Abstract
Pseudomonas aeruginosa translocates over solid surfaces by a type IV pilus-dependent form of multicellular motility known as twitching. We wondered whether cells utilize endogenous factors to organize twitching, and we purified from wild-type cells a lipid that caused directed movement. Wild-type P. aeruginosa, but not a pilJ pilus-deficient mutant, showed biased movement up gradients of phosphatidylethanolamine (PE) established in agar. Activity was related to the fatty acid composition of the lipid, as two synthetic PE species, dilauroyl and dioleoyl PE, were capable of directing P. aeruginosa motility while many other species were inactive. P. aeruginosa PE did not contain either laurate or oleate, implying that the native attractant species contains different fatty acids. Uniform concentrations of PE increased cell velocity, suggesting that chemokinesis may be at least partly responsible for directed movement. We speculate that PE-directed twitching motility may be involved in biofilm formation and pathogenesis.
Pseudomonas aeruginosa is a prevalent contaminant of ventilators and catheters, a common cause of nosocomial infections, and an opportunistic pathogen: lung infections with this organism are a leading cause of mortality in both cystic fibrosis and AIDS patients (27). The aggressiveness and persistence of this organism may be attributed to the formation of biofilm microcolonies which protect the component individuals and concentrate virulence factors (19). Recent studies have indicated that P. aeruginosa biofilm formation requires intercellular signaling, and coordinated motility has been implicated in the proper organization of three-dimensional, mushroom-shaped structures within the biofilm (7, 9, 22).
P. aeruginosa swims rapidly in liquid by means of flagella, and during biofilm formation swimming motility is involved in initial location and adherence to solid surfaces (22). Once attached to a surface, P. aeruginosa moves by the flagellum-independent surface motility known as twitching (6). Twitching cells move linearly along their long axis and are motile only in large groups (24). Twitching may be powered by retraction of type IV pili (20), and mutants defective in pilus biosynthesis genes lack twitching motility (21). Interestingly, mutations resulting in the loss of pili have also been identified in genes encoding homologs of the enteric Che chemotaxis proteins (8). In Escherichia coli, these proteins are essential for oriented movement within chemical gradients, suggesting that twitching motility may be chemically regulated. Twitching is absolutely essential for biofilm formation (22).
While directed movement in any surface motile organism is poorly understood, a chemoattractant has been discovered in the gliding bacterium Myxococcus xanthus (18). M. xanthus, a nonpathogenic soil bacterium, also forms a biofilm that requires both cell-cell signaling and surface motility (25). Gliding motility shares many features with twitching motility: movement is along the long axis of the cell, occurs in cell groups, and is dependent on the presence of type IV pili (16). M. xanthus cells migrate up gradients of phosphatidylethanolamine (PE) purified from their own cell membranes, and cells may secrete or otherwise present this chemical to neighboring cells in order to orchestrate group movement (18). The extensive similarities between P. aeruginosa and M. xanthus surface motility prompted us to search for an endogenous lipid produced by P. aeruginosa that functioned as a twitching chemoeffector.
Twitching motility.
In order to demonstrate twitching motility, P. aeruginosa PAO1 was grown to late log phase in L broth (10 g of tryptone, 5 g of yeast extract, and 5 g of NaCl per liter) and resuspended to 5 × 109 cells/ml in India ink-MOPS buffer {10 mM 3-(N-morpholino)propanesulfonic acid [pH 7.6], 8 mM MgSO4, 10% India ink}. One microliter of the cell suspension was spotted onto TPM agar (10 mM Tris HCl [pH 7.6], 8 mM MgSO4, 1 mM KHPO4-KH2PO4 buffer [pH 7.6], 1.5% Bacto agar) and incubated at 37°C for 24 h. The India ink absorbed to the agar surface and served as a reference point for the original inoculum. After 24 h, a halo of cells with a rough edge formed around the origin of the colony, suggesting that P. aeruginosa was capable of twitching at a surface-air interface (Fig. 1a). Zones of motility were measured using a Leitz light microscope at a magnification of ×100 with an ocular micrometer. Under these conditions, expansion of unstimulated swarms occurred at a rate of 31 μm/h.
FIG. 1.
P. aeruginosa biased twitching motility up PE gradients. Wild-type PAO1 P. aeruginosa (a and b) and a pilJ mutant (c and d) were spotted within mock gradients generated with chloroform (a and c) or PE gradients generated with 10 μg of PE purified from PAO1 in chloroform (b and d). Scale bar = 1 mm.
A pilJ mutant was examined to determine whether twitching motility was responsible for the observed swarm expansion. A pilJ mutant was constructed by gene replacement with a tetracycline resistance (Tcr) cassette; it completely lacks twitching motility and expresses no type IV pili, as determined by antipilin Western blotting. The mutation is nonpolar, as the pilJ locus expressed in trans restored twitching motility to the pilJ mutant (8). PilJ is homologous to the E. coli Tsr chemoreceptor methyl-accepting chemotaxis protein, and it is not clear why a mutation in this gene causes a twitching motility defect in P. aeruginosa (8). Nonetheless, the pilJ mutant produced a much smaller halo with a smooth edge, suggesting that the majority of the zone we observed in wild-type cells was due to twitching motility (Fig. 1c).
Purification and identification of twitching chemoeffectors.
A hydrophobic extract of P. aeruginosa PAO1 was prepared according to the guidelines of Bligh and Dyer to determine whether P. aeruginosa makes chemicals that effect twitching motility (4). A volume of 3.75 ml of methanol-chloroform (2:1) was added to 0.4 g of cells (wet weight) of late-log-phase P. aeruginosa PAO1 and was vortexed for 1 h. The suspension was centrifuged at 10,000 × g for 5 min and the supernatant was saved. The pellet was then reextracted with 4.75 ml of methanol-chloroform-water (2:1:0.8) and centrifuged again, and the supernatants were combined. Volumes of 2.5 ml of chloroform and 2.5 ml of water were added to the combined supernatants, vortexed, and centrifuged to separate the chloroform and aqueous layers. The chloroform layer containing hydrophobic material was collected and dried under nitrogen in a preweighed tube. The mass of the residue was determined and the residue was resuspended in chloroform.
The hydrophobic extract was used to create gradients in agar. A total of 100 μg of the extract was dried onto a 3-mm diameter Whatman no. 1 filter paper disk and placed on TPM agar. A disk with solvent alone served as a negative control. The plate was incubated at 32°C for 24 h to generate a gradient, after which P. aeruginosa cells, prepared as for the motility assay, were spotted 3 to 5 mm from the disk. Colonies spotted near the control disk produced uniform halos, but colonies near the disk containing extract migrated a distance twofold farther toward the disk than away from it (data not shown). The preferential motility suggested that P. aeruginosa contained an endogenous, hydrophobic twitching chemoeffector.
To identify the active molecules, the extract was fractionated by silica gel affinity chromatography (12). A total of 20 mg of extract in hexane–methyl tert-butyl ether (200:3) was loaded on a 2-g column of silica gel (Supelco) that had been washed with hexane. The column was then serially eluted with 12 ml of each solvent listed in Table 1. Each fraction was dried under nitrogen, weighed, and resuspended in chloroform. Cells migrated four- and threefold farther up gradients generated when 20 μg each of fractions 5 and 6, respectively, was spotted directly on the agar surface (Table 1). No biased motility was observed with any other fraction, regardless of concentration.
TABLE 1.
Purification of putative P. aeruginosa twitching motility attractants
| Fraction | Solventa | PMVb | Predicted eluatec |
|---|---|---|---|
| 1 | Hexane-MTBE (96:4) | 1.01 | Neutral lipids |
| 2 | Hexane-acetic acid (100:0.2) | 1.02 | |
| 3 | Hexane-MTBE-acetic acid (100:2:0.2) | 0.98 | Fatty acids |
| 4 | MTBE-MeOH-ammonium acetate (25:4:1) | 1.20 | PI |
| 5 | MTBE-MeOH-ammonium acetate (10:4:1) | 3.96 | PE |
| 6 | MTBE-MeOH-ammonium acetate (5:8:2) | 3.12 | PC |
Ammonium acetate was made by adding 2 parts each 1 mM ammonium hydroxide and 1 mM acetic acid (pH 8.6). MTBE, methyl tert-butyl ether.
The preferential migration value (PMV) is the distance cells moved up the gradient divided by the distance cells moved down the gradient when 20 μg of extract was applied in the chemotaxis assay. The values are the averages of 12 measurements.
Predicted eluates were determined previously (12). PI, phosphatidylinositol.
Fraction 5 (100 μg) was resolved by Silica Gel G thin-layer chromatography (TLC) in chloroform-methanol-water (65:25:4) until the solvent was 1 cm from the top of the 20-cm gel. After resolution, the plate was stained with iodine vapor, a nonspecific indicator of lipids, and with 0.5% ninhydrin (in 1-butanol and 3% acetic acid incubated at 100°C for 5 min), a color indicator of free amino groups (15). Fraction 5 contained only one iodine-reactive band, which had an Rf value of 0.53, was reactive with ninhydrin, and comigrated with a PE standard. The band was verified as PE by using chloroform-acetone-methanol-acetic acid-water (10:4:2:2:1) as an alternative solvent system. Sections of the plate were scraped, eluted with methanol, dried under nitrogen, and resuspended to a concentration of 1 mg/ml in chloroform. Each eluate (10 μl) was spotted directly on a TPM plate and incubated at 32°C for 24 h to generate a gradient. The fraction containing PE caused PAO1 to twitch threefold farther up the gradient than down the gradient (Fig. 1b). Other fractions displayed no activity.
While we focused most of our attention on fraction 5, fraction 6 also contained activity (Table 1) and was resolved with chloroform-methanol-water (65:25:4) by silica gel TLC. TLC analysis revealed four iodine-reactive bands. The most intense band was identified as PE because it had an Rf value of 0.5, reacted with ninhydrin, and comigrated with a PE standard. A second band was positively stained by ninhydrin and was identified as phosphatidylserine based on its Rf value of 0.15 (15). A third band did not react with ninhydrin and was identified as phosphatidylcholine (PC) because it had an Rf value of 0.20 and comigrated with a PC standard. The final band had an Rf value of 1.00 and stained weakly with ninhydrin. This band has not been identified. These observations were confirmed in an alternate solvent system, chloroform-acetone-methanol-acetic acid-water (10:4:2:2:1). The majority of fraction 6 was composed of PE, which likely accounted for the activity observed. However, the other bands were not isolated or tested directly.
Mechanism of PE-directed movement.
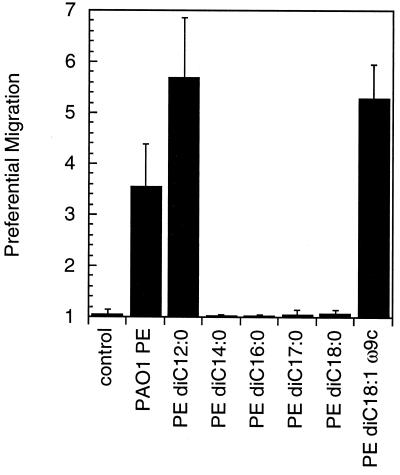
The pilJ mutant showed no preferential migration in the presence of purified PE (Fig. 1d). As the pilJ mutant is fully proficient in flagellum-mediated swimming motility, we concluded that directed movement to PE on surfaces requires twitching motility (8). We considered two possible explanations for the PE-dependent directed movement. Either PE could act as a lubricant to passively facilitate twitching motility, or PE could be a transduced stimulus mediated by a dedicated perception system to regulate motor output. The simplest way to address both possibilities was to determine the specificity of the PE response. One might expect that the surfactant properties of PE might be less dependent on the fatty acid side chains than is expected for a transduced stimulus. Six chemically synthesized PE species with different fatty acid side chains were tested to determine the specificity of the motility response. Only dilauroyl (di-C12:0) and dioleoyl (di-C18:1ω9c) PE induced directed motility, suggesting a significant degree of chemical specificity (Fig. 2). Because PE purified from P. aeruginosa likely contained a wide variety of chemical species, fatty acid analysis was conducted on PE purified from PAO1 (Microbial ID, Inc.) (Table 2). The two active synthetic chemicals cannot account for the activity of the extract, because the extract lacked both laurate and oleate under the growth conditions used here. Nonetheless, the specificity of the response argues against a passive mechanism for PE and favors the involvement of signal transduction.
FIG. 2.
P. aeruginosa biased twitching motility up synthetic dilauroyl and dioleoyl PE. Ten micrograms of each compound was tested. The preferential migration value is the distance migrated up the gradient divided by the distance migrated down the gradient. No preferential movement up the gradient produced a value of 1. Error bars show the standard deviations of 12 measurements.
TABLE 2.
Fatty acid composition of PAO1 PEa
| Fatty acid | % Composition |
|---|---|
| 13:02′-OH | 1.12 |
| 14:1ω5c | 1.10 |
| 14:0 | 3.28 |
| 15:1iso | 1.75 |
| 15:1iso 3′-OH | 1.19 |
| 15:1ω5c | 1.72 |
| 15:0iso | 1.17 |
| 16:1ω7c | 10.87 |
| 16:0 | 32.76 |
| 18:1ω7c | 34.00 |
| 18:0 | 6.64 |
| 19:0cycloω8c | 1.10 |
Fatty acids constituting less than 1% of PE were not included.
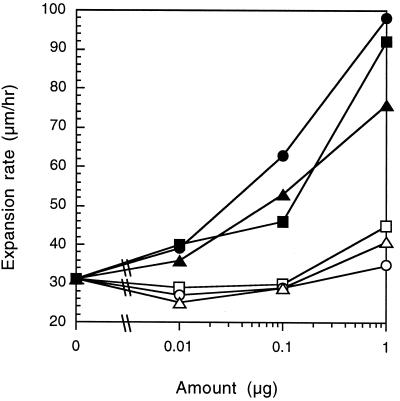
Twitching PAO1 individuals have the capacity to reverse direction within a group of cells, and directed movement may occur by chemotaxis (24). During chemotaxis, an effector excites a sensory system to suppress direction reversals and results in longer periods of movement toward an attractant (1). Preferential movement continues as long as the concentration continues to increase, because an adaptive response continually desensitizes the sensory system (5, 11). Alternatively, PE may increase the speed of twitching motility, and therefore directed movement may be the product of chemokinesis. Unlike chemotaxis, chemokinesis in Rhodobacter sphaeroides (23) and M. xanthus (28) does not show adaptation, and thus the effect on motility is independent of a gradient. To distinguish between chemotactic and chemokinetic responses in P. aeruginosa, cells were spotted onto a uniform concentration of PE. A total of 0.01, 0.1, or 1 μg of each of the six chemically synthesized PEs was delivered to TPM in 10 μl of chloroform and was incubated for 24 h at 32°C. Cells were then inoculated in the middle of the PE spot and incubated for 24 h at 32°C, and the swarm expansion rate was measured with a 30× dissecting microscope and an ocular micrometer. In the absence of a gradient, dilauroyl and dioleoyl PE enhanced twitching motility approximately threefold (Fig. 3). Unlike the gradient, uniform concentrations of dimyristoyl PE also enhanced swarm expansion. Nonetheless, dipalmitoyl, diheptadecanoyl, and distearoyl PE failed to increase the rate of swarm expansion and support the chemical specificity of the PE response. These results also suggest that directed movement is at least partly due to enhancement of cell velocity. A preferable way to measure chemotaxis and chemokinesis is to measure the reversal frequency and cellular speed of individual cells in response to PE. However, microscopic analysis of cell movement has proven difficult, as twitching P. aeruginosa is motile only in groups, and hence individuals are obscured by their neighbors.
FIG. 3.
P. aeruginosa twitching rate increased in the presence of uniform concentrations of some synthetic PE. The rate of PAO1 swarm expansion was measured when cells were spotted directly on top of the PE source. The following synthetic PEs were tested: dilauroyl PE (●), dimyristoyl PE (▴), dipalmitoyl PE (○), diheptadecanoyl PE (□), distearoyl PE (▵), and dioleoyl PE (■). Each point represents the average of three measurements.
The results presented here suggest that PE acts as a transduced stimulus to influence P. aeruginosa twitching motility, but the mechanism of signal transduction is not known. While it is interesting that PilJ is homologous to a methyl-accepting chemotaxis protein chemoreceptor, the complete lack of pili and twitching motility in a pilJ mutant prohibits any conclusions concerning the role of this system in sensory transduction. Directly upstream of pilJ in the P. aeruginosa chromosome lie pilG, pilH, and pilI, homologues of enteric cheY, another cheY gene, and cheW (chemotaxis genes), respectively (8). Although pilG and pilI mutants have the same twitching-deficient phenotype as pilJ mutants, pilH mutants express type IV pili but produce doughnut-shaped swirls on an agar surface, suggesting that control of twitching motility is dramatically altered (8). Since pilH is homologous to genes required for enteric chemotaxis and a pilH mutant has altered surface motility behavior, we wanted to examine the pilH mutant response to PE gradients. This mutant demonstrated no swarm expansion whatsoever in our assays. In fact, microscopic examination of the agar surface revealed extensive lysis; it appears that the starvation conditions of the assay are lethal to the pilH mutant. The pilH mutant could be very helpful in determining the role of these che homologues in PE signal transduction, but it unfortunately remained intractable under the assay conditions of this study.
The recent publication of the P. aeruginosa PAO1 genome sequence revealed four complete chemotaxis-like signal transduction systems, indicating an enormous complexity in motility control (26). One system controls swimming chemotaxis (17), and the pil system is essential for twitching (8), but the other two systems are novel and their roles are not understood. It is interesting that the third system is most related to that found in R. sphaeroides, an organism which responds chemokinetically to some chemicals (23), while the fourth system is homologous to the M. xanthus frz system, which controls gliding behavior and is involved in adaptation to PE during chemotaxis (3, 18). It will be interesting to determine the effects of mutations in the R. sphaeroides- and M. xanthus-like signal transduction systems on twitching motility and the response to PE. It is also worth noting that Foster et al. have suggested that type IV pili of enteropathogenic E. coli may bind PE directly (10). Perhaps PE binding to type IV pili increases either the rate or the force of pilus retraction.
Lipids are ideal signals for surface-borne organisms, as they adhere to surfaces and diffuse slowly. That P. aeruginosa biases twitching motility up PE gradients lends further support to the idea that surface-motile organisms utilize lipids as chemoeffectors. PE could conceivably regulate motility during M. xanthus fruiting-body formation and P. aeruginosa biofilm microcolony aggregation, as both processes are dependent on type IV pili (22). PE from P. aeruginosa membranes is active, suggesting that it may serve as an endogenous autoattractant and aggregation signal. It is also possible that P. aeruginosa might also direct twitching toward PE from host cells and use these signals for orientation during infection. Twitching motility has been identified in a variety of pathogenic organisms, including Acinetobacter calcoaceticus, Neisseria gonorrhoeae, Neisseria meningitidis, Moraxella bovis, and Pasteurella multocida (13, 14). Enteropathogenic E. coli may also possess twitching motility, as mutants deficient in type IV pilus production lack localized adherence or microcolony formation and are dramatically reduced in virulence (2). It is interesting to speculate that type IV pilus-dependent surface motility directed by lipid effectors may be a critical event in pathogenesis in these and other organisms.
Acknowledgments
This work was supported by grant MCB9601077 from the NSF and a Grant-in-Aid of Research from the National Academy of Sciences through Sigma Xi, The Scientific Research Society.
REFERENCES
- 1.Berg H C, Brown D A. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972;239:500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- 2.Bieber D, Ramer S W, Wu C Y, Murray W J, Tobe T, Fernandez R, Schoolnik G K. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science. 1998;280:2114–2118. doi: 10.1126/science.280.5372.2114. [DOI] [PubMed] [Google Scholar]
- 3.Blackhart B D, Zusman D R. “Frizzy” genes of Myxococcus xanthus are involved in control of frequency of reversal of gliding motility. Proc Natl Acad Sci USA. 1985;82:8767–8770. doi: 10.1073/pnas.82.24.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 5.Borkovich K A, Alex L A, Simon M I. Attenuation of sensory receptor signaling by covalent modification. Proc Natl Acad Sci USA. 1992;89:6756–6760. doi: 10.1073/pnas.89.15.6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley D E. A function of Pseudomonas aeruginosa PAO polar pili: twitching motility. Can J Microbiol. 1980;26:146–154. doi: 10.1139/m80-022. [DOI] [PubMed] [Google Scholar]
- 7.Costerton J W, Lewandowski Z, Caldwell D E, Korber D R, Lappin-Scott H M. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 8.Darzins A. Characterization of a Pseudomonas aeruginosa gene cluster involved in pilus biosynthesis and twitching motility: sequence similarity to the chemotaxis proteins of enterics and the gliding bacterium Myxococcus xanthus. Mol Microbiol. 1994;11:137–153. doi: 10.1111/j.1365-2958.1994.tb00296.x. [DOI] [PubMed] [Google Scholar]
- 9.Davies D G, Parsek M R, Pearson J P, Iglewski B H, Costerton J W, Greenberg E P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 10.Foster D B, Philpott D, Abul-Milh M, Huesca M, Sherman P M, Lingwood C A. Phosphatidylethanolamine recognition promotes enteropathogenic E. coli and enterohemorrhagic E. coli host cell attachment. Microb Pathog. 1999;27:289–301. doi: 10.1006/mpat.1999.0305. [DOI] [PubMed] [Google Scholar]
- 11.Guoyong L, Weis R M. Covalent modification regulates ligand binding to receptor complexes in the chemosensory system of Escherichia coli. Cell. 2000;100:357–365. doi: 10.1016/s0092-8674(00)80671-6. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton J G, Comai K. Rapid separation of neutral lipids, free fatty acids and polar lipids using prepacked silica sep-pak columns. Lipids. 1988;23:1146–1149. doi: 10.1007/BF02535281. [DOI] [PubMed] [Google Scholar]
- 13.Henrichsen J. The occurrence of twitching motility among Gram-negative bacteria. Acta Pathol Microbiol Scand Sect B. 1975;83:171–178. doi: 10.1111/j.1699-0463.1975.tb00089.x. [DOI] [PubMed] [Google Scholar]
- 14.Henriksen S D, Frøholm L O. A fimbriated strain of Pasteurella multocida with spreading and corroding colonies. Acta Pathol Microbiol Scand Sect B. 1975;83:129–132. doi: 10.1111/j.1699-0463.1975.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 15.Jennings W G. Lipids. In: Bernard F, Sherma J, editors. Thin layer chromatography: techniques and applications. 3rd ed. New York, N.Y: Marcel Dekker Inc.; 1994. pp. 245–285. [Google Scholar]
- 16.Kaiser D, Crosby C. Cell movement and its coordination in swarms of Myxococcus xanthus. Cell Motil. 1983;3:227–245. [Google Scholar]
- 17.Kato J, Nakamura T, Kuroda A, Ohtake H. Cloning and characterization of chemotaxis genes in Pseudomonas aeruginosa. Biosci Biotechnol Biochem. 1999;63:155–161. doi: 10.1271/bbb.63.155. [DOI] [PubMed] [Google Scholar]
- 18.Kearns D B, Shimkets L J. Chemotaxis in a gliding bacterium. Proc Natl Acad Sci USA. 1998;95:11857–11962. doi: 10.1073/pnas.95.20.11957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam J, Chan R, Lam K, Costerton J W. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun. 1980;28:546–556. doi: 10.1128/iai.28.2.546-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merz A J, So M, Sheetz M P. Pilus retraction powers bacterial twitching motility. Nature. 2000;407:98–102. doi: 10.1038/35024105. [DOI] [PubMed] [Google Scholar]
- 21.Nunn D, Bergman S, Lory S. Products of three accessory genes, pilB, pilC, and pilD, are required for biogenesis of Pseudomonas aeruginosa pili. J Bacteriol. 1990;172:2911–2919. doi: 10.1128/jb.172.6.2911-2919.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Toole G A, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 23.Packer H L, Gauden D E, Armitage J P. The behavioral response of anaerobic Rhodobacter sphaeroides to temporal stimuli. Microbiology. 1996;142:593–599. doi: 10.1099/13500872-142-3-593. [DOI] [PubMed] [Google Scholar]
- 24.Semmler A B T, Whitchurch C B, Mattick J S. A re-examination of twitching motility in Pseudomonas aeruginosa. Microbiology. 1999;145:2863–2873. doi: 10.1099/00221287-145-10-2863. [DOI] [PubMed] [Google Scholar]
- 25.Shimkets L J. Social and developmental biology of the myxobacteria. Microbiol Rev. 1990;54:473–501. doi: 10.1128/mr.54.4.473-501.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stover C K, Pham X Q, Erwin A L, Mizoguchi S D, Warrener P, Hickey M J, Brinkman F S L, Hufnagle W O, Kowalik D J, Lagrou M, Garber R L, Goltry L, Tolentino E, Westbrook-Wadman S, Yaun Y, Brody L L, Coulter S N, Folger K R, Kas A, Larbig K, Lim R, Spencer D, Wong G K-S, Wu Z, Paulsen I T, Reizer J, Saier M H, Hancock R E W, Lory S, Olson M V. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 27.Van Delden C, Iglewski B H. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis. 1998;4:551–560. doi: 10.3201/eid0404.980405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward M J, Mok K C, Zusman D R. Myxococcus xanthus displays Frz-dependent chemokinetic behavior during vegetative growth. J Bacteriol. 1998;180:440–443. doi: 10.1128/jb.180.2.440-443.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]