Abstract
The anaerobic bacterium Desulfobacterium cetonicum oxidized p-cresol completely to CO2 with sulfate as the electron acceptor. During growth, 4-hydroxybenzylsuccinate accumulated in the medium. This finding indicated that the methyl group of p-cresol is activated by addition to fumarate, analogous to anaerobic toluene, m-xylene, and m-cresol degradation. In cell extracts, the formation of 4-hydroxybenzylsuccinate from p-cresol and fumarate was detected at an initial rate of 0.57 nmol min−1 (mg of protein)−1. This activity was specific for extracts of p-cresol-grown cells. 4-Hydroxybenzylsuccinate was degraded further to 4-hydroxybenzoyl-coenzyme A (CoA), most likely via β-oxidation. 4-Hydroxybenzoyl-CoA was reductively dehydroxylated to benzoyl-CoA. There was no evidence of degradation of p-cresol via methyl group oxidation by p-cresol-methylhydroxylase in this bacterium.
The toxic aromatic compound p-cresol (4-methylphenol) is a constituent of disinfectants and preservatives and is used largely in the formulation of antioxidants and in the fragrance and dye industries (1). It originates mainly from coal gasification plants, fractionation of coal tar, and a variety of synthetic processes. p-Cresol is also formed from tyrosine by several anaerobic bacteria (16, 41, 44). Anaerobic degradation of p-cresol could be demonstrated with pure cultures of nitrate-reducing, iron-reducing, and sulfate-reducing bacteria and under methanogenic conditions (2, 7, 19, 25, 29, 37, 39, 43). It has been well established that denitrifying bacteria metabolize p-cresol, cognate to its degradation by aerobic bacteria (13 and references therein), through a sequence of oxidation reactions leading to 4-hydroxybenzoate, with water as the oxygen source (7, 14, 34). In the initial step, the methyl group of p-cresol is enzymatically oxidized, probably via formation of a quinone methide intermediate (13, 20), to form 4-hydroxybenzylalcohol. The latter is further converted to 4-hydroxybenzaldehyde by the same enzyme, p-cresol-methylhydroxylase (13, 22). The standard redox potential (E0′) of the couple 4-hydroxybenzylalcohol/p-cresol is in the range of +80 mV (calculated as described before [42]), and the methylhydroxylase reaction is coupled with the reduction of a c-type cytochrome with a midpoint potential of around +230 mV (21, 22).
It has been proposed that sulfate-reducing bacteria degrade p-cresol via methyl group oxidation, too (19, 28, 41). This would be surprising, since release of electrons at the redox potentials mentioned above would be difficult for these bacteria: transfer of electrons from the reduced cytochrome of p-cresol-methylhydroxylase to either adenosine 5′-phosphosulfate (E0′ = −60 mV [42]) or sulfite (E0′ = −116 mV [42]) as the electron acceptor would require a substantial energy input.
In the present study, we employed in vitro assays and physiological studies to examine sulfidogenic p-cresol degradation by Desulfobacterium cetonicum. With this bacterium, it was shown recently that anaerobic degradation of m-cresol proceeded via addition of the methyl group of m-cresol to fumarate to form 3-hydroxybenzylsuccinate (3-HBS) (32). This reaction is analogous to the anaerobic activation of toluene (4, 6, 33) and m-xylene (24) with fumarate to form benzylsuccinate or 3-methylbenzylsuccinate, respectively. The benzylsuccinate (derivative) is further converted in a β-oxidation-like scheme to benzoyl-coenzyme A (CoA) or a derivative thereof. The present results suggest that p-cresol is degraded via formation of 4-HBS rather than via methyl group oxidation by D. cetonicum.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
D. cetonicum 480 (17) (DSM 7267) was cultivated in a sulfide-reduced bicarbonate-buffered mineral salt water medium. Medium composition and growth conditions have been described previously in detail (23, 32). The amount of cell matter formed in growth tests was calculated via a gravimetrically determined conversion factor (0.1 optical density unit at 660 nm [OD660] = 22.6 mg/liter).
Enzyme assays.
Cell harvesting and preparation of cell extracts were carried out under anoxic conditions as reported before (23, 32). Enzyme assays were carried out at 30°C, and given activities are means of at least three independent measurements.
Formation of 4-HBS was monitored discontinuously in 2-ml Hungate vials under an N2 atmosphere in 50 mM potassium phosphate (pH 7.2) supplemented with 2.5 mM dithioerythritol and 0.2 mM titanium (III)-nitrilotriacetic acid (NTA) and containing 10.0 g of NaCl and 1.75 g of MgCl2 · 6 H2O per liter. Vials were sealed with butyl-rubber septa. The protein content varied between 1 and 3 mg/ml. All substrate stock solutions were prereduced with 2.5 mM dithioerythritol. The test was started by addition of p-cresol (100 μM) or fumarate (5 mM) to the assay mix (total volume, 1 ml). Samples were taken with gas-tight syringes (Macherey-Nagel, Düren, Germany), diluted in ice-cold 100 mM phosphoric acid to stop the reaction, and analyzed by high-pressure liquid chromatography (HPLC). 3-HBS-forming, 2-HBS-forming, and benzylsuccinate synthase activities were checked for with the same assay except that p-cresol was replaced with either m-cresol, o-cresol, or toluene. With benzylsuccinate synthase, the benzylsuccinate but not the toluene concentration was monitored over time. The oxygen sensitivity of 4-HBS-forming activity was checked by application of a gentle air stream over the cell extract for 2 min and rendering the assay anoxic again by flushing the gas phase for 5 min with N2 and by addition to Ti(III)-NTA (5 mM final concentration). Afterwards, the test was started by addition of p-cresol to the vials.
p-Cresol methylhydroxylase (EC 1.17.99.1) activity was checked under an N2 atmosphere according to Hopper (20). The assay mixture contained degassed 50 mM potassium phosphate (pH 7.2) containing 10.0 g of NaCl and 1.75 g of MgCl2 · 6H2O, 0.2 mM dichlorophenolindophenol, 0.3 mM phenazine methosulfate, 0.5 mM p-cresol, and cell extract (50 to 150 μg of protein).
The catabolism of 4-HBS was studied in assays for 4-HBS formation amended with a CoA source and/or a mixture of potential electron acceptors. Succinyl-CoA (2 mM), acetyl-CoA (2 mM), and free CoASH (0.5 mM) plus ATP (5 mM) were tested as CoA sources and NAD, NADP, and flavin adenine dinucleotide (FAD) (5 mM each) were added as electron acceptors at various times to the assay. Substrate and product concentrations were monitored by HPLC analysis. Samples were also analyzed after an alkaline treatment (KOH [pH 12], 70°C, 20 min). Dilution of substrate and product concentrations in the assays upon addition of a CoA source, electron acceptor, or KOH was taken into consideration.
4-Hydroxybenzoyl-CoA reductase activity was measured discontinuously by HPLC analysis (9). The reaction mixture contained 0.2 mM chemically synthesized 4-hydroxybenzoyl-CoA, 100 μM methylviologen, and 50 mM formate in 100 mM potassium phosphate buffer (pH 7.2) containing 10.0 g of NaCl and 1.75 g of MgCl2 · 6 H2O per liter, prereduced with H2-Pd catalyst. The test was started by addition of either 4-hydroxybenzoyl-CoA or formate to the assay mixture. Methylviologen was reduced in the formate-dehydrogenase reaction in the assay (23).
Fumarate reductase (EC 1.3.1.6) was checked for as described by Beh et al. (3).
Analytical methods.
The identification of 4-HBS in cultures grown on p-cresol was carried out basically as described earlier for the identification of 3-HBS in m-cresol-converting cultures (32). In brief, culture supernatant (1,000 ml) obtained by centrifugation was acidified to pH < 2 by addition of HCl (35%), degassed in order to remove hydrogen sulfide, and extracted thoroughly with diethyl ether. The ether fraction was concentrated at room temperature, derivatized with diazomethane, and analyzed by gas chromatography-mass spectrometry (GC/MS) (32). In addition, culture supernatant was analyzed by HPLC. Eluting compounds were scanned on-line with a photodiode array detector (Beckman-Coulter, Munich, Germany) in the wavelength range of 200 to 350 nm.
Other aromatic compounds and aliphatic acids were identified and quantified by HPLC analysis as described previously (10, 18). Sulfide in growth experiments was determined by the methylene blue formation reaction (11), and protein was quantified by the method of Bradford (8).
Synthesis of 4-HBS.
4-HBS was synthesized via the Stobbe reaction (40) essentially as described earlier for synthesis of 3-HBS (32). First, 4-benzyloxybenzylidene succinic acid was formed by condensation of 4-hydroxybenzaldehyde with diethyl succinic acid in a methanolic solution of sodium methoxide. 4-Benzyloxybenzylidene succinic acid was identified by 1H nuclear magnetic resonance (NMR) with spectra collected on a Bruker AC250 instrument (Bruker Analytik, Rheinstetten, Germany) in CD3SOCD3. Elemental analysis gave C = 68.8%, H = 5.2%; according to the formula C18H16O5, C = 69.2% and H = 5.1% would have been expected (melting point, 203 to 204°C). Subsequently, 4-benzyloxybenzylidene succinic acid was catalytically reduced with H2 to form 4-hydroxybenzylsuccinic acid. The latter was identified by 1H-NMR in CD3SOCD3. Elemental analysis gave C = 58.6%, H = 5.2%; according to the formula C11H12O5, C = 58.9% and H = 5.4% would have been expected (melting point, 178 to 180°C).
Other chemicals.
4-Hydroxybenzoyl-CoA was synthesized according to Wieland (15, 30) and purified by HPLC. Titanium(III)-NTA stock solutions contained 100 mM Ti3+ chelated by 150 mM NTA and were prepared as described elsewhere (31). All other chemicals and gases used were of reagent grade or better and from standard commercial sources.
RESULTS
Growth with p-cresol.
D. cetonicum oxidized p-cresol completely to CO2 with sulfate as the electron acceptor. Sulfide was produced concomitantly with substrate utilization and increase in optical density. Sulfide recovery was 96%, expressed as a percentage of theoretical production. After a lag phase of several days, D. cetonicum grew with a doubling time of 5.1 days. Growth was exponential even in the presence of 1.5 mM p-cresol. The in vivo substrate turnover rate was calculated to be 6.2 nmol min−1 (mg of protein)−1. D. cetonicum also grew with 4-hydroxybenzoate and 4-hydroxybenzaldehyde, but did not use 4-hydroxybenzylalcohol, o-cresol, o-, m-, or p-xylene, or 2-hydroxybenzoate. Growth with toluene, m-cresol, 3-hydroxybenzoate, and benzoate has been reported before (17, 32).
Identification of 4-HBS.
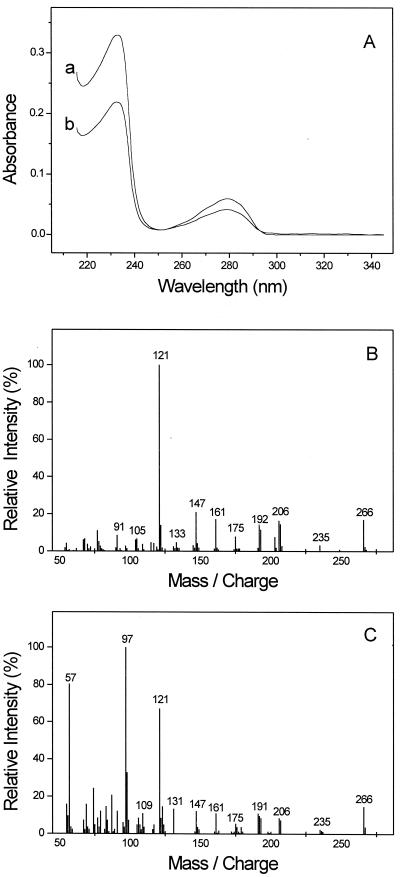
In cultures growing with p-cresol, a compound accumulated that was not detected while this strain was growing with other aromatic substrates, as analyzed by reversed-phase HPLC. The on-line UV spectrum of this compound (Fig. 1A) was similar to that of 3-HBS, which is an intermediate of m-cresol degradation by this bacterium (32). This observation indicated that 4-HBS is formed during degradation of p-cresol. Chemically synthesized 4-HBS coeluted with the compound from culture supernatant in an HPLC run at low methanol or acetonitrile concentrations (10%), and the UV spectra of these two substances were identical (Fig. 1A). After treatment of ethereal extract of culture supernatant with diazomethane, a compound was detected by GC/MS analysis which had an identical GC retention time and displayed essentially the same mass spectrum as chemically synthesized dimethyl ester of 4-methoxybenzylsuccinate (Fig. 1B and C). The mass spectrum of the standard (Fig. 1B) exhibits the molecular ion of derivatized 4-HBS at m/z 266 and apparently the methoxy tropylium ion (C8H9O+) at m/z 121. The overall pattern of fragment ions corresponds well with the described mass spectra of benzylsuccinate derivatives (4, 24, 32), taking the additional oxygen atom of 4-HBS into consideration. The spectrum of the compound from the culture supernatant (Fig. 1C) is basically the same as that of the standard, but two major additional peaks are observable at m/z 57 and 97. These might be attributed to coelution with another compound. Taken together, these findings confirmed that 4-HBS is a metabolite of p-cresol degradation by D. cetonicum and suggested that p-cresol degradation proceeds analogously to m-cresol degradation in this bacterium. A quantitative analysis revealed that the accumulating 4-HBS made up less than 5% of the overall p-cresol converted.
FIG. 1.
Identification of 4-HBS in supernatants of p-cresol-converting cultures of D. cetonicum. (A) On-line UV spectra of chemically synthesized 4-HBS (A) and of 4-HBS from culture supernatant (B) in acetonitrile (10%) and 10 mM potassium phosphate buffer (pH 2.2). The maxima are at 232 and 278 nm. (B and C) Mass analysis by GC/MS of chemically synthesized (B) 4-methoxybenzylsuccinate dimethyl ester and (C) 4-methoxybenzylsuccinate dimethyl ester obtained from culture supernatant; mass units are daltons.
In addition to detection of dimethyl ester of 4-methoxybenzylsuccinate by GC/MS analysis, a compound was observed in diazomethane-treated extract of culture supernatant that was tentatively identified as the dimethyl ester of 4-methoxyphenylitaconate (or a close analogue) by its mass spectrum (not shown). This compound had an apparent mass of 264 Da. The presence of most of the major fragment ions in the spectrum (m/z 249, 230, 219, 163, 145, 105, and 91) could be explained by loss of methyl, methylene, and carbonyl group(s) of the proposed parent compound. Due to lack of authentic standards, the nature of this compound could not be further elucidated. Furthermore, traces of 4-methoxybenzoic acid methyl ester were found by GC/MS analysis. 4-Hydroxybenzylalcohol and 4-hydroxybenzaldehyde (or their methylated derivatives) were not detected by HPLC or GC/MS analysis.
Formation of 4-HBS from p-cresol and fumarate.
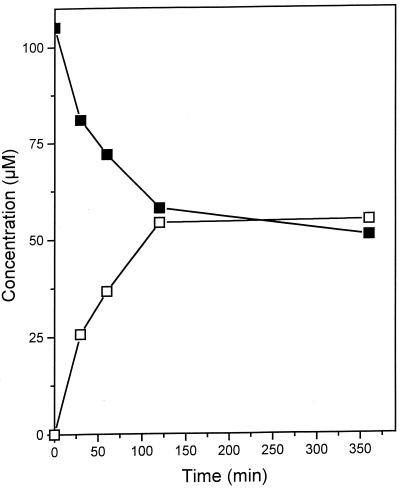
In anoxic cell extracts of p-cresol-grown cells, an activity was measured that converted p-cresol and fumarate, forming 4-HBS (Table 1). In the absence of either p-cresol or fumarate or with heat-denatured extract (90°C, 10 min), no formation of 4-HBS could be detected. A time course of a discontinuous assay for 4-HBS formation from p-cresol and fumarate is depicted in Fig. 2. During the reaction run, the activity decreased with time. The initial specific activity was 0.57 nmol min−1 (mg of protein)−1, thus being about 9% of the in vivo turnover rate. Formation of 4-HBS was observed only under strictly anoxic conditions. Treatment of the cell extract with a weak air stream for 2 min resulted in complete loss of the activity, and the activity could not be recovered by restoring reducing conditions. Applying a stream of N2 gas instead of air had no significant effect.
TABLE 1.
Specific activities of 4-HBS, 3-HBS, and benzylsuccinate formation in cell extracts of D. cetonicum grown on either p-cresol, m-cresol, or toluene
| Growth substrate | Mean sp act (nmol min−1 [mg of protein]−1)a ± SD
|
||
|---|---|---|---|
| 4-HBS formation | 3-HBS formation | Benzylsuccinate formation | |
| p-Cresol | 0.57 ± 0.33 | NDb | ND |
| m-Cresol | ND | 0.50 ± 0.16 | ND |
| Toluene | ND | ND | 0.34 ± 0.11 |
Values are means ± standard deviations of three independent measurements.
ND, not detectable.
FIG. 2.
Conversion of p-cresol (■) to 4-HBS (□) by cell extracts of D. cetonicum.
The 4-HBS-forming activity was the only reaction detected converting p-cresol. No indication of a p-cresol methylhydroxylase was found.
Specificity of the reaction.
In cell extracts of D. cetonicum grown with either p-cresol, m-cresol, or toluene, the formation of 4-HBS, 3-HBS, and benzylsuccinate from fumarate and the respective aromatic compound was determined. Formation of either one of the benzylsuccinate derivatives was detected only in extracts of cells grown with the corresponding substrate (Table 1). We also checked for an activity in cell extracts that reacted with o-cresol and fumarate to form 2-HBS. Regardless of the growth substrate, there was only a small decrease in o-cresol concentration with time (less than 5 μM/h) in the presence or in the absence of fumarate. With HPLC analysis, formation of several peaks could be detected; however, none had a retention time or on-line UV spectrum similar to that of either 4-HBS or 3-HBS.
Catabolism of 4-HBS.
The fate of 4-HBS was investigated in cell extracts by HPLC analysis. Assays for 4-HBS formation were amended with a CoA source (succinyl-CoA, acetyl-CoA, or free CoASH and ATP) and/or a mixture of electron acceptors (NAD, NADP, and FAD). Without the addition of a CoA source and electron acceptors, the 4-HBS detected made up at least 94% of the p-cresol converted. In the presence of succinyl-CoA and electron acceptors, significantly lower relative amounts of 4-HBS were detected (64 to 72% of p-cresol converted). Replacement of succinyl-CoA with acetyl-CoA or of CoASH plus ATP or omission of either succinyl-CoA or the electron acceptors led to a similar final concentration of 4-HBS (>90% of p-cresol converted) as in assays without any further addition. After addition of succinyl-CoA and electron acceptors, two compounds were detected in the reaction mixture that coeluted with 4-hydroxybenzoate and 4-hydroxybenzoyl-CoA. The on-line UV spectrum of the peak coeluting with 4-hydroxybenzoate was identical to that of authentic 4-hydroxybenzoate. Due to the small size of the peak coeluting with 4-hydroxybenzoyl-CoA, a reliable UV spectrum could not be recorded. However, after alkaline treatment of the sample, that peak was no longer detectable and the concentration of 4-hydroxybenzoate in the sample increased slightly. The total amount of 4-hydroxybenzoate in alkaline-treated samples (up to 9 μM) accounted for most of the difference in concentrations between p-cresol converted and 4-HBS detected. The formation of free 4-hydroxybenzoate in untreated samples was probably due to hydrolysis of 4-hydroxybenzoyl-CoA by the cell extract (19 nmol min−1 [mg of protein]−1).
In a separate assay, the reduction of 4-hydroxybenzoyl-CoA to benzoyl-CoA was measured at an activity of 6.8 nmol min−1 (mg of protein)−1. The reaction was not complete due to hydrolysis of 4-hydroxybenzoyl-CoA. Reduction of free 4-hydroxybenzoate was not detected.
Growth with p-cresol in the presence of aliphatic acids.
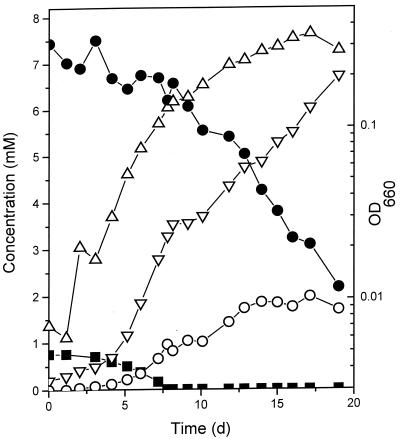
The influence of fumarate on the degradation of p-cresol by growing cultures of D. cetonicum was studied. In the presence of fumarate, growth with p-cresol was biphasic (Fig. 3). In the first phase, D. cetonicum metabolized p-cresol at a rate of 7 nmol min−1 (mg of protein)−1. Per mol of p-cresol, about 1 mol of fumarate was consumed (7.5 nmol min−1 [mg of protein]−1) and close to 1 mol of succinate accumulated in the medium (6.8 nmol min−1 [mg of protein]−1). After p-cresol depletion, D. cetonicum grew at the expense of fumarate (11 nmol min−1 [mg of protein]−1). Succinate was formed at a lower rate (4.6 nmol min−1 [mg of protein]−1) compared to the first growth phase. During both growth phases, small amounts of malate were excreted into the medium (not shown). In growth experiments with succinate or acetate in the presence of p-cresol, growth rates and yields were similar to those of cultures growing in the absence of an aliphatic acid (Table 2). During growth with the putative intermediate 4-hydroxybenzoate, there was no growth stimulation by fumarate compared to controls with succinate or acetate. D. cetonicum did not grow with p-cresol or 4-hydroxybenzoate as the electron donor and fumarate as the sole potential electron acceptor, and fumarate reductase was not detectable in D. cetonicum.
FIG. 3.
Degradation of p-cresol (■) in the presence of fumarate (●). Formation of sulfide (▿) and succinate (○) and increase in OD (▵) are also shown. d, days.
TABLE 2.
Growth rates and yields of D. cetonicum grown with p-cresol in the presence of aliphatic acids
| Addition to p-cresol | Growth rate (day−1) | Growth yield (g[dry wt] · [mol of p-cresol]−1)a |
|---|---|---|
| None | 0.14 | 32 |
| Fumarate | 0.18 | 40 |
| Succinate | 0.13 | 36 |
| Acetate | 0.14 | 33 |
Yields were calculated from OD660 values directly after p-cresol depletion in the medium via an experimentally determined conversion factor.
DISCUSSION
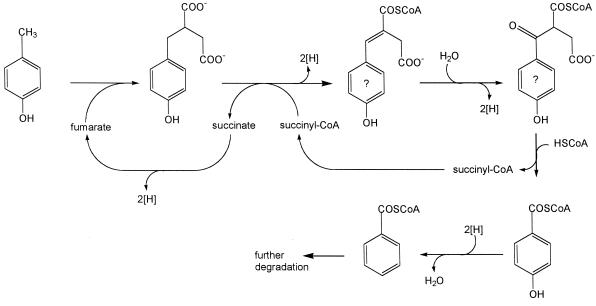
The present data strongly suggest that p-cresol degradation by D. cetonicum is initiated by formation of 4-HBS. This reaction, an addition of fumarate to the methyl group of p-cresol, and the proposed metabolism of 4-HBS to 4-hydroxybenzoyl-CoA through a β-oxidation-like scheme (Fig. 4) is analogous to anaerobic degradation of toluene (4, 6, 33), m-xylene (24), and m-cresol (32) in denitrifying and/or sulfate-reducing bacteria. Our results are therefore contrary to earlier studies on p-cresol metabolism by sulfate-reducing bacteria (19, 28). In those reports, it was proposed that these bacteria hydroxylate the methyl group of p-cresol as the initial reaction step. The in vitro formation of 4-hydroxybenzylalcohol or 4-hydroxybenzylaldehyde as reaction products, however, was not demonstrated in these reports. Our assumption that 4-HBS is a true intermediate of p-cresol degradation by D. cetonicum is supported by three lines of evidence.
FIG. 4.
Proposed initial reactions of p-cresol degradation by D. cetonicum. Compounds with a question mark (4-hydroxyphenylitaconyl-CoA and 4-hydroxybenzoylsuccinyl-CoA) were not identified.
(i) In cell extract of D. cetonicum, the formation of 4-HBS from p-cresol and fumarate could be detected at an initial activity of 0.57 nmol min−1 (mg of protein)−1 which accounted for about 9% of the in vivo turnover rate of p-cresol. This activity was found only in extracts of p-cresol-grown cells (Table 1), indicating that it is of specific physiological relevance for this degradation pathway. There was no evidence of the presence of p-cresol methylhydroxylase in D. cetonicum.
It can be envisioned that 4-HBS formation proceeds in a similar manner to formation of benzylsuccinate from toluene and fumarate. This reaction is most likely initiated by abstraction of an H atom from the methyl group of toluene by an enzyme-bound radical, to form benzyl radical (4, 5, 12, 26). The benzyl radical adds to the double bond of fumarate, and an H atom is donated back to the radical to form benzylsuccinate. The catalyzing enzyme, benzylsuccinate synthase, is irreversibly destroyed by molecular oxygen due to cleavage of the radical-harboring peptide chain (26). Therefore, it is not surprising that the 4-HBS-forming activity was also extremely oxygen sensitive. Similar to benzylsuccinate formation in cell extracts of the denitrifying bacterium Thauera aromatica and of Desulfobacula toluolica (6, 33), there was a severe loss of 4-HBS-forming activity with time (Fig. 2). This is unlike the 3-HBS- and benzylsuccinate-forming activities in cell extracts of D. cetonicum, which appeared to be rather stable. Since the experimental procedures for measuring the respective activities in D. cetonicum were the same, this might argue against inactivation by molecular oxygen as the sole reason for the decrease in 4-HBS-forming activity. It was speculated that the loss of an activating factor or additional cosubstrate accounts for the loss in benzylsuccinate-forming activity in T. aromatica and D. toluolica (6, 33). Whether this could also be the case in 4-HBS formation in cell extracts of D. cetonicum remains to be elucidated.
(ii) 4-HBS was metabolized further to 4-hydroxybenzoate and 4-hydroxybenzoyl-CoA in cell extracts in the presence of the electron acceptors NAD(P) and FAD and succinyl-CoA as a CoA source. The formation of 4-hydroxybenzoate was presumably due to hydrolysis of 4-hydroxybenzoyl-CoA by the cell extract. In assays in which succinyl-CoA was either omitted or replaced with acetyl-CoA or free CoASH plus ATP, only a little 4-HBS was converted and no formation of 4-hydroxybenzoyl-CoA or 4-hydroxybenzoate, respectively, could be detected. These findings indicate that 4-HBS is activated in a succinyl-CoA transferase reaction to 4-HBS-CoA. For benzylsuccinate oxidation to benzoyl-CoA in T. aromatica, involvement of a benzylsuccinate:succinyl-CoA CoA-transferase has already been reported (27). Under standard conditions, the oxidation of 4-HBS-CoA to 4-hydroxybenzoyl-CoA with pyridine nucleotides and FAD as electron acceptors is endergonic. In vitro, the reaction equilibrium was shifted to the product side by the exergonic hydrolysis of 4-hydroxybenzoyl-CoA. In vivo, the reductive dehydroxylation of 4-hydroxybenzoyl-CoA to benzoyl-CoA might pull the reaction forward. This reaction was reported to be irreversible in T. aromatica (9). The benzoyl-CoA formed is probably reduced further to aliphatic products.
Furthermore, an intermediate of the proposed pathway, 4-hydroxyphenylitaconate (resp. the CoA ester), could be tentatively identified in supernatants of p-cresol-grown cultures. This is equivalent to the identification of phenylitaconate in culture supernatants of toluene-grown cells (4).
(iii) Addition of fumarate to p-cresol-converting cultures significantly stimulated growth (Table 2, Fig. 3). Assuming a pathway of p-cresol degradation as outlined in Fig. 4, succinate has to be reoxidized to fumarate for 4-HBS formation. This oxidation is thermodynamically difficult for sulfate-reducing bacteria and might involve an energy-driven reversed electron transport (36). Such energy investment is not necessary if externally provided fumarate is used for 4-HBS formation. The increase in both growth rate and yield upon addition of fumarate and the detection of succinate in the medium strongly support the assumption of an energy-dependent oxidation of succinate to fumarate during growth with p-cresol. With the presumed intermediate 4-hydroxybenzoate, growth was not stimulated by addition of fumarate, and succinate was not detected in the culture supernatant.
It has been pointed out recently that the terminal electron-accepting system seems to largely influence the route by which various phenolic compounds are degraded under anoxic conditions (35). This also appears to be the case with anaerobic degradation of p-cresol. Whereas nitrate-reducing bacteria use an oxidation reaction for attacking the substrate, sulfate-reducing bacteria would have difficulties in disposing of electrons gained at a comparable high redox potential in this reaction and therefore apply a different degradation strategy via addition of the methyl group to fumarate.
ACKNOWLEDGMENTS
We thank A. M. Cook and coworkers for giving us the opportunity to use their HPLC system, Malin Beil for carrying out the GC/MS measurement, and Sigrid Welte for providing 1H-NMR data for 4-hydroxybenzylsuccinic acid and 4-benzyloxybenzylidene succinic acid.
This work was supported by grants from the Deutsche Forschungsgemeinschaft, the Fonds der Chemischen Industrie, and the University of Konstanz.
REFERENCES
- 1.Agency for Toxic Substances and Disease Registry. Toxicological profile for cresols (draft). U.S. Atlanta, Ga: Public Health Service, U.S. Department of Health and Human Services; 1990. [Google Scholar]
- 2.Bak F, Widdel F. Anaerobic degradation of phenol and phenol derivatives by Desulfobacterium phenolicum sp. nov. Arch Microbiol. 1986;146:177–180. [Google Scholar]
- 3.Beh M, Strauss G, Huber R, Stetter K-O, Fuchs G. Enzymes of the reductive citric acid cycle in the autotrophic eubacterium Aquifex pyrophilus and in the archaebacterium Thermoproteus neutrophilus. Arch Microbiol. 1993;160:306–311. [Google Scholar]
- 4.Beller H R, Spormann A M. Anaerobic activation of toluene and o-xylene by addition to fumarate in denitrifying strain T. J Bacteriol. 1997;179:670–676. doi: 10.1128/jb.179.3.670-676.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beller H R, Spormann A M. Analysis of the novel benzylsuccinate synthase reaction for anaerobic toluene activation based on structural studies of the product. J Bacteriol. 1998;180:5454–5457. doi: 10.1128/jb.180.20.5454-5457.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biegert T, Fuchs G, Heider J. Evidence that anaerobic oxidation of toluene in the denitrifying bacterium Thauera aromatica is initiated by formation of benzylsuccinate from toluene and fumarate. Eur J Biochem. 1996;238:661–668. doi: 10.1111/j.1432-1033.1996.0661w.x. [DOI] [PubMed] [Google Scholar]
- 7.Bossert I D, Young L Y. Anaerobic oxidation of p-cresol by a denitrifying bacterium. Appl Environ Microbiol. 1986;52:1117–1122. doi: 10.1128/aem.52.5.1117-1122.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 9.Breese K, Fuchs G. 4-Hydroxybenzoyl-CoA reductase (dehydroxylating) from the denitrifying bacterium Thauera aromatica. Eur J Biochem. 1998;251:916–923. doi: 10.1046/j.1432-1327.1998.2510916.x. [DOI] [PubMed] [Google Scholar]
- 10.Brune A, Schink B. Pyrogallol-to-phloroglucinol conversion and other hydroxyltransfer reactions catalyzed by cell extracts of Pelobacter acidigallici. J Bacteriol. 1990;172:1070–1076. doi: 10.1128/jb.172.2.1070-1076.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cline J D. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr. 1969;14:454–458. [Google Scholar]
- 12.Coschigano P W, Wehrman T S, Young L Y. Identification and analysis of genes involved in anaerobic toluene metabolism by strain T1: putative role of a glycine free radical. Appl Environ Microbiol. 1998;64:1650–1656. doi: 10.1128/aem.64.5.1650-1656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunane L M, Chen Z W, Shamala N, Mathews F S, Cronin C N, McIntire W S. Structures of the flavocytochrome p-cresol methylhydroxylase and its enzyme-substrate complex: gated substrate entry and proton relays support the proposed catalytic mechanism. J Mol Biol. 2000;295:357–374. doi: 10.1006/jmbi.1999.3290. [DOI] [PubMed] [Google Scholar]
- 14.Dangel W, Brackmann R, Lack A, Mohamed M, Koch J, Oswald B, Seyfried B, Tschech A, Fuchs G. Differential expression of enzyme activities initiating anaoxic metabolism of various aromatic compounds via benzoyl-CoA. Arch Microbiol. 1991;155:256–262. [Google Scholar]
- 15.Decker K. Die aktivierte Essigsäure. Stuttgart, Germany: Enke; 1959. pp. 63–66. [Google Scholar]
- 16.Elsden S R, Hilton M G, Waller J M. The end products of the metabolism of aromatic amino acids by Clostridia. Arch Microbiol. 1976;107:283–288. doi: 10.1007/BF00425340. [DOI] [PubMed] [Google Scholar]
- 17.Galushko A S, Rozanova E P. Desulfobacterium cetonicum sp. nov.: a sulfate-reducing bacterium which oxidizes fatty acids and ketones. Microbiology (Engl transl of Mikrobiologiya) 1991;60:742–746. [Google Scholar]
- 18.Galushko A S, Minz D, Schink B, Widdel F. Anaerobic degradation of naphthalene by a pure culture of a novel type of marine sulfate-reducing bacterium. Environ Microbiol. 1999;1:415–420. doi: 10.1046/j.1462-2920.1999.00051.x. [DOI] [PubMed] [Google Scholar]
- 19.Häggblom M M, Rivera M D, Bossert I D, Rogers J E, Young L Y. Anaerobic biodegradation of p-cresol under three reducing conditions. Microb Ecol. 1990;20:141–150. doi: 10.1007/BF02543873. [DOI] [PubMed] [Google Scholar]
- 20.Hopper D J. The hydroxylation of p-cresol and its conversion to p-hydroxybenzaldehyde in Pseudomonas putida. Biochem Biophys Res Commun. 1976;69:462–468. doi: 10.1016/0006-291x(76)90544-1. [DOI] [PubMed] [Google Scholar]
- 21.Hopper D J. Redox potential of the cytochrome c in the flavocytochrome p-cresol methylhydroxylase. FEBS Lett. 1983;161:100–102. doi: 10.1016/0014-5793(83)80738-8. [DOI] [PubMed] [Google Scholar]
- 22.Hopper D J, Bossert I D, Rhodes-Roberts M E. p-Cresol methylhydroxylase from a denitrifying bacterium involved in anaerobic degradation of p-cresol. J Bacteriol. 1991;173:1298–1301. doi: 10.1128/jb.173.3.1298-1301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janssen P H, Schink B. Metabolic pathways and energetics of the acetone-oxidizing, sulfate-reducing bacterium, Desulfobacterium cetonicum. Arch Microbiol. 1995;163:188–194. doi: 10.1007/BF00305352. [DOI] [PubMed] [Google Scholar]
- 24.Krieger C J, Beller H R, Reinhard M, Spormann A M. Initial reactions in anaerobic oxidation of m-xylene by the denitrifying bacterium Azoarcus sp. strain T. J Bacteriol. 1999;181:6403–6410. doi: 10.1128/jb.181.20.6403-6410.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuever J, Kulmer J, Jannsen S, Fischer U, Blotevogel K H. Isolation and characterization of a new spore-forming sulfate-reducing bacterium growing by complete oxidation of catechol. Arch Microbiol. 1993;159:282–288. doi: 10.1007/BF00248485. [DOI] [PubMed] [Google Scholar]
- 26.Leuthner B, Leutwein C, Schulz H, Hoerth P, Haehnel W, Schiltz E, Schraegger H, Heider J. Biochemical and genetic characterization of benzylsuccinate synthase from Thauera aromatica: a new glycyl radical enzyme catalysing the first step in anaerobic toluene metabolism. Mol Microbiol. 1998;28:615–628. doi: 10.1046/j.1365-2958.1998.00826.x. [DOI] [PubMed] [Google Scholar]
- 27.Leutwein C, Heider J. Anaerobic toluene-catabolic pathway in denitrifying Thauera aromatica: activation and beta-oxidation of the first intermediate, (R)-(+)-benzylsuccinate. Microbiology. 1999;145:3265–3271. doi: 10.1099/00221287-145-11-3265. [DOI] [PubMed] [Google Scholar]
- 28.Londry K L, Suflita J M, Tanner R S. Cresol metabolism by the sulfate-reducing bacterium Desulfotomaculum sp. strain Groll. Can J Microbiol. 1999;45:458–463. [PubMed] [Google Scholar]
- 29.Lovley D R, Lonergan D J. Anaerobic oxidation of toluene, phenol, and p-cresol by the dissimilatory iron-reducing organism GS-15. Appl Environ Microbiol. 1990;56:1858–1864. doi: 10.1128/aem.56.6.1858-1864.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merkel S, Eberhard A E, Gibson J, Harwood C S. Involvement of coenzyme A thioesters in anaerobic metabolism of 4-hydroxybenzoate by Rhodopseudomonas palustris. J Bacteriol. 1989;171:1–7. doi: 10.1128/jb.171.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moench T T, Zeikus J G. An improved preparation method for a titanium(III) media reductant. J Microbiol Methods. 1983;1:199–202. [Google Scholar]
- 32.Müller J A, Galushko A S, Kappler A, Schink B. Anaerobic degradation of m-cresol by Desulfobacterium cetonicum is initiated by formation of 3-hydroxybenzylsuccinate. Arch Microbiol. 1999;172:287–294. doi: 10.1007/s002030050782. [DOI] [PubMed] [Google Scholar]
- 33.Rabus R, Heider J. Initial reactions of anaerobic metabolism of alkylbenzenes in denitrifying and sulfate-reducing bacteria. Arch Microbiol. 1998;170:377–384. [Google Scholar]
- 34.Rudolphi A, Tschech A, Fuchs G. Anaerobic degradation of cresols by denitrifying bacteria. Arch Microbiol. 1991;155:238–248. doi: 10.1007/BF00252207. [DOI] [PubMed] [Google Scholar]
- 35.Schink B, Philipp B, Müller J. Anaerobic degradation of phenolic compounds. Naturwissenschaften. 2000;87:12–23. doi: 10.1007/s001140050002. [DOI] [PubMed] [Google Scholar]
- 36.Schirawski J, Unden G. Menaquinone-dependent succinate dehydrogenase of bacteria catalyzes reversed electron transport driven by the proton potential. Eur J Biochem. 1998;257:210–215. doi: 10.1046/j.1432-1327.1998.2570210.x. [DOI] [PubMed] [Google Scholar]
- 37.Schnell S, Bak F, Pfennig N. Anaerobic degradation of aniline and dihydroxybenzenes by newly isolated sulfate-reducing bacteria and description of Desulfobacterium anilini. Arch Microbiol. 1989;152:556–563. doi: 10.1007/BF00425486. [DOI] [PubMed] [Google Scholar]
- 38.Simon E J, Shemin D. The preparation of S-succinyl-CoA. J Am Chem Soc. 1953;75:2520. [Google Scholar]
- 39.Smolenski W J, Suflita J M. Biodegradation of cresol isomers in anoxic aquifers. Appl Environ Microbiol. 1987;53:710–716. doi: 10.1128/aem.53.4.710-716.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stobbe H. Monoarylfulgensäuren und ihre Fulgide. Liebigs Ann Chem. 1911;380:26–36. [Google Scholar]
- 41.Stone R W, Machamer H E, McAleer W J, Oakwood T S. Fermentation of tyrosine by marine bacteria. Arch Biochem. 1949;21:217–223. [PubMed] [Google Scholar]
- 42.Thauer R K, Jungermann K, Decker K. Energy conservation in anaerobic bacteria. Bacteriol Rev. 1977;41:100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tschech A, Fuchs G. Anaerobic degradation of phenol by pure cultures of newly isolated denitrifying pseudomonads. Arch Microbiol. 1987;148:213–217. doi: 10.1007/BF00414814. [DOI] [PubMed] [Google Scholar]
- 44.Yokoyama M T, Carlson J R. Production of skatole and para-cresol by a rumen Lactobacillus sp. Appl Environ Microbiol. 1981;41:71–76. doi: 10.1128/aem.41.1.71-76.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]