Abstract
A majority of lower extremities neuro‐ischaemic wounds (NIU) are related to: (a) only diabetes (DM); (b) only peripheral artery disease (PAD); (c) co‐existing diabetes and peripheral artery disease (DM‐PAD). This study aims to characterise the major clinical outcomes of forementioned three groups of lower extremity wound patients in Singapore. Patients hospitalised for lower extremity NIU between January 2014 and October 2017 in a tertiary hospital in Singapore were analysed. Patients' major limb amputation and mortality were assessed using Cox regression models. Cumulative survival and amputation‐free survival among the three classified groups were calculated using Kaplan‐Meier analysis. Compared with patients with only DM, those in the PAD group and the DM‐PAD group had higher risk of major limb amputation (adjusted hazard ratio: 2.47, 95% CI: 1.65‐3.70; adjusted hazard ratio: 2.01, 95% CI: 1.53‐2.65 respectively) and mortality (adjusted hazard ratio: 2.36, 95% CI: 1.57‐3.55; adjusted hazard ratio: 2.46, 95% CI: 1.86‐3.26 respectively). The 3‐year survival and amputation‐free survival were lowest in the DM‐PAD group (52.1% and 41.5% respectively), followed by the PAD group (53.3% and 44.6% respectively) and the DM group (74.2% and 68.5% respectively). Lower extremity NIU patients with PAD or DM‐PAD were found to have poorer clinical prognosis than those with DM only.
Keywords: amputation, diabetes mellitus, mortality, peripheral arterial disease, wounds
1. INTRODUCTION
Chronic wounds of the lower extremities represent a significant humanistic burden at an individual level, markedly associated with bodily pain, reduced mobility, poor psychosocial well‐being and low quality of life. 1 , 2 In addition, chronic wounds imposed a substantial economic burden onto the health care system globally. 3 , 4 , 5 A tertiary institution in Singapore reported that those with diabetic foot ulcer and ischaemic ulcers incurred a substantial amount of health care cost, with direct gross cost per annum amounting to SGD 1.68 million and SGD 0.43 million, respectively. 3
Patients presented with neuro‐ischaemic lower limb wounds (NIU) are closely related to diabetes (DM) and/or peripheral artery disease (PAD). They represent a heterogeneous group of patients with ulcers predominantly due to neuropathy, or ischaemia, or a mix of these two contributing components. 4 , 6 , 7 , 8 Both DM and PAD are prevalent in Singapore. 9 , 10 , 11 Patients present with both conditions (DM‐PAD) are also common as these two diseases are correlated. 12 , 13 Majority of the studies reporting clinical outcomes (lower extremity amputation and mortality) of NIU patients did not look into the subgroups (DM only, PAD only and those with DM‐PAD). 14 , 15 , 16 , 17 Some studies comparing patients with DM only to those with DM‐PAD provided conflicting conclusions. 7 , 12 , 18 , 19 , 20 , 21 , 22 To date, the characterisation of the clinical outcomes among the aforementioned three groups of patients is lacking in Asia.
Information about the clinical outcomes and health care resources required by these three categories of NIU patients is crucial for predicting their prognosis and designing future health care strategies. The primary aims of this study are: (a) to review the major clinical outcomes of lower extremity NIU individuals having DM only, PAD only and those with both DM and PAD in Singapore, a multi‐ethnic Asian city state; (b) to identify factors associated with worse clinical outcomes. This study will also report the health care resources and cost associated with these three categories of NIU patients.
2. METHODS
2.1. Date extraction
This study is a retrospective electronic record review of patients with chronic lower extremity wounds from a tertiary level university hospital that has 1200 hospital beds, with a catchment area of more than 1 million Singaporeans in the western part of Singapore. 23 All patients' retrievable electronic data from 1 January 2013 to 31 October 2017 were extracted by the Academic Informatic Office of the hospital. New NIU cases were identified based on the diagnosis code of patients' first wound‐related hospitalization. To exclude patients with existing wounds, a 1‐year free from wound‐related hospitalization period was used. Thus, new cases were identified from 1 January 2014 onwards.
Three groups of patients with NIU who are 21‐year‐old or above were retrieved and identified based on their admission International Classification of Diseases Tenth Revision (ICD‐10) diagnosis codes (Appendix A and B) in the electronic records. These are patients with diabetes only (DM), patients with peripheral arterial disease only (PAD) and patients with both diabetes and peripheral arterial disease (DM‐PAD). The matrix of grouping the identified patients based on their ICD‐10 codes is listed in Appendix B. The study has been approved by the National Health Group Domain Specific Review Board (DSRB 2019/00917).
2.2. Study data
The data of individual's demographics, co‐morbidities, dressing services utilised, surgical procedures, limb salvage outcome, length of stay in the hospital, outpatient visits, hospital bill and mortality information were retrieved from the electronic record system based on ICD‐10 diagnosis codes, surgical codes and service codes. Inpatient bedside dressing includes minor debridement and dressing done by podiatrists and nurses. Revascularization included lower limb arterial angioplasty, stenting, surgical bypass and endarterectomy. Minor amputations included single or multiple toe disarticulations, ray amputations and transmetatarsal amputations. Major amputations include below‐knee amputation, through‐knee amputations and above‐knee amputations. The hospital length of stay (LOS) per admission was defined as the individual patient's average number of wound‐related inpatient stay per admission during the study period. We considered hospital bill per admission as the individual patient's average amount billed to the patient per admission before government subsidy in Singapore dollars (1 Singapore Dollar=0.76 USD) during the study period. The total outpatient cost was defined as the total amount of bill incurred from patients' visit to the vascular clinic, diabetic clinic and orthopaedic clinic as well as outpatient podiatry visits.
2.3. Statistical analysis
All data were analysed using R software, version 3.1.2. Patients' demographics, co‐morbidities and inpatient resource consumptions were summarised and compared by the three patient groups stated. The Kolmogorov‐Smirnov test was used to examine normality. Age was reported as mean and standard deviation and compared by ANOVA test. Other continuous variables were reported as median accompanied by interquartile range and compared by Kruskal‐Wallis test. Categorical variables were reported as numbers and proportions. They were compared using the Chi‐square test. All tests were two‐tailed, and P values ≤ .05 were considered to indicate statistical significance.
In the variable selection for multivariable regression models, we considered three sets of clinically plausible covariates in the comparison of the clinical outcomes among the three patient groups: basic characteristics, co‐morbidities and surgical procedures. Basic characteristics included age, gender, race and smoking history; co‐morbidities included ischaemic heart disease (IHD), congestive heart failure (CHF), hypertension, hyperlipidaemia, chronic kidney disease (CKD)/end stage renal failure (ESRF) requiring dialysis, cerebral vascular disease, infection requiring intravenous antibiotic; procedures included wound debridement, minor amputation and major amputation (only for mortality) and revascularization. Multicollinearity was checked by variance inflation factor (VIF), with VIF <2 for every variable. Akaike information criterion (AIC) was used as the selection criteria with a combination of both forward selection and backward elimination stepwise approach.
Multivariable Cox regression models were used to assess the association between risk factors and the two major clinical outcomes (mortality and major limb amputation). Proportional hazard assumption was checked using Schoenfeld residuals plots. Kaplan‐Meier was used to display adjusted cumulative survival and major amputation‐free survival. Log‐rank statistics were used to compare the subset of patients.
3. RESULTS
3.1. Baseline characteristics, co‐morbidities and resource consumption
Based on the relevant ICD10 diagnosis codes, 1722 patients (640, 134 and 948 from DM, PAD and DM‐PAD group respectively) were identified, and their corresponding data were extracted from 1 January 2013 to 31 October 2017. After removing those patients who had wound‐related admission in 2013, 1223 patients were enrolled in the study: DM (n = 469), PAD (n = 99) and DM‐PAD (n = 655).
The baseline characteristics and co‐morbidities of enrolled patients are presented in Table 1. Patients in DM group were younger, followed by DM‐PAD group and PAD group (P < .001). The PAD group had the highest proportion of Chinese patients, whereas the frequency of Malay patients was highest among DM group (P < .001). Compared with the other two groups, DM‐PAD patients had a higher percentage of cardiovascular co‐morbidities, including IHD, CHF, hypertension, hyperlipidaemia, CKD/ESRF and cerebral vascular disease (P < .001). More patients with concomitant DM‐PAD required insulin for DM control, compared with DM group (P < .01).
TABLE 1.
Patients' baseline characteristics and inpatient resource consumption of patients with DM, PAD and DM‐PAD
| DM | PAD | DM‐PAD | P | |
|---|---|---|---|---|
| Patients, n (% of all) | 469 (38.3) | 99 (8.1%) | 655 (53.6%) | |
| Baseline characteristics | ||||
| Age (years), mean (SD) | 66.3 (13.3) | 76.1 (17.0) | 71.0 (12.2) | <.001*** |
| Men, n (%) | 284 (60.6) | 56 (56.6) | 374 (57.1) | .48 |
| Race, n (%) | ||||
| Chinese | 192 (40.9) | 64 (64.6) | 340 (51.9) | <.001*** |
| Malay | 141 (30.1) | 15 (15.2) | 127 (19.4) | |
| Indian | 72 (15.4) | 10 (10.1) | 113 (17.3) | |
| Others | 64 (13.6) | 10 (10.1) | 75 (11.5) | |
| Smoking history, n (%) | 127 (27.1) | 36 (36.4) | 189 (28.9) | .08 |
| Co‐morbidities | ||||
| Ischaemic heart disease, n (%) | 87 (18.6) | 29 (29.3) | 293 (44.7) | <.001*** |
| Congestive heart failure, n (%) | 69 (14.7) | 19 (19.2) | 165 (25.2) | <.001*** |
| Hypertension, n (%) | 365 (77.8) | 60 (60.6) | 574 (87.6) | <.001*** |
| Hyperlipidaemia, n (%) | 319 (68.0) | 22 (22.2) | 533 (81.4) | <.001*** |
| Renal function, n (%) | ||||
| Normal | 224 (47.8) | 52 (52.5) | 179 (27.3) | <.001*** |
| Chronic kidney disease | 180 (38.4) | 21 (21.2) | 261 (39.8) | |
| End stage renal failure | 65 (13.9) | 26 (26.3) | 215 (32.8) | |
| Cerebral vascular disease, n (%) | 24 (5.1) | 11 (11.1) | 84 (12.8) | <.001*** |
| Infection requires intravenous antibiotic, n (%) | 432 (92.1) | 86 (86.9) | 613 (93.6) | .06 |
| DM controlled by insulin, n (%) | 136 (29.0) | — a | 242 (36.9) | <.01** |
| Procedures and resource consumption | ||||
| Number of bedside wound dressing, median (IQR) | 2.0 (3.0) | 3.0 (4.0) | 5.0 (9.0) | <.001*** |
| Wound debridement, n (%) | 105 (22.4) | 9 (9.1) | 127 (19.4) | <.001*** |
| Minor amputation, n (%) | 88 (18.8) | 14 (14.1) | 214 (32.7) | <.001*** |
| Revascularization, n (%) | — a | 42 (42.4) | 381 (58.2) | <.01** |
| Number of hospital admissions, median (IQR) | 1.0 (0.0) | 1.0 (0.0) | 2.0 (2.0) | <.001*** |
| Length of inpatient stay per admission (days), median (IQR) | 6.0 (10.0) | 13.0 (19.9) | 13.2 (19.9) | <.001*** |
| Inpatient bill per visit (Singapore Dollar), median (IQR) | 4853.9 (9948.0) | 14060.1 (20276.7) | 13497.0 (20457.5) | <.001*** |
| Outpatient bill (Singapore Dollar), median (IQR) | 1672.9 (4936.5) | 2474.3 (5466.9) | 4933.5 (9411.3) | <.001*** |
Abbreviations: DM, diabetes; IQR, interquartile range; PAD, peripheral artery disease; SD, standard deviation.
No insulin therapy is required in PAD group; No revascularization is required in DM group.
**P<.01; ***P<.001.
In terms of surgical and interventional procedures, the rate of wound debridement was lowest in the PAD group compared with the other two groups (P < .001). Minor amputation was most frequently done in patients with DM‐PAD, followed by DM group and PAD group (P < .001). Higher percentage of patients in DM‐PAD group received revascularization procedure than PAD group (P < .01), while DM only group did not require any revascularization procedure. The number of bedside wound dressing received by the DM‐PAD group is more than twice of that received by the DM only group and the PAD only group (P < .001).
The number of hospital admissions experienced by the DM‐PAD group was twice the number of the other two groups (P < .001). NIU patients with ischaemic component (PAD and DM‐PAD groups) had a more than 2‐fold increase in the average LOS and inpatient bill per admission, compared with the DM group (P < .001). Similarly, patients in the DM‐PAD group had the highest outpatient bill, followed by the PAD group and the DM group (P < .001).
3.2. Major limb amputation
Table 2 lists the results of the univariable and multivariable Cox regression analysis of predictors of major limb amputation. In univariable analysis, patients in the PAD group and the DM‐PAD group had a hazard ratio of 2.88 (95% CI: 1.97‐4.21; P < .001) and 2.20 (95% CI: 1.73‐2.81; P < .001) for major limb amputation respectively, compared with patients in the DM group. After adjustment of covariates selected by the stepwise regression, the major amputation risk among the PAD group (adjusted HR: 2.47, 95% CI: 1.65‐3.70; P < .001) and the DM‐PAD group (adjusted HR: 2.01, 95% CI: 1.53‐2.65; P < .001) remained significantly higher relative to the DM group.
TABLE 2.
Multivariable cox regression analysis of predictors of major limb amputation
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Crude HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |
| Wound group (reference: DM) | — | — | — | — |
| PAD group | 2.88 (1.97‐4.21) | <.001*** | 2.47 (1.65‐3.70) | <.001*** |
| DM‐PAD group | 2.20 (1.73‐2.81) | <.001*** | 2.01 (1.53‐2.65) | <.001*** |
| Age (above 70 years old) | 1.76 (1.42‐2.19) | <.001*** | 1.44 (1.15‐1.81) | <.01** |
| Ischaemic heart disease | 2.08 (1.70‐2.55) | <.001*** | 1.48 (1.18‐1.84) | <.001*** |
| Renal function (reference: normal) | — | — | — | — |
| Chronic kidney disease | 1.89 (1.45‐2.47) | <.001*** | 1.37 (1.04‐1.82) | .03* |
| End stage renal failure | 2.54 (1.92‐3.36) | <.001*** | 1.96 (1.45‐2.65) | <.001*** |
| Wound debridement | 0.51 (0.37‐0.72) | <.001*** | 0.66 (0.47‐0.94) | .02* |
| Minor amputation | 0.67 (0.51‐0.87) | <.01** | 0.77 (0.58‐1.01) | .06 |
| Revascularization | 1.09 (0.88‐1.35) | .43 | 0.70 (0.54‐0.89) | <.01** |
Note: All variables that were included in the multivariable regressions are displayed in the table.
Abbreviations: CI, confidence interval; DM, diabetes; HR, hazard ratio; PAD, peripheral artery disease.
P ≤ .05; **P ≤ .01; ***P ≤ .001.
Furthermore, the risk for major amputation was elevated for those aged above 70 years (adjusted HR:1.44, 95% CI: 1.15‐1.81; P < .01), history of IHD (adjusted HR:1.48, 95% CI: 1.18‐1.84; P < .001), presence of CKD (adjusted HR:1.37, 95% CI: 1.04‐1.82; P = .03) or ESRF (adjusted HR:1.96, 95% CI: 1.45‐2.65; P < .001).
Wound debridement and revascularization were independently associated with lesser risk of major amputation, with an adjusted hazard ratio of 0.66 (95% CI: 0.47‐0.94; P = .02) and 0.70 (95% CI: 0.54‐0.89; P < .01), respectively. Though less precisely estimated, minor amputation was also potentially a protective factor for major limb event (adjusted HR: 0.77, 95% CI: 0.58‐1.01; P = .06).
3.3. All‐cause mortality
Table 3 lists the results of the univariable and multivariable Cox regression analysis of predictors of all‐cause mortality. In the crude analysis, the hazard ratio for the all‐cause mortality were 2.83 (95% CI: 1.94‐4.14; P < .001) in the PAD group and 2.27 (95% CI: 1.78‐2.88; P < .001) in the DM‐PAD group, compared with those in the DM group. After adjustment of the selected covariates, the PAD and DM‐PAD groups still showed increased risk of mortality by an adjusted hazard ratio of 2.36 (95% CI: 1.57‐3.55; P < .001) and 2.46 (95% CI: 1.86‐3.26; P < .001), respectively, compared with the DM group.
TABLE 3.
Cox regression analysis of predictors of mortality
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Crude HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |
| Wound group (reference: DM) | — | — | — | — |
| PAD group | 2.83 (1.94‐4.14) | <.001*** | 2.36 (1.57‐3.55) | <.001*** |
| DM‐PAD group | 2.27 (1.78‐2.88) | <.001*** | 2.46 (1.86‐3.26) | <.001*** |
| Age (above 70 years old) | 2.55 (2.05‐3.17) | <.001*** | 2.01 (1.60‐2.53) | <.001*** |
| Race (reference: Chinese) | — | — | — | — |
| Malay | 0.72 (0.56‐0.94) | .01 | 0.98 (0.75‐1.28) | .90 |
| Indian | 0.64 (0.47‐0.87) | <.01** | 0.71 (0.52‐0.98) | .04* |
| Others | 0.54 (0.38‐0.77) | <.001*** | 0.71 (0.49‐1.02) | .07 |
| Ischaemic heart disease | 2.18 (1.78‐2.67) | <.001*** | 1.38 (1.10‐1.74) | <.01* |
| Congestive heart failure | 2.16 (1.74‐2.69) | <.001*** | 1.54 (1.22‐1.95) | <.001*** |
| Renal function (reference: normal) | — | — | — | — |
| Chronic kidney disease | 2.25 (1.73‐2.93) | <.001*** | 1.64 (1.23‐2.18) | <.001*** |
| End stage renal failure | 2.55 (1.93‐3.36) | <.001*** | 2.12 (1.56‐2.88) | <.001*** |
| Dyslipidaemia | 1.04 (0.82‐1.30) | .77 | 0.77 (0.60‐1.00) | .05* |
| Wound debridement | 0.37 (0.27‐0.52) | <.001*** | 0.54 (0.38‐0.76) | <.001*** |
| Minor amputation | 0.52 (0.40‐0.68) | <.001*** | 0.59 (0.45‐0.79) | <.001*** |
| Revascularization | 0.95 (0.76‐1.17) | .61 | 0.57 (0.44‐0.73) | <.001*** |
Note: All variables that were included in the multivariable regressions are displayed in the table.
Abbreviations: CI, confidence interval; DM, diabetes; HR, hazard ratio; PAD, peripheral artery disease.
P ≤ .05; **P ≤ .01; ***P ≤ .001.
Also, the risk of mortality was relevantly associated with age above 70 years old (adjusted HR:2.01, 95% CI: 1.60‐2.53; P < .001), IHD (adjusted HR: 1.38, 95% CI: 1.10‐1.74; P < .01), CHF (adjusted HR: 1.54, 95% CI: 1.22‐1.95; P < .001), CKD (adjusted HR: 1.64, 95% CI: 1.23‐2.18; P < .001) and ESRF (adjusted HR: 2.12, 95% CI: 1.56‐2.88; P < .001). Interestingly, the presence of dyslipidaemia associated with decreased risk of mortality (adjusted HR: 0.77, 95% CI: 0.60‐1.00; P = .05).
In terms of surgical interventions, wound debridement (adjusted HR: 0.54, 95% CI: 0.38‐0.76; P < .001), minor amputation (adjusted HR: 0.59, 95% CI: 0.45‐0.79; P < .001) were associated with lower risk of mortality. Despite being insignificant in univariable analysis, revascularisation was significantly associated with lower risk of mortality in the multivariable analysis (adjusted HR: 0.57, 95% CI: 0.44‐0.73; P < .001).
3.4. Survival analysis
The adjusted cumulative survival and amputation‐free survival of the three groups over the study period are displayed by the Kaplan‐Meier plot in Figure 1. The 12‐, 24‐ and 36‐month cumulative survival was 86.7%, 79.5% and 74.2% in DM group, 72.4%, 60.8% and 53.3% for PAD group, and 71.5%, 59.7% and 52.1% for DM‐PAD group.
FIGURE 1.
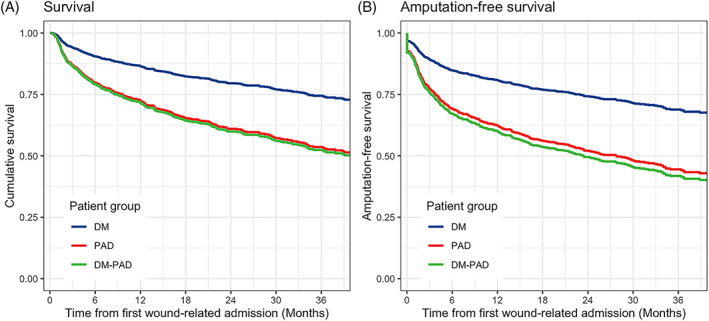
Adjusted Kaplan‐Meier survival curves for survival (A) and amputation‐free survival (B) in diabetes only (DM) group, peripheral arterial disease (PAD) group and DM‐PAD group. Log‐rank statistics were used to compare a subset of patients
The amputation‐free survival at 12‐,24‐ and 36‐month was 80.8%, 74.1% and 68.5%, respectively, in DM group, 62.7%, 52.3% and 44.6% in PAD group, and 60.0%, 49.3% and 41.5% in DM‐PAD group (Figure 1B). Patients in the PAD group and the DM‐PAD group had worse outcomes in cumulative survival (P < .001) and amputation‐free survival (P < .001), compared with patients with DM only.
4. DISCUSSION
NIUs include a wide spectrum of ulcers with varying degree of neuropathy and ischaemia caused by DM and PAD, respectively. Despite a large number of studies reported limb loss and mortality rates in NIU patients, 7 , 12 , 18 , 19 , 20 , 21 , 22 majority of these studies included a heterogenous group of patients. Only few studies compared NIU patients with predominantly neuropathy (DM only), predominantly ischaemia (PAD only) and the combination of two (DM‐PAD). 7 , 12 It is important to find out the magnitude of the difference in clinical outcomes between these three categories of NIU patients, so as to guide the urgency of referral to specialists' care as well as decision of treatment strategy. The results of the EURODIALE study 12 suggested the presence of PAD has a detrimental impact on wound healing. In the current study, the longer‐term clinical outcomes (limb loss, overall survival and amputation‐free survival) are similar between PAD group and DM‐PAD group, but significantly worse than DM group. As such, assessment for presence of any lower limb ischaemia should be the very first evaluation for all NIU patients. Nevertheless, PAD is often under‐diagnosed in diabetic patients as the classic symptom of intermittent claudication usually does not exist. 15 Meticulous clinical examination and proper application of non‐invasive lower limb perfusion studies are essential.
We noticed the results of the current study are in contrary to the findings of a study that analysed the German national health insurance data. 7 Maylar et al 7 concluded that patients with diabetic foot syndrome (DFS) had the worst cumulative survival and amputation‐free survival rate compared with the PAD‐DM group and the PAD group. We suspect this discrepancy could be due to the difference in the NIU aetiology classification method. Over 90% of the patients in the diabetic foot syndrome group had a Rutherford classification of stage 5 or 6, which indicates patients also had peripheral artery disease. 7 Whereas the corresponding group in our study consisted of NIU relating to DM without ischaemic component. With the distinctive classification of lower extremity wound type in our study, the picture of the clinical and economic burden of DM and/or PAD is clearer.
The wound ischaemia foot infection (WIfI) score proposed in the year 2014 considered three main aspects of wounds: (a) wound extent, (b) degree of ischaemia and (c) severity of foot infection to define the disease burden. 24 Subsequent studies confirmed the above‐mentioned three components are important prognostic factors for major limb amputation in this heterogenous patient population, whether diabetic or not. 25 , 26 , 27 , 28 Wound healing in NIU patients with DM only mainly determined by the wound extent and wound infection severity. 29 Thus, the management for this group of patients focuses on effective wound dressing, wound debridement, glycaemic and infection control and proper offloading. Data in the current study also indicated higher percentage of patients required surgical wound debridement in DM group. In the PAD group and the DM‐PAD group, all patients had a certain degree of lower limb ischaemia on top of the wound extent and wound infection. Therefore, these two groups of patients likely had higher WIfI score and poorer outcome prognosis. For NIU patients with ischaemia component, revascularization becomes one of the main treatment strategies, while wound management and infection control also require high attention. In particular, patients in the DM‐PAD group tend to have more severe and diffuse arterial obstruction relative to the PAD group. 22 This may explain the observation of higher percentage of patients in DM‐PAD groups required revascularization and minor amputation compared with PAD only group. Addressing the multifaceted care needed by the DM‐PAD NIU patients, the International Working Group on the diabetic Foot (IWGDF) recommended an integrated team composed of vascular surgeons, endocrinologists, orthopaedic surgeons, podiatrists and wound nurses to improve the prognosis of this subgroup of patients. 30
To the best of our knowledge, this is the first study in an Asian population to evaluate the clinical outcome difference in NIU patients with different aetiologies. Apart from indicating NIU patients with ischaemic component (PAD and DM‐PAD groups) had higher risk of limb loss and death, this study also illustrates a great magnitude of difference in health care resources consumption and health care cost required by patients with lower limb ischaemia compared with those with DM only. The median LOS and median inpatient bill per admission of NIU patients with ischaemic component (PAD and DM‐PAD groups) were more than twice that of DM only patients. The median number of hospital admissions of DM‐PAD group also significantly more than the other two groups. The median outpatient bill of DM‐PAD groups was about two times that of PAD group and three times that of DM group. This indicates the complexity of disease, requirement of multi‐modality treatments are higher for NIU patients with lower limb ischaemia. This category of patients demands a streamline of coordinated multi‐disciplinary care. In view of the poorer prognosis and higher need of multi‐modality treatment for NIU patients with lower limb ischaemia, primary health clinicians should refer this category of patients to tertiary care promptly once diagnosis is established. A more efficient referral pathway between primary and tertiary care is also needed. 31 , 32 , 33 The health care economic data of the current study also could form a benchmark information for cost‐effectiveness evaluation of future service reform for NIU patients of different categories.
In terms of co‐morbidities, the presence of cardiovascular disease and ESRF being the risk factors of major limb amputation and mortality in NIU patients is in line with existing evidence. 34 , 35 , 36 , 37 , 38 , 39 Moreover, cardiovascular disease is more prevalent in NIU patients with PAD as both share similar atherosclerosis risk factor profiles 14 ; even after adjustment for known atherosclerosis‐related risk factors, patients with PAD were still prospectively associated with higher risk of all‐cause mortality compared with those having wounds without ischaemic component. 14 , 16 , 40 , 41 , 42 Also, patients with ESRF tend to have more extensive arterial calcifications, impaired microcirculatory perfusion and higher likelihood of drug‐resistant wound infection. 43 , 44 Heart‐renal DM‐PAD patients, therefore, are the most fragile subgroup of patients with lower extremity wounds, owing to more difficult arterial disease and higher risk of cardiovascular mortality. Interestingly, a history of dyslipidaemia was associated with lower risk of mortality. It is likely that diagnosis of dyslipidaemia was a proxy for statin prescription in the local setting. Accumulating evidences have suggested that statin use reduces the risk of all‐cause mortality among patients with PAD. 45 , 46 , 47 The protective effect of dyslipidaemia showed in this study might be due to statin usage.
While using electronic administrative data could enable the health care professionals to obtain a bird‐eye view on the clinical outcomes and health economics of certain disease category efficiently, it also has some inherent limitations. Residual confounders are expected from the administrative data, ie, other co‐morbidities, duration of diabetes, HbA1c and severity of arterial obstruction. Furthermore, some important clinical variables were not available, such as wound duration before the patients' admission, any wound recurrence after complete healing. A longer look‐back period could improve the accuracy of identifying patients with newly developed NIU, but it might reduce the size of cohorts. 48 We did, however, use a 1‐year look‐back period to ascertain previous wound‐related admissions to balance the need to maximise the number of cases available for analysis while minimizing misclassification.
This study also heavily depends on the accurate entry of ICD codes and procedure codes. Physicians' clinical coding practice may vary in different settings. In the authors' institution, PAD was diagnosed based on: absence of one or more lower extremity pulse(s); abnormal ankle‐brachial index (ABI) or toe‐brachial index (TBI). Although ABI and TBI studies are available in the institution, the results were unfortunately not linked to the main electronic database.
Furthermore, the results of this study might have limited generalizability to the community population with lower extremity NIU as the data were extracted from hospitalised patients only, representing a more morbid group compared with patients who can be managed in community clinics.
5. CONCLUSION
Patients having lower extremity NIU due to PAD, whether with or without DM, sustain higher likelihood of major limb amputation and mortality, compared to those with DM only. Multifaceted approach with efficient referral pathway is necessary to mitigate the potential poor prognosis.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENT
We would like to thank Mrs. Doris Fok for providing professional edits to the manuscript. All authors contributed to and approved the final manuscript.
Appendix A. LIST OF INTERNATIONAL CLASSIFICATION OF DISEASE, TENTH REVISION (ICD‐10) CODES USED FOR PATIENT'S IDENTIFICATION
| Category | Diagnosis description | ICD‐10 codes |
|---|---|---|
| Section A—Wounds related to diabetes | Type 2 diabetes mellitus with foot ulcer due to multiple causes | E1173 |
| Unspecified diabetes mellitus with foot ulcer due to multiple causes | E1473 | |
| Other specified diabetes mellitus with foot ulcer due to multiple causes | E1373 | |
| Type 1 diabetes mellitus with foot ulcer due to multiple causes | E1073 | |
| Section B—Wounds related to peripheral artery disease | Atherosclerosis of arteries of extremities with ulceration | I7023 |
| Atherosclerosis of arteries of extremities with gangrene | I7024 | |
| Section C—Wounds related to both diabetes and peripheral artery disease | "Type 1 diabetes mellitus with peripheral angiopathy, with gangrene" | E1052 |
| "Type 2 diabetes mellitus with peripheral angiopathy, with gangrene" | E1152 | |
| "Unspecified diabetes mellitus with peripheral angiopathy, with gangrene" | E1452 | |
| Section D—Peripheral arterial disease | "Atherosclerosis of arteries of extremities, unspecified" | I7020 |
| "Impaired glucose regulation with peripheral angiopathy, without gangrene" | E0951 | |
| Other specified peripheral vascular diseases | I738 | |
| "Peripheral vascular disease, unspecified" | I739 | |
| Atherosclerosis of arteries of extremities with intermittent claudication | I7021 | |
| Section E—Both diabetes and peripheral arterial disease | "Type 1 diabetes mellitus with peripheral angiopathy, without gangrene" | E1051 |
| "Type 2 diabetes mellitus with peripheral angiopathy, without gangrene" | E1151 | |
| "Unspecified diabetes mellitus with peripheral angiopathy, without gangrene" | E1451 | |
| "Other specified diabetes mellitus with peripheral angiopathy, without gangrene" | E1351 |
Appendix B. CLASSIFICATION OF LOWER EXTREMITIES WOUNDS INTO GROUPS 1 TO 3
| Groups | Group 1: Wounds relating to diabetes only (DM) | Group 2: Wounds relating to peripheral artery disease only (PAD) | Group 3: Wounds relating to both diabetes and peripheral artery disease (DM‐PAD) |
|---|---|---|---|
| Classification Criteria | Section A only | Section B only (without Section A, C, E) |
|
Meng L, Graves N, Du RC, et al. Major limb amputation and mortality in patients with neuro‐ischaemic lower extremity wounds managed in a tertiary hospital: Focus on the differences among patients with diabetes, peripheral arterial disease and both. Int Wound J. 2022;19(6):1298-1308. doi: 10.1111/iwj.13724
Funding information We would like to acknowledge the funding supported from the Agency for Science, Technology and Research (A*STAR) under its Industry Alignment Fund‐Pre‐Positioning Programme (IAF‐PP) grant number H1901a00Y9 as part of the Wound Care Innovation for the Tropics (WCIT) Programme.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Olsson M, Järbrink K, Divakar U, et al. The humanistic and economic burden of chronic wounds: a systematic review. Wound Repair Regen. 2019;27(1):114‐125. [DOI] [PubMed] [Google Scholar]
- 2. Kirsner R, Vivas A. Lower‐extremity ulcers: diagnosis and management. Br J Dermatol. 2015;173(2):379‐390. [DOI] [PubMed] [Google Scholar]
- 3. Tan B, Tan E, Chong S, Chang Y, Song C, Lee V. An economic evaluation of chronic wound management in a tertiary hospital. Wound Pract Res. 2016;24(3):130‐136. [Google Scholar]
- 4. Lo ZJ, Lim X, Eng D, et al. Clinical and economic burden of wound care in the tropics: a 5‐year institutional population health review. Int Wound J. 2020;17(3):790‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health. 2018;21(1):27‐32. [DOI] [PubMed] [Google Scholar]
- 6. Star A. Peripheral arterial disease: Differentiating lower extremity wounds: Arterial, venous, neurotrophic. Paper presented at: Seminars in interventional radiology; 2018. [DOI] [PMC free article] [PubMed]
- 7. Malyar NM, Freisinger E, Meyborg M, et al. Amputations and mortality in in‐hospital treated patients with peripheral artery disease and diabetic foot syndrome. J Diabetes Complications. 2016;30(6):1117‐1122. [DOI] [PubMed] [Google Scholar]
- 8. Hussain MA, Al‐Omran M, Salata K, et al. Population‐based secular trends in lower‐extremity amputation for diabetes and peripheral artery disease. CMAJ. 2019;191(35):E955‐E961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Population Health survery 2016/2017: Ministry of Health, Singapore; 2017. https://www.moh.gov.sg/resources-statistics/reports/national-population-health-survey-2016-17. Accessed March 04, 2021.
- 10. Subramaniam T, Nang EEK, Lim SC, et al. Distribution of ankle—brachial index and the risk factors of peripheral artery disease in a multi‐ethnic Asian population. Vasc Med. 2011;16(2):87‐95. [DOI] [PubMed] [Google Scholar]
- 11. Ministry of Health . New stratergic framework to empower clinical management of diabetic foot complications. https://www.moh.gov.sg/news-highlights/details/new-strategic-framework-to-empower-clinical-management-of-diabetic-foot-complications. Accessed March 12, 2021.
- 12. Prompers L, Schaper N, Apelqvist J, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia. 2008;51(5):747‐755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barnes JA, Eid MA, Creager MA, Goodney PP. Epidemiology and risk of amputation in patients with diabetes mellitus and peripheral artery disease. Arterioscler Thromb Vasc Biol. 2020;40(8):1808‐1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116(9):1509‐1526. [DOI] [PubMed] [Google Scholar]
- 15. Campia U, Gerhard‐Herman M, Piazza G, Goldhaber SZ. Peripheral artery disease: past, present, and future. Am J Med. 2019;132(10):1133‐1141. [DOI] [PubMed] [Google Scholar]
- 16. Sartipy F, Sigvant B, Lundin F, Wahlberg E. Ten year mortality in different peripheral arterial disease stages: a population based observational study on outcome. Eur J Vasc Endovasc Surg. 2018;55(4):529‐536. [DOI] [PubMed] [Google Scholar]
- 17. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hao D, Hu C, Zhang T, Feng G, Chai J, Li T. Contribution of infection and peripheral artery disease to severity of diabetic foot ulcers in Chinese patients. Int J Clin Pract. 2014;68(9):1161‐1164. [DOI] [PubMed] [Google Scholar]
- 19. Yildiz PA, Özdil T, Dizbay M, Tunçcan ÖG, Hizel K. Peripheral arterial disease increases the risk of multidrug‐resistant bacteria and amputation in diabetic foot infections. Turk J Med Sci. 2018;48(4):845‐850. [DOI] [PubMed] [Google Scholar]
- 20. Yammine K, Hayek F, Assi C. A meta‐analysis of mortality after minor amputation among patients with diabetes and/or peripheral vascular disease. J Vasc Surg. 2020;72(6):2197‐2207. [DOI] [PubMed] [Google Scholar]
- 21. Freisinger E, Malyar NM, Reinecke H, Lawall H. Impact of diabetes on outcome in critical limb ischemia with tissue loss: a large‐scaled routine data analysis. Cardiovasc Diabetol. 2017;16(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thiruvoipati T, Kielhorn CE, Armstrong EJ. Peripheral artery disease in patients with diabetes: epidemiology, mechanisms, and outcomes. World J Diabetes. 2015;6(7):961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National University Hospital about NUH: overview. https://www.nuh.com.sg/About-NUH/Pages/overview.aspx. Accessed February 19, 2021.
- 24. Mills JL Sr, Conte MS, Armstrong DG, et al. The society for vascular surgery lower extremity threatened limb classification system: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014;59(1):220‐234.e222. [DOI] [PubMed] [Google Scholar]
- 25. Hicks CW, Canner JK, Mathioudakis N, et al. The Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification independently predicts wound healing in diabetic foot ulcers. J Vasc Surg. 2018;68(4):1096‐1103. [DOI] [PubMed] [Google Scholar]
- 26. Monteiro‐Soares M, Boyko EJ, Jeffcoate W, et al. Diabetic foot ulcer classifications: a critical review. Diabetes Metab Res Rev. 2020;36:e3272. [DOI] [PubMed] [Google Scholar]
- 27. Robinson WP, Loretz L, Hanesian C, et al. Society for Vascular Surgery Wound, Ischemia, foot Infection (WIfI) score correlates with the intensity of multimodal limb treatment and patient‐centered outcomes in patients with threatened limbs managed in a limb preservation center. J Vasc Surg. 2017;66(2):488‐498.e482. [DOI] [PubMed] [Google Scholar]
- 28. van Reijen NS, Ponchant K, Ubbink DT, Koelemay MJ. Editor's choice–the prognostic value of the WIfI classification in patients with chronic limb threatening ischaemia: a systematic review and meta‐analysis. Eur J Vasc Endovasc Surg. 2019;58(3):362‐371. [DOI] [PubMed] [Google Scholar]
- 29. Tecilazich F, Veves A. Role of peripheral neuropathy in the development of foot ulceration and impaired wound healing in diabetes mellitus. Nutritional and Therapeutic Interventions for Diabetes and Metabolic Syndrome. London, UK: Elsevier; 2018:95‐104. [Google Scholar]
- 30. Schaper NC, van Netten JJ, Apelqvist J, et al. Practical Guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36:e3266. [DOI] [PubMed] [Google Scholar]
- 31. Meloni M, Izzo V, Manu C, et al. Fast‐track pathway: an easy‐to‐use tool to reduce delayed referral and amputations in diabetic patients with foot ulceration. Diabetic Foot. 2019;22(2):39. [Google Scholar]
- 32. Meloni M, Martinez JLL, Ahluwalia R, et al. Effectiveness of fast‐track pathway for diabetic foot ulcerations. Acta Diabetol. 2021;58:1351‐1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nickinson AT, Dimitrova J, Houghton JS, et al. Does the introduction of a vascular limb salvage service improve one year amputation outcomes for patients with chronic limb‐threatening ischaemia? Eur J Vasc Endovasc Surg. 2021;61(4):612‐619. [DOI] [PubMed] [Google Scholar]
- 34. Adeleye OO, Ugwu ET, Gezawa ID, Okpe I, Ezeani I, Enamino M. Predictors of intra‐hospital mortality in patients with diabetic foot ulcers in Nigeria: data from the MEDFUN study. BMC Endocr Disord. 2020;20(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shatnawi NJ, Al‐Zoubi NA, Hawamdeh HM, Khader YS, Garaibeh K, Heis HA. Predictors of major lower limb amputation in type 2 diabetic patients referred for hospital care with diabetic foot syndrome. Diabetes Metab Syndr Obes. 2018;11:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gazzaruso C, Gallotti P, Pujia A, Montalcini T, Giustina A, Coppola A. Predictors of healing, ulcer recurrence and persistence, amputation and mortality in type 2 diabetic patients with diabetic foot: a 10‐year retrospective cohort study. Endocrine. 2021;71(1):59‐68. [DOI] [PubMed] [Google Scholar]
- 37. Shin JY, Roh S‐G, Sharaf B, Lee N‐H. Risk of major limb amputation in diabetic foot ulcer and accompanying disease: a meta‐analysis. J Plast Reconstr Aesthet Surg. 2017;70(12):1681‐1688. [DOI] [PubMed] [Google Scholar]
- 38. Meloni M, Izzo V, Giurato L, Cervelli V, Gandini R, Uccioli L. Impact of heart failure and dialysis in the prognosis of diabetic patients with ischemic foot ulcers. J Clin Transl Endocrinol. 2018;11:31‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu L, Qian H, Gu J, Shi J, Gu X, Tang Z. Heart failure in hospitalized patients with diabetic foot ulcers: clinical characteristics and their relationship with prognosis. J Diabetes. 2013;5(4):429‐438. [DOI] [PubMed] [Google Scholar]
- 40. Alahdab F, Wang AT, Elraiyah TA, et al. A systematic review for the screening for peripheral arterial disease in asymptomatic patients. J Vasc Surg. 2015;61(3):42S‐53S. [DOI] [PubMed] [Google Scholar]
- 41. Hirsch AT, Criqui MH, Treat‐Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317‐1324. [DOI] [PubMed] [Google Scholar]
- 42. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR. Inter‐society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(1):S5‐S67. [DOI] [PubMed] [Google Scholar]
- 43. Boufi M, Ghaffari P, Allaire E, Fessi H, Ronco P, Vayssairat M. Foot gangrene in patients with end‐stage renal disease: a case control study. Angiology. 2006;57(3):355‐361. [DOI] [PubMed] [Google Scholar]
- 44. Vr F, Jirkovská A, Vr P, Boucek P, Skibová J. Comparison of microbial findings and resistance to antibiotics between transplant patients, patients on hemodialysis, and other patients with the diabetic foot. J Diabetes Complications. 2004;18(2):108‐112. [DOI] [PubMed] [Google Scholar]
- 45. Kokkinidis DG, Arfaras‐Melainis A, Giannopoulos S, et al. Statin therapy for reduction of cardiovascular and limb‐related events in critical limb ischemia: a systematic review and meta‐analysis. Vasc Med. 2020;25(2):106‐117. [DOI] [PubMed] [Google Scholar]
- 46. Pastori D, Farcomeni A, Milanese A, et al. Statins and major adverse limb events in patients with peripheral artery disease: a systematic review and meta‐analysis. Thromb Haemost. 2020;120(05):866‐875. [DOI] [PubMed] [Google Scholar]
- 47. Antoniou GA, Fisher RK, Georgiadis GS, Antoniou SA, Torella F. Statin therapy in lower limb peripheral arterial disease: systematic review and meta‐analysis. Vascul Pharmacol. 2014;63(2):79‐87. [DOI] [PubMed] [Google Scholar]
- 48. Kolossvary E, Ferenci T, Kovats T. Potentials, challenges, and limitations of the analysis of administrative data on vascular limb amputations in health care. Vasa. 2019;49:87‐97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


