transcriptional regulators on the O2R element and found the 5′-GATAA-3′ sequence, which is the binding site for GATA family transcriptional activation factors Gln3p and probably Gat1p (Fig. 3A) (5, 13). Although the 5′-GATAA-3′ sequence is on the complementary strand of the O2R element, it is known that the 5′-GATAA-3′ sequence functions in an orientation-independent manner (12). The GATA family of DNA-binding proteins are present in organisms from S. cerevisiae to humans (29, 30). These binding proteins contain one or more characteristic C4 zinc finger motifs and bind to DNA sequences with sequence GATA at their cores (11). In vertebrates, six GATA factors, GATA-1, GATA-2, GATA-3, GATA-GT1, GATA-GT2, and GATA-5, have been identified and are involved in regulation of various genes such as those encoding globins, erythropoietin receptors, preproendothelin-1, T-cell receptors, and the gastric proton pump (29). To determine whether the 5′-GATAA-3′ sequence on the O2R element functions in O2 repression, we mutated the 5′-GATAA-3′ sequence to 5′-ACGCC-3′. As shown in Fig. 3B, this mutation resulted in a decrease in the level of anaerobic derepression from 7.3- to 3.3-fold, indicating that the 5′-GATAA-3′ sequence is important in O2 repression. Since it is known that there are only four GATA factors, i.e., transcriptional activators Gln3p and Gat1p and transcriptional repressors Dal80p and Deh1p, in S. cerevisiae (11), Gln3p and Gat1p could be candidates for the transcriptional activator acting on the O2R element. These four proteins respond to the nitrogen source signal to regulate nitrogen catabolite-repressed genes (11) but have not been reported to be involved in transcriptional regulation by O2 or UFAs. However, in vertebrates, GATA-1 has been demonstrated to play a major role in the regulation of various specific erythroid genes, for example, genes encoding globins and heme biosynthetic enzymes involved in terminal erythroid differentiation in vivo (14, 36, 37, 47, 49, 50). This finding appears to imply a relationship between GATA factors and O2 signals.
FIG. 3.
(A) The O2R element contains a 5′-GATAA-3′ sequence (arrow) on the complementary strand. (B) The 5′-GATAA-3′ sequence plays an important role in O2 repression. Dotted box, O2R element (nt −356 to −307); solid box, O2R in which the 5′-GATAA-3′ sequence is changed to 5′-ACGCC-3′ by site-directed mutagenesis. The conditions used for measurement of rAPase activity in cells of the wild-type strain (SH5143) harboring the respective reporter genes and the symbols employed are as described in the legend to Fig. 2.
In S. cerevisiae, transcription of ATF1, encoding alcohol acetyltransferase, is also repressed by O2 and UFA (18). Fujiwara et al. identified a 51-bp region (nt −150 to −100) responsible for O2 repression and an 18-bp region (nt −85 to −68) responsible for UFA repression of ATF1 (17). The O2-responsive 51-bp region contains a Rox1p binding site, and O2 repression was partially abolished in the rox1 null mutant (17). There is no homology between the O2R element and the 51-bp region of ATF1, and the 51-bp region does not contain the 5′-GATAA-3′ sequence. From these facts, we conclude that the O2 repression mechanism of OLE1 transcription is different from that of ATF1 transcription.
O2 and UFA signals act on the O2R element.
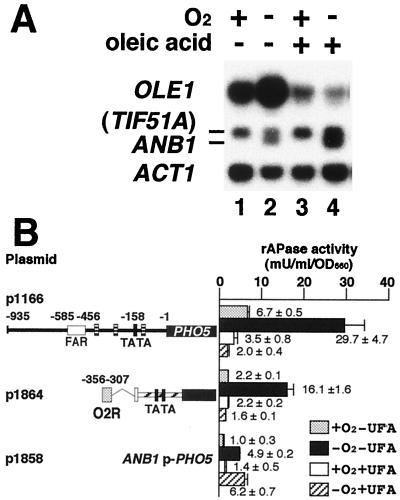
To further clarify the relationship between O2 and UFA repression mechanisms, we investigated the effect of UFAs on anaerobic derepression of OLE1 transcription. Northern blot analysis showed that anaerobic derepression of OLE1 transcription (Fig. 4A, lanes 1 and 2) did not occur in the presence of UFA (Fig. 4A, lanes 3 and 4). This suggests that UFA repression is epistatic to anaerobic derepression. From a physiological viewpoint, this result is reasonable because, if there is a sufficient amount of UFA in the medium, cells do not have to produce UFA even under anaerobic conditions.
FIG. 4.
(A) Anaerobic derepression of OLE1 transcription is repressed by oleic acid. Cells of the wild-type strain (SH5141) were inoculated into YPDA medium (lanes 1 and 2) or YPDA medium containing 1 mM oleic acid (lanes 3 and 4) and cultivated at 30°C aerobically (lanes 1 and 3) or anaerobically (lanes 2 and 4). Total RNA was prepared from cells in the logarithmic growth phase. The RNA samples (each 10 μg of RNA) were subjected to Northern blot hybridization as described in the legend to Fig. 1A. (B) The O2R element is responsible for repression by UFA. Cells of SH5143 (wild type) harboring one copy of the respective reporter genes integrated at the ura3 locus were cultivated aerobically without oleic acid (dotted bars), anaerobically without oleic acid (solid bars), aerobically with oleic acid (open bars), or anaerobically with oleic acid (hatched bars) and subjected to an rAPase assay as described in the legend to Fig. 2.
However, these results are different from those of Kwast et al. (26). They reported that OLE1 transcription is derepressed when cells are shifted to anaerobic conditions even in the presence of UFAs. We think that this difference may be due to the source or concentration of UFA because we used free oleic acid at a concentration of 1 mM while they used Tween 80, which is an oleic acid ester, at a concentration of 0.1% (vol/vol) (approximately 0.6 mM). Our experimental conditions might be more highly repressive than theirs. If an excess amount of UFA is incorporated by cells under their experimental conditions, the anaerobic derepression may not occur, as was the case in our study.
As UFA repression of OLE1 transcription depends on the FAR element under aerobic conditions, the FAR element has been considered to be the sole element which responds to UFA signals in the OLE1 promoter (10). To determine whether UFA repression of OLE1 transcription under anaerobic conditions also depends on the FAR element, we examined the effect of UFAs on the O2R element only. Unexpectedly, despite not being included within the FAR element, the O2R element was responsible for this UFA repression (Fig. 4B). The fact that the short 50-bp O2R element responds to both O2 and UFA signals implies that the signal transduction pathways of O2 and UFA merge in the ultimate step of the pathways.
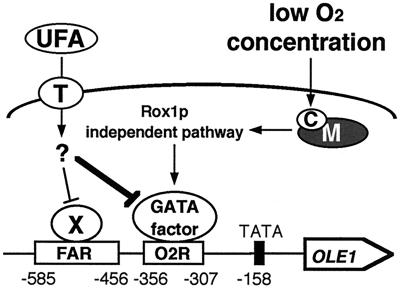
Our data together with those of other researchers (10) suggest that the FAR element plays a major role in the production of UFAs under aerobic conditions (Fig. 2; p1787), while the O2R element plays a major role under anaerobic conditions (Fig. 2; p1864) because it has a higher potential for transcriptional activation than the FAR element. Based on these findings we propose a model for OLE1 transcriptional regulation by O2 and UFA signals (Fig. 5). According to our model, under aerobic conditions in the absence of UFAs, OLE1 transcription is derepressed mainly by unknown transcriptional activators acting on the FAR element. Under anaerobic conditions in the absence of UFAs, a different transcriptional activator which acts on the newly identified O2R element plays a major role in further derepression of OLE1 transcription for the efficient use of limiting O2 by cells. The GATA factors Gln3p and Gat1p are candidates for the unknown activator. The anaerobic signal is transmitted in a Rox1p-independent manner. The mitochondrial respiratory chain is involved in the anaerobic induction of OLE1 transcription, and cytochrome c oxidase is likely the hemoprotein sensor for O2 (26). On the other hand, under aerobic conditions in the presence of UFAs, the UFAs are transported into cells by an unidentified transporter located at the cellular membrane and repress OLE1 transcription by inhibiting activators acting on the FAR element. Under anaerobic conditions in the presence of UFAs, the UFAs repress OLE1 transcription by inhibiting both FAR- and O2R-dependent transcriptional activators.
FIG. 5.
A model for signal transduction pathways of O2 and UFA regulating OLE1 transcription. X, unknown transcriptional activators acting on the FAR element; M and C, mitochondrial respiratory chain and cytochrome c oxidase, respectively; T, unknown fatty acid transporter located at the cellular membrane. Arrows and blunt arrows, positive and negative interactions, respectively. UFA repression is epistatic to anaerobic derepression (thick line). See Discussion for details.
In S. cerevisiae, the hypoxic genes fall into at least two classes (53). One class comprises single-copy genes and includes OLE1, ERG11, CPR1, HEM13, and SUT1. These genes encode enzymes expressed at low levels under aerobic conditions, but expression levels increase as oxygen becomes limiting. The second class represents gene pairs, where one gene is expressed under aerobic conditions while the other gene is expressed under hypoxic conditions. These gene pairs include COX5a/COX5b, CYC1/CYC7, AAC2/AAC3, and TIF51a/ANB1 (25, 53). The products of gene pairs COX5a/COX5b and CYC1/CYC7 have been shown to influence the maximal turnover number of holocytochrome c oxidase, with the hypoxic isoforms increasing this rate (2, 7). On the basis of these facts, we argue that cells regulate the expression of single-copy hypoxic genes by switching on regulatory elements of the genes, such as the FAR and O2R elements in the case of OLE1, instead of switching on the expression of the gene pairs responding to environmental O2 signals.
ACKNOWLEDGMENTS
We thank Y. Mukai of our laboratory for valuable comments.
This work was partially supported by a Grant-in-Aid for Scientific Research (no. 08456054) to S.H. from the Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
- 1.Alexandre H, Rousseaux I, Charpentier C. Relationship between ethanol tolerance, lipid composition and plasma membrane fluidity in Saccharomyces cerevisiae and Kloeckera apiculata. FEMS Microbiol Lett. 1994;124:17–22. doi: 10.1111/j.1574-6968.1994.tb07255.x. [DOI] [PubMed] [Google Scholar]
- 2.Allen L A, Zhao X J, Caughey W, Poyton R O. Isoforms of yeast cytochrome c oxidase subunit V affect the binuclear reaction center and alter the kinetics of interaction with the isoforms of yeast cytochrome c. J Biol Chem. 1995;270:110–118. doi: 10.1074/jbc.270.1.110. [DOI] [PubMed] [Google Scholar]
- 3.Balasubramanian B, Lowry C V, Zitomer R S. The Rox1 repressor of the Saccharomyces cerevisiae hypoxic genes is a specific DNA-binding protein with a high-mobility-group motif. Mol Cell Biol. 1993;13:6071–6078. doi: 10.1128/mcb.13.10.6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berben G, Dumont J, Gilliquet V, Bolle P A, Hilger F. The YDp plasmids: a uniform set of vectors bearing versatile gene disruption cassettes for Saccharomyces cerevisiae. Yeast. 1991;7:475–477. doi: 10.1002/yea.320070506. [DOI] [PubMed] [Google Scholar]
- 5.Blinder D, Magasanik B. Recognition of nitrogen-responsive upstream activation sequences of Saccharomyces cerevisiae by the product of the GLN3 gene. J Bacteriol. 1995;177:4190–4193. doi: 10.1128/jb.177.14.4190-4193.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bossie M A, Martin C E. Nutritional regulation of yeast Δ-9 fatty acid desaturase activity. J Bacteriol. 1989;171:6409–6413. doi: 10.1128/jb.171.12.6409-6413.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke P A, Poyton R O. Structure/function of oxygen-regulated isoforms in cytochrome c oxidase. J Exp Biol. 1998;201:1163–1175. doi: 10.1242/jeb.201.8.1163. [DOI] [PubMed] [Google Scholar]
- 8.Carratu L, Franceschelli S, Pardini C L, Kobayashi G S, Horvath I, Vigh L, Maresca B. Membrane lipid perturbation modifies the set point of the temperature of heat shock response in yeast. Proc Natl Acad Sci USA. 1996;93:3870–3875. doi: 10.1073/pnas.93.9.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi J Y, Martin C E. The Saccharomyces cerevisiae FAT1 gene encodes an acyl-CoA synthetase that is required for maintenance of very long chain fatty acid levels. J Biol Chem. 1999;274:4671–4683. doi: 10.1074/jbc.274.8.4671. [DOI] [PubMed] [Google Scholar]
- 10.Choi J Y, Stukey J, Hwang S Y, Martin C E. Regulatory elements that control transcription activation and unsaturated fatty acid-mediated repression of the Saccharomyces cerevisiae OLE1 gene. J Biol Chem. 1996;271:3581–3589. doi: 10.1074/jbc.271.7.3581. [DOI] [PubMed] [Google Scholar]
- 11.Coffman J A, Rai R, Loprete D M, Cunningham T, Svetlov V, Cooper T G. Cross regulation of four GATA factors that control nitrogen catabolic gene expression in Saccharomyces cerevisiae. J Bacteriol. 1997;179:3416–3429. doi: 10.1128/jb.179.11.3416-3429.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham T S, Dorrington R A, Cooper T G. The UGA4 UASNTR site required for GLN3-dependent transcriptional activation also mediates DAL80-responsive regulation and DAL80 protein binding in Saccharomyces cerevisiae. J Bacteriol. 1994;176:4718–4725. doi: 10.1128/jb.176.15.4718-4725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunningham T S, Svetlov V V, Rai R, Smart W, Cooper T G. Gln3p is capable of binding to UASNTR elements and activating transcription in Saccharomyces cerevisiae. J Bacteriol. 1996;178:3470–3479. doi: 10.1128/jb.178.12.3470-3479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans T, Felsenfeld G. The erythroid-specific transcription factor Eryf1: a new finger protein. Cell. 1989;58:877–885. doi: 10.1016/0092-8674(89)90940-9. [DOI] [PubMed] [Google Scholar]
- 15.Faergeman N J, DiRusso C C, Elberger A, Knudsen J, Black P N. Disruption of the Saccharomyces cerevisiae homologue to the murine fatty acid transport protein impairs uptake and growth on long-chain fatty acids. J Biol Chem. 1997;272:8531–8538. doi: 10.1074/jbc.272.13.8531. [DOI] [PubMed] [Google Scholar]
- 16.Fujimori K, Anamnart S, Nakagawa Y, Sugioka S, Ohta D, Oshima Y, Yamada Y, Harashima S. Isolation and characterization of mutations affecting expression of the Δ9- fatty acid desaturase gene, OLE1, in Saccharomyces cerevisiae. FEBS Lett. 1997;413:226–230. doi: 10.1016/s0014-5793(97)00846-6. [DOI] [PubMed] [Google Scholar]
- 17.Fujiwara D, Kobayashi O, Yoshimoto H, Harashima S, Tamai Y. Molecular mechanism of the multiple regulation of the Saccharomyces cerevisiae ATF1 gene encoding alcohol acetyltransferase. Yeast. 1999;15:1183–1197. doi: 10.1002/(SICI)1097-0061(19990915)15:12<1183::AID-YEA444>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 18.Fujiwara D, Yoshimoto H, Sone H, Harashima S, Tamai Y. Transcriptional co-regulation of Saccharomyces cerevisiae alcohol acetyltransferase gene, ATF1 and Δ-9 fatty acid desaturase gene, OLE1 by unsaturated fatty acids. Yeast. 1998;14:711–721. doi: 10.1002/(SICI)1097-0061(19980615)14:8<711::AID-YEA263>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Gallwitz D, Sures I. Structure of a split yeast gene: complete nucleotide sequence of the actin gene in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1980;77:2546–2550. doi: 10.1073/pnas.77.5.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez C I, Martin C E. Fatty acid-responsive control of mRNA stability. J Biol Chem. 1996;271:25801–25809. doi: 10.1074/jbc.271.42.25801. [DOI] [PubMed] [Google Scholar]
- 21.Guillemin K, Krasnow M A. The hypoxic response: huffing and HIFing. Cell. 1997;89:9–12. doi: 10.1016/s0092-8674(00)80176-2. [DOI] [PubMed] [Google Scholar]
- 22.Hazel J R. Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu Rev Physiol. 1995;57:19–42. doi: 10.1146/annurev.ph.57.030195.000315. [DOI] [PubMed] [Google Scholar]
- 23.Hereford L, Fahrner K, Woolford J, Jr, Rosbash M, Kaback D B. Isolation of yeast histone genes H2A and H2B. Cell. 1979;18:1261–1271. doi: 10.1016/0092-8674(79)90237-x. [DOI] [PubMed] [Google Scholar]
- 24.Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwast K E, Burke P V, Poyton R O. Oxygen sensing and the transcriptional regulation of oxygen-responsive genes in yeast. J Exp Biol. 1998;201:1177–1195. doi: 10.1242/jeb.201.8.1177. [DOI] [PubMed] [Google Scholar]
- 26.Kwast K E, Burke P V, Staahl B T, Poyton R O. Oxygen sensing in yeast: evidence for the involvement of the respiratory chain in regulating the transcription of a subset of hypoxic genes. Proc Natl Acad Sci USA. 1999;96:5446–5451. doi: 10.1073/pnas.96.10.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowry C V, Cerdan M E, Zitomer R S. A hypoxic consensus operator and a constitutive activation region regulate the ANB1 gene of Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:5921–5926. doi: 10.1128/mcb.10.11.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowry C V, Lieber R H. Negative regulation of the Saccharomyces cerevisiae ANB1 gene by heme, as mediated by the ROX1 gene product. Mol Cell Biol. 1986;6:4145–4148. doi: 10.1128/mcb.6.12.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeda M, Kubo K, Nishi T, Futai M. Roles of gastric GATA DNA-binding proteins. J Exp Biol. 1996;199:513–520. doi: 10.1242/jeb.199.3.513. [DOI] [PubMed] [Google Scholar]
- 30.Marzluf G A. Genetic regulation of nitrogen metabolism in the fungi. Microbiol Mol Biol Rev. 1997;61:17–32. doi: 10.1128/mmbr.61.1.17-32.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizuno T, Nakazawa N, Remgsamrarn P, Kunoh T, Oshima Y, Harashima S. The Tup1-Ssn6 general repressor is involved in repression of IME1 encoding a transcriptional activator of meiosis in Saccharomyces cerevisiae. Curr Genet. 1998;33:239–247. doi: 10.1007/s002940050332. [DOI] [PubMed] [Google Scholar]
- 32.Mortimer R K, Contopoulou C R, King J S. Genetic and physical maps of Saccharomyces cerevisiae, edition 11. Yeast. 1992;8:817–902. doi: 10.1002/yea.320081002. [DOI] [PubMed] [Google Scholar]
- 33.Mortimer R K, Johnston J R. Genealogy of principal strains of the yeast genetic stock center. Genetics. 1986;113:35–43. doi: 10.1093/genetics/113.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukai Y, Harashima S, Oshima Y. Function of the Ste signal transduction pathway for mating pheromones sustains MATα1 transcription in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:2050–2060. doi: 10.1128/mcb.13.4.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ntambi J M. Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J Lipid Res. 1999;40:1549–1558. [PubMed] [Google Scholar]
- 36.Orkin S H. GATA-binding transcription factors in hematopoietic cells. Blood. 1992;80:575–581. [PubMed] [Google Scholar]
- 37.Pevny L, Simon M C, Robertson E, Klein W H, Tsai S F, D'Agati V, Orkin S H, Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991;349:257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- 38.Rose M D, Winston F, Hieter P. Methods in yeast genetics: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- 39.Sakumoto N, Mukai Y, Uchida K, Kouchi T, Kuwajima J, Nakagawa Y, Sugioka S, Yamamoto E, Furuyama T, Mizubuchi H, Ohsugi N, Sakuno T, Kikuchi K, Matsuoka I, Ogawa N, Kaneko Y, Harashima S. A series of protein phosphatase gene disruptants in Saccharomyces cerevisiae. Yeast. 1999;15:1669–1679. doi: 10.1002/(SICI)1097-0061(199911)15:15<1669::AID-YEA480>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 41.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaffer J E, Lodish H F. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell. 1994;79:427–436. doi: 10.1016/0092-8674(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 43.Semenza G L, Jiang B H, Leung S W, Passantino R, Concordet J P, Maire P, Giallongo A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271:32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 44.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart L C, Yaffe M P. A role for unsaturated fatty acids in mitochondrial movement and inheritance. J Cell Biol. 1991;115:1249–1257. doi: 10.1083/jcb.115.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stukey J E, McDonough V M, Martin C E. The OLE1 gene of Saccharomyces cerevisiae encodes the Δ9 fatty acid desaturase and can be functionally replaced by the rat stearoyl-CoA desaturase gene. J Biol Chem. 1990;265:20144–20149. [PubMed] [Google Scholar]
- 47.Tanabe A, Furukawa T, Ogawa Y, Yamamoto M, Hayashi N, Tokunaga R, Taketani S. Involvement of the transcriptional factor GATA-1 in regulation of expression of coproporphyrinogen oxidase in mouse erythroleukemia cells. Biochem Biophys Res Commun. 1997;233:729–736. doi: 10.1006/bbrc.1997.6532. [DOI] [PubMed] [Google Scholar]
- 48.Toh-e A, Ueda Y, Kakimoto S I, Oshima Y. Isolation and characterization of acid phosphatase mutants in Saccharomyces cerevisiae. J Bacteriol. 1973;113:727–738. doi: 10.1128/jb.113.2.727-738.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsai S F, Martin D I, Zon L I, D'Andrea A D, Wong G G, Orkin S H. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989;339:446–451. doi: 10.1038/339446a0. [DOI] [PubMed] [Google Scholar]
- 50.Vieille-Grosjean I, Huber P. Transcription factor GATA-1 regulates human HOXB2 gene expression in erythroid cells. J Biol Chem. 1995;270:4544–4550. doi: 10.1074/jbc.270.9.4544. [DOI] [PubMed] [Google Scholar]
- 51.Wang G L, Semenza G L. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watkins P A, Lu J F, Steinberg S J, Gould S J, Smith K D, Braiterman L T. Disruption of the Saccharomyces cerevisiae FAT1 gene decreases very long-chain fatty acyl-CoA synthetase activity and elevates intracellular very long-chain fatty acid concentrations. J Biol Chem. 1998;273:18210–18219. doi: 10.1074/jbc.273.29.18210. [DOI] [PubMed] [Google Scholar]
- 53.Zitomer R S, Carrico P, Deckert J. Regulation of hypoxic gene expression in yeast. Kidney Int. 1997;51:507–513. doi: 10.1038/ki.1997.71. [DOI] [PubMed] [Google Scholar]