Abstract
Inguinal and incisional hernias are the two most common types of hernias caused by abdominal wall weakness and defects in connective tissue. The structure of the extracellular matrix, mainly collagen and metalloproteinases (MMPs), and their regulators have been studied extensively and found to play a significant role in the pathophysiology of hernias. One of the regulators of MMPs, tissue inhibitor metalloproteinases (TIMPs), bind to MMPs and inhibit its activity significantly shifting the balance towards collagen synthesis rather than degradation. Due to their importance in collagen metabolism, their metabolism might be significant in the aetiology of hernias. Our study used immunohistochemical techniques to investigate the possible effects of TIMP 1 and 2 on the samples taken from the abdominal walls of patients with inguinal and incisional hernias, compared them with control patients, and reviewed the literature. In this study, samples of 90 patients (30 patients from control, inguinal hernia, and incisional hernia groups) were taken and analysed. These samples were stained with TIMP‐1 Ab‐2 and TIMP2 Ab‐5 (Clone 3A4) antibodies and evaluated under ×100 magnification. The degree of staining was classified as (a): No staining (0), (b): Staining less than 10% (I), (c): Staining between 10% and 50% (II), (d): Staining more than 50% (III). Statistical analyses were done. No significant difference was found between groups in terms of patient demographics. Smoking and family history of hernia was not found to be associated with TIMP expression. TIMP1 expression was significantly higher in the incisional and inguinal hernia group than in the control group (P < .05), while the level of TIMP2 was higher in the control group. (P < .05). TIMP1 and TIMP2 levels did not significantly differ between incisional and inguinal hernia groups. We found significantly increased TIMP‐1 levels in tissue samples from patients with hernia supporting its suggested role in hernia pathophysiology. Local alterations in MMP and TIMP levels might play a role in the pathogenesis of hernias. Thus detection of TIMP in tissues can be important for clinical use after further validation studies. In the era of molecular medicine, detecting TIMP levels in hernia patients can impact clinical practice.
Keywords: incisional hernia, inguinal hernia, matrix metalloproteinase
1. INTRODUCTION
Incisional hernias are abdominal wall hernias encountered often after surgical procedures. Their incidence used to be 20%. However, current studies report an incidence of 10%. 1 Incisional hernias relapse 30% to 50% following primary repair. This ratio decreases from 0% to 15% following mesh repair. 2 Obesity, type of incision, type of suture material, presence of wound infection, older age, malnutrition, presence of ascites, long term usage of corticosteroids, diabetes mellitus, connective tissue diseases, smoking, chronic systemic diseases such as malignancy or chronic obstructive pulmonary disease, emergency operations and postoperative sepsis are the most common etiologic factors of hernias. 3 , 4 Studies in recent years have focused on metalloproteinases (MMPs), their inhibitors, and their metabolism in the aetiology of hernias.
2. BIOLOGICAL FACTORS
2.1. Disorders of collagen metabolism
Collagen is the primary component of the extracellular matrix. Type 1 collagen is the mature and the most potent collagen, while type 3 is the immature isoform. Hernias and recurrence are more common in patients with connective tissue disorders such as Marfan, Ehler Danlos, and osteogenesis imperfecta. 5 These findings led the researchers to investigate the importance of collagen metabolism in the aetiology of hernias.
Friedman et al Showed the increased synthesis of type 3 collagen and decreased type 1/type 3 collagen in patients with inguinal hernia. 6 Later, various studies showed that the extracellular matrix primarily comprises type 3 collagen. 7 Finally, Nikolov and Beltschev performed a study with the electron microscope. They showed disorganisation in microfibrils' wrapped structure, forming of dysplastic collagen microfibrils, collagenophagia, and intracellular and extracellular collagenolysis in patients with direct inguinal hernia. 8
2.2. Matrix metalloproteinases
They are the essential enzymes responsible for the extracellular matrix's degradation (ECM). 9 Remodelling of collagen is concurrent and continuous in ECM. In collagen remodeling, collagenases are released from endothelial cells, fibroblasts, inflammatory cells (macrophage, neutrophils), and keratinocytes. 10 Lysis of different collagen types is controlled by the balance between MMPs and their inhibitors. 11
MMP family is an essential member of extracellular proteinases. Their role has been shown in various physiological and pathologic processes. These enzymes are crucial to the turnover of ECM, tissue remodelling, angiogenesis, and morphogenesis. They are also known to participate in cell migration, invasion, proliferation, and apoptosis. 12 MMP activity is controlled by gene expression, regulation of zymogens, inhibition of active enzymes with specific inhibitors, α‐2 macroglobulin, and tissue inhibitors of metalloproteinases (TIMPs). 13
MMPs are secreted from various cells. MMP‐1, macrophages, monocytes, fibroblasts, keratinocytes, chondrocytes, hepatocytes, and many tumour cells. MMP‐8; chondrocytes, synovial fibroblasts, and endothelial cells. MMP‐3 and 10; fibroblastic reticular cells, normal and transformed squamous epithelial cells. MMP‐9; keratinocytes, monocytes, polymorphonuclear leukocytes, and malignant cells. 12
In a Turkish study, levels of MMP‐1, 2, and 9 were significantly increased in hernia patients compared to the control patients. 14
2.3. Structure and functions of tissue inhibitor of metalloproteinases (TIMPs)
The proteolytic activity of MMP can be controlled by both non‐specific (α‐2 macroglobulin, α‐1 anti‐protease) and specific inhibitors. 15 Inhibitor activity of TIMPs is the result of their noncovalent binding with MMPs. Four different types of TIMPs have been defined so far. 16
TIMP‐1; is a glycoprotein. Fibroblast growth factor, platelet‐derived growth factor, phorbol esters, and interleukin 11 are exciters of TIMP‐1 expression in fibroblasts. 17 TIMP‐1 binds to pro‐MMP‐9, a member of the gelatinase family. This proMMP‐9/TIMP‐1 complex inhibits all the active MMPs and forms a more stable complex of proMMP‐9/TIMP‐1/MMP triad. 18
TIMP‐2; is a non‐glycolysed protein that is isolated from melanoma cells for the first time. It inhibits MMPs except for MMP‐9. It is secreted with proMMP‐2, mainly from fibroblasts. Conversely, it is secreted solely from alveolar macrophages. 17
In our study, we used immunohistochemical techniques to investigate the possible effects of TIMP 1 and 2, which are thought to take a role in the remodelling of ECM, on the samples taken from the abdominal walls of patients with inguinal and incisional hernias, compared them with control patients and reviewed the literature.
3. MATERIALS AND METHODS
Following the local Ethics Committee's approval, 90 patients were included in the study prospectively. We operated on them for various abdominal hernias from January to December 2014 in our clinic. Patients were divided into three equal groups; the first group consisted of 30 inguinal hernia patients, the second group of incisional hernia patients, and the third group established the control patients. The Control group consisted of patients who operated on other disorders rather than hernia and malignancy. All of the patients signed informed consent, expressing the study and the procedure.
The inguinal hernia group consisted of patients with direct inguinal hernia (excluding recurrent cases and patients with a bilateral hernia). The incisional hernia group consisted of patients who had a hernia following a laparotomy (patients who underwent surgery for malignancy were excluded). Patients with diabetes, connective tissue disease, pregnancy, and steroid therapy were excluded from the study in both groups.
Sampling: 1 × 1 cm2 tissue samples were obtained from the fascia layers of abdominal walls (anterior rectus sheath and linea alba) of the patients with incisional hernia and control patients. Samples of the same size were obtained from patients with direct inguinal hernia's intact part of the transverse fascia. All of the samples were sent to the pathology department in a 10% formaldehyde solution.
Patients in the second and third groups were operated under general anaesthesia, while patients with inguinal hernias were operated under spinal anaesthesia. The control group underwent explorative laparotomy, peptic ulcus perforation, open cholecystectomy, mechanical intestinal obstruction, open appendectomy, and open splenectomy. Patients with any hernias were excluded from the control group.
Evaluation: Immunohistochemical evaluation was performed using polyclonal or monoclonal antibodies against various antigens on the tissue. 19 We used TIMP1 Ab‐2 (Clone 102D1) (Thermo) and TIMP2 Ab‐5 (Clone 3A4) (Thermo) as antibodies.
Tissue samples from 90 individuals were sent to the pathology department in 1% formaldehyde solution, and the evaluation was performed as pathologist‐blinded by a single pathologist. The samples were deparaffinised by full automated BenchMark Ultra IHC/ISH (Ventana). Following the antigens' reveal with retrieval solution, the slides were incubated with ultraview copper for 4 minutes and with haematoxylin for 8 minutes for background staining. Lastly, the slides were washed with water‐based balsam. Later, the samples were analysed using a light microscope. The samples were scanned fully with a 100x microscope. The ratio of stained cells in each region to the total number of cells in the same region was calculated. Those ratios were divided into the number of the sample areas, so an average of staining for each case was determined.
The slides were evaluated as; (a): No staining (0), (b): Staining less than 10% (I), (c): Staining between 10% and 50% (II), (d): Staining more than 50% (III) (Figure 1A‐D).
FIGURE 1.
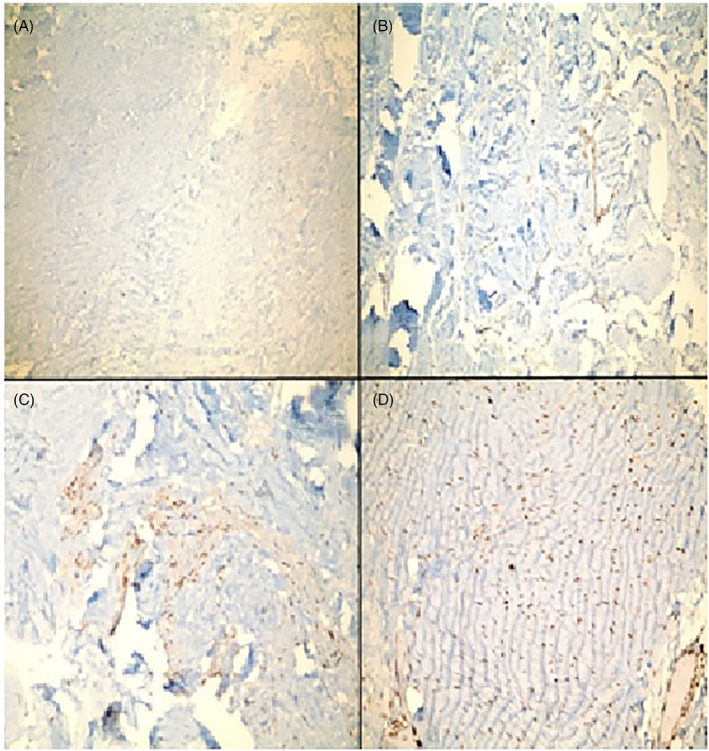
(A‐D) Microscopic images of samples after immunohistochemical staining. (A) TIMP 2 without immune staining, which we accepted as score 0. (B) Immune staining of TIMP 2, less than 10% and accepted as score 1. (C) Immune staining of TIMP 1, between 10% and 50% and accepted as score 2. (D) Immune staining of TIMP 1, over 50% and accepted as score 3. TIMP, tissue inhibitor metalloproteinases
3.1. Statistics
The descriptive statistics of the data, mean average, standard deviation, median, minimum‐maximum ratio, and frequency, were used. The distribution of the variants was evaluated with the Kolmogorov Smirnov test. ANOVA test was performed for the analysis of quantitative data and Chi‐square test for qualitative data. SPSS 22.0 was used for overall analysis.
4. RESULTS
We evaluated and compared the demographics of 30 individuals in each group (Table 1). The mean age was 55.4 ± 8.1 in the inguinal hernia group, 53.2 ± 10.4 in the incisional hernia group, and 49.0 ± 14.2 in the control group. The female/male ratio was 8/22, 7/23, and 7/23 and in the groups, respectively. There were not any significant differences between groups in terms of gender or age. Patients' mean body mass index score (BMI) was 28.1 ± 3.7 in the inguinal hernia group, 28.5 ± 2.4 in the incisional hernia group, and 27.1 ± 3.5 in the control group. There were not any statistically significant differences between the groups in means of BMI score.
TABLE 1.
Demographics of each group
| Control | Incisional | Inguinal | P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Mean ± s.s. | 49.0 ± 14.2 | 53.2 ± 10.4 | 55.4 ± 8.1 | .086 | ||||||
| Med(Min‐Mak) | 49 | 21.0‐74.0 | 52 | 37.0‐73.0 | 33.0‐71.0 | ||||||
| Sex | Female | n (%) | 7 | 23.3% | 7 | 23.3% | 8 | 26.7% | .942 | ||
| Male | n (%) | 23 | 76.7% | 23 | 76.7% | 22 | 73.3% | ||||
| BMI | Mean ± s.s. | 27.1 ± 3.5 | 28.5 ± 2.4 | 28.1 ± 3.7 | .230 | ||||||
| Med(Min‐Mak) | 28 | 21.0‐33.9 | 29 | 23.3‐33.4 | 21.0‐37.9 | ||||||
| Smoking | − | n (%) | 14 | 46.7% | 10 | 33.3% | 12 | 40.0% | .574 | ||
| + | n (%) | 16 | 53.3% | 20 | 66.7% | 18 | 60.0% | ||||
| Family | − | n (%) | 26 | 86.7% | 27 | 90.0% | 27 | 90.0% | .894 | ||
| + | n (%) | 4 | 13.3% | 3 | 10.0% | 3 | 10.0% | ||||
Note: ANOVA/Chi‐square test.
Abbreviations: ANOVA, analysis of variance; TIMP, tissue inhibitor metalloproteinases.
Sixteen patients in the control group, 20 patients in the incisional hernia group, and 18 patients in the inguinal hernia group were active smokers. Smoking was not found to affect TIMP staining.
The positive family history was 3/30, 3/30, and 4/30 in the groups, respectively. The results were not significantly different between the groups.
Levels of TIMP‐1 were significantly higher in groups 1 and 2 compared to the control group (P < .05). The difference between the first two groups was not significant (Table 2). Levels of TIMP‐2 were significantly lower in groups 1 and 2 compared to the control group (P < .05). There were not any statistically significant differences in groups 1 and 2 (Table 3).
TABLE 2.
TIMP‐1 levels in groups 1 to 3
| Control | Incisional | Inguinal | P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TIMP‐1 | 0 | n (%) | 9 | 30.0% | 0* | 0% | 2 | 6.7%* | .001 |
| I | n (%) | 15 | 50.0% | 12 | 40.0% | 14 | 46.7% | ||
| II | n (%) | 6 | 20.0% | 11 | 36.7% | 13 | 43.3% | ||
| III | n (%) | 0 | 0.0% | 7 | 23.3% | 1 | 3.3% | ||
Note: Chi‐Square test/Fischer test.
Bold values indicate statistically significant values.
Abbreviation: TIMP, tissue inhibitor metalloproteinases.
P < .05 (compared with controls).
TABLE 3.
TIMP‐2 levels in groups 1 to 3
| Control | Incisional | Inguinal | P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TIMP‐2 | 0 | n (%) | 11 | 36.7% | 19 | 63.3%* | 21 | 70%* | .022 |
| I | n (%) | 15 | 50.0% | 11 | 36.7% | 9 | 30.0% | ||
| II | n (%) | 4 | 13.3% | 0 | 0.0% | 0 | 0.0% | ||
Note: Chi‐Square test.
Bold values indicate statistically significant values.
Abbreviation: TIMP, tissue inhibitor metalloproteinases.
P < .05 (compared with controls).
The distribution of staining levels of TIMP‐2 in group 1 was; (0) in 21 and (I) in 9 patients. The scores were (0) in 19 and (I) in 11 patients in the second group. In the control group, the levels were found as; (0) in 11, (I) in 15, and (II) in 4 patients.
5. DISCUSSION
In recent years, studies focused on metabolic leading points in inguinal and incisional hernia aetiology rather than types of repair. Collagen fibres and metalloproteinase activity with tissue inhibitors that regulate collagen metabolism are the main subjects that concern the researchers investigating hernia aetiology. 20
Collagenases are enzymes responsible for breaking the peptide bond in collagen and released from endothelial cells, fibroblasts, inflammatory cells, and keratinocytes. The ratio of collagen types is balanced by the activities of MMP and TIMP in the body. TIMP binds to the active part of MMP and inhibits covalent interaction. 11 MMP expressions are regulated by extracellular matrix interaction, cytoskeleton transitions, growth factors, cytokines, and various hormonal activities. 21 MMP activity is under the control of especially alfa‐2 macroglobulin activity and TIMPs. 13 MMP‐2 breaks down mainly type IV, V, VII, X, XI and gelatin, proteoglycan, fibronectin, and elastin. High serum levels of MMP‐2 are related to basal membrane degradation, tumour invasion, and systemic metastases. 22 Bellon et al determined high levels of MMP‐2 in the fascia of patients with direct inguinal hernia. 5
The most essential function of TIMP is to inhibit the functions of MMP. It consists of four multifunctional protein groups and supplies the balance between synthesis and breaking down ECM proteins. 23 There are partially‐defined complex interactions between MMP, TIMP, cytokines, and growth factors that determine inflammation and remodelling mediators. 13 TNF‐α plays a role in the pathogenesis of chronic inflammation in vivo and is one of MMP protein production's essential inductors. 24 Read has shown that the substances in cigarette smoke inhibit antiproteases and increase the ratio of protease/antiprotease in blood. 25
In a study conducted in Turkey in 2005, TIMP‐2 levels were lower in patients with direct hernia than the patients with indirect hernia and the control group. 26 However, their results were similar to our study. In our study, the levels were significantly decreased in the hernia groups compared to the control group, but there was no statistically significant difference between the hernia groups.
In 2009, a prospective study in Spain evaluated MMP/TIMP levels' transitions and inflammatory findings in patients with incisional hernias. They performed zymography, polymerase chain reaction (PCR), and immunologic staining techniques. 27 As a result, they determined ECM loss, an increase of MMP‐1 in the aponeurotic tissue, MMP‐2,9,4 in the skeletal muscle, and a decrease of MMP‐3,13, TIMP‐4 in the aponeurotic tissue, TIMP‐3 in the skeletal muscle. In addition, they showed increased levels of TNF‐α and IL‐6 in the incisional hernia group.
A similar prospective study was conducted in Poland in 2010, and serum levels of MMP‐2 and TIMP‐2 were evaluated in hernia patients. 28 MMP‐2 was found to be higher in hernia groups, being highest in the direct inguinal hernia group TIMP‐2 was found to be significantly higher only in the recurrent hernia group. Concurrent and statistically significant elevated MMP‐2 and TIMP‐2 made the researchers think it was the underlying aetiology of extracellular matrix degradation in the recurrent group. Our study determined high levels of TIMP‐1 in the tissue samples, while TIMP‐2 levels were decreased, conversely to this study.
Another study group investigated MMP‐2 and MMP‐9 levels with TIMP‐1 and TIMP‐2 in plasma and tissues of patients with inguinal hernia and compared with the control group in 2011, using ELISA. 29 In the control group, plasma levels of MMP‐2, MMP‐9, and TIMP‐1 were significantly higher than in the inguinal hernia group, while TIMP‐2 levels were decreased. In addition, MMP‐2 and MMP‐9 were increased in the hernia group in tissue samples, while TIMP‐1 and TIMP‐2 decreased in the control group. Our study found reduced levels of TIMP‐2 in tissue samples of hernia patients similar to these results, but TIMP‐1 levels were higher than controls.
In a more recent and more far‐reaching study in 2013, in 72 patients with incisional hernia out of 305 patients with a history of laparotomy, serum levels of MMP‐1, MMP‐2, MMP‐3, MMP‐7, MMP‐6, MMP‐9, MMP‐10, MMP‐12, MMP‐13 and TIMP‐1, TIMP‐2, TIMP‐4 were evaluated. 30 Levels of TIMP‐1 and MMP‐9 were found to be higher, but the increase was not statistically significant. Still, as a conclusion, the researchers stated that local alterations in MMP and TIMP levels might play a role in the pathogenesis. In our study, we found significantly increased TIMP‐1 levels in tissue samples.
We think that TIMPs may have a role in the aetiology of abdominal wall hernias through collagen metabolism and their crucial role in tissue degradation and remodelling, so they may also have pathophysiological importance. Although there has been published literature about this issue, the number of studies is insufficient and needs further research.
6. CONCLUSION
Our prospective study compared inguinal hernia, incisional hernia, and patients operated for other disorders rather than a hernia. No statistically significant difference was reported in means of demographics, family history, BMI, or smoking. We found that TIMP‐1 levels were significantly lower, and TIMP‐2 levels were significantly higher in inguinal and incisional hernia groups when compared to the control group. Thus, after further verification with randomised controlled trials and other prospective studies on big patient groups, TIMP levels can be used in clinical practice. Depending on its levels, treatment and follow‐up algorithms may be reconsidered. Patients with high TIMP‐1 may be approached carefully and closely followed up for recurrence in the future.
We conclude that our study can help achieve a better understanding of the histopathological aetiology of the disease and may lead to patient‐based prophylactic and therapeutic approaches in the future. This study may be one of the footsteps towards more comprehensive studies of the future.
CONFLICT OF INTEREST
The authors have no conflict of interest.
AUTHOR CONTRIBUTIONS
Drafting of the manuscript: Uğur Durukan; Analysis and interpretation of the data: Orhan Agcaoglu, Salih Nafiz Karahan, İbrahim Ozata; Study conception and design: Emre Ozoran, Yigit Duzkoylu; Esra Pasaoglu; Critical revision of the manuscript: Acar Aren.
Durukan U, Agcaoglu O, Ozoran E, et al. The role of tissue inhibitor of metalloproteinases in the aetiology of inguinal and incisional hernias. Int Wound J. 2022;19(6):1502-1508. doi: 10.1111/iwj.13746
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Bender JS. Open retrofascial incisional hernia repair is a safe and effective operation. Am J Surg. 2016;211(3):589‐592. [DOI] [PubMed] [Google Scholar]
- 2. Van't Riet M, de Vos van Steenwijk PJ, Bonthuis F, et al. Prevention of adhesion to prosteticmesh: comparison of barriers using an incisional hernia model. Ann Surg. 2003;237:123‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lau B, Kim H, Haigh PI, Tejirian T. Obesity increases the odds of acquiring and incarcerating noninguinal abdominal wall hernias. Am Surg. 2012;78(10):1118‐1121. [PubMed] [Google Scholar]
- 4. Davis JE. Major ambulatory surgery of the general surgical patients: management of the breast disease and hernias of the abdominal wall. Surg Clin North Am. 1987;67:733‐760. [DOI] [PubMed] [Google Scholar]
- 5. Bellon JM, Bajo A, Ga‐Honduvilla N, et al. Fibroblasts from the transversalis fascia of young patients with direct inguinal hernias show constituve MMP‐2 overexpression. Ann Surg. 2001;233:287‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Friedman DW, Boyd CD, Norton P, et al. Increases in type III collagen gene expression and protein synthesis in patients with inguinal hernias. Ann Surg. 1993;218:754‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Casanova AB, Trindade EN, Trindade MR. Collagen in the transversalis fascia of patients with indirect inguinal hernia: a case‐control study. Am J Surg. 2009;198:1‐5. [DOI] [PubMed] [Google Scholar]
- 8. Nikolov S, Beltschev B. Several ultrastructural peculiarities of the fascia transversalis in direct inguinal hernias of senile men. Anat Anz. 1990;170:265‐272. [PubMed] [Google Scholar]
- 9. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562‐573. [DOI] [PubMed] [Google Scholar]
- 10. Elkington PT, Green JA, Friedland JS. Analysis of matrix metalloproteinase secretion by macrophages. Methods Mol Biol. 2009;531:253‐265. [DOI] [PubMed] [Google Scholar]
- 11. Kleiner DE, Stetler‐Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol. 1999;43(Suppl 1):42‐51. [DOI] [PubMed] [Google Scholar]
- 12. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases structure, function, and biochemistry. Circ Res. 2003;92:827‐839. [DOI] [PubMed] [Google Scholar]
- 13. Clark IM, Swingler TE, Sampieri CL, Edwards DR. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol. 2008;40:1362‐1378. [DOI] [PubMed] [Google Scholar]
- 14. Aren A, Gokce AH, Gokce FS, Dursun N. Roles of matrix metalloproteinases in the etiology of inguinal hernia. Hernia. 2011;15(6):667‐671. [DOI] [PubMed] [Google Scholar]
- 15. Woessner FJ. Matrix metalloproteinase and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145‐2154. [PubMed] [Google Scholar]
- 16. Matrisian LM. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990;6(4):121‐125. [DOI] [PubMed] [Google Scholar]
- 17. Curran S, Murray GI. Matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 1999;189(3):300‐308. [DOI] [PubMed] [Google Scholar]
- 18. Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18(5):1135‐1149. [DOI] [PubMed] [Google Scholar]
- 19. Rosai J. Special techniques in surgical pathology. Ackerman's Surgical Pathology. Vol 1. 8th ed. New York, NY; Mosby; 1996:34‐35. [Google Scholar]
- 20. Rosch R, Klinge V, Si ZY, Junge K, Klosterhalfen B, Schumpelick V. A role for the collagen I/III and MMP‐1/13 genes in primary inquinal hernia. BMC Med Genet. 2002;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee MH, Murphy G. Matrix metalloproteinases at a glance. J Cell Sci. 2004;117:4015‐4016. [DOI] [PubMed] [Google Scholar]
- 22. Kugler A. Matrix metalloproteinases and their inhibitors. Anticancer Res. 1999;19:1589‐1592. [PubMed] [Google Scholar]
- 23. Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74(2):111‐112. [PubMed] [Google Scholar]
- 24. Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200:448‐464. [DOI] [PubMed] [Google Scholar]
- 25. Read RC. “Blood protease”/antiprotease imbalance in patients with acguired herniation prob. Gen Surg. 1995;12:41‐46. [Google Scholar]
- 26. Abci I, Bilgi S, Altan A. Role of TIMP‐2 in fascia transversalis on development of inguinal hernias. J Investig Surg. 2005;18(3):123‐128. [DOI] [PubMed] [Google Scholar]
- 27. Jordi GM, Ramon D, Maria TQ, et al. MMPs/TIMPs and inflammatory signalling de‐regulationin human incisional hernia tissues. J Cell Mol Med. 2009;13(11–12):4432‐4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smigielski J, Brocki M, Kuzdak K, Kołomecki K. Serum MMP 2 and TIMP 2 in patients with inguinal hernias. Eur J Clin Investig. 2011;41(6):584‐588. [DOI] [PubMed] [Google Scholar]
- 29. Antoniou GA, Tentes IK, Antoniou SA, Simopoulos C, Lazarides MK. Matrix metalloproteinase imbalance in inguinal hernia formation. J Investig Surg. 2011;24(4):145‐150. [DOI] [PubMed] [Google Scholar]
- 30. Henriksen NA, Sørensen LT, Jorgensen LN, Ågren MS. Circulating levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with incisional hernia. Wound Repair Regen. 2013;21:661‐666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


