Abstract
Deoxyribonucleic acid (DNA) hydrogels combine the properties of DNAs and hydrogels, and adding functionalized DNAs is key to the wide application of DNA hydrogels. In stimuli-responsive DNA hydrogels, the DNA transcends its application in genetics and bridges the gap between different fields. Specifically, the DNA acts as both an information carrier and a bridge in constructing DNA hydrogels. The programmability and biocompatibility of DNA hydrogel make it change macroscopically in response to a variety of stimuli. In order to meet the needs of different scenarios, DNA hydrogels were also designed into microcapsules, beads, membranes, microneedle patches, and other forms. In this study, the stimuli were classified into single biological and non-biological stimuli and composite stimuli. Stimuli-responsive DNA hydrogels from the past five years were summarized, including but not limited to their design and application, in particular logic gate pathways and signal amplification mechanisms. Stimuli-responsive DNA hydrogels have been applied to fields such as sensing, nanorobots, information carriers, controlled drug release, and disease treatment. Different potential applications and the developmental pro-spects of stimuli-responsive DNA hydrogels were discussed.
Keywords: Stimuli-responsive, DNA hydrogel, Biological stimuli, Non-biological stimuli, Composite stimuli
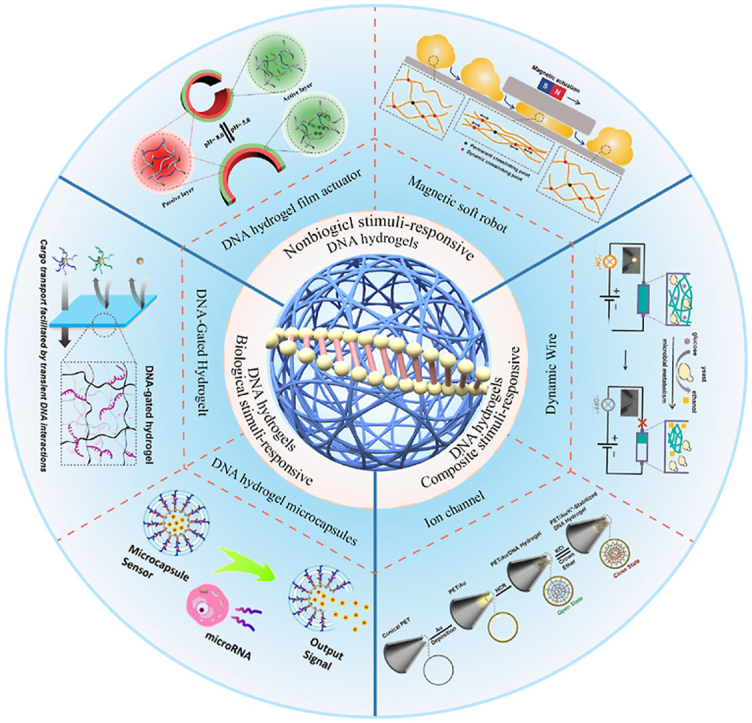
Graphical abstract
Highlights
-
•
DNA hydrogel, favored by researchers, combines properties of DNA and hydrogels.
-
•
Both DNA and skeleton, having many response characteristics, can respond to stimuli.
-
•
Sensing, nano robots, information carriers, drug delivery, and disease treatment uses.
-
•
Three stimulus response types: single biological, single abiotic and compound.
1. Introduction
Hydrogels are materials with a three-dimensional network structure that can be formed by chemical or physical cross-linking, and composed of any water-soluble or hydrophilic polymer [1]. However, restricted by biocompatibility, only some polymers can synthesize hydrogel. For instance, poly(lactic-co-glycolic acid) (PLGA), polyethylene glycol (PEG), and some natural polymers (such as polysaccharides, proteins, and DNAs) are used as scaffolds. The rheological property of hydrogels is adjudged based on the transformation from solid-liquid state (Sol-Gel transition) of the material system, by comparing the storage modulus (G′) with the loss modulus (G″) (If G' < G″, the system is equivalent to viscous liquid. If G' > G″, the system is equivalent to solid.) [2,3]. Compared to gels, hydrogels swell up to hundreds of times more in water on a dry weight basis, due to their hydrophilicity. Polymers are liable to dissolve in water without cross-linking, but gels formed after cross-linking show a specific shape. In the last decades, hydrogels have been rapidly developed and applied as drug delivery carriers [4], sensors [5], and scaffolds in disease treatment and in tissue engineering [6] with long-term progress. Hydrogels display various advantages, including high binding affinity, adjustable pore size, water-absorbing swelling, absorbance ability, biodegradability, and relative mechanical stability, which are expected to play a more prominent role in the environment [7]. Especially, recent studies have shifted the potential applications and development of hydrogels from the traditional areas such as cell/tissue scaffolds [6], drug/gene delivery carriers [4], wound dressing [8], and contact lenses [9] to emerging areas such as wearable devices [10], electronic skins [11], soft robots [12], and artificial intelligence sensors [13].
Since Watson and Crick proposed the DNA double helix structure in 1953, showing how it was composed of just four types of monomeric nucleotides whose arrangement forms the genetic information, the cognition and research on DNAs have increased rapidly. In 1970s, the synthesis has expanded the function of DNAs from pure biomolecules to attractive chemical materials. Therefore, oligonucleotides became a programmable and predictable polymer in material science. In chemical engineering, nucleic acids with specific sequences are being explored as a cornerstone in making functional hydrogels [14]. The base sequences, composed of nucleic acids, provide considerable structural and functional information to biopolymers. The functional information of nucleic acid coding and the switchable trigger function has been extensively applied in various aspects of developing DNA nanotechnology [15,16], involving the development of DNA-based circuit pathways [17], DNA machines [18], nucleic acid-based sensors [19], and carriers used for controlled drug release [20]. Seeman completed the first work using DNA as building block in nanotechnology by assembling DNAs into four-arm Holliday junction and lattice [21], proving that DNA could be designed into artificial structures. Since then, DNA has been applied in many fields other than biology, leading to the production of different nanostructures, nanomachines, and intelligent materials. Furthermore, in 1996, Nagahara et al. from the National Cerebral and Cardiovascular Center in Japan first designed and synthesized the DNA hybrid hydrogels by covalently attaching oligodeoxyribonucleotides to the side groups of polymers based on intermolecular cross-linking formed by hydrogen bonding in base pairs [22]. In 2006, Luo's research group from Cornell University produced pure DNA hydrogels under physiological conditions by using ligases on branched DNA [23], which carved up a new direction of studies on DNA hydrogel. Undoubtedly, introducing DNAs into hydrogels contributes to the programmability of the material. As a material constructing DNA hydrogels, DNAs can be subjected to physical cross-linking, such as hydrogen-bond interaction [23], electrostatic interaction [24], coordination [25], physical entanglement [26] and π-π accumulation [27], and other non-covalent interactions. The DNA hydrogels formed after physical cross-linking are susceptible to external stimuli, resulting in the change of structures. By contrast, chemical DNA hydrogels linked by covalent bonds [28] or formed in enzymatic reaction [29] are more stable in structure, which expands the application of DNA hydrogels in biomedicine.
Simple “intelligence” is provided by producers to stimuli-responsive polymers, which enables polymers to substantially change their structure in response to one or multiple signals, thereby altering their physicochemical property and realizing the goal of designers [[30], [31], [32]]. DNA hydrogels with the properties of both DNA and hydrogels possess designability and adjustability. This study introduced three types of stimuli, namely, 1) nonbiological stimuli, such as temperature, pH (ions), light, and magnetism, 2) biological stimuli, such as nucleic acids, toxins, adenosine triphosphates (ATPs), proteins, bacteria, viruses, and some small molecules, and 3) composite stimuli, such as nonbiological interactions, biological interactions, and interactions between biological and nonbiological organisms. Triggered by external factors, DNA hydrogel changes in appearance, volume, and other physicochemical properties on the basis of Watson-Crick complementary base pairing between long DNA strands or characteristic response behaviors of other functional nucleic acid structures [33,34]. The design strategy, molecular structure, and action mechanism of the stimuli-responsive DNA hydrogels over the recent five years have been overviewed.
2. Nonbiological stimuli-responsive DNA hydrogels
When DNA hydrogels respond to nonbiological stimuli like temperature, light, pH, and magnetism, their physicochemical structure will experience Gel-Sol or Sol-Gel transition, and the cross-linking structure may open under stimuli. Especially, DNA in the hydrogels serves as a cross-linking bridge or provides structural information at this moment when they can identify the specific stimuli and change in structure. Interestingly, in some work, the response to nonbiological stimuli was found to originate from the properties of nanoparticles embedded in the DNA hydrogels, such as gold nanoparticles (AuNPs), gold nanorods (AuNRs), and magnetic nanoparticles (MNPs). The embedded substances respond to the stimuli and give feedback to the whole DNA hydrogel. This type of DNA hydrogels has been devised and applied in controlled drug release and cell culture and as sensors, intelligent robots, and information storage carriers.
2.1. Temperature-responsive DNA hydrogels
Temperature is a usual external stimulus. The temperature responsive DNA hydrogels function by DNA strand hybridization and thermal dissociation, temperature sensitivity of hydrogels, and action on DNA hydrogels by temperature responses of nanoparticles embedded in the DNA hydrogels. In 1996, Nagahara S and Matsuda T et al. prepared the first thermosensitive DNA hydrogel from succinimide copolymer by utilizing the hybridization and thermal dissociation between DNA complementary base pairs [22]. Further, in 2016, Hu and coworkers used DNA structures that are more sensitive to temperature to design a temperature-responsive hybrid double-layer hydrogel consisting of T-A•T triplex crosslinking units bridged non responsive polyacrylamide hydrogel I and T-A•T bridged thermosensitive poly (N-isopropylacrylamide) hydrogel II [35]. At 25 °C, the double-layer hydrogel maintained its linear structure, but it would harden due to the Gel-Sol transition when heated to 45 °C; the hardness of the gel increased, leading to the bending of the composite structure. Cooling system could lead to the Sol-Gel transition, so the reversibility and switchable property of the linear double-layer structure could be reproduced. The bending curvature was controlled by the width and length of the double-layer hybrid. pH responsive DNA hydrogels can be produced by altering the primer of the structure in accordance with the same principle, which is also described hereafter. Predictably, hydrogel materials with mechanical shape modulation will have a greater impact on the development of mechanical responsive sensors and actuators.
Temperature stimuli-responsive DNA hydrogels can be further applied in controlled drug release. The drug is wrapped in DNA hydrogel, which changes the gel to sol under temperature stimulation to release the drug. The formulation must be capable of coping with complex environmental changes and maintaining the stability and sensitivity of materials. Specifically, liposomes as multivalent cross-linkers (CL) [36] are grafted onto DNA copolymer chains via the hydrophobic interaction between the liposomes and cholesterol groups modified on the DNA strand using the thermal sensitivity of liposomes and gels. Danya Lyu et al. reported a kind of DNA hydrogel, that underwent the stimuli-responsive Sol-Gel transition because of the opening of DNA sequence motif, in the presence of the restriction enzyme EcoR I or temperature variations [37], facilitating the controlled release of liposomes. Meanwhile, the point of transition of the release curve in this system for the liposomes was found to be 63 °C. This DNA hydrogel has good self-healing property and can be further applied to the development of other targeted controlled-release systems.
When the thermoplasmonic properties of AuNPs or AuNRs was activated in the polyacrylamide DNA hydrogels upon irradiation, the whole system would become temperature responsive because the thermal mass would be heated in hydrogels. The disassociation of the heat-induced nucleic acid duplex led to the shapeless state of the hydrogel substrate, so the stiffness of hydrogels changed [38]. Further, in order to make the deformation reversible, Wang et al. reported a type of DNA hydrogel with thermoplasmonic properties loaded with AuNPs or AuNRs which collaboratively cross-linked in the polyacrylamide DNA hydrogels via bis-acrylamide and DNA duplex or borate, glucosamine and DNA duplex [39], respectively. Under irradiation at λ = 532 nm or λ = 808 nm, the heating of thermal mass in the hydrogels and heat-induced dehybridization of DNA dimers promoted the formation of hydrogels of low stiffness. Opening or closing irradiation could cause the switch between high and low stiffness of DNA hydrogels in a short time. This kind of DNA hydrogel exhibited shape memory and self-healing and mechanical property, and could act as drug release tool, sensor, and information storage device.
2.2. Ions (pH)-responsive DNA hydrogels
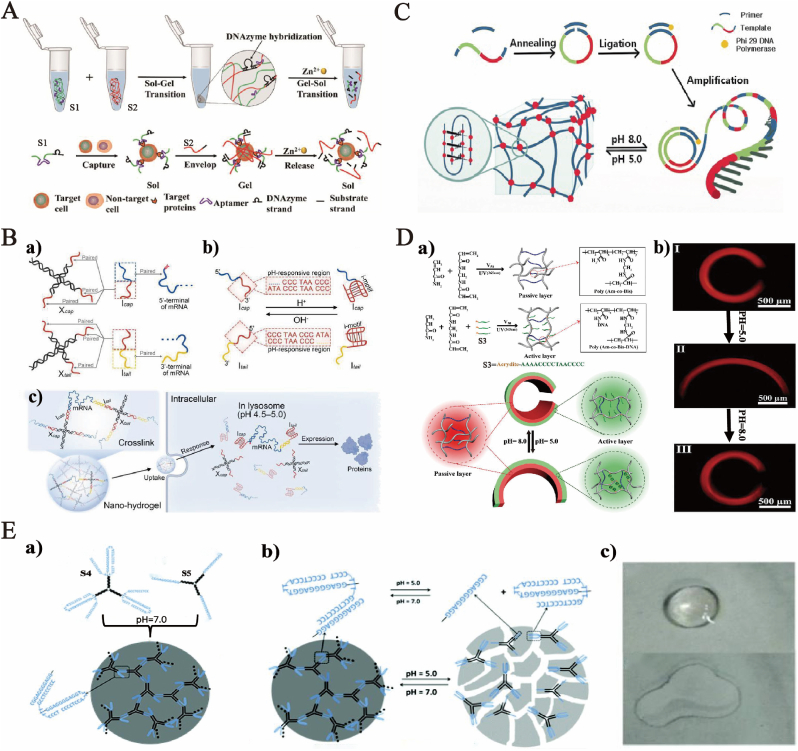
Predictably, the presence of ions, including protons and metal ions, will change the pH of the solution. Most of the metal ion-responsive DNA hydrogels depend on the ions-specific responsive DNA sequence, which forms the specific DNA secondary structure. For instance, K+ transforms G-rich DNA into G-quadruplex. The affinity of 18-crown-6 for K+ is higher than that of G-rich sequence, which can dissociate the K+-stabilized G-quadruplex cross-linked structure and lead to the transition from the hydrogel state to the solution state [40]. Wang et al. introduced Pb2+-specific DNAzyme into DNA hydrogels. The DNAzyme will form a G-quadruplex structure in the presence of Pb2+, otherwise it is a single stranded state, which also leads to the transformation of DNA hydrogels between gel sol [41]. In the same way, researchers constructed an acid resistant DNA hydrogel containing a-motif (parallel double strand at pH 1.2–3.0) and g-motif (G-quadruplex at pH 4.0–6.0) [42]. The DNA hydrogel successfully passed through gastric juice, duodenal juice, and intestinal juice in order to prepare a new carrier for oral insulin. Based on the combination of cytosine rich DNA sequence and Ag+, the C–Ag+-C structure is formed. This process will lead to the contraction of the hydrogel. Using this characteristic, the researchers combined it with the microfluidic heating system for the repeated detection of Ag+ [43]. Furthermore, the shrinking hydrogel can change the contained photonic crystal interval, thus enabling the quantitative detection of the target [44]. Hg2+ transforms the thymidine-rich sequence folds into a T-Hg2+-T hairpin structure [45] in the presence of mercury ions. The mercury ions can interfere with the metabolism and function of cells by binding to sulfhydryl groups. Compared to traditional means, DNA hydrogels are more efficient, rapid, and sensitive in detection. To reduce the cost of synthesizing DNA hydrogels, Li et al. attached a 5′ adenine (A5) block to DNA sequences with different functions to obtain diblock DNA sequences [46]. The incorporation rate of these sequences in polyacrylamide hydrogels was 75% at −20 °C, which was basically the same as that of acrydite-modified DNA sequences in the hydrogels; therefore, production cost could be considerably decreased. In addition, DNA hydrogels can also be used to detect other metal ions by introducing the specific DNAzyme. A type of Zn2+ responsive DNA hydrogel was used for selective capture [47], in-situ package, and mild release of target cells after Sol-Gel-Sol transition triggered by DNAzyme (Fig. 1A). The DNA hydrogel was produced from the cross-linking of strand S1 with surface markers of specifically recognizing target cells and DNAzyme sequences and strand S2 with DNAzymes through rolling circle amplification (RCA). S1 could specifically capture target cells which were released through the specific cleavage (Sol-Gel transition) of Zn2+-induced DNAzyme hydrogels.
Fig. 1.
(A) Synthesis of DNAzyme-triggered Zn2+-responsive DNA hydrogel. Copyright 2021, American Chemical Society. (B) Schematic of mRNA delivery by pH-responsive DNA nano-hydrogel, (a) Schematic of the “X”-shaped DNA scaffold, (b) Structural changes of the pH-responsive Itail and Icap, and (c) DNA nano-hydrogel-assisted mRNA delivery and its intracellular pH-responsive release. Copyright 2021, Wiley-VCH. (C) Preparation of pH-responsive DNA hydrogel by RCA method; the I-motif structure was destroyed when pH changed from 5.0 to 8.0. Copyright 2017, Wiley-VCH. (D) Composite diagram of pH-responsive smart bilayer DNA hydrogel film actuators; the actuators deform reversibly when the pH changes. Copyright 2020, Wiley-VCH. (E) Schematic of pH-controlled self-assembly DNA hydrogel. (a) “Y”-shaped DNA scaffolds synthesize DNA when pH is 7, (b) DNA hydrogel dissociated under acidic conditions, and (c) Gel state and liquid state of DNA hydrogel. Copyright 2018, The Royal Society of Chemistry.
The response of DNA hydrogels to pH is due to the change in DNA conformation, which is dominated by constructing I-motif in the DNA sequences. K. Gehring and J.L. Leroy et al. first put forth the I-motif structure [48]. At present, I-motif forming sequences (IFSs), have been extensively researched. Moreover, the triple helix C-G·C+ is usually brought into DNA hydrogels to realize the pH-triggered formation or disassociation. Laura Heinen et al. neutralized two enzymes antagonistic to pH regulation in the feedback-controlled biocatalytic reaction network (BRN) and coupled them to the pH-responsive DNA hydrogels [49], forming the hydrogel system with obvious programmable lag time and life in the closed system. BRN can switch the “opening” and “closing” of temporary gel state at a precise time and control the orthogonal internal time by adjusting the programmable non-linear pH changes. Fu X synthesized a kind of pure pH-responsive DNA hydrogel for the messenger ribonucleic acid (mRNA) transmission in cells based on the following synthesis techniques. Firstly, modifying the transmitted mRNA as shown in Figure (Fig. 1B), Fu et al. stabilized the mRNA by synthesizing it through in vitro-transcription (IVT) [50]. The nanohydrogels involved two “X"-shaped DNA scaffolds as shown in Fig. 1B a. Specifically, Itail and Icap were designed to involve I-motif sequences which could hybridize with Xtail and Xcap, respectively, in the neutral or alkaline environment and fold into I-motif structure after dehybridization under the acidic condition (Fig. 1B b). Therefore, nanohydrogels are pH-responsive. When the nanohydrogels enter the lysosome (pH 4.5–5.0) of cells through endocytosis, they are expected to maintain the nanostructure in the extracellular physiological environment and decompose and release mRNA to express the encoded proteins (Fig. 1B c). This system shows huge potential in the development of mRNA medicines and treatment of cancers and other diseases by further introducing the target identifying molecular elements. Similarly, Xu et al. amplified IFSs through RCA to make them periodically sequence on the RCA strand [51], which served as a process of driving gels by CLs (Fig. 1C). To achieve this goal, the closed units of IFSs needed to form an effective intermolecular I-motif structure under the acidic condition. Besides, when the pH value was elevated form 5.0 (acidic) to 8.0 (alkaline), the I-motif structure would be damaged, thereby achieving the Gel-Sol transition. Also, a poly(Adenine) segment 30 (green) was embedded as spacer for different motifs to ensure the normal function of IFSs (red). DNA hybrid hydrogels can be utilized to construct the programmable pH-responsive film actuator, which can effect reversible and significant microscopic shape change via the sequences of DNA cross-linking units (Fig. 1D). Bi et al. adopted the step-by-step photolithographic technology to realize the parallel reassembly of double-layer hydrogel films (passive layer and active layer)in heterogeneous patterning shape made up of polyacrylamide (Poly(Am-co-Bis)) and DNA CL (Poly(Am-co-Bis-DNA)) [52]. As a proof of concept, cross-linked DNA S3 based on DNA I-motif was introduced into the active layer of the double-layer film. In this way, the polymer is formed or dissociated under pH stimulation, which leads to the redistribution of internal stress in the double-layer structure, and finally leads to reversible deformation. Taking advantage of DNA programmability and abundant functional sequence motif, these film actuators can be potentially applied in intelligent biological sensors, biological medical instruments, soft robots, and adaptable electronic devices.
Compared with the traditional I-motif responsive pH, Lu et al. introduced two kinds of triple helix structures on the basis of protonated cytosine-guanine-cytosine (C-G·C+) and thymidine (T-A•T) and built a type of pH-controlled bidirectionally pure DNA hydrogel [53], as shown in Fig. 1E a. Both types of Y-shaped modules (S4 and S5) had three interlocked sticky ends formed by the complementary pairing, and the stoichiometry of self-assembly S4 and S5 was mixed. At pH 7.0, C-G·C+ structure at the sticky end of module S4 would break to hybridize with the sticky end of module S5 through A-T/C-G base pairs. With the accumulation of these hybrid structures, hydrogel would be formed. When the pH was adjusted into 5.0 (Fig. 1E b & c) by adding hydrochloric acid, the hydrogel dissolved into liquid state, proving that the pH-activated reversible hydrogel was successfully synthesized. Aptamer with fluorescein (FAM) and black hole quencher 1 (BHQ1) were introduced into the hydrogel to transform the formation and dissociation of hydrogels into a fluorescence signal output. This strategy provides a fluorescence-based method for the rapid visual supervision of hydrogel state and casts a new light on the formation of hydrogels.
In addition, we noticed that Liu's research group combined DNA Nanomotor [54], Kinetically interlocking multiple-units [55] and I-motif into DNA hydrogels, which greatly improved the mechanical strength and pH response efficiency of hydrogels. Further, by using branched DNA and a programmable link [56], Liu's group built a DNA hydrogel for different responses, whose mechanical properties could be adjusted by three orders of magnitude, and branched out to achieve a good response to K+ by introducing G-four-strand sequences. These new DNA hydrogel construction methods provide a good basis for 3D printing and tissue engineering.
The construction of ion-responsive DNA hydrogel requires us to discover more specific DNA sequences and structures. Compared with many ion sensors, DNA hydrogel shows its unique advantages, especially when combined with other signal amplification mechanisms, and the detection limit of ions is very low. Since the discovery of I-motif structure, it has been utilized extensively in the construction of DNA nanomaterials based on acid response. In comparison, the three-fold structure of protonated C-G·C+ and T-A•T provides new ideas to apply pH-responsive DNA hydrogels in specific acid-base environments. pH-responsive hydrogels have made some progress in cell engineering, controlled drug delivery, and preparation of membrane brakes. The coupling of enzymes that antagonize pH regulation and pH-responsive DNA hydrogel have also predicted the autonomous life cycle of composite materials.
2.3. Photon-responsive DNA hydrogels
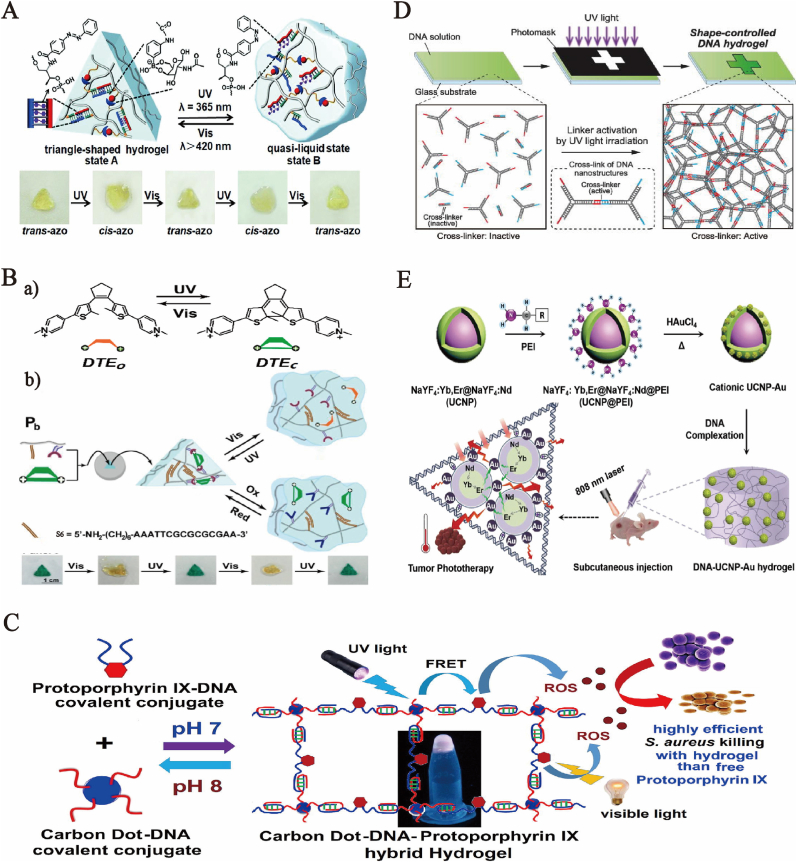
At present, azobenzenes and arylazopyrazoles [57] are the only photoisomerization compounds that can interact with DNA and control the stability of the double-stranded nucleic structure. Developing a new type of photoisomerization switch that interacts with nucleic acids (especially the convertible switch working in visible light or the infrared region) is a vital challenge. With light as the switch to control Sol-Gel transition, the photons-responsive DNA hydrogels are also developed and applied in the controlled drug release. Specifically, Liu et al. functionalized two polyacrylamide chains using phenylboronic acid ligand and glucosamine, respectively, and modified and functionalized the polyacrylamide chains by nucleic acid stay-chain. A designed single strand and a DNA strand against azobenzene functionalization were used to further hybridize with DNA incorporated with polyacrylamide chains, to guarantee the synergistic stability of hard hydrogels bridged by glucosamine-borate and photons-responsive trans-azobenzene-tethered DNA (t-azoDNA) units [58]. As shown in Fig. 2A, azobenzene underwent trans-to-cis photoisomerization (λ = 365 nm), and cis-azobenzene (cis-azo) units lacked the affinity to double-stranded nucleic acids, resulting in the dissociation of double-stranded area between the photoactive domain in the DNA strand related to cis-azo and partial structural domain as a part of the DNA stay-chain. The disassociation of double-stranded area reduced hydrogel stiffness. Photoisomerization of the cis-azo unit to the trans-azo state (λ > 420 nm) restored the stiff hydrogel that is cooperatively stabilized by the two cross-linking motifs. In this study, the development of the photons-responsive DNA hydrogel used for the convertible, controlled release of Adriamycin was also explored. Photostimulation-controlled hydrogel stiffness has paved a smooth path for the application of hydrogel substrates in controlled drug delivery and the preparation of “mechanical” hydrogels as actuators. However, a more extensive light response effect and simplified synthesis process of composites was required. Researchers found that bipyridinium dithienylethene (DTE) was characterized by cyclic and reversible photoisomerization, switching between the closed state (DTEc), the electron acceptor, and the open isomer (DTEo) that lacks electron acceptor properties (Fig. 2 B a). Li et al. designed a multi-triggered DNA/DTE carboxymethyl cellulose (CMC) hybrid hydrogel [59]. The stiff-to-soft transition of the hydrogel could be achieved by means of the photoisomerization of photo-induced DTE and donor receptor interactions and S6/S6 duplexes (Fig. 2 B) In addition, photon-responsive DNA hydrogels have also been developed and applied in sterilization. They are loaded with drugs for treatment using the coating capability of DNA hydrogels and the drugs are released by stimuli to achieve sterilization. S Kumari first used DNA-hybrid hydrogels to load drugs for photodynamic therapy (PDT) to simultaneously restrain the aggregation-induced self-quenching and controlled release [60] (Fig. 2C), thereby designing a type of photo-induced carbon dot (CD)-DNA-protoporphyrin hybrid hydrogel with antibacterial activity. CD and protoporphyrin IX (PpIX) were covalently bounded to the 5′-phosphoric acid end of the C-rich single-stranded DNA (ssDNA) to induce the self-assembly of DNA strand via I-motif into CD-DNA-PpIX hybrid hydrogel under neutral condition. Reactive oxygen species (ROS) was produced by the direct irradiation of PpIX through visible light or the activation of Forster Radius energy transfer (FRET) from CD (donor), and the killing effect on the Gram-positive bacteria (Staphylococcus aureus) was also tested. However, compared with other DNA hydrogels used in diagnosis and treatment, the system lacks the use of the basic characteristics of hydrogels, and the biocompatibility of the hydrogels can be further improved. However, it can be predicted that the non-covalent interaction between CD and hydrogel matrix provides a direction for combined treatment of tumors.
Fig. 2.
(A) Scheme of light-induced shape-memory transition from glucosamine borate ester bridge and trans azobenzene stabilized double crosslinking agent hydrogel. Copyright 2019, The Royal Society of Chemistry. (B) (a) Scheme of photoisomerization between DTEo and DTEc. (b) Schematic of preparation and light-induced shape-memory properties of DNA hydrogel. Copyright 2018, American Chemical Society. (C) Carbon dot-DNA-protoporphyrin hybrid hydrogel for sustained photoinduced antimicrobial activity. Copyright 2019, Elsevier. (D) Conceptual illustration of the photolithographic formation of shape-controlled DNA-motif hydrogels based on the photo-activated self-assembly of DNA nanostructures. Copyright 2019, American Institute of Physics. (E) Schematic of injectable and NIR-Responsive DNA–Inorganic Hybrid Hydrogels. Copyright 2020, Wiley-VCH.
Keonwook Nam et al. designed a multifunction X-shaped DNA nanostructure (X-DNA) [61], which was self-assembled into cell adhesion and photo-cross-linking part with stoichiometric ratio. With polyethylene glycol diacrylate (PEGDA) as a structural carrier, the hybrid hydrogel could be produced through rapid photo-cross-linking under the mild reaction condition, following which X-DNA was combined with cells. This DNA hydrogel could be utilized to strengthen cell proliferation and targeted specific adhesion in the 3D cell culture.
In addition to adding photon-responsive chemical substances in the aforementioned DNA hydrogels, DNA hydrogels can become photon-responsive via photon-responsive DNA sequences. Interestingly, the photon-responsive DNA hydrogels are further combined with photolithographic technology to prepare the patterning DNA hydrogels in a controllable shape, expanding the development of the hydrogel memory system. Yu Kasahara et al. reported a photolithographic method for the photo-activated self-assembly DNA hydrogels to control the shape based on Y-shaped DNA nanostructure (Y-motif) [62] (Fig. 2D). The authors designed two Y-shaped.
DNA with sticky ends, which are complementary to hairpin DNA (cross-linker, CL) covered with photolysis sequences. Under ultraviolet light irradiation at 300–350 nm wavelength, the DNA sequence covered in the inactivated linker was dissociated from the CL DNA sequence. Later, the inactivated CL returned to the complete CL DNA with cross-linking activity. From then on, the complete CL DNA would be called “activated CL,” thereby self-assembling and forming the DNA-motif hydrogel with grid structure. Subsequently, the authors demonstrated the controllability of the DNA-motif hydrogel shape using photolithographic technology. Further, the hybridization chain reaction (HCR) triggered by the association of the nucleic acid-binding chain with continuous hydrogel substrates led to the stress-induced ordered orthogonal shape change on the patterning domain, causing the ordered shape of the combination of cell aggregates and this pattern. This triggering process was completed under photostimulation. On thisbasis, Huang et al. introduced a series of photolithographic patterning of polyacrylamide hydrogel films, or patterning of biphotonic laser scanning confocal microscopy, on the basis of o-nitrobenzyl phosphate nucleic acid. The cyclically alternate round patterning area surrounded with continuous hydrogel substrates would be produced by these films [63]. This DNA hydrogel film leads to membrane expansion and anisotropic stress through HCR, which can guide HeLa cells to deposit and proliferate in the microcontainer. Equally important, the membrane is expected to regulate the shape of proliferating cells and the pattern of cells.
In addition to the aforementioned two types of photon-responsive mechanisms, the response of nanoparticle coated-DNA hydrogels to light can be utilized to make the entire system photon-responsive, and this response acts on the system in turn to achieve the final goal. Liu B et al. designed a kind of near infrared (NIR)-responsive injectable up-conversion mediated by DNA and Au nanoparticle hybrid (DNA-UCNP-Au) hydrogel [64], which could be utilized for oncotherapy (Fig. 2E). The hydrogel could be prepared in three steps. Firstly, UCNPs were produced by solvothermal synthesis. Secondly, they were superficially functionalized by polyethylene glycol (PEI) to form the core @ shell structure. At last, the attained UCNP@PEI was used to restore HAuCl4 and form UCNP–Au nanoparticles. The cationic properties of UCNP–Au nanoparticles allowed them to electrostatically complex with salmon sperm DNA (smDNA) to form DNA-UCNP-Au hydrogel, which has obvious inhibitory effect on tumor in mice under NIR light irradiation.
Due to the addition of isomeric compounds, the construction of a photo-response DNA hydrogel is less tedious compared to the design of DNA nanostructures that can be cross-linked and dissociated under light induction; however, it also reduces the accuracy of response to light. The study of embedding light-responsive nanoparticles to construct DNA responsive hydrogel from inside to outside is also surprising. In particular, the mechanism of additional thermal effects provides a new solution for tumor therapy. Looking forward, it can be predicted that these switchable functions of photo-responsive hydrogels will be used to develop shape memory hydrogels and self-healing hydrogel matrices [65]. For example, light-induced control of hydrogel stiffness has led to the development of switchable controlled release drug materials, or “mechanical” hydrogels that can convert light energy into actuators, and functional bodies such as molecular robots and artificial cells. In the near future, the internal and external synergies produced by light-responsive DNA hydrogel under light stimulation are believed to play a greater role in clinical treatment.
2.4. Magnetism-responsive DNA hydrogels
In recent years, researchers have been working to develop responsive DNA hydrogels that can be further manipulated without physical contact for clinical applications, such as killing specific pathogens and removing tumor cells at designated locations. Compared with the remote triggering of hydrogels, such as light and temperature, the functionalized natural polymer hydrogels from magnetic materials have become a new type of intelligent drug responsive device due to advantages such as rapid response and remote control. Currently reported magnetism-responsive DNA hydrogels are often produced by embedding magnetic nanoparticles in the DNA hydrogels or coupling magnetic nanoparticles with DNA or complementary DNA that constitutes DNA hydrogels; this makes DNA hydrogels magnetism-responsive due to the properties of magnetic nanoparticles. For instance, Ma et al. designed a magnetism-responsive DNA hydrogel [66]. DNA modifying MNPs and Y-shaped DNA were connected through DNA, so the MNPs could be incorporated into the DNA hydrogel by hybridization instead of physical cross-linking. As a result, MNPs existing in the DNA hydrogel caused the external non-contact stimuli (such as magnetic field) to manipulate the deformation of the hydrogel into various shapes, and the movement towards certain direction or even out of the surface. This hydrogel provides new ideas for cell culture and biological medicine.
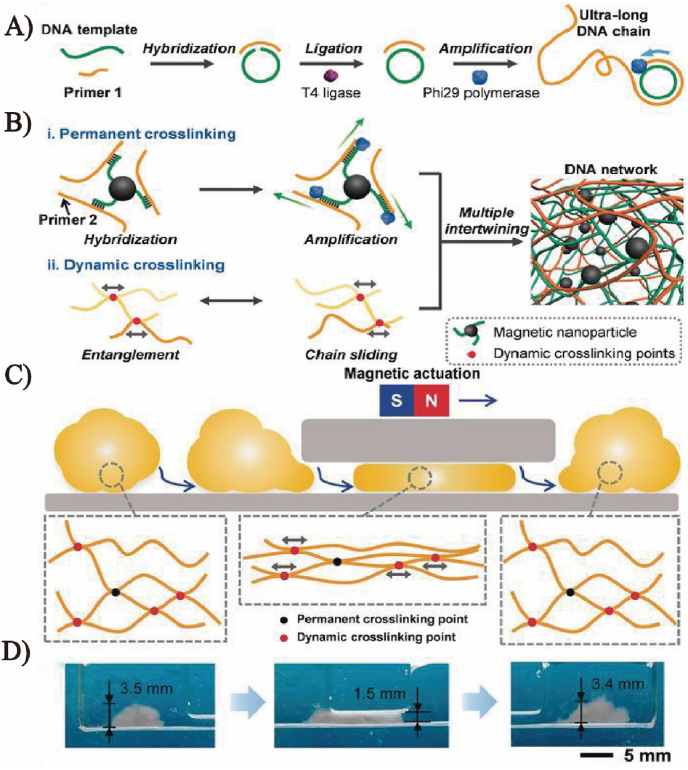
Fig. 3 shows a magnetic DNA hydrogel soft robot [67]. The ultra-long ssDNA was synthesized based on RCA, containing the capture sequence that could hybridize with the ssDNA on the modifying magnetic nanoparticles for the second amplification. Through this process, the stable magnetic DNA hydrogel could be obtained. This DNA robot could cross narrow pathways or the groove-like maze under magnetic actuation by adjusting and restoring its shape. It is worth noting that these pathways were smaller than the robot, and the robot could restore to its original shape after deformation. The sound navigation and biocompatibility of DNA robots make them an intelligent transporting tool for living cells, and they have a promising future in diagnosis and treatment, implantable medical devices, and minimally invasive surgeries. Moreover, researchers found that the enzymatic activity, cascade reaction efficiency, and the stability for temperature, long-term storage, and organic solvent of encapsulated multienzyme magnetic DNA hydrogels have been significantly improved. Researchers also revealed in subsequent studies that the coated multienzyme could be used to detect low-concentration glucose [68], showing great selectivity.
Fig. 3.
Schematic of magnetic driven DNA hydrogel synthesis. (A) Enzymatic amplification of RCA to produce ultralong DNA strand products. (B) Permanent crosslinking and dynamic crosslinking. Green strands represent the DNA chain product of secondary amplification. (C) Schematic of the behavior of a robot when it strikes and passes through an obstacle. (D) The characterization of shape adaptability of the DNA robot. Copyright 2019, Wiley-VCH.
In addition to the abovementioned nonbiological stimuli, researchers designed a force change-responsive DNA hydrogel and applied it in the effective capture of T lymphocytes of humans and the adjustable activation of T-cell receptors (TCRs). Alexander S et al. developed a functionalized hydrogel based on nucleic acid nanoassembly (NAN) [69] to explore the source of the initial mechanical stimuli (presynaptic and microvilli-based TCR activation) during immune surveillance. The authors proved the effective capture of human T lymphocytes and the adjustable activation of TCR by combining NAN connectors of different lengths with polyacrylamide hydrogels with different shear moduli. Relying on the rational design of DNA and response skeleton, nonbiological stimulation responsive DNA hydrogels are designed into microspheres, films, vesicles, etc. by adding effective loads and targeted driving, they have made great progress in controlled drug release, sensors, intelligent robots, cell culture, information storage carriers, etc. However, it is not difficult to see that accurate response sources and faster response mechanisms are still the directions in which research is required.
3. Biological stimuli-responsive DNA hydrogels
As research delves deeper into DNA hydrogels, they are endowed with ever-increasing intelligent features. Polymers become programmable due to the principle of complementary base pairing. Aptamers, which play an important role in designing biological stimuli responsive DNA hydrogels, have been characterized by strong binding capacity, high specificity, and a broad target range (many small molecular compounds, proteins, polypeptides, and bacteria are involved). For the above two reasons, DNA hydrogels have been applied more extensively with controllable binding and dissociation, and the impacts brought by non-specificity are eliminated. New ideas have been proposed for the logic pathway design of DNA hydrogels, but undeniably, the follow-up difficulty still prevails in designing feasible logical pathways. Biological stimuli-responsive DNA hydrogels have developed prosperously in various aspects like nucleic acid detection, in vivo transport, sensors, diagnosis and treatment of diseases.
3.1. Nucleic acid-responsive DNA hydrogels
3.1.1. DNA-responsive DNA hydrogels
Clearly, the response mechanism of DNA hydrogels to nucleic acids can be very easily established on cross-linked primers, among which the interaction between complementary sequences is commonly utilized. Using this principle, a series of polymer polymerized motor gels with polymer as skeleton was constructed [70]. By inserting the cascade drive effect generated by DNA hairpin, the system responded to swelling. It provides an opportunity to create cell growth and split gel. Further, Gu et al. reported a DNA-gated hydrogel strategy for the selective transport of macromolecular substances in line with the gating principle [71] as follows: Under DNA-DNA interactions, DNA strands bind to the hydrogel via covalent interactions, and only the cargo marked with the DNA sequences complementary to the DNAs in the DNA hydrogel can reversibly bind to these gated DNA strands, and thus cross barriers. This strategy could be successfully implemented with the following two prerequisites: first, the DNA-DNA interactions generated enough attractive force to conveniently allocate the cargo into the hydrogel. Second, such interactions were instantaneous enough to facilitate the cargo flow and diffusion in the hydrogel. The authors further explored the DNA-DNA binding strength and how the cargo valency (i.e., the number of DNA strands on the cargo) influenced the transport selectivity in a synthesis system. This strategy can be transformed to realize field-mediated directional transport, and meanwhile, popularized to other scaffolds. Furthermore, researchers found that DNA strand exchange reactions can effectively eliminate the interface between DNA hydrogel bricks [72]. The dynamic nature of DNA assembly ensures the good cell permeability of the gel and the migration of cells between different hydrogel bricks.
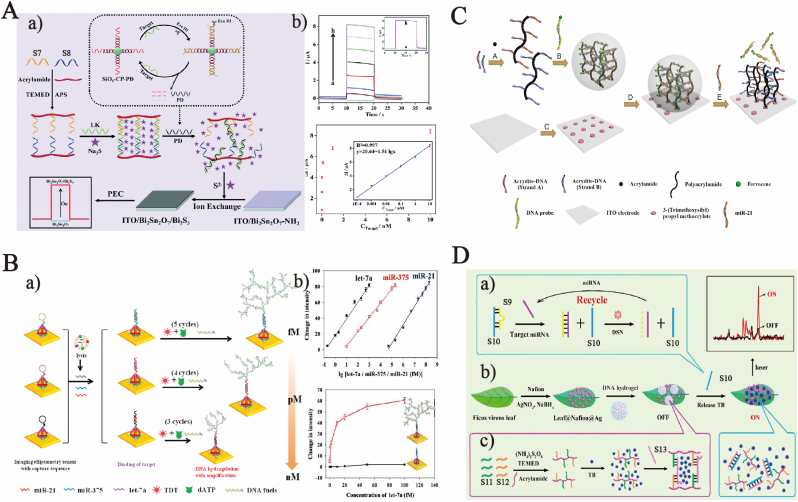
Responses to DNAs can also be achieved by specific capture probes. Realizing that the detection of promyelocytic leukemia/retinoic acid receptor alpha (PML/RARα) can provide a molecular basis for the diagnosis and illness monitoring of acute promyelocytic leukemia (APL), Guo et al. designed a streptavidin (SA)-encapsulated and target-triggered self-assembled DNA hydrogel [73], which was then combined with the surface plasma resonance (SPR) biosensing technology for the detection of PML/RARα. The results showed that the SA aptamer-based hydrogel was formed through the self-assembly between primers. The SPR signal was strengthened by both the synthesis of ultra-high molecular weight complexes and the introduction of the SA aptamer, which substantially increased the limit of detection (LOD) for PML/RARα, to 45.22 fM. Especially, the lower LOD for DNA detection was greatly lowered due to the recyclability of nucleic acids during the reaction and by combining the DNA hydrogel that serves as a signal amplification mechanism. Li et al. reported a photoelectrochemical (PEC) sensor [74] based on DNA hydrogel and S2−-sensitizing-Bi2Sn2O7 by the anion-exchange reaction and applied it to the ultrasensitive detection of p53 gene DNA (Fig. 4 A). Moreover, the author designed a recycling mechanism for the recycling production of product strands (PDs), connected the capture chloroplast (CP) DNA onto carboxyl-modified SiO2 microspheres via covalent bonds, and hybridized CP with PD to obtain SiO2-CP-PD. The target DNA, if existing, will be hybridized with the CP on SiO2 microspheres, with 3′-end of the CP strand exposed. Exonuclease III (Exo III) tends to act upon the flat or depressed substrate at the 3′ end of dsDNAs. In this study, therefore, Exo III catalyzed the gradual removal of mononucleotides from 3′ hydroxyl terminal of the CP strand to release the target and PD. The target DNA was continuously utilized in the next cycle to generate a mass of PDs. Subsequently, the cross-linked triple-stranded DNA (tsDNA) hydrogel which was formed by the acrydite-modified S7 and S8 under the catalysis of APS and TEMED embedding sodium sulfide (Na2S) was designed and synthesized. The Linker DNA in the DNA hydrogel specifically bound to PDs, and as a result, Na2S in the hydrogel was released to Bi2Sn2O7/Bi2S3 electrode and applied to the follow-up PEC sensing. The LOD and detection range for p53 gene were 20 fM and 100 fM-10 nm, respectively. This strategy provides a good reference for using stimulus responsive DNA hydrogels as signal amplification mechanisms, but it is also worth noting that cumbersome reaction processes often require more accurate experimental operations.
Fig. 4.
(A) (a) Schematic of target-switchable DNA hydrogels coupled with a Bi2Sn2O7/Bi2S3 heterojunction based on in situ anion exchange for photoelectrochemical detection of DNA. (b) PEC response of sensor to different target concentrations. Copyright 2021, The Royal Society of Chemistry. (B) (a) Schematic of imaging ellipsometry biosensor based on DNA hydrogelation for multiplexed exosomal miRNA detection. (b) Linear range of the IES for the multiplexed detection of let-7a, miR-375, and miR-21. Copyright 2020, American Chemical Society. C. Schematic of the electrochemical biosensing platform based on hybrid DNA hydrogel using for miR-21detection. Copyright 2018, Elsevier. (D) (a) Schematic of target miRNA 155-Induced Duplex-Specific Nuclease Signal Amplification Possess, (b) schematic of SERS Platform construction, and (c) preparation of TB Trapped 3D DNA Hydrogel. Copyright 2017, American Chemical Society.
To improve the stability of primer crosslinking in DNA hydrogel, Cheol Am Hong et al. reported an enzyme-free quantum dot (QD)-DNA hydrogen based on FRET to detect target DNAs and realized the efficient energy transfer from the QD donor to the quenched receptor [75]. A single target DNA molecule could mediate the opening of hairpin loops multiple times, thereby enhancing the sensitivity of target DNA detection (to 6 fM) with shorter detection time (1 h). Finally, the universality of this strategy was proved by detecting the target gene of Klebsiella pneumoniae carbapenemase (KPC).
3.1.2. RNA-responsive DNA hydrogels
At present, microRNAs (miRNAs) are not thoroughly understood. Some miRNAs are correlated with the development of diseases and disruption of vital processes, because they play significant roles in regulating cell growth and tissue differentiation. This is also demonstrated by the site analysis of miRNAs on genomes. In general, miRNAs are detected using their specific recognition primers as probes, because DNA hydrogels can be expanded to a dendritic shape [76] by the hybridization and cross-linking between DNAs. In this way, the drug loading capacity of DNA hydrogels can be strengthened to a great extent. Zou et al. established an elliptically polarized imaging sensor [77] for the sensitive detection of let-7a, miR-375 and miR-21 by combining dendritic DNA hydrogels with the amplification technology (Fig. 4 B). Specifically, tetrahedral DNA probes (TDPs) were immobilized on an Au film through Au–S bonds as the sensor substrate. Next, the hairpin structure of the capture sequence was opened under the presence of, for example, miR-21. Subsequently, 3′-OH exposed in TDPs was extended using tATP terminal deoxynucleotidyl transferase (TDT) and hybridized with DNA fuels. After 3 cycles, a dendritically fractal 3D micro-hydrogel was generated on the Au film. Similar hydrogels could be formed by miR-375 (concentration: pM) and let-7a fuels (fM) through 4 and 5 cycles, respectively. Moreover, the concentration of target miRNAs can be quantified according to the strength change of the elliptically polarized imaging sensor. Furthermore, Deng et al. prepared a dendritic DNA hydrogel with dynamically controllable terminals through the repeated catalytic action of TDT on the basis of nucleic acid amplification [78]. TDT overhung the free 3′-OH terminal to the homopolymeric adenosine base pair and alternate with the worn branches of complementary seed oligonucleotides. Next, the target miRNAs were detected by introducing a capture probe responding uniquely to the target miRNAs into the DNA hydrogel as a switch of TDT.
There is another DNA hydrogel commonly applied to detection. It is prepared by taking a specific target DNA as the Linker and cross-linking it with its two complementary single-stranded DNAs (ssDNAs) to form a hybridized tsDNA hydrogel. The target, if present, binds to the target DNA (such as aptamer), which results in the fracture of the hydrogel, and thus the detection goal is reached by combining other detection mechanisms. Geraint J. Langford et al. designed an acrylamide moiety Morpholino Oligonucleotides (MOs) cross-linked DNA hydrogel [79] for the first time. MO CLs were selectively cut by the microRNA-based short target analyte, namely, the ssDNA sequence, to cause obvious swelling that can be optically measured. Compared with the traditional DNA cross-linking implemented to produce DNA hydrogels, MO cross-linking showed better thermal stability without any salt, and the sensitivity was also greatly improved. With the mature application of cross-linked tsDNA hydrogels, researchers have combined this strategy with other mechanisms to prepare DNA hydrogels, which are used for drug loading as a single-step signal amplification mechanism, and the drugs released are applied to the subsequent reaction. For instance, Liu et al. reported an electrochemical biosensor based on a hybridized DNA hydrogel immobilized on indium tin oxide/polyethylene terephthalate (ITO/PET) electrode [80] to detect the lung cancer-specific miRNA, miR-21 (Fig. 4C). The author grafted acrydite-modified DNA A and B to the acrylamide monomer to form a polyacrylamide gel (PAAG), the two strands were complementary with the ferrocene-labeled DNA probe, and primers were cross-linked to form a hybridized DNA hydrogel immobilized on the 3-trimethoxysilyl propyl methacrylate (KH570) modified ITO electrode. MiR-21, when present, was hybridized with the recognition probe, and the DNA hydrogel would collapse due to the fracture of primer chains in it, thereby losing ferrocene tags and reducing the current. The LOD reached 5 nM (1 pmol) if cyclic voltammetry (CV) and differential pulse voltammetry (DPV) were adopted. Similarly, Si et al. converted the detection of target miRNA into the glucose detection of portable glucometers [81], making the detection more portable and sensitive. Different nanoparticles were successfully encapsulated in DNA hydrogels, which benefited from the three strands hybridization strategy. Therefore, DNA hydrogels could be combined with Raman substrates. Different multi-component nucleic acid enzymes (MNAzymes) [82] were used to detect different miRNAs. He et al. designed a target-switchable responsive DNA hydrogel to detect miRNAs by controlling the capture and release of Raman signal molecules [83] (Fig. 4D). In this DNA hydrogel design strategy, ssDNA (S9) and target miRNA-155 were completely complementary, where the former carried S9 away from the released DNA (S10). Next, duplex-specific nuclease (DSN) was introduced to pyrolyze the double strands of DNA-RNA hydride (miRNA-S1) and release miRNAs to induce the next cycle, thus acquiring a large amount of ssDNA S10 for later use. Such DNA hydrogels were formed by the Linker ssDNA S13 and its two complementary ssDNA strands S11 and S12 and they could embed Raman signal molecules. S10 complementary to this Linker ssDNA resulted in the collapse of the DNA hydrogel and release of Raman signal molecules, which can be used for detection.
Clearly, the sensing detection of RNA based on the principle of complementary base pairing is also a common strategy adopted by researchers. Li et al. designed a multi-signal amplification fluorescence detection strategy for miR-141 [84], which combined DNA walker and DNA hydrogel, and achieved detection through the chemiluminescence in the detection system. Chang et al. reported a DNA-based hydrogel microcapsular sensor [85] that realized the detection through the competitive displacement between nucleic acids. A recognition molecule of target miRNA was loaded in the microcapsule, and the bridging DNA was designed into a cage-like sandwich type structure, which facilitated the loading of QDs as the read-out signal. The linear detection range of this sensor for miR-141 was 2.5–50 μM, with the LOD of 1.69 μM. Meng et al. reported a DNA hydrogel that detected different miRNAs by introducing DNA-modified AuNPs and their complementary fluorescence DNA sequences [86]. The strand displacement reaction was triggered through transfection of specific miRNAs into cells to activate the DNAzyme-aided target recycle and strengthen the corresponding imaging fluorescence intensity. The results showed that this strategy could generate different fluorescence intensities through double signal amplification and evaluate the abundance of multiple tumor-related miRNAs in different cells.
Depending on the combination of complementary sequences between primers and specific recognition, the recognition and detection of nucleic acid using primers as probes has become rapid and sensitive. The detection limit of nucleic acids has become very low due to the application of DNA hydrogels combined with SPR, PEC, FRET, SERS and other sensing technology, and nucleic acid amplification technology as a signal amplification mechanism. Based on the molecular transport platform constructed by the nucleic acid response to DNA hydrogel, a model system was constructed to understand how cargo-barrier interactions affects transport process fundamentally in molecular transportation. Dendritic DNA hydrogels formed by crosslinking natural growth between DNA greatly increased drug loading. However, designing and producing controlled dendritic or even DNA hydrogels in other shapes is still the focus of further research.
3.2. Toxin-responsive DNA hydrogels
A more sensitive, rapid, accurate, and portable method for toxin detection has always been the research direction. At present, major developments have been made to detect common toxins in everyday life, including biological detection, mass spectrometry, fluorescence detection, and enzyme-linked immunosorbent assay. However, the synergistic effect of aptamer-based DNA hydrogel as a detection element and other analytical methods has greatly improved the detection efficiency, especially with portable instruments, allowing the detection of toxins whenever and wherever it is required. Hao et al. reported a self-assembled fluorescence DNA hydrogel based on ochratoxin A (OTA) aptamer and RCA to detect OTA [87], with an LOD of 0.01 ng/mL and a linear range of 0.05–100 ng/mL. Moreover, the DNA hydrogel was also effective in detecting actual beef samples. According to another study, the gel membrane prepared by heating DNA hydrogels renders a brand-new idea to apply DNA hydrogels in detection. Besides, the membrane thickness is also adjustable based on the thermal reversibility principle of DNA hydrogels. Li et al. prepared a DNA hydrogel membrane in a capillary tube for cocaine detection [88]. The thickness (0.15 ± 0.01 mm) of this hydrogel membrane was regulated through the time (3 s) of thermal capillary tube in the hydrogel. Such hydrogels were formed by triple-strand (cocaine aptamer and its two complementary ssDNA strands) cross-linking. Cocaine, if present, was cross-linked with its aptamer to cause the pyrolysis of hydrogel membrane, thereby enhancing the permeability of the membrane. The flow velocity of sample solution in the capillary tube varied with the permeability of the DNA hydrogel. The LOD for cocaine was calculated as 1.17 nM through the relation between time and the distance in the capillary tube. The detection sensitivity was higher than that of similar DNA hydrogels, and it can also be used to detect other substances by changing the cross-linking DNA and aptamer. Tang et al. designed an aflatoxin B1 (AFB1)-responsive DNA hydrogel with readings given by an electronic balance [89]. In this DNA hydrogel, linear hyaluronic acid grafted ssDNA compounds served as scaffolds and the aptamer of AFB1 and polyethyleneimine as CLs. Upon adding Exonuclease I, the compound of AFB1 and its aptamer was cut so that the released AFB1 reacted with the hydrogel once again, achieving the recycling. Moreover, the platinum nanoparticles (PtNPs) encapsulated in the hydrogel resulted in the release of O2 in the system by catalyzing H2O2, and then the system weight was reduced, with the readings given by an electronic balance.
In comparison, the construction of DNA hydrogel membrane combined with capillary elements provides us with a more convenient way of thinking, and this method is also widely applicable. With enough research and development, these methods can potentially be integrated into industrialized production. Improving the stability of toxin-responsive DNA hydrogels under conditions of environmental changes and reducing the pretreatment of samples to achieve the same high effective detection effect, are important problems that require consideration.
3.3. ATP-responsive DNA hydrogels
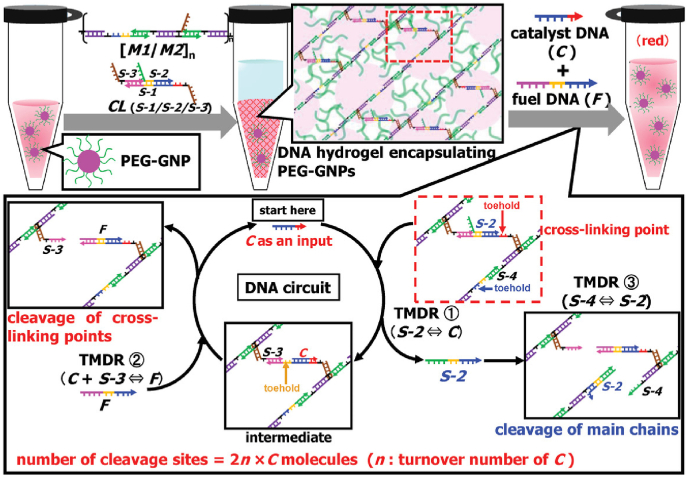
ATP is an unstable high-energy phosphate compound and a ubiquitous energy carrier in cells. In general, DNA hydrogels responsive to ATP bind to adenosine triphosphate through the single stranded aptamer sequence in the linker and undergo conformational transformation [90]. DNA nanodevices based on ATP aptamers can be activated by ultraviolet light, which expands the remote control and detection of ATP in vivo [91]. Endowed with the dynamic programming function, stimuli-responsive DNA hydrogels allow a brand-new development direction, so multiple sites in a network can be cut by one molecule input by specific biomolecules. Accurate detection results are achieved fast when this strategy is applied to prepare ATP stimuli-responsive DNA hydrogels. Motoi Oishi∗ and Kazuki Nakatani reported a dynamically programmed, stimuli responsive DNA hydrogel for ATP detection [92] (Fig. 5). This design strategy relied upon entropy-driven catalytic reactions through cascading toehold-mediated DNA displacement reactions (TMDRs). The DNAs constituting the hydrogel are subjected to pyrolysis according to the varying DNA input. This DNA hydrogel, which consists of long dsDNA ([M1/M2] n) and CL, was mainly formed by hybridizing the overhung ssDNA at both ends of dsDNA ([M1/M2] n) and the protrusions at both ends of CL, and it achieved visualization by encapsulating AuNPs. With catalytic DNA (C) as the input, the TMDR was triggered on the cross-linking point of three DNA strands constituting the CL. As a result, one DNA strand was released from the cross-linking point, forming an intermediate with a new toehold and effecting the gel-sol transformation in the system. ATP was detected by combining the ATP aptamer and the structural transformant that consists of the ATP aptamer and pre-catalytic DNA (pre-C). In the absence of ATP, pre-C bound to the ATP aptamer to keep this system in gel state. On the contrary, pre-C was dissociated if ATP competitively bound to its aptamer, and the system was converted into a sol state. Meanwhile, AuNPs were released, and the LOD within 30 min was 5.6 × 10−6 M. Compared with biocatalytic cleavage responsive DNA hydrogels, dynamically programmed DNA hydrogels can synchronize the catalytic cleavage of cross-linking points and main chains, which is an obvious advantage.
Fig. 5.
Schematic of the principle of a dynamically programmed DNA hydrogel based on a DNA circuit system through cascading toehold-mediated DNA displacement reactions (TMDRs). Copyright 2019, Wiley-VCH.
Ji et al. reported a DNA hydrogel based on silica nanoparticles (SiNPs) and fluorescent probes for ATP detection and imaging in cells [93], based on the following design idea: amido bond-modified DNA1 and DNA2 were fluorescently modified using FAM and Cy3 and connected to methacrylic acids, and the polymethylacrylic acid (PMA) compound of DNAs was synthesized through the photopolymerization method. Afterwards, the PMA-DNA1 modified SiNPs and PMA-DNA2 were hybridized through the ATP aptamer to form a nanohydrogel with a nuclear shell. Without the target ATP, the fluorophores of separately labeled donor (FAM) and receptor (Cy3) were connected, and FRET was under “ON” state. On the contrary, FRET was converted into “OFF” state if ATP binds to its aptamer.
Compared with traditional stimuli responsive DNA hydrogel, dynamic programming DNA hydrogel as an enzyme free signal amplifier can achieve rapid gel-sol transformation, making its ATP detection efficiency much higher than that of other sensors. The detection and imaging of ATP in DNA cells with SiNPs and fluorescent probes showed that the functionalized DNA hydrogels possessed excellent biological characteristics and imaging properties. The encapsulation of DNA hydrogels also enhanced the stability of nanoparticles in response to environmental changes.
3.4. Protein-responsive DNA hydrogels
Aptamers play significant roles in detecting proteins that serve as the markers of tumors or cancers. Similarly, the detection of some antigens and enzymes through aptamer-based DNA hydrogels has aroused high interest from researchers. In general, such proteins are detected by specific aptamers. Zhang et al. combined biorthogonal “click chemistry” and aptamer to prepare a DNA hydrogel for the specific adsorption and capture of proteins [94], in which patterned proteins could be formed. Four-arm thiol and four-arm maleimide-functionalized PEG were blended through thiol-Michael addition reaction to form a PEG hydrogel. Then, the acrylamide-modified protein-specific aptamer DNA covalently bound into the PEG hydrogel, and the release of proteins was controlled by adding the complementary DNA (cDNA) strands of the aptamer. This strategy is applicable to the controllable protein transfer. Furthermore, Jiang et al. blocked one end of the capillary tube with this DNA hydrogel under the cutting action of endonuclease on DNA strands [95], to serve as a filter membrane separator and control the molecular flow into the capillary tube. The reading of distance in the capillary tube was reached by the hydrogel transformation, and the detection sensitivity for UDG reached 0.02 mU/mL. Sun et al. developed a cascading logic quadruple signal amplification strategy based on DNA hydrogels and nanomaterials to detect mucin 1 protein (MUC1) and the circulating tumor cells (CTCs) of breast cancer [96]. Specifically, the substrate, namely tsDNA, acted upon mycobacterium smegmatis porin A (M2MspA) nanopores by relying upon the cutting action of DNAzymes to effect the characteristic translocation events directly influencing MUC1 and the number of CTCs. This strategy strengthened the antijamming capability and improves the detection sensitivity.
The toehold tether of hairpin DNAs will be opened during the competitive binding of DNAs, thereby inducing HCR. This mechanism has been used in DNA hydrogels to successfully realize targeted drug delivery and intracellular drug tracking. Ji et al. connected hairpin DNAs covalently onto PMA via amido bonds and blended them to form a carcinoembryonic antigen (CEA)-responsive nanohydrogel [97]. Given that the edges of a DNA tetrahedron can be methylated by DNA adenine methyltransferase (Dam), Gao et al. constructed a tetrahedral DNA-based hydrogel as a real-time detection equipment for Dam [98], and finally read the quantitative value through a personal glucometer (PGM), with the direct LOD lowered to 0.001 U/mL.
3.5. Bacteria and virus-responsive DNA hydrogels
Viral replication and proliferation rely upon parasitic living cells. In general, viruses only contain one kind of nucleic acids, DNAs or RNAs; therefore, viral stimuli-responsive DNA hydrogels are usually constructed using primer probes capable of specific virus recognition. Especially, the detection time is shortened and the detection sensitivity is improved, owing to the massive amplification of DNAs through RCA and PCR. Wonhwi Na et al. subtly combined DNA hydrogels with microfluidic devices to detect multiple viruses [99]. Specifically, many microbead-like DNA hydrogels are formed during RCA on the surface of the Sepharose bead. This system is beneficial because microbeads are filled into a microtubule, which may contribute to the significant increase in the surface area of RCA and the reduction of the corresponding flow area on the cross section, thus shortening the detection time and substantially enhancing the detection sensitivity. Moreover, different viruses are detected by replacing different primer probes, and the devices are simple to operate and portable. Hwang-soo Kim et al. designed a microfluidic system based on RCA DNA hydrogels to detect SARS-CoV-2 [100], and innovatively shortened the detection time using microscale gaps formed in meshes. Such gaps could be very easily and quickly blocked off by RCA hydrogels. A target could be rapidly (within 5 or 15 min) detected using DNAs with the concentration of 3.0 or 30 aM. Compared with the RCA technology requiring circular DNA molecules, PCR, which is also a DNA amplification technology, is capable of substantially increasing trace DNAs. Chen et al. constructed a cage-like tetrahedral DNA hydrogel based on tetrahedral DNAs and PCR for the visual detection of Salmonella [101]. A 3D structural connecting arm was designed and constructed on a tetrahedral structure to achieve spatial orientation. Next, PCR was performed using Salmonella genome as the template to acquire many target sequences. This design is ingenious because the stiff joint PCR products carrying blockers are uniformly distributed on the spatially isolated DNA tetrahedral nanostructure, which substantially reduces the interactions between strands and promotes the hybridization. Moreover, this tetrahedron, synthesized in a one-step one-pot fashion within several minutes, is of very high homogeneity and productivity, and ensures a very high repeatability of the detection.
Comparatively, bacterial stimuli-responsive DNA hydrogels have been used as antibacterial materials. Antibacterial materials are loaded on DNA hydrogels because of their good biocompatibility and stability in different environments. Furthermore, DNA hydrogels can also be formed by the electrostatic interaction between anionic DNAs and cationic polypeptides, and meanwhile, some antibacterial agents can be embedded. Sybil Obuobi et al. reported a DNA hydrogel prepared through the high binding affinity between polyanionic DNA nanostructure and cationic antimicrobial peptides (AMPs) to respond to the pathogenic Staphylococcus aureus infection [102]. The author produced a DNA hydrogel by hybrid cross-linking of complementary sequences between Y-scaffold and L-linker and the electrostatic interaction between anionic DNA and cationic polypeptides, and immobilized AMPs into the hydrogel. This responsive hydrogel exhibits powerful antibacterial activity, along with a very good recovery effect on skin wounds.
3.6. Some small molecule-responsive DNA hydrogels
Small molecule-responsive DNA hydrogels, which are mostly established by introducing aptamers, have prospered due to their high specificity and sensitivity. However, most of them have only been applied to in vitro detection, which is highly inadequate, and in vivo transport and detection of some small molecules continue to be problematic. RCA products are rich in guanines with an intermolecular quadruplex configuration, providing enough cross-linking force for the formation of hydrogels. Huang et al. reported an RCA technology-based hydrogel [103] that catalyzed horseradish peroxidase (HRP) for glucose detection. Mao et al. synthesized a surface-immobilized pure DNA hydrogel by combining linear RCA (LRCA) [104], which immobilized primers on the surface, with the surface primer induction (SPI) strategy, and then applied it to biosensing. The results showed that the lower LOD for H2O2 in blood serum and bilirubin, a biomarker for jaundice, was 22 nM and 32 nM, respectively. Tang et al. produced a DNA hydrogel by cross-linking malathion aptamer, which served as a cross-linking strand, and its two complementary ssDNA strands, and embedded catalase into this hydrogel [105]. In the presence of the target, the hydrogel is disintegrated, and catalase is released to oxidize H2O2 in the supernatant so as to release O2, which participated in the follow-up reaction of the heating system with the following reaction formula 4Fe(s) + 3O2(g)→ 2Fe2O3(s), where ΔH = −1648.4 kJ mol−1. The temperature change was detected using a thermometer, and the lower LOD for malathion was 0.032 pgmL−1. Similarly, by changing the target aptamer in these methods, they can be popularized for the detection of other targets. Hydrogel microcapsules with loading mechanisms have aroused high attention among researchers in the past years. With unique advantages in both drug loading and degradation, the microcapsules have attained new heights in combining the programmability of DNAs. Different stimuli are taken as “keys” to enable DNA hydrogel microcapsules to output different signals. Liao et al. proposed a method of constructing stimuli responsive DNA polyacrylamide hydrogel microcapsules [106] using a predesigned nucleic acid hairpin unit-modified polyacrylamide strand and a single-strand tether that finally renders the designated hybridization and recognition functions. Next, cocaine was detected by adding a cocaine aptamer. The preliminary study indicated that the ATP-containing anticancer drug Adriamycin or pH-responsive microcapsules have a selective cytotoxic effect on MDA-MB-231 cancer cells. Also worthy of our attention is the electrochemical biosensor based on DNA hydrogel. Mao et al. constructed a three-dimensional electron transmitter by using DNA hydrogel to construct scaffolds to improve the efficiency of electron transfer [107]. Through the introduction of DNAzyme, the effective catalysis of H2O2 is realized, which provides potential support for breaking through the limitations of two-dimensional electrodes.
In general, the application of bio stimuli-responsive DNA hydrogel is mainly focused on sensing, thus deriving its application in disease diagnosis, antibacterial effects, and so on. Utilizing bio responsive DNA hydrogel as a step signal amplification mechanism or combined with a portable instrument has expanded its application. The crosslinked dendritic hydrogel greatly improved the drug loading of hydrogel. As a microcarrier, DNA polyacrylamide microcapsule has switching permeability to its shell, which shows us its controllability of load release and indicating that it could play an important role in future clinical treatment. Furthermore, to observe the conformational change of DNA hydrogel under micro stimulus, Joshua Fern et al. reported a DNA strand-displacement controller for directing the time scale and degree of DNA hydrogel expansion [108], which enables the dramatic changes in the material size when the specific biomolecular input was smaller than 100 nM. Moreover, this controller can instruct swelling in response to small molecules or execute a logic. How the DNA strand-displacement controller in the hydrogel regulates the material size by releasing, amplifying, and integrating stimuli and releasing reaction-guiding signals has been demonstrated.
4. Composite class-responsive DNA hydrogels
As research delves deeper into the stimuli-responsive DNA hydrogels, conventional stimuli, single-stimulus patterns, and simple response mechanisms fail to meet researchers' demands for smart materials. Besides, more DNA hydrogels responding to multiple stimuli have attracted attention from researchers, including multi-biological-stimuli-responsive, multi-nonbiological-stimuli-responsive, biological stimuli-responsive, and composite nonbiological stimuli-responsive DNA hydrogels. Such DNA hydrogels, usually with a complex design, have been applied to cell simulation, circuit design, nanochannels, drug delivery, and information storage.
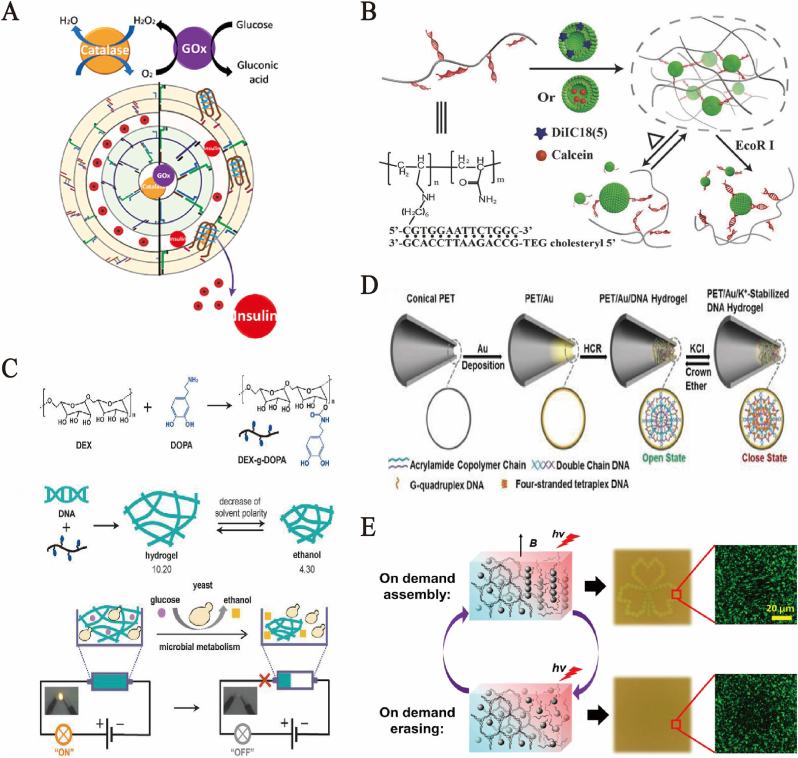
With unique and fascinating properties, DNA hydrogels have been successfully prepared into cell simulators and “artificial pancreas.” Compared to traditional cell engineering and tissue engineering at the cellular level, the DNA hydrogel mimics possessed some functions of cells or organs, and had obvious advantages in terms of time and cost. They were useful hybrid materials for synthetic biology. Henrike Niederholtmeyer et al. designed a cell nucleus-like DNA hydrogel chamber in artificial cell mimics [109], under multiple stimuli, which could express and exhibit proteins and exchange with adjacent cell mimics through diffused protein signals. Microfluidic technology is used to produce cell mimics with porous polymer films containing man-made hydrogel chambers. Such a hydrogel chamber is similar to the nucleus of eukaryotic cells, because it contains the genetic materials of cell mimics used for protein synthesis and can isolate transcription factors. Simulators can perform gene expression and exchange through diffused protein signals by collaboratively responding to multiple signal stimuli. Besides simulating cell functions, DNA hydrogels are also used to simulate “artificial pancreas” for the controlled release of insulin. Amit Fischer et al. designed a system as shown in (Fig. 6A), in which the stable internal water chamber in the stimuli responsive hydrogel layer provides internal microcapsules and loads CaCO3 and tetramethyl rhodamine-dextran (TMR-D) [110]. Moreover, the internal microcapsules are separated from the external water chamber, which generates microcapsules through the external stimuli responsive hydrogel layer in the microcapsule system and loads QDs. With high stiffness, the hydrogel is capable of preventing the load leakage in internal and external water chambers. In the hydrogel, response mechanisms are provided by different primers and primers with hairpin and I-motif structures, i.e., capsule release mechanism and two different triggers: Zn2+-ions and/or pH. Specifically, Zn2+ ions, if used to treat the microcapsule-microcapsule system, will lower the cross-linking degree of the internal hydrogel, further reduce its hardness, and cause load mixing in the two water chambers. When the microcapsule protein-microcapsule system is placed at pH 5.5, the I-motif structure is relocated in the external hydrogel and the double-stranded nucleic acid bridge is isolated. This process reduces the stiffness of the external hydrogel layer, thus allowing the external water chamber to release the load while not releasing the load in the internal liquid accumulator. Furthermore, this method was used as an “artificial pancreas” for insulin release, with the operation controlled via pH value. Next, glucose oxidase (Gox) was loaded in the internal chamber and insulin in the external chamber, glucose permeated via the two hydrogel layers, so Gox catalyzed the aerobic oxidation of glucose into gluconic acids.
Fig. 6.
(A) Schematic of carboxymethyl cellulose (CMC) stimuli-responsive nucleic acid-based hydrogel microcapsule system. Copyright 2020, American Chemical Society. (B) Schematic of the liposome–DNA hydrogel and its stimuli-responsive release behavior. Copyright 2018, Wiley-VCH. (C) Molecular design and synthesis route of DNA/DEX-g-DOPA hydrogel. (a) Synthesis route of DEX-g-DOPA. (b) Preparation of nanofiber-assembled hydrogel with volumetric responsiveness upon solvent polarity. (c) Electric circuit switched by a microbial metabolism process which produced ethanol using DNA/DEX-g-DOPA hydrogel as dynamic wires. Copyright 2020, Wiley-VCH. (D) Schematic of ion channel preparation process based on DNA hydrogel. Copyright 2018, Wiley-VCH. (E) Laser-patterning demonstrations of in situ assembly of DNA supramolecular Photonic hydrogels. Copyright 2021, American Chemical Society.
DNA hydrogels have been extensively applied to both in vitro and in vivo biomedicines. For example, DNA hydrogels may be used to prevent the distant relapse of tumors as a platform of regulating tumor immune microenvironment. Wu et al. reported a photothermal composite responsive degradable DNA hydrogel [111] for delivering dual nucleotide adjuvants and photoresponsive components. Moreover, the DNA hydrogels collaboratively responding to enzymes and heat have been developed for injection and in vivo medical application. Danya Lyu et al. reported a stimuli-responsive DNA hydrogel with liposomes as the non-covalent CL [37] (Fig. 6B), wherein the release was controlled by functional small molecular carriers. This DNA hydrogel showed both enzymatic and thermal responsiveness. Moreover, the recognition sites of restriction endonuclease were encoded in DNA; therefore, this hydrogel was enzyme-responsive. In addition, the hydrogel showed favorable thermal responsiveness after addition of liposomes, which was highly correlated with the melting temperature (Tm) of DNAs and could be regulated through salts. As for this strategy, different controlled release systems can be developed at destinations. Such systems are very suitable for all kinds of in vitro and in vivo biomedical applications due to its seal-healing and injectable properties and good biocompatibility.
In addition, multiple stimuli have been considered as the keys to control DNA hydrogels. For composite stimuli-responsive DNA hydrogels, specific switches are opened by different keys combined action or a switch can be opened by multiple keys. This strategy is usually used for circuits or gating devices. Furthermore, such multi-stimuli-responsive nucleic acid hydrogels are applicable to the delivery, transport, and controlled release of drugs. Han et al. reported an ultrasoft and dynamic nanofiber-assembled DNA/dopamine-grafted glucan hydrogel composed of natural long-strand DNA and dopamine-grafted glucan (DEXg-DOPA) [112] (Fig. 6C). In this hydrogel, double networks with permanent and dynamical synergistic action were integrated. A series of dynamic hydrogel circuits have been fabricated using this unique DNA hydrogel as a dynamical connecting line, which are triggered by polar (like water) and nonpolar [like petroleum ether (MSO)] solvents as switches. When soaked in MSO, the hydrogel is subjected to volume shrinkage within several seconds and rapid aggregation into a compact form. When resoaked in water, the hydrogel recovers its initial shape and experiences bidirectional volume expansion. To demonstrate its application, a series of dynamic hydrogel-based circuits were fabricated to regulate cell adhesion and control drug delivery. Wu et al. reported a DNA hydrogel-based nanochannel as a stimuli responsive scaffold [113] (Fig. 6D) with a high ion flux and the function of regulating selective ion transport. This asymmetric conical nanochannel was prepared through surfactants in combination with the ion track etching method, and the DNA hydrogel network was assembled to the gold-plated tip side through HCR, thus realizing a high ion flux and a high rectification ratio. The DNA hydrogel, with a G-quadruplex structure, kept a high stiffness in the presence of K+. Moreover, the G-quadruplex structure was dissociated into a hydrogel state with low stiffness under the 18-crown-6-ether stimulus, which provides the gating mechanism for the nanochannel. Under a low pH value, the hydrogel matrix also rendered pH responsiveness due to the protonation of nucleobases. Alternatively, the transport direction of cations or anions was accurately controlled and the gating characteristics were regulated by combining the DNA hydrogel state and pH stimulus. This mechanism study carves a path for practically utilizing hydrogel networks in microfluidic systems, sensors, and seawater desalination equipment.
Furthermore, light-, heat-, and magnetism-responsive DNA hydrogels have been applied to various fields like chemical sensing, molecular diagnosis, and information storage. Researchers designed a double-layer hydrogel structure consisting of a thermosensitive DNA hydrogel and an external DNA hydrogel combination formed by half I-motif sequence cytosine chain [35], which underwent physical deformation and recovery under the combined stimulation of temperature and pH. DNA hydrogels with shape memory function have been applied in information storage. Dong et al. reported a DNA hydrogel encapsulating MNPs [114] (Fig. 6E) which generated structural colors under the actions of heating and magnetic field. Next, spatially controlled photonic crystal coloring was implemented through the photolithographic technology under near-infrared illumination. Without any external magnetic field, colors were “erased” by lighting or heating the hydrogel. Under lighting conditions, MNPs will generate heat, and the hydrogel was locally melted in case of their migration and alignment with the external magnetic field to form a periodic PC structure. After removal of lighting conditions, the DNA strands will be rehybridized due to the fast heat dissipation. Under the joint action of lighting, heating, and magnetic fields, this strategy can reach a high spatial resolution (up to 10 μm) and realize direct inscription of colors and patterns, fast inscription (<1 s), and erasure (<10 s) without needing any mask. Compared with organic dyes, this strategy is more stable in coloring. Compared with other PC rewritable methods, this strategy has a faster response time, which also opens a new way for the application of stimulus responsive DNA hydrogels.
In comparison, complex stimuli-responsive DNA hydrogels often have more complex design pathways, such as multi compartment microcapsules, sandwich DNA hydrogels, dynamic collaborative network DNA hydrogels, etc., which provide new ideas for the construction of hydrogel-based circuits, flexible electronic devices, multiple gating devices, etc. But the logic control ability and signal output mode of strengthening systems in response to multiple stimuli are still the hotspot in contemporary studies.
5. Conclusion and perspectives
Stimuli-responsive DNA hydrogels can also be applied in various forms including microencapsulation, microbead, injectable property, membrane structure, and dendritic structure. However, certain requirements cannot be met by the functions of pure stimuli-responsive DNA hydrogels. Therefore, recent studies have focused on combining stimuli-responsive DNA hydrogels with other mechanisms. For instance, with CDs as carriers, the stimuli-responsive DNA hydrogels achieve FRET; with PEC, they improve the sensitivity of detection by measuring chemiluminescence; with QDs, they achieve high-efficiency FRET from QD donors to quenching receptors; with the modified Au film for the preparation of an oval polarization sensor, they realize the sensitive detection and imaging of the target; with microfluidic chips or capillary tubes, they can be used to prepare portable detection devices. In addition, embedding stimuli-responsive DNA hydrogels with different nanoparticles alsoendows the system with the same properties of nanoparticles. Integrating metal/semiconductor nanoparticles/nanorods with distinctive optical, electronic, or catalytic properties with stimuli-responsive hydrogels helps construct new hybrid composites with switchable functions. For example, the hot viscosity characteristic of AuNP/AuNP can be found. The response of UCNP to light acts upon the photothermal effect induced by the whole material. Besides, the DNA hydrogels embedded with MNPs can be used to build soft robots. Mechanism, characteristics and application of different stimuli-responsive DNA hydrogel was presented in Table 1.
Table 1.
Mechanism, characteristics, and application of different stimuli-responsive DNA hydrogel.
| Type | Stimulus | Characteristic | Mechanism | Application | Reference |
|---|---|---|---|---|---|
| Nonbiological stimuli- responsive DNA hydrogels | Temperature | T-A•T structure DNA PNIPAM hydrogel | T-A•T structure and PNIPAM have temperature sensitivity | Sensors and actuators | [35] |
| Liposomes grafted onto DNA as crosslinkers | Liposomes are heat sensitive | Controlled drug release | [37] | ||
| DNA hydrogel embedding AuNP or AuNR. | Thermoplasmonic properties of AuNPs and AuNRs | Controlled drug release and sensors | [39] | ||
| Ions/pH | Introduction of G-rich sequence into DNA hydrogel | K+, Pb2+, and acidity convert G-rich sequence into G-quadruplex | Ion detection and drug release | [[40], [41], [42], [43], [44], [45],115] | |
| Specific DNAzyme | DNAzyme folded into hairpin structure in the presence of Hg2+ or Zn2+ | Ion detection and drug release | [46,47] | ||
| I-motif structure | Response to acidic environment | PH detection and drug release | [[49], [50], [51], [52]] | ||
| C-G•C+ and T-A•T structure | Response to acidic environment | PH detection and drug release | [53] | ||
| Photon | t-azoDNA and hairpin DNA with photolytic sequence | t-azoDNA and hairpin DNA with photolytic sequence have photo-responsiveness | Drug release Information storage | [58,62] | |
| DNA/DTE carboxymethyl cellulose hybrid hydrogel | DTE is characterized by cyclic and reversible photoisomerization | Controlled drug release | [59] | ||
| CD and PpIX covalently bind to C-rich single stranded DNA | PpIX is photo-responsive | Sterilization | [60] | ||
| UCNPs and AuNPs hybrid DNA hydrogel | UCNP–Au nanoparticles respond to NIR | Tumor therapy | [64] | ||
| Magnetism | sDNA modified by MNPs grafted onto DNA hydrogels | Magnetic responsiveness of MNPs | Clinical medicine and nano soft robots | [66,67] | |
| Encapsulated multienzyme magnetic DNA hydrogel | MNPs DNA hydrogel improves enzyme activity | Detection | [68] | ||
| Mechanical stress | Isothermal toe mediated RNA/DNA heteroduplex | The Nan connector adjusts the 3D force momentum along the TCR mechanical axis, and the hydrogel helps adjust the 2D shear modulus | Efficient capture of T lymphocytes and regulated activation of TCR | [69] | |
| Biological stimuli-responsive DNA hydrogels | Nucleic acid | DNA motor hydrogel and DNA gated hydrogel | Complementary base pairing | Cell culture and molecular transport | [[70], [71], [72]] |
| DNA hydrogels as signal amplification mechanisms | Capture probes specifically recognize targets | Detection | [[73], [74], [75],116] | ||
| Dendritic DNA hydrogel | DNA self-assembly and expanded drug loading of dendritic hydrogels | Detection and drug loading | [[76], [77], [78]] | ||
| Toxin | Construction of POCT with aptamer DNA hydrogel or DNA hydrogel film as detection element | The aptamer specifically recognizes the target and causes the hydrogel to cleave | Detection and sensing | [[86], [87], [88], [89]] | |
| ATP | Dynamically programmable DNA hydrogel | Aptamer recognizes ATP cleavage network sites | Detect ATP | [91,92] | |
| DNA hydrogels based on SiNPs and fluorescent probes | Aptamer recognizes ATP and turns off FRET | ATP detection and imaging | [93] | ||
| Protein | Biorthogonal “click chemistry” prepare aptamer DNA hydrogel | Aptamer recognition, and aptamer complementary chain control protein release | Specifically adsorb and capture proteins | [94] | |
| DNA hydrogel as signal amplification element | Aptamer specific recognition | Detection | [95,96] | ||
| Bacteria and viruses | Production of DNA hydrogel beads by microfluidic equipment | RCA amplification recognition probe increases sensitivity | Detection | [100] | |
| DNA hydrogel entrapment of AMPS | AMPs are antibacterial | Antibacterial | [102] | ||
| Small molecules | DNA hydrogel of aptamers as bridging chains | The aptamer specifically recognizes the target, causing the hydrogel to cleave and release signal molecules | Detection and sensing | [105,117,118] | |
| Composite class-response DNA hydrogels | Zn2+ and/or pH | Multi compartment DNA hydrogel microcapsule | Hierarchical DNA structure responds to different stimuli | “Artificial pancreas" | [110] |
| Enzymes and temperature | Liposome crosslinked DNA hydrogel | The recognition site of restriction enzyme is encoded in DNA | Injectable DNA hydrogel and controlled drug release | [37] | |
| Water or petroleum ether | DNA/dopamine grafted dextran hydrogel | Dynamic hydrogel circuit | Regulating cell adhesion and controlled drug delivery | [112] | |
| Light, heat, and magnetism | Photonic crystal DNA hydrogel | Photoresist controlled coloring of photonic crystals | Information carrier | [114] |
Although great progress has been made in developing stimuli-responsive DNA hydrogels, there remains much room for its improvement. Limited by the materials, the stability of stimuli responsive DNA hydrogels remains a considerable challenge. Particularly, the resistance of DNA to harsh environments or organic solvents often limits the specific applications of DNA hydrogels. The limitations can be summarized as follows: 1) In biosensing, DNA hydrogels are of insufficient sensitivity and respond slowly to low-abundance target substances; missed detection occurs in the simultaneous detection of multiple targets. 2) DNAs in stimuli-responsive DNA hydrogels usually need to be chemically modified and coupled with different groups to obtain special properties. Efficient methods must be developed to modify DNAs at a low cost. Industrializing the low-cost and high-efficient production of DNA hydrogels is also a long-term challenge. 3) The binding kinetics of stimuli-responsive DNA hydrogels and their dissociation and release kinetics in the face of external stimulus require in-depth exploration. The accuracy of drug release and anti-interference to external stimulus can be improved by precise kinetic properties. 4) The application of stimuli-responsive DNA hydrogels is mainly limited to in vitro detection of markers related to human diseases, because the structural backbones of DNA hydrogels are mostly toxic polyacrylamides. Moreover, the toxicological properties of some stimuli-responsive DNA hydrogels controlling drug release must be thoroughly evaluated before clinical use. 5) Some stimuli responses, such as temperature, pH, and ultraviolet light, are produced under strict environmental conditions, which are not feasible in vivo. 6) The logic control capability and accuracy of output mode of the system under multi-stimuli--responsive system should be improved and strengthened.
In the face of the above difficulties, in the field of sensing, make rational use of the signal amplification mechanism, develop more green and non-toxic chemicals as DNA hydrogel frameworks in the medical field, and deeply understand the dissociation dynamics of hydrogels in the face of stimuli. Similarly, we can't neglect the research on aspects such as mechanical strength and self-healing properties of DNA hydrogels, which paves the way for the development of wearable devices and implantable components. In addition, the study of stimulation responsive DNA hydrogel loading can also provide us with new surprises, such as the development of conductive and catalytic DNA hydrogels. In summary, more sensitive and rapid stimuli-responsive DNA hydrogel sensors are expected to be developed for mass producing non-toxic and low-cost responsive DNA hydrogels. We expect that 3D printing technology will contribute to the preparation of DNA hydrogels in future, and the development of responsive DNA hydrogels with regards to orthogonal collaborative multifunctionalization and intellectualization. We also look forward to its application in synthetic biology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the National Key Research and Development Program of China [grant number 2021YFA0910204].
Contributor Information
Shiping Yang, Email: shipingy@shnu.edu.cn.
Shuang Li, Email: liza3320@163.com.
Zhixian Gao, Email: gaozhx@163.com.
Data availability
Data will be made available on request.
References
- 1.Huang Q., Zou Y., Arno M.C., Chen S., Wang T., Gao J., Dove A.P., Du J. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem. Soc. Rev. 2017;46:6255–6275. doi: 10.1039/c6cs00052e. [DOI] [PubMed] [Google Scholar]
- 2.Byrne M.E., Park K., Peppas N.A. Molecular imprinting within hydrogels. Adv. Drug Deliv. Rev. 2002;54:149–161. doi: 10.1016/s0169-409x(01)00246-0. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed E.M. Hydrogel: preparation, characterization, and applications: a review. J. Adv. Res. 2015;6:105–121. doi: 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu H., Guo H., Li D., Hou Y., Kuang T., Ding J. Intravesical hydrogels as drug reservoirs. Trends Biotechnol. 2020;38:579–583. doi: 10.1016/j.tibtech.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Zhang C., Zhou Y., Han H., Zheng H., Xu W., Wang Z. Dopamine-triggered hydrogels with high transparency, self-adhesion, and thermoresponse as skinlike sensors. ACS Nano. 2021;15:1785–1794. doi: 10.1021/acsnano.0c09577. [DOI] [PubMed] [Google Scholar]
- 6.Liu X., Steiger C., Lin S., Parada G.A., Liu J., Chan H.F., Yuk H., Phan N.V., Collins J., Tamang S., Traverso G., Zhao X. Ingestible hydrogel device. Nat. Commun. 2019;10:493. doi: 10.1038/s41467-019-08355-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y., Zhu Y., Hu Y., Zeng G., Zhang Y., Zhang C., Feng C. How to construct DNA hydrogels for environmental applications: advanced water treatment and environmental analysis. Small. 2018;14 doi: 10.1002/smll.201703305. [DOI] [PubMed] [Google Scholar]
- 8.Zhao X., Wu H., Guo B., Dong R., Qiu Y., Ma P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials. 2017;122:34–47. doi: 10.1016/j.biomaterials.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Xu J., Li X., Sun F. Cyclodextrin-containing hydrogels for contact lenses as a platform for drug incorporation and release. Acta Biomater. 2010;6:486–493. doi: 10.1016/j.actbio.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Lim C., Hong Y.J., Jung J., Shin Y., Sunwoo S.H., Baik S., Park O.K., Choi S.H., Hyeon T., Kim J.H., Lee S., Kim D.H. Tissue-like skin-device interface for wearable bioelectronics by using ultrasoft, mass-permeable, and low-impedance hydrogels. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abd3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu J., Zhao X., Liang Y., Zhang T., Ma P.X., Guo B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials. 2018;183:185–199. doi: 10.1016/j.biomaterials.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 12.Shin S.R., Migliori B., Miccoli B., Li Y.C., Mostafalu P., Seo J., Mandla S., Enrico A., Antona S., Sabarish R., Zheng T., Pirrami L., Zhang K., Zhang Y.S., Wan K.T., Demarchi D., Dokmeci M.R., Khademhosseini A. Deerfield Beach, Fla.; 2018. Electrically Driven Microengineered Bioinspired Soft Robots, Advanced Materials; p. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X., He L., Li Y., Chao M., Li M., Wan P., Zhang L. Healable, degradable, and conductive MXene nanocomposite hydrogel for multifunctional epidermal sensors. ACS Nano. 2021;15:7765–7773. doi: 10.1021/acsnano.1c01751. [DOI] [PubMed] [Google Scholar]
- 14.Kahn J.S., Hu Y., Willner I. Stimuli-responsive DNA-based hydrogels: from basic principles to applications. Acc. Chem. Res. 2017;50:680–690. doi: 10.1021/acs.accounts.6b00542. [DOI] [PubMed] [Google Scholar]
- 15.Roh Y.H., Ruiz R.C., Peng S., Lee J.B., Luo D. Engineering DNA-based functional materials. Chem. Soc. Rev. 2011;40:5730–5744. doi: 10.1039/c1cs15162b. [DOI] [PubMed] [Google Scholar]
- 16.Wang F., Lu C.H., Willner I. From cascaded catalytic nucleic acids to enzyme-DNA nanostructures: controlling reactivity, sensing, logic operations, and assembly of complex structures. Chem. Rev. 2014;114:2881–2941. doi: 10.1021/cr400354z. [DOI] [PubMed] [Google Scholar]
- 17.Tang Q., Lai W., Wang P., Xiong X., Xiao M., Li L., Fan C., Pei H. Multi-mode reconfigurable DNA-based chemical reaction circuits for soft matter computing and control. Angew. Chem. 2021;60:15013–15019. doi: 10.1002/anie.202102169. [DOI] [PubMed] [Google Scholar]
- 18.Kolpashchikov D.M. Evolution of hybridization probes to DNA machines and robots. Acc. Chem. Res. 2019;52:1949–1956. doi: 10.1021/acs.accounts.9b00098. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y., Ali S., White R.J. Electrocatalytic mechanism for improving sensitivity and specificity of electrochemical nucleic acid-based sensors with covalent redox tags-Part I. ACS Sens. 2020;5:3833–3841. doi: 10.1021/acssensors.0c02362. [DOI] [PubMed] [Google Scholar]
- 20.Umeki Y., Saito M., Takahashi Y., Takakura Y., Nishikawa M. Retardation of antigen release from DNA hydrogel using cholesterol-modified DNA for increased antigen-specific immune response. Adv. Healthcare Mater. 2017;6 doi: 10.1002/adhm.201700355. [DOI] [PubMed] [Google Scholar]
- 21.Mueller J.E., Kemper B., Cunningham R.P., Kallenbach N.R., Seeman N.C. T4 endonuclease VII cleaves the crossover strands of Holliday junction analogs. Proc. Natl. Acad. Sci. U.S.A. 1988;85:9441–9445. doi: 10.1073/pnas.85.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagahara S., Matsuda T. Hydrogel formation via hybridization of oligonucleotides derivatized in water-soluble vinyl polymers. Polym. Gels Netw. 1996;4:111–127. [Google Scholar]
- 23.Um S.H., Lee J.B., Park N., Kwon S.Y., Umbach C.C., Luo D. Enzyme-catalysed assembly of DNA hydrogel. Nat. Mater. 2006;5:797–801. doi: 10.1038/nmat1741. [DOI] [PubMed] [Google Scholar]
- 24.Basu S., Pacelli S., Feng Y., Lu Q., Wang J., Paul A. Harnessing the noncovalent interactions of DNA backbone with 2D silicate nanodisks to fabricate injectable therapeutic hydrogels. ACS Nano. 2018;12:9866–9880. doi: 10.1021/acsnano.8b02434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang K., Yam V.W. Platinum(ii) non-covalent crosslinkers for supramolecular DNA hydrogels. Chem. Sci. 2020;11:3241–3249. doi: 10.1039/c9sc05910e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J.B., Peng S., Yang D., Roh Y.H., Funabashi H., Park N., Rice E.J., Chen L., Long R., Wu M., Luo D. A mechanical metamaterial made from a DNA hydrogel. Nat. Nanotechnol. 2012;7:816–820. doi: 10.1038/nnano.2012.211. [DOI] [PubMed] [Google Scholar]
- 27.Kim M.G., Shon Y., Miao W., Lee J., Oh Y.K. Biodegradable graphene oxide and polyaptamer DNA hybrid hydrogels for implantable drug delivery. Carbon. 2016;105:14–22. [Google Scholar]
- 28.Basu S., Pacelli S., Paul A. Self-healing DNA-based injectable hydrogels with reversible covalent linkages for controlled drug delivery. Acta Biomater. 2020;105:159–169. doi: 10.1016/j.actbio.2020.01.021. [DOI] [PubMed] [Google Scholar]
- 29.Han S., Park Y., Kim H., Nam H., Ko O., Lee J.B. Double controlled release of therapeutic RNA modules through injectable DNA-RNA hybrid hydrogel. ACS Appl. Mater. Interfaces. 2020;12:55554–55563. doi: 10.1021/acsami.0c12506. [DOI] [PubMed] [Google Scholar]
- 30.Wang D., Hu Y., Liu P., Luo D. Bioresponsive DNA hydrogels: beyond the conventional stimuli responsiveness. Acc. Chem. Res. 2017;50:733–739. doi: 10.1021/acs.accounts.6b00581. [DOI] [PubMed] [Google Scholar]
- 31.Vázquez-González M., Willner I. Stimuli-responsive biomolecule-based hydrogels and their applications. Angew. Chem. 2020;59:15342–15377. doi: 10.1002/anie.201907670. [DOI] [PubMed] [Google Scholar]
- 32.Shao Y., Jia H., Cao T., Liu D. Supramolecular hydrogels based on DNA self-assembly. Acc. Chem. Res. 2017;50:659–668. doi: 10.1021/acs.accounts.6b00524. [DOI] [PubMed] [Google Scholar]
- 33.Nöll T., Wenderhold-Reeb S., Schönherr H., Nöll G. Pristine DNA hydrogels from biotechnologically derived plasmid DNA. Angew. Chem. 2017;56:12004–12008. doi: 10.1002/anie.201705001. [DOI] [PubMed] [Google Scholar]
- 34.Sontakke V.A., Yokobayashi Y. Programmable macroscopic self-assembly of DNA-decorated hydrogels. J. Am. Chem. Soc. 2022;144:2149–2155. doi: 10.1021/jacs.1c10308. [DOI] [PubMed] [Google Scholar]
- 35.Hu Y., Kahn J.S., Guo W., Huang F., Fadeev M., Harries D., Willner I. Reversible modulation of DNA-based hydrogel shapes by internal stress interactions. J. Am. Chem. Soc. 2016;138:16112–16119. doi: 10.1021/jacs.6b10458. [DOI] [PubMed] [Google Scholar]
- 36.Chen Z., Liu F., Chen Y., Liu J., Wang X., Chen A.T., Deng G., Zhang H., Liu J., Hong Z., Zhou J. Targeted delivery of CRISPR/Cas9-Mediated cancer gene therapy via liposome-templated hydrogel nanoparticles. Adv. Funct. Mater. 2017;27 doi: 10.1002/adfm.201703036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyu D., Chen S., Guo W. Liposome crosslinked polyacrylamide/DNA hydrogel: a smart controlled-release system for small molecular payloads. Small. 2018;14 doi: 10.1002/smll.201704039. [DOI] [PubMed] [Google Scholar]
- 38.Chang G., Wang Y., Gong B., Xiao Y., Chen Y., Wang S., Li S., Huang F., Shen Y., Xie A. Reduced graphene oxide/amaranth extract/AuNPs composite hydrogel on tumor cells as integrated platform for localized and multiple synergistic therapy. ACS Appl. Mater. Interfaces. 2015;7:11246–11256. doi: 10.1021/acsami.5b03907. [DOI] [PubMed] [Google Scholar]
- 39.Wang C., Liu X., Wulf V., Vázquez-González M., Fadeev M., Willner I. DNA-based hydrogels loaded with Au nanoparticles or Au nanorods: thermoresponsive plasmonic matrices for shape-memory, self-healing, controlled release, and mechanical applications. ACS Nano. 2019;13:3424–3433. doi: 10.1021/acsnano.8b09470. [DOI] [PubMed] [Google Scholar]
- 40.Hu S., Du X., Bi Y., He P.-P., Mu Y., Liu C., Gao Q., Yin M., Guo W. Smart hydrogels based on self-assembly of one short single-stranded DNA for functional surface patterning. ACS Appl. Polymer Mater. 2022;4:5199–5208. [Google Scholar]
- 41.Wang X., Chen C., Waterhouse G.I.N., Qiao X., Xu Z. Ultra-sensitive detection of streptomycin in foods using a novel SERS switch sensor fabricated by AuNRs array and DNA hydrogel embedded with DNAzyme. Food Chem. 2022;393 doi: 10.1016/j.foodchem.2022.133413. [DOI] [PubMed] [Google Scholar]
- 42.Hu Y., Gao S., Lu H., Ying J.Y. Acid-resistant and physiological pH-responsive DNA hydrogel composed of A-motif and i-motif toward oral insulin delivery. J. Am. Chem. Soc. 2022;144:5461–5470. doi: 10.1021/jacs.1c13426. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida K., Hayashi T., Takinoue M., Onoe H. Repeatable detection of Ag(+) ions using a DNA aptamer-linked hydrogel biochemical sensor integrated with microfluidic heating system. Sci. Rep. 2022;12:9692. doi: 10.1038/s41598-022-13970-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Q., Wang S., Huang T., Xiao F., Wu Z., Yu R. Construction and research of multiple stimuli-responsive 2D photonic crystal DNA hydrogel sensing platform with double-network structure and signal self-expression. Anal. Chem. 2022;94:5530–5537. doi: 10.1021/acs.analchem.1c04390. [DOI] [PubMed] [Google Scholar]
- 45.Pi K., Liu J., Van Cappellen P. A DNA-based biosensor for aqueous Hg(II): performance under variable pH, temperature and competing ligand composition. J. Hazard Mater. 2020;385 doi: 10.1016/j.jhazmat.2019.121572. [DOI] [PubMed] [Google Scholar]
- 46.Li Y., Gao H., Qi Z., Huang Z., Ma L., Liu J. Freezing-assisted conjugation of unmodified diblock DNA to hydrogel nanoparticles and monoliths for DNA and Hg(2+) sensing. Angew. Chem. 2021;60:12985–12991. doi: 10.1002/anie.202102330. [DOI] [PubMed] [Google Scholar]
- 47.Hou M., Yin X., Jiang J., He J. DNAzyme-triggered sol-gel-sol transition of a hydrogel allows target cell enrichment. ACS Appl. Mater. Interfaces. 2021;13:15031–15039. doi: 10.1021/acsami.1c02262. [DOI] [PubMed] [Google Scholar]
- 48.Gehring K., Leroy J.L., Guéron M. A tetrameric DNA structure with protonated cytosine.cytosine base pairs. Nature. 1993;363:561–565. doi: 10.1038/363561a0. [DOI] [PubMed] [Google Scholar]
- 49.Heinen L., Heuser T., Steinschulte A., Walther A. Antagonistic enzymes in a biocatalytic pH feedback system Program autonomous DNA hydrogel life cycles. Nano Lett. 2017;17:4989–4995. doi: 10.1021/acs.nanolett.7b02165. [DOI] [PubMed] [Google Scholar]
- 50.Fu X., Chen T., Song Y., Feng C., Chen H., Zhang Q., Chen G., Zhu X. mRNA delivery by a pH-responsive DNA nano-hydrogel. Small. 2021;17 doi: 10.1002/smll.202101224. [DOI] [PubMed] [Google Scholar]
- 51.Xu W., Huang Y., Zhao H., Li P., Liu G., Li J., Zhu C., Tian L. DNA hydrogel with tunable pH-responsive properties produced by rolling circle amplification. Chemistry. 2017;23:18276–18281. doi: 10.1002/chem.201704390. [DOI] [PubMed] [Google Scholar]
- 52.Bi Y., Du X., He P., Wang C., Liu C., Guo W. Smart bilayer polyacrylamide/DNA hybrid hydrogel film actuators exhibiting programmable responsive and reversible macroscopic shape deformations. Small. 2020;16 doi: 10.1002/smll.201906998. [DOI] [PubMed] [Google Scholar]
- 53.Lu S., Wang S., Zhao J., Sun J., Yang X. A pH-controlled bidirectionally pure DNA hydrogel: reversible self-assembly and fluorescence monitoring. Chem. Commun. 2018;54:4621–4624. doi: 10.1039/c8cc01603h. [DOI] [PubMed] [Google Scholar]
- 54.Zhou X., Li C., Shao Y., Chen C., Yang Z., Liu D. Reversibly tuning the mechanical properties of a DNA hydrogel by a DNA nanomotor. Chem. Commun. 2016;52:10668–10671. doi: 10.1039/c6cc04724f. [DOI] [PubMed] [Google Scholar]
- 55.Shi J., Zhu C., Li Q., Li Y., Chen L., Yang B., Xu J.F., Dong Y., Mao C., Liu D. Kinetically interlocking multiple-units polymerization of DNA double crossover and its application in hydrogel formation. Macromol. Rapid Commun. 2021;42 doi: 10.1002/marc.202100182. [DOI] [PubMed] [Google Scholar]
- 56.Yang B., Zhao Z., Pan Y., Xie J., Zhou B., Li Y., Dong Y., Liu D. Shear-thinning and designable responsive supramolecular DNA hydrogels based on chemically branched DNA. ACS Appl. Mater. Interfaces. 2021;13:48414–48422. doi: 10.1021/acsami.1c15494. [DOI] [PubMed] [Google Scholar]
- 57.Stricker L., Fritz E.C., Peterlechner M., Doltsinis N.L., Ravoo B.J. Arylazopyrazoles as light-responsive molecular switches in cyclodextrin-based supramolecular systems. J. Am. Chem. Soc. 2016;138:4547–4554. doi: 10.1021/jacs.6b00484. [DOI] [PubMed] [Google Scholar]
- 58.Liu X., Zhang J., Fadeev M., Li Z., Wulf V., Tian H., Willner I. Chemical and photochemical DNA "gears" reversibly control stiffness, shape-memory, self-healing and controlled release properties of polyacrylamide hydrogels. Chem. Sci. 2019;10:1008–1016. doi: 10.1039/c8sc04292f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Z., Davidson-Rozenfeld G., Vazquez-Gonzalez M., Fadeev M., Zhang J., Tian H., Willner I. Multi-triggered supramolecular DNA/bipyridinium dithienylethene hydrogels driven by light, redox, and chemical stimuli for shape-memory and self-healing applications. J. Am. Chem. Soc. 2018;140:17691–17701. doi: 10.1021/jacs.8b10481. [DOI] [PubMed] [Google Scholar]
- 60.Kumari S., Rajit Prasad S., Mandal D., Das P. Carbon dot-DNA-protoporphyrin hybrid hydrogel for sustained photoinduced antimicrobial activity. J. Colloid Interface Sci. 2019;553:228–238. doi: 10.1016/j.jcis.2019.06.034. [DOI] [PubMed] [Google Scholar]
- 61.Nam K., Im B.I., Kim T., Kim Y.M., Roh Y.H. Anisotropically functionalized aptamer-DNA nanostructures for enhanced cell proliferation and target-specific adhesion in 3D cell cultures. Biomacromolecules. 2021;22:3138–3147. doi: 10.1021/acs.biomac.1c00619. [DOI] [PubMed] [Google Scholar]
- 62.Kasahara Y., Sato Y., Masukawa M.K., Okuda Y., Takinoue M. Photolithographic shape control of DNA hydrogels by photo-activated self-assembly of DNA nanostructures. APL Bioeng. 2020;4 doi: 10.1063/1.5132929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang F., Chen M., Zhou Z., Duan R., Xia F., Willner I. Spatiotemporal patterning of photoresponsive DNA-based hydrogels to tune local cell responses. Nat. Commun. 2021;12:2364. doi: 10.1038/s41467-021-22645-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu B., Sun J., Zhu J., Li B., Ma C., Gu X., Liu K., Zhang H., Wang F., Su J., Yang Y. Injectable and NIR-responsive DNA-inorganic hybrid hydrogels with outstanding photothermal therapy. Adv. Mater. 2020;32 doi: 10.1002/adma.202004460. [DOI] [PubMed] [Google Scholar]
- 65.Li C., Chen P., Shao Y., Zhou X., Wu Y., Yang Z., Li Z., Weil T., Liu D. A writable polypeptide-DNA hydrogel with rationally designed multi-modification sites. Small. 2015;11:1138–1143. doi: 10.1002/smll.201401906. [DOI] [PubMed] [Google Scholar]
- 66.Ma X., Yang Z., Wang Y., Zhang G., Shao Y., Jia H., Cao T., Wang R., Liu D. Remote controlling DNA hydrogel by magnetic field. ACS Appl. Mater. Interfaces. 2017;9:1995–2000. doi: 10.1021/acsami.6b12327. [DOI] [PubMed] [Google Scholar]
- 67.Tang J., Yao C., Gu Z., Jung S., Luo D., Yang D. Super-soft and super-elastic DNA robot with magnetically driven navigational locomotion for cell delivery in confined space. Angew. Chem. 2020;59:2490–2495. doi: 10.1002/anie.201913549. [DOI] [PubMed] [Google Scholar]
- 68.Song J., He W., Shen H., Zhou Z., Li M., Su P., Yang Y. Self-assembly of a magnetic DNA hydrogel as a new biomaterial for enzyme encapsulation with enhanced activity and stability. Chem. Commun. 2019;55:2449–2452. doi: 10.1039/c8cc09717h. [DOI] [PubMed] [Google Scholar]
- 69.Zhovmer A.S., Chandler M., Manning A., Afonin K.A., Tabdanov E.D. Programmable DNA-augmented hydrogels for controlled activation of human lymphocytes. Nanomed. Nanotechnol. Biol. Med. 2021;37 doi: 10.1016/j.nano.2021.102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi R., Fern J., Xu W., Jia S., Huang Q., Pahapale G., Schulman R., Gracias D.H. Multicomponent DNA polymerization motor gels. Small. 2020;16 doi: 10.1002/smll.202002946. [DOI] [PubMed] [Google Scholar]
- 71.Gu Y., Distler M.E., Cheng H.F., Huang C., Mirkin C.A. A general DNA-gated hydrogel strategy for selective transport of chemical and biological cargos. J. Am. Chem. Soc. 2021;143:17200–17208. doi: 10.1021/jacs.1c08114. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y., Shao Y., Ma X., Zhou B., Faulkner-Jones A., Shu W., Liu D. Constructing tissuelike complex structures using cell-laden DNA hydrogel bricks. ACS Appl. Mater. Interfaces. 2017;9:12311–12315. doi: 10.1021/acsami.7b01604. [DOI] [PubMed] [Google Scholar]
- 73.Guo B., Wen B., Cheng W., Zhou X., Duan X., Zhao M., Xia Q., Ding S. An enzyme-free and label-free surface plasmon resonance biosensor for ultrasensitive detection of fusion gene based on DNA self-assembly hydrogel with streptavidin encapsulation. Biosens. Bioelectron. 2018;112:120–126. doi: 10.1016/j.bios.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 74.Li H., Cai Q., Yan X., Jie G. Target-switchable DNA hydrogels coupled with a Bi2Sn2O7/Bi2S3 heterojunction based on in situ anion exchange for the "signal-on" photoelectrochemical detection of DNA. Nanoscale. 2021;13:7678–7684. doi: 10.1039/d1nr00573a. [DOI] [PubMed] [Google Scholar]
- 75.Hong C.A., Park J.C., Na H., Jeon H., Nam Y.S. Short DNA-catalyzed formation of quantum dot-DNA hydrogel for enzyme-free femtomolar specific DNA assay. Biosens. Bioelectron. 2021;182 doi: 10.1016/j.bios.2021.113110. [DOI] [PubMed] [Google Scholar]
- 76.Zhao M.L., Zeng W.J., Chai Y.Q., Yuan R., Zhuo Y. An affinity-enhanced DNA intercalator with intense ECL embedded in DNA hydrogel for biosensing applications. Anal. Chem. 2020;92:11044–11052. doi: 10.1021/acs.analchem.0c00152. [DOI] [PubMed] [Google Scholar]
- 77.Zou L., Wu Z., Liu X., Zheng Y., Mei W., Wang Q., Yang X., Wang K. DNA hydrogelation-enhanced imaging ellipsometry for sensing exosomal microRNAs with a tunable detection range. Anal. Chem. 2020;92:11953–11959. doi: 10.1021/acs.analchem.0c02345. [DOI] [PubMed] [Google Scholar]
- 78.Deng S., Yan J., Wang F., Su Y., Zhang X., Li Q., Liu G., Fan C., Pei H., Wan Y. In situ terminus-regulated DNA hydrogelation for ultrasensitive on-chip microRNA assay. Biosens. Bioelectron. 2019;137:263–270. doi: 10.1016/j.bios.2019.04.053. [DOI] [PubMed] [Google Scholar]
- 79.Langford G.J., Raeburn J., Ferrier D.C., Hands P.J.W., Shaver M.P. Morpholino oligonucleotide cross-linked hydrogels as portable optical oligonucleotide biosensors. ACS Sens. 2019;4:185–191. doi: 10.1021/acssensors.8b01208. [DOI] [PubMed] [Google Scholar]
- 80.Liu S., Su W., Li Y., Zhang L., Ding X. Manufacturing of an electrochemical biosensing platform based on hybrid DNA hydrogel: taking lung cancer-specific miR-21 as an example. Biosens. Bioelectron. 2018;103:1–5. doi: 10.1016/j.bios.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 81.Si Y., Li L., Wang N., Zheng J., Yang R., Li J. Oligonucleotide cross-linked hydrogel for recognition and quantitation of MicroRNAs based on a portable glucometer readout. ACS Appl. Mater. Interfaces. 2019;11:7792–7799. doi: 10.1021/acsami.8b21727. [DOI] [PubMed] [Google Scholar]
- 82.Si Y., Xu L., Wang N., Zheng J., Yang R., Li J. Target MicroRNA-responsive DNA hydrogel-based surface-enhanced Raman scattering sensor arrays for MicroRNA-marked cancer screening. Anal. Chem. 2020;92:2649–2655. doi: 10.1021/acs.analchem.9b04606. [DOI] [PubMed] [Google Scholar]
- 83.He Y., Yang X., Yuan R., Chai Y. Switchable target-responsive 3D DNA hydrogels as a signal amplification strategy combining with SERS technique for ultrasensitive detection of miRNA 155. Anal. Chem. 2017;89:8538–8544. doi: 10.1021/acs.analchem.7b02321. [DOI] [PubMed] [Google Scholar]
- 84.Li C., Li H., Ge J., Jie G. Versatile fluorescence detection of microRNA based on novel DNA hydrogel-amplified signal probes coupled with DNA walker amplification. Chem. Commun. 2019;55:3919–3922. doi: 10.1039/c9cc00565j. [DOI] [PubMed] [Google Scholar]
- 85.Chang W.H., Lee Y.F., Liu Y.W., Willner I., Liao W.C. Stimuli-responsive hydrogel microcapsules for the amplified detection of microRNAs. Nanoscale. 2021;13:16799–16808. doi: 10.1039/d1nr05170a. [DOI] [PubMed] [Google Scholar]
- 86.Meng X., Zhang K., Dai W., Cao Y., Yang F., Dong H., Zhang X. Multiplex microRNA imaging in living cells using DNA-capped-Au assembled hydrogels. Chem. Sci. 2018;9:7419–7425. doi: 10.1039/c8sc02858c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hao L., Wang W., Shen X., Wang S., Li Q., An F., Wu S. A fluorescent DNA hydrogel aptasensor based on the self-assembly of rolling circle amplification products for sensitive detection of ochratoxin A. J. Agric. Food Chem. 2020;68:369–375. doi: 10.1021/acs.jafc.9b06021. [DOI] [PubMed] [Google Scholar]
- 88.Li Y., Ma Y., Jiao X., Li T., Lv Z., Yang C.J., Zhang X., Wen Y. Control of capillary behavior through target-responsive hydrogel permeability alteration for sensitive visual quantitative detection. Nat. Commun. 2019;10:1036. doi: 10.1038/s41467-019-08952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang L., Huang Y., Lin C., Qiu B., Guo L., Luo F., Lin Z. Highly sensitive and selective aflatoxin B1 biosensor based on Exonuclease I-catalyzed target recycling amplification and targeted response aptamer-crosslinked hydrogel using electronic balances as a readout. Talanta. 2020;214 doi: 10.1016/j.talanta.2020.120862. [DOI] [PubMed] [Google Scholar]
- 90.Liu H., Cao T., Xu Y., Dong Y., Liu D. Tuning the mechanical properties of a DNA hydrogel in three phases based on ATP aptamer. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19061633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao J., Gao J., Xue W., Di Z., Xing H., Lu Y., Li L. Upconversion luminescence-activated DNA nanodevice for ATP sensing in living cells. J. Am. Chem. Soc. 2018;140:578–581. doi: 10.1021/jacs.7b11161. [DOI] [PubMed] [Google Scholar]
- 92.Oishi M., Nakatani K. Dynamically programmed switchable DNA hydrogels based on a DNA circuit mechanism. Small. 2019;15 doi: 10.1002/smll.201900490. [DOI] [PubMed] [Google Scholar]
- 93.Ji X., Wang J., Niu S., Ding C. Size-controlled DNA-cross-linked hydrogel coated silica nanoparticles served as a ratiometric fluorescent probe for the detection of adenosine triphosphate in living cells. Chem. Commun. 2019;55:5243–5246. doi: 10.1039/c9cc01832h. [DOI] [PubMed] [Google Scholar]
- 94.Zhang Z., Du J., Li Y., Wu J., Yu F., Chen Y. An aptamer-patterned hydrogel for the controlled capture and release of proteins via biorthogonal click chemistry and DNA hybridization. J. Mater. Chem. B. 2017;5:5974–5982. doi: 10.1039/c7tb00883j. [DOI] [PubMed] [Google Scholar]
- 95.Jiang C., Wang F., Zhang K., Min T., Chen D., Wen Y. Distance-based biosensor for ultrasensitive detection of uracil-DNA glycosylase using membrane filtration of DNA hydrogel. ACS Sens. 2021;6:2395–2402. doi: 10.1021/acssensors.1c00624. [DOI] [PubMed] [Google Scholar]
- 96.Sun K., Chen P., Yan S., Yuan W., Wang Y., Li X., Dou L., Zhao C., Zhang J., Wang Q., Fu Z., Wei L., Xin Z., Tang Z., Yan Y., Peng Y., Ying B., Chen J., Geng J. Ultrasensitive nanopore sensing of mucin 1 and circulating tumor cells in whole blood of breast cancer patients by analyte-triggered triplex-DNA release. ACS Appl. Mater. Interfaces. 2021;13:21030–21039. doi: 10.1021/acsami.1c03538. [DOI] [PubMed] [Google Scholar]
- 97.Ji X., Lv H., Sun X., Ding C. Green-emitting carbon dot loaded silica nanoparticles coated with DNA-cross-linked hydrogels for sensitive carcinoembryonic antigen detection and effective targeted cancer therapy. Chem. Commun. 2019;55:15101–15104. doi: 10.1039/c9cc07831b. [DOI] [PubMed] [Google Scholar]
- 98.Gao X., Li X., Sun X., Zhang J., Zhao Y., Liu X., Li F. DNA tetrahedra-cross-linked hydrogel functionalized paper for onsite analysis of DNA methyltransferase activity using a personal glucose meter. Anal. Chem. 2020;92:4592–4599. doi: 10.1021/acs.analchem.0c00018. [DOI] [PubMed] [Google Scholar]
- 99.Na W., Nam D., Lee H., Shin S. Rapid molecular diagnosis of infectious viruses in microfluidics using DNA hydrogel formation. Biosens. Bioelectron. 2018;108:9–13. doi: 10.1016/j.bios.2018.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim H.S., Abbas N., Shin S. A rapid diagnosis of SARS-CoV-2 using DNA hydrogel formation on microfluidic pores. Biosens. Bioelectron. 2021;177 doi: 10.1016/j.bios.2021.113005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen X., Xie Y., Zhang Y., Li C., Xu W. Programmable 3D rigid clathrate hydrogels based on self-assembly of tetrahedral DNA and linker PCR products. Chem. Commun. 2020;56:13181–13184. doi: 10.1039/d0cc05898j. [DOI] [PubMed] [Google Scholar]
- 102.Obuobi S., Tay H.K., Tram N.D.T., Selvarajan V., Khara J.S., Wang Y., Ee P.L.R. Facile and efficient encapsulation of antimicrobial peptides via crosslinked DNA nanostructures and their application in wound therapy. J. Contr. Release. 2019;313:120–130. doi: 10.1016/j.jconrel.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 103.Huang Y., Xu W., Liu G., Tian L. A pure DNA hydrogel with stable catalytic ability produced by one-step rolling circle amplification. Chem. Commun. 2017;53:3038–3041. doi: 10.1039/c7cc00636e. [DOI] [PubMed] [Google Scholar]
- 104.Mao X., Chen G., Wang Z., Zhang Y., Zhu X., Li G. Surface-immobilized and self-shaped DNA hydrogels and their application in biosensing. Chem. Sci. 2018;9:811–818. doi: 10.1039/c7sc03716c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tang J., Liu L., Gao S., Qin J., Liu X., Tang D. A portable thermal detection method based on the target responsive hydrogel mediated self-heating of a warming pad. Chem. Commun. 2021;57:9862–9865. doi: 10.1039/d1cc03733a. [DOI] [PubMed] [Google Scholar]
- 106.Liao W.C., Lilienthal S., Kahn J.S., Riutin M., Sohn Y.S., Nechushtai R., Willner I. pH- and ligand-induced release of loads from DNA-acrylamide hydrogel microcapsules. Chem. Sci. 2017;8:3362–3373. doi: 10.1039/c6sc04770j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mao X., Mao D., Chen T., Jalalah M., Al-Assiri M.S., Harraz F.A., Zhu X., Li G. DNA hydrogel-based three-dimensional electron transporter and its application in electrochemical biosensing. ACS Appl. Mater. Interfaces. 2020;12:36851–36859. doi: 10.1021/acsami.0c08064. [DOI] [PubMed] [Google Scholar]
- 108.Fern J., Schulman R. Modular DNA strand-displacement controllers for directing material expansion. Nat. Commun. 2018;9:3766. doi: 10.1038/s41467-018-06218-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Niederholtmeyer H., Chaggan C., Devaraj N.K. Communication and quorum sensing in non-living mimics of eukaryotic cells. Nat. Commun. 2018;9:5027. doi: 10.1038/s41467-018-07473-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fischer A., Lilienthal S., Vazquez-Gonzalez M., Fadeev M., Sohn Y.S., Nechushtai R., Willner I. Triggered release of loads from microcapsule-in-microcapsule hydrogel microcarriers: en-route to an "artificial pancreas. J. Am. Chem. Soc. 2020;142:4223–4234. doi: 10.1021/jacs.9b11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu Y., Li Q., Shim G., Oh Y.K. Melanin-loaded CpG DNA hydrogel for modulation of tumor immune microenvironment. J. Contr. Release. 2021;330:540–553. doi: 10.1016/j.jconrel.2020.12.040. [DOI] [PubMed] [Google Scholar]
- 112.Han J., Cui Y., Han X., Liang C., Liu W., Luo D., Yang D. Super-soft DNA/Dopamine-Grafted-Dextran hydrogel as dynamic wire for electric circuits switched by a microbial metabolism process. Adv. Sci. 2020;7 doi: 10.1002/advs.202000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu Y., Wang D., Willner I., Tian Y., Jiang L. Smart DNA hydrogel integrated nanochannels with high ion flux and adjustable selective ionic transport. Angew. Chem. 2018;57:7790–7794. doi: 10.1002/anie.201803222. [DOI] [PubMed] [Google Scholar]
- 114.Dong Y., Combs J.D., Cao C., Weeks E.R., Bazrafshan A., Rashid S.A., Salaita K. Supramolecular DNA photonic hydrogels for on-demand control of coloration with high spatial and temporal resolution. Nano Lett. 2021;21:9958–9965. doi: 10.1021/acs.nanolett.1c03399. [DOI] [PubMed] [Google Scholar]
- 115.Jang K., Westbay J.H., Asher S.A. DNA-crosslinked 2D photonic crystal hydrogels for detection of adenosine actuated by an adenosine-binding aptamer. ACS Sens. 2022;7:1648–1656. doi: 10.1021/acssensors.1c02424. [DOI] [PubMed] [Google Scholar]
- 116.Lim J., Hwang J.-S., Beom Seo S., Kang B., Jang S., Uk Son S., Ki J., Kim J.-S., Kang T., Jung J., Han T.-S., Lim E.-K. Janus hydrogel-based fuel stimulant powered amplification for multiple detections of miRNA biomarkers in gastric cancer. Chem. Eng. J. 2022;448 [Google Scholar]
- 117.Zheng M., Liu H., Ye J., Ni B., Xie Y., Wang S. Target-responsive aptamer-cross-linked hydrogel sensors for the visual quantitative detection of aflatoxin B1 using exonuclease I-Triggered target cyclic amplification. Food Chem. X. 2022;15 doi: 10.1016/j.fochx.2022.100395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sun Y., Qi S., Dong X., Qin M., Zhang Y., Wang Z. Colorimetric aptasensor targeting zearalenone developed based on the hyaluronic Acid-DNA hydrogel and bimetallic MOFzyme. Biosens. Bioelectron. 2022;212 doi: 10.1016/j.bios.2022.114366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.