Abstract
Being sessile organisms, plants cannot escape unwanted changes in the environment. The rapid human population explosion caused significant environmental problems. Heavy metals produced through various sources can cause severe damage to living organisms. The study was planned to evaluate four grass species’ morpho-physiological growth characteristics and phytoremediation capabilities under chromium (Cr) and lead stress (Pb) in the arid climate. Typha angustifolia, Tragus roxburghii, Aeluropus logopoides, and Cenchrus ciliaris grass species were used for the study. One-year-old stubbles from the Cholistan desert were used for the experiment. Cr treatments in the form of K2Cr2O7 were applied at 0, 20, 40, and 80 mg L–1, whereas Pb was applied as PbCl2 at 0, 50, 200, and 500 mg L–1 as control, low, moderate and high-stress, respectively. After 6 weeks of heavy metals treatments, plants were harvested and analyzed for growth performance and phytoremediation capabilities. Results depicted that, regarding morphological attributes, T. angustifolia performed better, followed by C. ciliaris; no clear pattern was observed for T. roxburghii and A. logopoides. The CO2 assimilation rate (Co2d) and water use efficiency (WUE) increased as the heavy metal stress increased in all species under both metals. In contrast, total chlorophyll content was higher under low stress. Other physiological parameters, such as relative humidity (RHd), net photosynthetic rate (A), transpiration rate (E), stomatal conductance (Gs), leaf internal CO2 concentration (Ci) and membrane stability index (MSI) gradually decreased as the Cr, and Pb stress levels increased among all the species. Moreover, Cr and Pb absorption contents of T. angustifolia were higher than the other three species at each stress level. Overall, T. angustifolia thrived against heavy metals stress and showed higher biomass, maximum photosynthetic measurements, WUE and higher metal absorption among all the selected species. Results concluded that although all the selected species behaved fine under stress conditions, T. angustifolia performance was better; thus, it can be used to remediate the soil near industrial estates.
Keywords: productivity, biochemical attributes, gas exchange, heavy metals, dry climate, Cholistan
Introduction
Abiotic stresses are assessed to be the main cause of the yield reduction in plants and thus, are considered a prodigious constraint to crop productivity (Yousaf et al., 2018; Gilani et al., 2020; Ali et al., 2021; Ahmad et al., 2022; Hussain et al., 2022; Nawaz et al., 2022; Panikar et al., 2022). Plants are often sensitive to the low and high accessibility of different heavy metal ions as essential micronutrients (Awad et al., 2021; Javed et al., 2021; Koleva et al., 2022; Shabbir et al., 2022; Xiao et al., 2022). Heavy metals are elements with a large atomic mass and are at least five times denser than water (Tchounwou et al., 2012; Singhal et al., 2022). They are not biodegradable in natural systems and are ready to mount health dangers when they exceed permissible concentrations (Javed et al., 2020; De Conti et al., 2021; Abbas et al., 2022). The key sources of heavy metal emissions are industries such as mining, painting, car-making, smelters, and metal refining (Singh et al., 2011). Rapid population increase, excessive industrialization, and challenging farming practices contribute to significant environmental problems and large quantities of hazardous waste being generated and disposed of in the soil (Lin et al., 2022). Heavy metals like lead (Pb), arsenic (As), cadmium (Cd), copper (Cu), zinc (Zn), nickel (Ni), chromium (Cr), and mercury (Hg) are continuously being introduced into the atmosphere by removing metropolitan sludge and industrial waste from agricultural and agrochemical soils, are the main components of inorganic pollutants (Gunatilake, 2015; Javad et al., 2022).
In recent years, compelling work into emerging cost-effective remediation techniques has contributed to public concerns about human health and environmental bullying resulting from soil contamination. The basic methods to cure polluted soils are cost-effective to install and maintain, namely chemical, physical and microbiological processes. A new green technique called phytoremediation has been developed, which uses plants to clean up soil, water, and the environment (Saha et al., 2017). Phytoremediation is dirt-free, uncomplicated, economical, and environmentally friendly, and the by-products will most notably have a range of other applications (De Conti et al., 2021). However, many restrictions on phytoremediation for pollution management involve a further study of plant and site-specific soil conditions. Environmental factors also decide phytoremediation’s effectiveness, as plants’ survival and development are badly affected by the harsh environment, toxicity, and soil characteristics in contaminated areas. Eventually, various forms of soil and water contaminants need different kinds of plant remediation vegetation, which adds to the complexity of the technology and requires a wide range of research activities before specific plants are released to commercialization (De Conti et al., 2021).
Due to their widespread toxicity, Cr and Pb, among heavy metals, have become more seriously toxic to plant functions (Nawaz et al., 2021; Shah et al., 2022). High soil Cr exposure causes oxidizing damage to plants, resulting in reactive oxygen species (ROS) formation by removing iron (Fe) from proteins and inhibiting the chloroplast and mitochondrial electron transport chains in plants (Scandalios, 2005). In addition, toxicity also influences the inhibition of the photosynthesis system and photosynthetic transportation of electrons (Romero-Puertas et al., 2007). Pb is not biodegradable in agricultural fields and can remain in the environment for 150–5,000 years (Saxena et al., 1999). Therefore, it is almost impossible to extract Pb from the soil once it reaches the soil system. It has a growth-inhibitor effect in plants, influencing photosynthesis and oxidative stress physiological and metabolic functions (Nouman et al., 2020).
Heavy metals (essential and non-essential) usually produce common toxic effects on plants, such as inhibition of growth, low biomass accumulation, photosynthesis reduction, senescence, altered water balance, and nutrient assimilation, which ultimately cause plant death (Singh et al., 2016). Therefore, this research is driven by the hope of decreasing the entry of heavy metals into crop plants, reducing the risk of contamination in animals and humans. The study’s objectives were to (1) evaluate four grass species (Typha angustifolia, Tragus roxburghii, Aeluropus logopoides and Cenchrus ciliaris) morpho-physiological growth characteristics and (2) phytoremediation capabilities under Chromium (Cr) and Lead stress (Pb). (3) To recommend the best-adapted plant species for phytoremediation in the arid climate.
Materials and Methods
Description of the study area
The total area of Cholistan is 26,000 km2, stretching from southern Bahawalpur of Punjab to the deserts of Nara and Thar in Sindh between latitudes 27° 42 N and 29° 45 N and longitude 69° 52E and 75° 24E, which is about 112 m above sea level. The desert of Cholistan is situated in a sub-tropical area, and its climate is arid to semi-arid. The mean annual rainfall ranges between 100 and 250 mm. The mean summer temperature is 34–38°C, and the average winter temperature is 15–20°C. The highest temperature could reach 51.6°C. Extreme temperatures and long droughts occasionally affect monsoon rainfall. These areas have very low humidity and high evaporation rates.
Experimental site and design
The experiment was conducted in the field area of the department of Forestry, Range and Wildlife Management, the Islamia University of Bahawalpur, from September to October 2019. The grass stumps of Typha angustifolia, Tragus roxburghii, Aeluropus logopoides, and Cenchrus ciliaris were collected from the Cholistan rangeland. The plants were trimmed to the uniform size of the stump, which was 10.16 cm. The stumps were placed in well-prepared plastic pots. Four stumps were planted per pot, plants were assigned to control, and different heavy metal stress treatments.
Four treatments with three replications per species were used, making it a total of 24 planted stumps. The control plants were irrigated with 300 mL of tap water four times a week. Stumps under heavy metals treatment were similarly treated with 300 mL water containing a solution of heavy metal salts of Pb and Cr (the concentrations are shown in Table 1). Cr treatments in the form of K2Cr2O7 were applied at 0, 20, 40, and 80 μmol/L, whereas Pb was applied as PbCl2 at 0, 50, 200, and 500 μmol/L as control, low, moderate, and high-stress treatments, respectively. Cr and Pb salt concentrations are shown in Table 1.
TABLE 1.
Treatment concentration levels of chromium (K2Cr2O7) and lead (PbCl2) salts.
| Stress level | K2Cr2O7 (mg/L) | PbCl2 (mg/L) | ||
| Control | T1 | 0 | T1 | 0 |
| Low stress | T2 | 113.15 | T2 | 67.14 |
| Moderate stress | T3 | 226.28 | T3 | 268.15 |
| High stress | T4 | 452.56 | T4 | 536.28 |
The experimental design was randomized to prevent the effects of microclimatic variations, and all the pots were rotated during the experiment. The experiment was carried out under normal atmospheric conditions with average temperature and humidity were 31.2 ± 2°C and 50 ± 4%, respectively. The Plants were harvested after 6 weeks of heavy metals stress.
Analysis of morphological parameters
After harvesting, samples were divided into two parts; one was kept for morphological parameters analysis, and the other was stored to analyze the physiological parameters. The morphological parameters such as leaves count, root and shoot length, and fresh and dry biomass were evaluated for T. angustifolia, T. roxburghii, A. logopoides, and C. ciliaris grass species under Cr and Pb stress. The root and shoot lengths were measured with a scale. Fresh biomass was measured immediately after harvesting. Representative samples of all the replicates were obtained for dry weight measurement. Dry weight was determined after oven-drying at 80°C until constant weight in the lab. The electrical balance was used to take the fresh and dry weight of the samples. Values were tabulated as a mean of three biological replicates for all the parameters.
Physiological parameters
Physiological parameters were calculated, including chlorophyll content, membrane stability index (MSI), and leaf gas exchange.
Chlorophyll content and membrane stability index
Chlorophyll contents were measured using the chlorophyll meter’s pad value. For MSI, one gram of fresh sample has been taken from pre-washed and autoclaved test tubes. In test tubes, 10 mL of ultrapure distilled water was applied and incubated at 45°C for 35 min. Incubation was done in the water bath, which was preheated. Electrical conductivity (C1) was calculated via a conductivity meter after incubation. A similar experiment was repeated for 15 min at 100°C. The second conductivity was measured as (C2). MSI was calculated using the formula below;
Leaf gas exchange measurements
For gas exchange parameters like net CO2 assimilation rate (μmol m–2 s–1), net photosynthetic rate (A), transpiration rate (mmolm–2 s–1), and stomatal conductance (mmol s–2 s–1), a portable infrared gas exchange analyzer was used. Well-lit leaves from 10 plants were carefully selected and placed in a 6 cm3 leaf chamber. The leaf chamber temperature was set at 20°C during the test, and the relative humidity was set to 400 μmol–1 as well as the CO2 reference. All measurements have taken place between 10:00 a.m. and 2:00 p.m. with the maximum light availability. As a combination of net CO2 assimilation rate and transpiration rate, effective water usage was determined (WUE) (Rasheed et al., 2015). All gas-exchange measurements were carried out at a leaf temperature of 25 ± 0.1°C, a leaf to air vapor pressure deficit of < 1.5 kPa, and a constant gas flow rate of 500 μmol s–1. To decrease the gas-exchange measurements time, the ambient CO2 concentration was increased to no more than 700 μmol mol–1.
Determination of chromium and lead content
A subset of 10 plants was randomly selected from the control and heavy metal treatment. Samples were washed and air-dried at 105°C for 24 h in fluidized bed dryer. The dried matter was then pulverized with Mortimer and pestle. Pyrex digestive pipes 0.5 g of powdered plant specimens were weighed and transferred to 100 mL (10 mL (HNO3; HClO4; 7:3 ratio) and 1,800°C heated digestive mixture). The temperature slowly rose until all HNO3 signs vanished. White flumes were exposed. Digestion lasted 30 min. Upon digestion, the tube rack was removed from the warm plate and cooled for 2 h. The resulting plant solutions were then diluted with distilled water up to 100 mL, and each diluted solution was filtered using Watman-42 filter paper and stored in sampling bottles (Guo et al., 2015). A blank solution (containing all reagents except plant samples) was also digested with other samples. The heavy metals concentration was calculated through the atomic absorption spectrophotometer (UV-Visible Spectrophotometer Carry-60) with 4 standards using distilled water with concentration ranges of 0.5, 1.0, 1.5, 2.0 ppm and a standard deviation of ± 0.01 mg/kg and with standard compared.
Statistical analysis
Two-way Analysis of Variance (ANOVA) was performed at a 5% probability level to investigate the treatments and species effect for all the studied parameters measured. A Pearson correlation was used to observe the association and intensity of the parameter in different species under various treatments. Values from a completely randomized design are indicated as mean ± SD, and the significant differences were compared using post hoc Tukey’s HSD test. Statistical analysis was performed using (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.) and figures were created using origin software.
Results
Morphological parameters
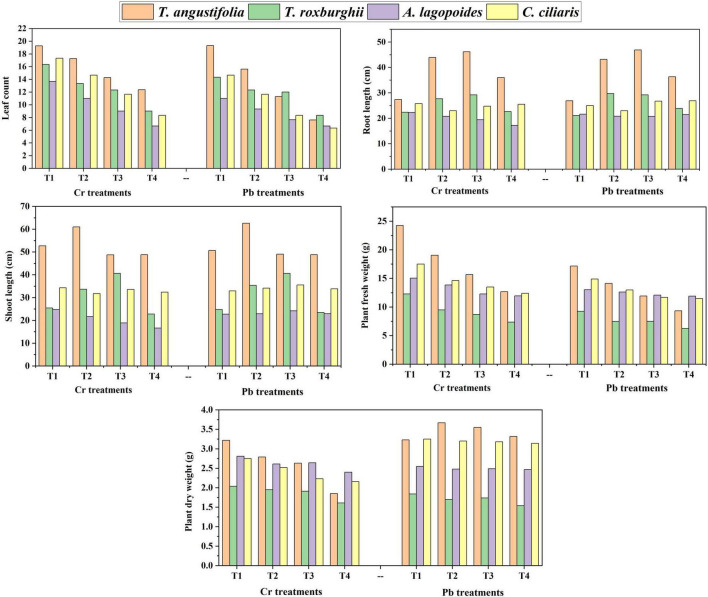
Columns in Figure 1 showed the values of all the morphological parameters for four grass species against Cr and Pb stresses varied significantly (Figure 1 and Supplementary Table 1). Leaf count decreased as the heavy metal stress increased in all the species. Interestingly root length and shoot length were higher under heavy metal stress conditions (T1 and T2) than in the control treatment. Plant fresh weight consistently decreased as the heavy metal stress increased in all the species. Plant dry weight also decreased as the Cr stress increased; however, no particular pattern was observed against Pb stress. Overall, T. angustifolia performed better, followed by C. ciliaris; no clear pattern was observed for T. roxburghii and A. logopoides (Figure 1 and Supplementary Table 1).
FIGURE 1.
Mean values of morphological parameters (leaf count, root length, shoot length, plant fresh weight and plant dry weight) against Chromium (Cr) and Chromium (Pb) concentrations in Typha angustifolia, Tragus roxburghii, Aeluropus lagopoides and Cenchrus ciliaris selected grass species.
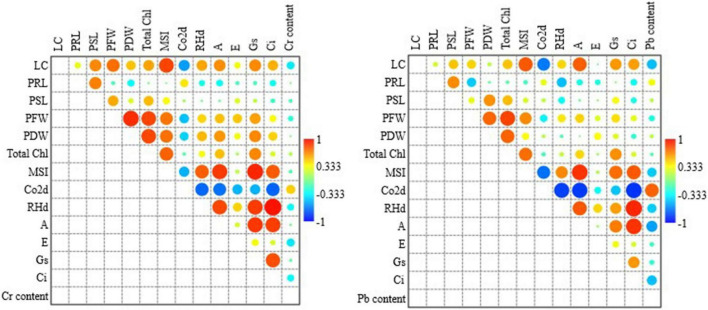
Across all grass species and treatments, leaf count was significantly correlated to shoot length and fresh biomass production. Root length was correlated to shoot length. Shoot length was also associated with fresh and dry biomass production. Moreover, there was a positive relationship between fresh and dry biomass production (Figure 2).
FIGURE 2.
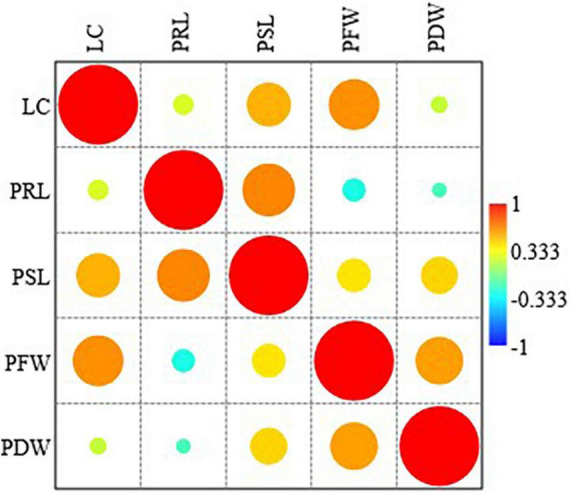
Pearson correlation analysis among the morphological parameters across all the treatments and species. LC, Leaf count; PRL, plant root length; PSL, plant shoot length; PFW, plant fresh weight and PDW, plant dry weight.
Gas exchange measurements
CO2 assimilation rate (Co2d) was higher under Cr stress conditions than in the control treatment. Although no massive difference was observed, the Co2d increased as the Cr stress increased in all species. Co2d was observed to be slightly higher than T. angustifolia followed by T. roxburghii. All other gas exchange parameters, such as relative humidity (RHd), net photosynthetic rate (A), transpiration rate (E), stomatal conductance (Gs) and leaf internal CO2 concentration (Ci), were higher under control treatment, and gradually decreased as the Cr stress levels increased among all the species (Table 2 and Supplementary Table 3). In comparing species, all the gaseous exchange parameters under Cr stress were higher in C. ciliaris followed by T. angustifolia, T. roxburghii and A. lagopoides (Table 2 and Supplementary Table 2).
TABLE 2.
Leaf gas exchange measurements against chromium (Cr) concentrations in Typha angustifolia, Tragus roxburghii, Aeluropus lagopoides, and Cenchrus ciliaris grass species.
| Treatment | Co2d (mol m–2 s–1) | RHd | A (μmol CO2 m–2 s–1) | E (mmol H2O m–2s–1) | Gs (mmol H2O m–2 s–1) | Ci (ppm) |
| Typha angustifolia | ||||||
| T1 | 12.59 ± 0.15 | 2.87 ± 0.02 | 4.24 ± 0.07 | 2.52 ± 0.13 | 23.22 ± 0.52 | 666.3 ± 14.52 |
| T2 | 19.14 ± 0.11 | 2.55 ± 0.14 | 3.91 ± 0.13 | 2.25 ± 0.05 | 24.05 ± 0.03 | 615.0 ± 6.74 |
| T3 | 19.55 ± 3.31 | 2.16 ± 0.11 | 3.20 ± 0.06 | 2.17 ± 0.05 | 21.74 ± 0.07 | 596.0 ± 7.21 |
| T4 | 19.56 ± 0.29 | 1.93 ± 0.04 | 3.14 ± 0.03 | 2.17 ± 0.04 | 20.06 ± 0.07 | 550.6 ± 3.51 |
| Tragus roxburghii | ||||||
| T1 | 12.55 ± 0.46 | 2.79 ± 0.15 | 4.29 ± 0.01 | 2.33 ± 0.01 | 23.27 ± 0.11 | 679.3 ± 3.78 |
| T2 | 18.69 ± 0.68 | 2.67 ± 0.08 | 3.98 ± 0.02 | 2.23 ± 0.01 | 22.98 ± 0.11 | 656.6 ± 6.65 |
| T3 | 18.76 ± 1.74 | 2.55 ± 0.08 | 3.27 ± 0.23 | 2.18 ± 3.46 | 21.18 ± 0.69 | 633.6 ± 6.42 |
| T4 | 18.85 ± 0.02 | 2.08 ± 0.09 | 2.87 ± 0.11 | 2.15 ± 0.01 | 19.88 ± 0.18 | 582.3 ± 14.64 |
| Aeluropus lagopoides | ||||||
| T1 | 14.07 ± 0.94 | 2.75 ± 0.05 | 4.71 ± 0.06 | 2.26 ± 0.05 | 23.86 ± 0.10 | 663.3 ± 8.32 |
| T2 | 18.23 ± 0.01 | 2.60 ± 0.08 | 4.22 ± 0.23 | 2.19 ± 1.52 | 23.62 ± 0.15 | 655.3 ± 5.50 |
| T3 | 17.53 ± 0.24 | 2.45 ± 0.05 | 3.79 ± 0.31 | 2.17 ± 1.21 | 21.92 ± 0.23 | 629.6 ± 17.03 |
| T4 | 19.03 ± 0.28 | 2.29 ± 0.01 | 3.13 ± 0.13 | 2.16 ± 0.03 | 20.50 ± 0.48 | 601.0 ± 2.64 |
| Cenchrus ciliaris | ||||||
| T1 | 12.50 ± 0.44 | 2.76 ± 0.77 | 4.35 ± 0.08 | 2.26 ± 0.15 | 23.28 ± 0.14 | 676.0 ± 4.58 |
| T2 | 17.76 ± 0.11 | 2.66 ± 0.02 | 4.26 ± 0.08 | 2.17 ± 0.77 | 22.87 ± 0.08 | 650.6 ± 1.15 |
| T3 | 17.82 ± 0.16 | 2.57 ± 0.03 | 3.24 ± 0.06 | 2.20 ± 0.05 | 22.62 ± 0.04 | 640.6 ± 6.02 |
| T4 | 17.84 ± 0.12 | 2.45 ± 0.02 | 3.14 ± 0.15 | 2.55 ± 0.20 | 21.83 ± 0.14 | 594.0 ± 12.76 |
Values are mean ± SD. “Co2d” is the CO2 assimilation rate, “RHd” is relative humidity, “A” is net photosynthetic rate, “E” is transpiration rate, “Gs” is stomatal conductance, and “Ci” is leaf internal CO2 concentration.
Regarding Pb stress, Co2d was higher under Pb stress conditions than in the control treatment. Significant differences were observed among species, and treatment and Co2d increased as the Pb stress increased among all species. All other gas exchange parameters gradually decreased as the Pb stress levels increased among all the species. While comparing species, all the gaseous exchange parameters under Pb stress were higher in T. roxburghii followed by C. ciliaris (Table 3 and Supplementary Table 2).
TABLE 3.
Leaf gas exchange measurements against lead (Pb) Concentrations in Typha angustifolia, Tragus roxburghii, Aeluropus lagopoides, and Cenchrus ciliaris grass species.
| Treatment | Co2d (mol m–2 s–1) | RHd | A (μmol CO2 m–2 s–1) | E (mmol H2O m–2s–1) | Gs (mmol H2O m–2 s–1) | Ci (ppm) |
| Typha angustifolia | ||||||
| T1 | 9.48 ± 0.03 | 2.63 ± 0.02 | 4.17 ± 0.01 | 2.22 ± 0.08 | 22.3 ± 0.13 | 644.3 ± 17.95 |
| T2 | 16.21 ± 0.07 | 2.26 ± 0.05 | 3.77 ± 0.20 | 2.13 ± 0.01 | 26.88 ± 3.18 | 610.3 ± 4.04 |
| T3 | 17.04 ± 0.06 | 1.86 ± 0.05 | 3.12 ± 0.96 | 2.11 ± 0.01 | 20.74 ± 0.07 | 589.3 ± 9.01 |
| T4 | 19.81 ± 0.60 | 1.65 ± 0.01 | 2.77 ± 0.17 | 2.07 ± 0.04 | 19.06 ± 0.07 | 548.6 ± 1.52 |
| Tragus roxburghii | ||||||
| T1 | 9.64 ± 0.18 | 2.70 ± 0.01 | 4.29 ± 0.01 | 2.23 ± 0.01 | 23.18 ± 0.01 | 662.6 ± 2.08 |
| T2 | 12.88 ± 0.50 | 2.54 ± 0.77 | 3.94 ± 0.05 | 2.13 ± 0.01 | 22.98 ± 0.01 | 649.6 ± 1.52 |
| T3 | 14.14 ± 0.07 | 2.40 ± 0.01 | 3.18 ± 0.01 | 2.08 ± 0.78 | 21.18 ± 0.69 | 621.3 ± 0.57 |
| T4 | 17.23 ± 0.01 | 1.94 ± 0.05 | 2.95 ± 0.06 | 2.04 ± 0.05 | 19.91 ± 0.12 | 595.3 ± 3.05 |
| Aeluropus lagopoides | ||||||
| T1 | 9.12 ± 0.11 | 2.63 ± 0.02 | 4.12 ± 0.04 | 2.20 ± 0.01 | 22.90 ± 0.07 | 652.6 ± 2.30 |
| T2 | 15.21 ± 0.07 | 2.55 ± 0.06 | 3.87 ± 0.17 | 2.09 ± 0.64 | 22.73 ± 0.15 | 642.0 ± 2.0 |
| T3 | 16.22 ± 0.04 | 2.40 ± 0.06 | 3.13 ± 0.01 | 2.07 ± 0.21 | 20.92 ± 0.23 | 620.3 ± 18.47 |
| T4 | 18.25 ± 0.02 | 2.17 ± 0.66 | 3.03 ± 0.09 | 2.04 ± 0.02 | 19.66 ± 0.01 | 576.3 ± 4.04 |
| Cenchrus ciliaris | ||||||
| T1 | 9.62 ± 0.27 | 2.76 ± 0.07 | 4.26 ± 0.01 | 2.23 ± 0.01 | 23.15 ± 0.77 | 659.0 ± 6.55 |
| T2 | 14.15 ± 0.06 | 2.64 ± 0.02 | 4.16 ± 0.08 | 2.17 ± 0.30 | 22.88 ± 0.10 | 649.0 ± 2.0 |
| T3 | 15.10 ± 0.16 | 2.53 ± 0.01 | 3.08 ± 0.01 | 2.51 ± 0.39 | 22.66 ± 0.01 | 634.0 ± 4.0 |
| T4 | 15.47 ± 0.42 | 2.44 ± 0.03 | 2.98 ± 0.11 | 2.48 ± 0.15 | 21.79 ± 0.15 | 585.0 ± 14.1 |
Values are mean ± SD. “Co2d” is the CO2 assimilation rate, “RHd” is relative humidity, “A” is net photosynthetic rate, “E” is transpiration rate, “Gs” is stomatal conductance, and “Ci” is leaf internal CO2 concentration.
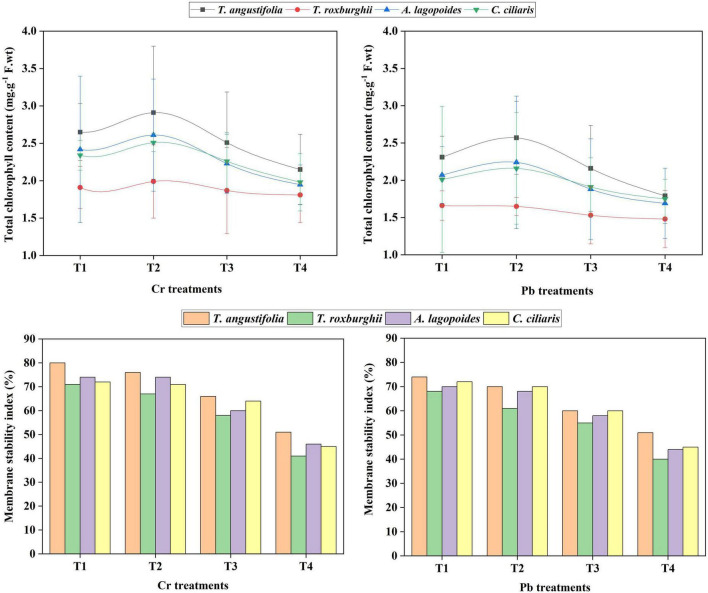
Chlorophyll content and membrane stability index
Significant differences were observed for total chlorophyll content among all the treatments for most of the species except for T. roxburghii against both heavy metals. Total chlorophyll content was in order of T. angustifolia > A. lagopoides > C. ciliaris > T. roxburghii against both heavy metals. In terms of MSI, Pb had a more sever effect on MSI compared to Cr in all the species. Overall, membrane stability index decreased as the heavy metal stress increased. T. angustifolia showed better MSI followed by C. ciliaris, A. lagopoides and T. roxburghii (Figure 3 and Supplementary Table 3).
FIGURE 3.
Total chlorophyll contents measurements and membrane stability index against Chromium (Cr) and lead (Pb) Concentrations in Typha angustifolia, Tragus roxburghii, Aeluropus lagopoides and Cenchrus ciliaris grass species.
Water use efficiency
No significant difference was observed among all the treatments for T. angustifolia after applying both Cr (P = 0.29) and Pb (P = 0.42) treatments. Although significant differences were observed among treatments for the remaining species, no clear patterns were seen. Overall, WUE was higher under heavy metals stress conditions than the control for all the species for both Cr and Pb. While comparing species, the WUE was in the order of T. angustifolia > A. lagopoides > T. roxburghii > C. ciliaris under both Cr and Pb stress (Table 4 and Supplementary Table 4).
TABLE 4.
Water use efficiency (μmolm–2s -/mmolm–2 s –1) of Typha angustifolia, Tragus roxburghii, Aeluropus lagopoides, and Cenchrus ciliaris grass species against Chromium (Cr) Concentrations.
| Treatment | Chromium (Cr) stress | Lead (Pb) stress |
| Typha angustifolia | ||
| T1 | 2.77 ± 0.50 | 3.02 ± 1.02 |
| T2 | 3.50 ± 0.01 | 3.13 ± 0.05 |
| T3 | 3.61 ± 0.06 | 3.36 ± 0.10 |
| T4 | 3.69 ± 0.08 | 3.45 ± 0.01 |
| Tragus roxburghii | ||
| T1 | 1.75 ± 0.06 | 1.87 ± 0.76 |
| T2 | 2.76 ± 0.01 | 1.92 ± 0.04 |
| T3 | 2.85 ± 0.03 | 1.96 ± 0.10 |
| T4 | 3.16 ± 0.03 | 1.97 ± 0.77 |
| Aeluropus lagopoides | ||
| T1 | 2.15 ± 0.04 | 2.69 ± 0.03 |
| T2 | 2.44 ± 0.03 | 2.76 ± 0.7 |
| T3 | 2.65 ± 0.06 | 2.74 ± 0.03 |
| T4 | 2.69 ± 0.05 | 2.78 ± 0.04 |
| Cenchrus ciliaris | ||
| T1 | 1.75 ± 0.06 | 1.84 ± 0.01 |
| T2 | 1.96 ± 0.01 | 1.84 ± 0.02 |
| T3 | 1.85 ± 0.03 | 1.77 ± 0.01 |
| T4 | 2.06 ± 0.03 | 1.93 ± 0.03 |
Values are mean ± SD.
Phytoremediation of chromium and chromium
Cr and Pb concentrations were evaluated in four grass species after 6 weeks of treatment. Statistical analyses showed that changes in Cr concentration significantly affect Cr absorption by all four grass species (Table 5). T. angustifili, T. roxburghii, A. lagopoides, and C. ciliaris revealed comparable increments in metal content compared to control. It was observed that the Cr contents of T. angustifolia were higher than the other three species at each level of Cr stress. In terms of species, at 80 μM, the highest Cr intake was observed in all four grass species.
TABLE 5.
Absorption of Cr and Pb by the leaves of Typha angustifolia, Tragus roxburghii, Aeluropus lagopoides, and Cenchrus ciliaris selected grass species.
| Treatments | Typha angustifolia | Tragus roxburghii | Aeluropus lagopoides | Cenchrus ciliaris |
| Cr Concentrations | ||||
| T1 | 0.0024 ± 0.09 | 0.0018 ± 0.04 | 0.0211 ± 0.02 | 0.0012 ± 0.03 |
| T2 | 0.0109 ± 0.09 | 0.0090 ± 0.06 | 0.0114 ± 0.07 | 0.0096 ± 0.07 |
| T3 | 0.0111 ± 0.08 | 0.0093 ± 0.02 | 0.0102 ± 0.04 | 0.0081 ± 0.05 |
| T4 | 0.0121 ± 0.05 | 0.0096 ± 0.05 | 0.0103 ± 0.06 | 0.0083 ± 0.05 |
| Pb Concentrations | ||||
| T1 | 0.0023 ± 0.05 | 0.0016 ± 0.15 | 0.0020 ± 0.72 | 0.0151 ± 0.08 |
| T2 | 0.0146 ± 0.08 | 0.0141 ± 0.01 | 0.0141 ± 0.03 | 0.0136 ± 0.52 |
| T3 | 0.0149 ± 0.05 | 0.0142 ± 0.08 | 0.0143 ± 0.04 | 0.0138 ± 0.51 |
| T4 | 0.0152 ± 1.52 | 0.0143 ± 1.38 | 0.0145 ± 0.05 | 0.0139 ± 0.07 |
Values are mean ± SD.
Statistical analyses revealed a highly significant effect of Pb stress on Pb uptake among all species (Table 5). As the Pb stress increased, T. angustifolia exhibited more absorption in Pb than Aeluropus lagopoides, Tragus roxburghii, and Cenchrus ciliaris, respectively. Like Cr, at the highest stress level of Pb, all the grass species showed the highest uptake (Table 5).
Association between the studied parameters
Across all grass species and treatments of Cr concentrations, total chlorophyll content and MSI were strongly correlated to leaf count and fresh and dry biomass. CO2 assimilation rate was negatively correlated to leaf count and MSI. Net photosynthetic rate was positively correlated to MSI, RHd, leaf count, total chlorophyll content and dry biomass, where it was negatively correlated to CO2 assimilation rate. Stomatal conductance and leaf internal CO2 concentration were positively correlated to leaf count, biomass, MSI, RHd, photosynthetic rate and whereas negatively correlated to CO2 assimilation rate; Moreover, Stomatal conductance and leaf internal CO2 concentration were also correlated to each other. Apart from a negative correlation with CO2 assimilation rate, there was no association found for transpiration rate with any other parameter (Figure 4).
FIGURE 4.
Pearson correlation analysis among all the studied parameters across all the treatments and species against Cr (left side figure) and Pb (right side figure) concentrations. LC, Leaf count; PRL, plant root length; PSL, plant shoot length; PFW, plant fresh weight; PDW, plant dry weight; Chl content, chlorophyll content; MSI, membrane sustainability index; CO2d, CO2 assimilation rate; RHd, relative humidity; A, net photosynthetic rate; E, transpiration rate; Gs, is stomatal conductance; and Ci, is leaf internal CO2 concentration.
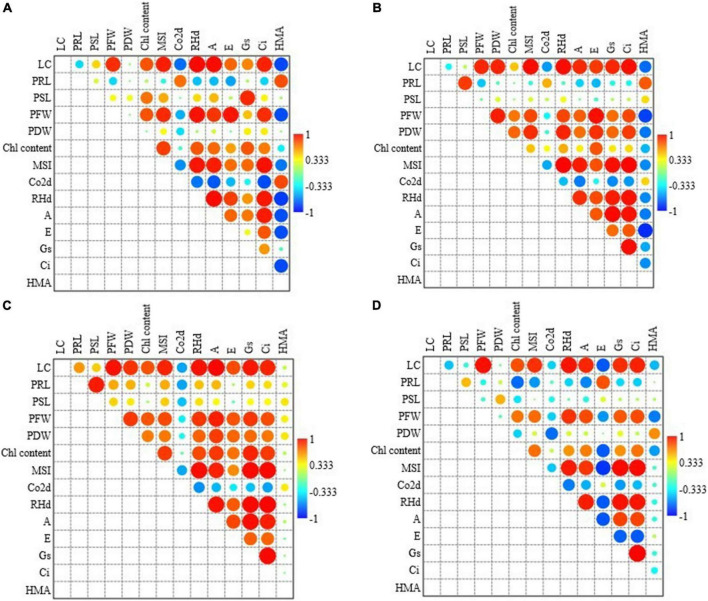
Across all grass species and treatments of Pb concentrations, total chlorophyll content was strongly correlated to fresh and dry biomass. MSI was correlated leaf count, fresh biomass and total. CO2 assimilation rate was negatively correlated to leaf count and MSI. Net photosynthetic rate was positively correlated to leaf count, MSI and RHd whereas it was negatively correlated to CO2 assimilation rate. Stomatal conductance has a weak positive association with leaf count, total chlorophyll content, correlated to leaf count, MSI, CO2 assimilation rate and photosynthetic rate. Leaf internal CO2 concentration had a strong positive association with MSI, RHd and photosynthetic rate and a negative association with CO2 assimilation rate. There was no association found for transpiration rate with any other parameter. Pb absorption was positively associated with MSI and negatively correlated to leaf count, photosynthetic rate, and internal CO2 concentration (Figure 4). Species-wise correlation is shown in Figure 5.
FIGURE 5.
Pearson correlation analysis for (A) Typha angustifolia, (B) Tragus roxburghii, (C) Aeluropus lagopoides, and (D) Cenchrus ciliaris grass species among all the studied parameters across both heavy metals concentrations. LC, Leaf count; PRL, plant root length; PSL, plant shoot length; PFW, plant fresh weight; PDW, plant dry weight; Chl content, chlorophyll content; MSI, membrane sustainability index; CO2d, CO2 assimilation rate; RHd, relative humidity; A, net photosynthetic rate; E, transpiration rate; Gs, is stomatal conductance; and Ci, is leaf internal CO2 concentration.
Discussion
Due to industrial wastewater, heavy metals pollution in Pakistan is worsening, negatively impacting the region’s overall biodiversity (Arooj et al., 2018; Hussain et al., 2018). Heavy metal pollution leads to the injection of toxic material into the food chain (Kadam et al., 2018). In this study, grass species showed varied growth behavior against the different concentrations of heavy metals; generally, their growth decreased with the elevated heavy metals concentrations.
Morphological and physiological parameters are considered good indicators for estimating plant tolerance against heavy metal and nutrient deficiency stresses (Jiang et al., 2015; Rashid et al., 2020a,b; Faiz et al., 2022). In this study, root and shoot lengths under low and moderate Cr and Pb stress either increased or did not show a big change compared to control and high stress; however, leaf count and biomass production decreased in all species. Based on the high toxicity of Cr and Pb heavy metals, their accumulation could lead to soil contamination. Once the soil gets contaminated, it will affect crop growth, production and yield quality. Our results support Ho et al. (2013), who stated that under excessive heavy metal stress, Lycopersicon esculentum showed no effect in root and shoot length; however, overall fruit production reduced. These results also correlate with Rév et al. (2017), who reported decreased root growth and shoot water content in wheatgrass cultivar Szarvasi-1 energy grass under heavy metal stress. In contrast, shoot growth and root water content were unaffected. Zhang et al. (2014) studied different morphological parameters under heavy metal (Zn, Cr, Pb, Ni) stress in king grass (Pennisetum purpureum) and concluded that fresh root weight was affected by increasing concentration of Cr and Pb. Similar results were observed by Yao et al. (2012), in which Pb exhibited a significant effect on fresh weight reduction (25%).
Our results contradict Richard and Weidhaas (2014) that Sesbania drummondii reduced root length under heavy metal stress. The possible reason is the less nutrient availability in the contaminated soil, and the roots have to grow longer to reach for nutrients under low and medium stress. However, under high stress, the root lengths were significantly reduced. Under high-stress conditions, Cr enters the root cells and interacts with nucleic acid by stimulating the aggregation of chromosomes. This ultimately resulted in the inhibition of DNA replication during cell division, leading to root growth cessation and biomass production (Uchman et al., 2017).
Heavy metals possess a toxic effect on physiological parameters in plants. Different heavy metal elements, including Cu, Cr, Cd, and Zn have been found to lower the net photosynthesis rates, WUE and stomatal conductance. In this study, apart from CO2 assimilation, other gaseous exchange activities (RHd, photosynthetic rate, transpiration rate, stomatal conductance and leaf internal CO2 concentration) were lowered with stress increase in all the species. Heavy metals cause the rate of CO2 production to decrease and subsequently diminish, thus reducing the leaf’s internal CO2 concentration, which eventually affects the gaseous exchange process, especially the photosynthetic rate. Leaf transpiration rate lessened due to reduced stomatal opening (Haider et al., 2021), which could be induced by direct/indirect interaction of heavy metals toxicity with guard cells (Rucińska-Sobkowiak, 2016). Long-term exposure to the extreme toxicity of heavy metals mostly results in water deficiency. Heavy metals considerably reduce the leaf gaseous exchange parameters, which may result in water stress due to the low water flow from the soil to the leaves (Rai et al., 2016). This reduces leaf turgor and stomatal conductance (Yadav, 2010).
Heavy metals, such as Cd, Pb and Zn, affect membrane stability by reducing cell membrane permeability (Rucińska-Sobkowiak, 2016), followed by a disturbance in the ionic balance of the cell. This leads to reduced chlorophyll concentrations (Petrovic and Krivokapic, 2020) and photosystem II activity (Rai et al., 2016), which limits photosynthesis, thus leading to metabolic disruptions (Muhammad et al., 2021). Apart from grasses and agricultural species, Chandra and Kang (2016) also presented similar results in forest tree species: gaseous exchange activities reduced with the increasing concentration of heavy metals.
Chlorophyll content in leaf tissue and membrane stability of plants is influenced by various environmental stresses such as salinity, drought, cold and heat etc. Various heavy metals (i.e., mercury (Hg), copper (Cu), Cr, cadmium (Cd), and zinc (Zn) decreased the chlorophyll content in various plant species in most cases. In this study, total chlorophyll content was higher under low stress water deficits. Moreover, increased chlorophyll contents under low stress are paired with increased CO2 assimilation and WUE (Wang et al., 2012; Rasheed and Delagrange, 2016).
Cadmium (Cd) and lead (Pb), among heavy metals, have become more seriously toxic to plant functions due to their widespread toxicity. The use of green plant species to treat and control wastes (metals, metalloids, salts, sewage, sludge and some xenobiotic contaminants etc.) in the water, soil, and air, has gained global importance in the new field of ecology. Grasses are considered the best tool for detoxifying heavy metals in the soil and water. In this study, T. angustifolia showed higher absorption of Cr than other species. Pb absorption by grasses was in the order of T. angustifolia. A. lagopoides > T. roxburghii, and C. ciliaris. Caldelas et al. (2012) reported that Iris pseudacorus L. cano remediate heavy metals from soil. Similar results were studied by Khan et al. (2021) in their review article and found that ornamental species are well adapted to absorb heavy metals from soil.
Conclusion
The present research was used to characterize the impact of high metal stress on Cholistan grasses. Heavy metals have a significant effect on plant production that not only reduces overall production but also leads to serious damage and even causes plant mortality. T. angustifolia morphological attributes were higher than other species under both heavy metals. Under both Cr and Pb stress, apart from Co2d and WUE, other physiological parameters, including RHd, A, E, Gs, Ci, and MSI, gradually decreased as the stress increased among all the species. In contrast, total chlorophyll content was higher under low stress. WUE was in the order of T. angustifolia > A. lagopoides > T.roxburghii > C. ciliaris under both Cr and Pb stress. Cr uptake of T. angustifolia was higher than the other three kinds of grasses in normal growth conditions. A similar trend was observed at 20, 40, and 80 μmol/L. Increasing Pb stress levels showed significant increments in Pb absorption of all species. Local species that naturally revegetate can be much cheaper than planting/management phytoremediation. Therefore, due to better growth, increased chlorophyll level, increased CO2 assimilation, WUE, and higher heavy metals absorption capacity, we may infer that T. angustifolia could be suitable for phytoremediation in heavy metal soils.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
TF, HB, and MRa: conceptualization. HB and RS: methodology. HB and MRi: fieldworks and chemical analysis. HB, AS, and MRi: data analysis. TF, HB, and UK: writing—original draft preparation. TF and BA: validation. TF, MRa, and BA: writing—review and editing. MRa: supervision. KQ and MJ: funding. All authors have read and agreed to the published version of the manuscript.
Funding
We extend our sincere appreciation to King Abdullah University of Science and Technology (KAUST), Thuwal, Saudi Arabia, for supporting the current research. This research was also financially supported by the Central South University of Forestry and Technology and the Hunan Province Educational Department (No. 70702-45200003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor declared a past co-authorship with one of the author RS.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.997120/full#supplementary-material
References
- Abbas A., Shah A. N., Shah A. A., Nadeem M. A., Alsaleh A., Javed T., et al. (2022). Genome-wide analysis of invertase gene family, and expression profiling under abiotic stress conditions in potato. Biology (Basel) 11:539. 10.3390/biology11040539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad I., Jabeen B., Nawaz M. F., Asif M., Rashid M. H. U., Hussain M., et al. (2022). Influence of salinity on the morphological behavior and ionic response of different commercially important bamboo species. Fresenius Environ. Bull. 31 668–676. [Google Scholar]
- Ali A., Chu N., Ma P., Javed T., Zaheer U., Huang M. T., et al. (2021). Genome-wide analysis of mitogen-activated protein (MAP) kinase gene family expression in response to biotic and abiotic stresses in sugarcane. Physiol. plant. 171 86–107. 10.1111/ppl.13208 [DOI] [PubMed] [Google Scholar]
- Arooj S., Arfan M., Masood A., Abid A., Fatima T., Khalid N., et al. (2018). Assessment of salicylic acid in morpho-physiological attributes of maize (zea mays) subjected to nickel stress. Fresenius Environ. Bull. 27 6519–6524. [Google Scholar]
- Awad M., El-Desoky M. A., Ghallab A., Kubes J., Abdel-Mawly S. E., Danish S., et al. (2021). Ornamental plant efficiency for heavy metals phytoextraction from contaminated soils amended with organic materials. Molecules 26:3360. 10.3390/molecules26113360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldelas C., Bort J., Febrero A. (2012). Ultrastructure and subcellular distribution of Cr in Iris pseudacorus L. using TEM and X-ray microanalysis. Cell Boil. Toxicol. 28 57–68. 10.1007/s10565-011-9205-7 [DOI] [PubMed] [Google Scholar]
- Chandra R., Kang H. (2016). Mixed heavy metal stress on photosynthesis, transpiration rate, and chlorophyll content in poplar hybrids. For. Sci. Technol. 12 55–61. 10.1080/21580103.2015.1044024 [DOI] [Google Scholar]
- De Conti L., Marques A. C. R., Ceretta C. A., Tarouco C. P., Nicoloso F. T., Ferreira P. A. A., et al. (2021). Tolerance and phytoremediation potential of grass species native to South American grasslands to copper-contaminated soils. Int. J. Phytoremediation 23 726–735. 10.1080/15226514.2020.1852528 [DOI] [PubMed] [Google Scholar]
- Faiz S., Shah A. A., Naveed N. H., Nijabat A., Yasin N. A., Batool A. I., et al. (2022). Synergistic application of silver nanoparticles and indole acetic acid alleviate cadmium induced stress and improve growth of Daucus carota L. Chemosphere 290:133200. 10.1016/j.chemosphere.2021.133200 [DOI] [PubMed] [Google Scholar]
- Gilani M. M., Tigabu M., Liu B., Farooq T. H., Rashid M. H. U., Ramzan M., et al. (2020). Seed germination and seedling emergence of four tree species of southern China in response to acid rain. J. Forestry Res. 32 471–481. 10.1007/s11676-020-01102-0 [DOI] [Google Scholar]
- Gunatilake S. K. (2015). Methods of removing heavy metals from industrial wastewater. Methods 1:14. [Google Scholar]
- Guo B., Dai S., Wang R., Guo J., Ding Y., Xu Y. (2015). Combined effects of elevated CO2 and Cd-contaminated soil on the growth, gas exchange, antioxidant defense, and Cd accumulation of poplars and willows. Environ. Exp. Bot. 1 1–10. 10.1016/j.envexpbot.2015.02.002 [DOI] [Google Scholar]
- Haider F. U., Liqun C., Coulter J. A., Cheema S. A., Wu J., Zhang R., et al. (2021). Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 211 111–887. 10.1016/j.ecoenv.2020.111887 [DOI] [PubMed] [Google Scholar]
- Ho Y. N., Hsieh J. L., Huang C. C. (2013). Construction of a plant–microbe phytoremediation system: Combination of vetiver grass with a functional endophytic bacterium, Achromobacter xylosoxidans F3B, for aromatic pollutants removal. Bioresour. Technol. 1 43–47. 10.1016/j.biortech.2013.02.051 [DOI] [PubMed] [Google Scholar]
- Hussain M. I., Khan Z. I., Farooq T. H., Al Farraj D. A., Elshikh M. S. (2022). Comparative Plasticity Responses of Stable Isotopes of Carbon (δ13C) and Nitrogen (δ15N). Ion Homeostasis and Yield Attributes in Barley Exposed to Saline Environment. Plants 11:1516. 10.3390/plants11111516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain Z., Arslan M., Malik M. H., Mohsin M., Iqbal S., Afzal M. (2018). Treatment of the textile industry effluent in a pilot-scale vertical flow constructed wetland system augmented with bacterial endophytes. Sci. Total Environ. 645 966–973. 10.1016/j.scitotenv.2018.07.163 [DOI] [PubMed] [Google Scholar]
- Javad S., Shah A. A., Ramzan M., Sardar R., Javed T., Al-Huqail A. A., et al. (2022). Hydrogen sulphide alleviates cadmium stress in Trigonella foenum-graecum by modulating antioxidant enzymes and polyamine content. Plant Biol. 24 618–626. 10.1111/plb.13393 [DOI] [PubMed] [Google Scholar]
- Javed S. A., Arif M. S., Shahzad S. M., Ashraf M., Kausar R., Farooq T. H., et al. (2021). Can different salt formulations revert the depressing effect of salinity on maize by modulating plant biochemical attributes and activating stress regulators through improved N Supply? Sustainability 13:8022. 10.3390/su13148022 [DOI] [Google Scholar]
- Javed T., Shabbir R., Ali A., Afzal I., Zaheer U., Gao S. J. (2020). Transcription factors in plant stress responses: Challenges and potential for sugarcane improvement. Plants 9:491. 10.3390/plants9040491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J. H., Ge G., Gao K., Pang Y., Chai R. C., Jia X. H., et al. (2015). Calcium signaling involvement in cadmium-induced astrocyte cytotoxicity and cell death through activation of MAPK and PI3K/Akt signaling pathways. Neurochem. Res. 40 1929–1944. 10.1007/s11064-015-1686-y [DOI] [PubMed] [Google Scholar]
- Kadam S. K., Chandanshive V. V., Rane N. R., Patil S. M., Gholave A. R., Khandare R. V., et al. (2018). Phytobeds with Fimbristylis dichotoma and Ammannia baccifera for treatment of real textile effluent: An in situ treatment, anatomical studies and toxicity evaluation. Environ. Res. 160 1–11. 10.1016/j.envres.2017.09.009 [DOI] [PubMed] [Google Scholar]
- Khan A. H. A., Kiyani A., Mirza C. R., Butt T. A., Barros R., Ali B., et al. (2021). Ornamental plants for the phytoremediation of heavy metals: Present knowledge and future perspectives. Environ. Res. 195:110780. 10.1016/j.envres.2021.110780 [DOI] [PubMed] [Google Scholar]
- Koleva L., Umar A., Yasin N. A., Shah A. A., Siddiqui M. H., Alamri S., et al. (2022). Iron oxide and silicon nanoparticles modulate mineral nutrient homeostasis and metabolism in cadmium-stressed phaseolus vulgaris. Front. Plant Sci. 13:806781. 10.3389/fpls.2022.806781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. D., Ho J. R., Yang B. Y., Ko C. H., Chang F. C. (2022). Life cycle assessment of heavy metal contaminated sites: Phytoremediation and soil excavation. Int. J. Phytoremediation 24 334–341. 10.1080/15226514.2021.1937933 [DOI] [PubMed] [Google Scholar]
- Muhammad I., Shalmani A., Ali M., Yang Q. H., Ahmad H., Li F. B. (2021). Mechanisms regulating the dynamics of photosynthesis under abiotic stresses. Front. Plant Sci. 11:2310. 10.3389/fpls.2020.615942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz M. F., Rashid M. H. U., Arif M. Z., Sabir M. A., Farooq T. H., Gul S., et al. (2021). Ecophysiological response of Eucalyptus camaldulensis to dust and lead pollution. New Zealand J. Forestry Sci. 13:1010. 10.33494/nzjfs512021x145x [DOI] [Google Scholar]
- Nawaz M. F., Rashid M. H. U., Saeed-Ur-Rehman M., Gul S., Farooq T. H., Sabir M. A., et al. (2022). Effect of dust types on the eco-physiological response of three tree species seedlings: Eucalyptus camaldulensis. Conocarpus erectus and Bombax ceiba. Atmosphere 13:1010. 10.3390/atmos13071010 [DOI] [Google Scholar]
- Nouman W., Bashir T., Gul R., Gull T., Olson M. E., Shaheen M., et al. (2020). Metalliferous conditions induce regulation in antioxidant activities, polyphenolics and nutritional quality of Moringa oleifera L. Int. J. Phytoremediation 22 1348–1361. 10.1080/15226514.2020.1775547 [DOI] [PubMed] [Google Scholar]
- Panikar S., Nanthini A. U. R., Umapathy V. R., SumathiJones C., Mukherjee A., Prakash P., et al. (2022). Morphological, chemoprofile and soil analysis comparison of Corymbia citriodora (Hook.) KD Hill and LAS Johnson along with the green synthesis of iron oxide nanoparticles. J. King Saud Univ. Sci. 34:102081. 10.1016/j.jksus.2022.102081 [DOI] [Google Scholar]
- Petrovic D., Krivokapic S. (2020). The effect of Cu, Zn, Cd, and Pb accumulation on biochemical parameters (proline, chlorophyll) in the water caltrop (Trapa natans L.), Lake Skadar, Montenegro. Plant 9:1287. 10.3390/plants9101287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai R., Agrawal M., Agrawal S. B. (2016). “Impact of heavy metals on physiological processes of plants: With special reference to photosynthetic system,” in Plant responses to xenobiotics, (Singapore: Springer; ), 127–140. 10.1007/978-981-10-2860-1_6 [DOI] [Google Scholar]
- Rasheed F., Delagrange S. (2016). Acclimation of Betula alleghaniensis Britton to moderate soil water deficit: Small morphological changes make for important consequences in crown display. Tree Physiol. 36 1320–1329. 10.1093/treephys/tpw064 [DOI] [PubMed] [Google Scholar]
- Rasheed F., Dreyer E., Richard B., Brignolas F., Brendel O., Le Thiec D. (2015). Vapour pressure deficit during growth has little impact on genotypic differences of transpiration efficiency at leaf and whole-plant level: An example from Populus nigra L. Plant Cell Environ. 38 670–684. 10.1111/pce.12423 [DOI] [PubMed] [Google Scholar]
- Rashid M. H. U., Farooq T. H., Iqbal W., Asif M., Islam W., Lin D. C., et al. (2020a). Role of indole acetic acid on growth and biomass production of athel tree (Tamarix aphylla) by using different cutting lengths. Appl. Ecol. Environ. Res. 18 3805–3816. 10.15666/aeer/1803_38053816 [DOI] [Google Scholar]
- Rashid M. H. U., Tigabu M., Chen H., Farooq T. H., Ma X., Wu P. (2020b). Calcium-mediated adaptive responses to low phosphorus stress in Chinese fir. Trees 34 825–834. 10.1007/s00468-020-01961-4 [DOI] [Google Scholar]
- Rév A., Tóth B., Solti Á, Sipos G., Fodor F. (2017). Responses of Szarvasi-1 energy grass to sewage sludge treatments in hydroponics. Plant Physiol. Biochem. 118 627–633. 10.1016/j.plaphy.2017.07.027 [DOI] [PubMed] [Google Scholar]
- Richard T., Weidhaas J. (2014). Dissolution, sorption, and phytoremediation of IMX-101 explosive formulation constituents: 2, 4-dinitroanisole (DNAN), 3-nitro-1, 2, 4-triazol-5-one (NTO), and nitroguanidine. J. Hazard. Mater. 15 561–569. 10.1016/j.jhazmat.2014.08.042 [DOI] [PubMed] [Google Scholar]
- Romero-Puertas M. C., Corpas F. J., Rodríguez-Serrano M., Gómez M., Luis A., Sandalio L. M. (2007). Differential expression and regulation of antioxidative enzymes by cadmium in pea plants. J. Plant Physiol. 164 1346–1357. 10.1016/j.jplph.2006.06.018 [DOI] [PubMed] [Google Scholar]
- Rucińska-Sobkowiak R. (2016). Water relations in plants subjected to heavy metal stresses. Acta Physiol. Plant 38:257. 10.1007/s11738-016-2277-5 [DOI] [Google Scholar]
- Saha P., Shinde O., Sarkar S. (2017). Phytoremediation of industrial mines wastewater using water hyacinth. Int. J. Phytoremediation 19 87–96. 10.1080/15226514.2016.1216078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena P. K., KrishnaRaj S., Dan T., Perras M. R., Vettakkorumakankav N. N. (1999). “Phytoremediation of heavy metal contaminated and polluted soils,” in Heavy metal stress in plants, (Berlin: Springer; ), 305–329. 10.1007/978-3-662-07745-0_14 [DOI] [Google Scholar]
- Scandalios J. G. (2005). Oxidative stress: Molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Biol. Res. 38 995–1014. 10.1590/S0100-879X2005000700003 [DOI] [PubMed] [Google Scholar]
- Shabbir R., Javed T., Hussain S., Ahmar S., Naz M., Zafar H., et al. (2022). Calcium homeostasis and potential roles to combat environmental stresses in plants. S. Afr. J. Bot. 148 683–693. 10.1016/j.sajb.2022.05.038 [DOI] [Google Scholar]
- Shah A. A., Shah A. N., Tahir M. B., Abbas A., Javad S., Ali S., et al. (2022). Harzianopyridone supplementation reduced chromium uptake and enhanced activity of antioxidant enzymes in vigna radiata seedlings exposed to chromium toxicity. Front. Plant Sci. 13:881561. 10.3389/fpls.2022.881561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Gautam N., Mishra A., Gupta R. (2011). Heavy metals and living systems: An overview. Indian J. Pharmacol. 43:246. 10.4103/0253-7613.81505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Parihar P., Singh R., Singh V. P., Prasad S. M. (2016). Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 6:1143. 10.3389/fpls.2015.01143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal R. K., Fahad S., Kumar P., Choyal P., Javed T., Jinger D., et al. (2022). Beneficial elements: New players in improving nutrient use efficiency and abiotic stress tolerance. Plant Growth Regul. 1–29. 10.1007/s10725-022-00843-835892116 [DOI] [Google Scholar]
- Tchounwou P. B., Yedjou C. G., Patlolla A. K., Sutton D. J. (2012). Heavy metal toxicity and the environment. Molecular, clinical and environmental toxicology. Berlin: Springer, 133–164. 10.1007/978-3-7643-8340-4_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchman W., Skorek-Osikowska A., Werle S. (2017). Evaluation of the potential of the production of electricity and heat using energy crops with phytoremediation features. Appl. Therm. Eng. 126 194–203. 10.1016/j.applthermaleng.2017.07.142 [DOI] [Google Scholar]
- Wang Y., Papanatsiou M., Eisenach C., Karnik R., Williams M., Hills A., et al. (2012). Systems dynamic modeling of a guard cell Cl- channel mutant uncovers an emergent homeostatic network regulating stomatal transpiration. Plant Physiol. 160 1956–1967. 10.1104/pp.112.207704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Li Y., Shi Y., Li Z., Zhang X., Liu T., et al. (2022). Combined toxicity of zinc oxide nanoparticles and cadmium inducing root damage in Phytolacca americana L. Sci. Total Environ. 806:151211. 10.1016/j.scitotenv.2021.151211 [DOI] [PubMed] [Google Scholar]
- Yadav S. K. (2010). Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 76 167–179. 10.1016/j.sajb.2009.10.007 [DOI] [Google Scholar]
- Yao Z., Li J., Xie H., Yu C. (2012). Review on remediation technologies of soil contaminated by heavy metals. Procedia Environ. Sci. 1 722–729. 10.1016/j.proenv.2012.10.099 [DOI] [Google Scholar]
- Yousaf M. S., Farooq T. H., Ahmad I., Gilani M. M., Rashid M. H., Gautam N. P., et al. (2018). Effect of drought stress on the growth and morphological traits of Eucalyptus camaldulensis and Eucalyptus citriodora. PSM Biol. Res. 3 85–91. [Google Scholar]
- Zhang X., Zhang X., Gao B., Li Z., Xia H., Li H., et al. (2014). Effect of cadmium on growth, photosynthesis, mineral nutrition and metal accumulation of an energy crop, king grass (Pennisetum americanum × P. purpureum). Biomass Bioenergy 67 179–187. 10.1016/j.biombioe.2014.04.030 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.