Abstract
Rationale:
Transplant renal artery stenosis (TRAS) is a well-recognized and potentially reversible cause of resistant hypertension post transplantation and can affect 1% to 23% of recipients. Stenosis of the iliac segment proximal to the transplant renal artery (proximal TRAS) causing dysfunction of the transplanted kidney is less common with reported incidence of 2% to 3%. Presentation typically occurs between 3 months and 2 years post transplant but may happen at any time. Noninvasive investigations such as Doppler ultrasound, computed tomography (CT) angiogram, and magnetic resonance angiogram are useful in initial evaluation, but definitive diagnosis of hemodynamically significant stenosis often requires formal angiogram. Transplant renal artery stenosis should be suspected in any kidney transplant recipient with worsening hypertension and/or deterioration in kidney function which is otherwise unexplained. We present the case of a kidney transplant recipient with resistant hypertension and impaired graft function, secondary to severe impairment of graft blood flow from proximal iliac system occlusion.
Presenting concerns of the patient:
A 74-year-old female 15 years post live donor kidney transplant presented with graft dysfunction (serum Cr 229 μmol/L) and resistant hypertension, requiring use of 8 antihypertensive medications. On physical examination, blood pressure was 160/92 mm Hg with no tenderness over the renal graft in the right lower abdominal quadrant and no audible bruit in kidney allograft area.
Diagnosis:
Transplant Doppler ultrasound showed reversal of flow in the right external iliac artery suggestive of ipsilateral proximal iliac occlusion. Pre-procedure CT demonstrated severe atherosclerotic burden within the aorta and bilateral iliac systems. The anastomosed right renal artery appeared patent.
Interventions:
Conventional angiogram showed occlusion of the right common and proximal external iliac arteries with retrograde perfusion of the transplant kidney via the contralateral left iliac system and aorta. Subintimal recanalization of the right iliac system was performed with angioplasty and kissing stent placement at the aortic bifurcation with stents extending into the proximal right external iliac artery. Post deployment angiogram demonstrated renewed patency of the right iliac system, with restoration of antegrade perfusion to the transplant kidney.
Outcomes:
The patient’s blood pressure decreased significantly after the procedure, with improvement in graft function. After 6 months, the patient continued to have optimally controlled blood pressure (on 3 medications) and stable graft function (serum Cr 74 μmol/L).
Teaching points:
Our case describes proximal TRAS and the contribution of renal hypoperfusion to hypertension and impaired graft function, with the potential for reversibility.
Keywords: TRAS, proximal TRAS, resistant hypertension, post-renal transplant, PTA
Abrégé
Justification:
La sténose de l’artère du rein transplanté (SART) est une cause bien connue et potentiellement réversible d’hypertension résistante qui touche de 1 à 23% des receveurs après l’intervention. La sténose du segment iliaque proximal de l’artère du rein transplanté (SARTprox), laquelle cause un dysfonctionnement du greffon, est moins fréquente (incidence rapportée: 2 à 3%). Elle se produit généralement entre 3 mois et 2 ans après la transplantation, mais peut se produire à tout moment. Les examens non invasifs tels que l’échographie Doppler, l’angiographie par tomodensitométrie (TDM) et l’angiographie par résonance magnétique sont utiles pour l’évaluation initiale, mais le diagnostic définitif d’une sténose hémodynamiquement significative nécessite souvent une angiographie formelle. Les SART doivent être suspectées chez tout receveur d’une greffe rénale présentant une aggravation de l’hypertension et/ou une détérioration de la fonction rénale, autrement inexpliquées. Nous présentons le cas d’une receveuse souffrant d’hypertension résistante et d’une altération de la fonction du greffon résultant d’une grave altération du flux sanguin dans le greffon causée par l’occlusion du système iliaque proximal.
Présentation du cas:
Une femme de 74 ans, greffée 15 ans auparavant avec un rein de donneur vivant, présentait un dysfonctionnement du greffon (Cr sérique: 229 µmol/L) et une hypertension résistante nécessitant huit médicaments antihypertenseurs. À l’examen physique, la pression artérielle était de 160/92 mm Hg et la patiente ne présentait aucune sensibilité au-dessus du greffon rénal dans le quadrant inférieur droit de l’abdomen, ni bruit audible au niveau de l’artère rénale.
Diagnostic:
L’échographie Doppler du greffon a montré une inversion du flux dans l’artère iliaque externe droite, ce qui suggérait une occlusion iliaque proximale ipsilatérale. La TDM avant l’intervention avait montré une charge athérosclérotique sévère dans l’aorte et les systèmes iliaques bilatéraux. L’artère rénale droite anastomosée semblait non obstruée.
Intervention:
L’angiographie conventionnelle a montré une occlusion de l’artère iliaque commune droite et de l’artère iliaque externe proximale, avec une perfusion rétrograde du rein transplanté via le système iliaque gauche controlatéral et l’aorte. La recanalisation sous-intimale du système iliaque droit a été réalisée par angioplastie et on a procédé à la pose d’une endoprothèse au niveau de la bifurcation aortique avec des extenseurs s’étendant dans l’artère iliaque externe droite proximale. L’angiographie post-déploiement a démontré une perméabilité renouvelée du système iliaque droit, avec restauration de la perfusion antérograde vers le rein transplanté.
Résultats:
Après la procédure, la pression artérielle de la patiente s’est abaissée significativement et la fonction du greffon s’est améliorée. Après 6 mois, la pression artérielle demeurait bien contrôlée (avec trois médicaments) et la fonction du greffon était stable (Cr sérique: 74 µmol/L).
Enseignements tirés:
Notre cas décrit une SARTprox et la contribution de l’hypoperfusion rénale à l’hypertension et à l’altération de la fonction du greffon, avec un potentiel de réversibilité.
Introduction
Resistant hypertension is common in patients with advanced chronic kidney disease or on chronic dialysis. Resistant hypertension is known to improve post-kidney transplantation as the patient achieves good kidney function. In patients with kidney transplants, delayed and/or chronic allograft dysfunction, use of calcineurin inhibitors, glucocorticoid therapy, weight gain, and renal artery stenosis are risk factors associated with a higher incidence of post-transplant hypertension. 1 Transplant renal artery stenosis (TRAS) 2 typically presents between 3 months and 2 years post transplant but may happen at any time. Diagnostic criteria 2 for proximal TRAS include low pulsatility index (<1.0), pulsus parvus et tardus, and max velocity within the iliac artery proximal to the graft greater than 200 cm/s with monophasic flow profile within the iliac artery distal from the transplant artery.
In this case report, we describe a patient post kidney transplant with resistant hypertension and graft dysfunction related to occlusion of the iliac segment proximal to the transplant renal artery, which was reversed following iliac recanalization with angioplasty and stenting.
Presenting Concerns
A 74-year-old Caucasian woman with past medical history significant for live donor kidney transplant 15 years earlier and baseline serum creatinine of 90 to 100 µmol/L presented to hospital with a 2-week history of decreased oral intake and worsening fatigue. The patient had a history of suspected focal and segmental glomerulosclerosis as native kidney disease, hypertension, dyslipidemia, and chronic obstructive pulmonary disease. There was no history of diabetes mellitus, coronary artery disease, or previous stroke. She had a 28 pack-year history of cigarette smoking, with no history of alcohol consumption or illicit drug use. There was no family history of kidney disease.
Her history was otherwise unremarkable, with no fever or infectious symptoms. There was no history of nonsteroidal anti-inflammatory drug use or over-the-counter medications. The patient had no complaints of intermittent claudication, abdominal pain or vomiting, and there was no history of gross hematuria or lower urinary tract symptoms.
Immunosuppressive medications consisted of extended-release tacrolimus with plasma level of 4.5 µg/L (target 4-6 µg/L) and oral prednisone 7.5 mg daily. She was not taking antimetabolites in view of a history of chronic diarrhea.
The patient had been regularly followed in the outpatient kidney transplant clinic, and blood pressure was well controlled on 2 antihypertensive medications until 2 years prior to admission. Subsequently, she was noted to have uncontrolled hypertension needing progressive escalation of antihypertensive drugs. At the time of admission, her blood pressure remained above target on maximally tolerated doses of amlodipine, metoprolol, clonidine, perindopril, spironolactone, hydralazine, terazosin, and indapamide.
Clinical Findings
Physical examination revealed an elderly frail patient with body mass index (BMI) of 19.9 kg/m2. Her blood pressure was 160/92 mm Hg right upper limb, with pulse 62 beats/min. Her cardiopulmonary and abdominal examinations were unremarkable. Specifically, peripheral arterial pulses were palpable and symmetric bilaterally with no abdominal graft tenderness, no bruit in kidney allograft area, and no erythema or rashes.
Diagnostic Focus and Assessment
Initial blood work demonstrated serum sodium 135 mmol/L, potassium 6.1 mmol/L, bicarbonate 16 mmol/L, anion gap 11 mmol/L, random blood glucose 7.8 mmol/L, urea 24.1 mmol/L, and creatinine 229 µmol/L, with estimated glomerular filtration rate (eGFR) of 17 mL/min per 1.73 m2 via CKD-EPI equation. 3 Urinalysis was negative for blood and protein, with urine albumin–creatinine ratio 4.6 mg/mmol and protein-creatinine ratio 12 mg/mmol.
Her hyperkalemia was managed by intracellular shifting with intravenous (IV) dextrose and insulin and with discontinuance of perindopril, spironolactone, and trimethoprim/sulfamethoxazole. Indapamide was discontinued because of suspected volume contraction.
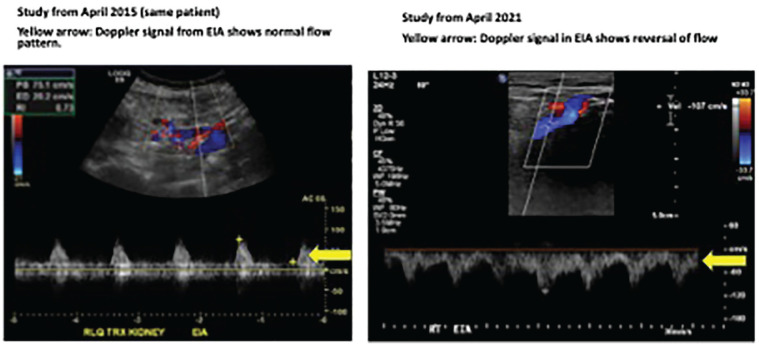
An ultrasound Doppler of the transplant kidney performed 3 months before admission showed good perfusion of the renal parenchyma. Pulse Doppler signal for arcuate vessels showed normal waveforms with resistive indices ranging between 0.5 and 0.64 (normal < 0.8). The main renal artery was patent with peak systolic velocity of 116 cm/s (normal < 200 cm/s). The external iliac artery systolic velocity was 107 cm/s (normal 100-150 cm/s). However, the external iliac artery showed reversal of flow (Figure 1).
Figure 1.
Ultrasound guided Doppler of transplant kidney: Image on left depicts normal flow in study performed 6 years prior to admission. Image on right shows reversal of flow in external iliac artery 6 years later.
A computed tomography (CT) performed prior to admission showed significant atherosclerotic burden within the aorta and bilateral iliac systems. There was moderate to severe stenosis within the distal abdominal aorta. The right common and proximal external iliac artery was occluded although the anastomosed right renal artery appeared to be patent.
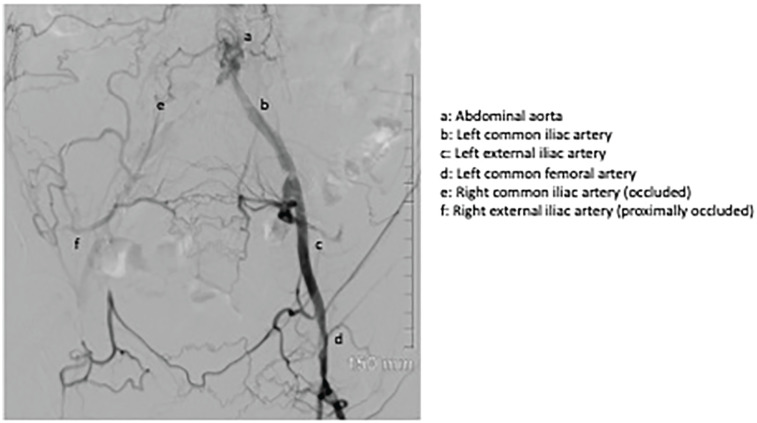
An interventional radiologist was consulted during the admission, and a conventional angiogram was performed which demonstrated multifocal moderate-severe stenosis within the distal abdominal aorta. The right common and proximal external iliac arteries were completely occluded with reconstitution of flow within the distal right external iliac artery via collaterals from the left iliac system and the aorta (Figure 2). The renal artery anastomosis appeared patent, being fed in a retrograde fashion by the reconstituted right external iliac artery. Moderate ostial stenosis of the proximal left common iliac artery and severe bilateral common femoral artery stenoses were also noted.
Figure 2.
Diagnostic angiogram: Image depicts completely occluded right common and proximal external iliac arteries with reconstitution of flow within the distal right external iliac artery via collaterals from the left iliac system and the aorta.
Therapeutic Focus and Assessment
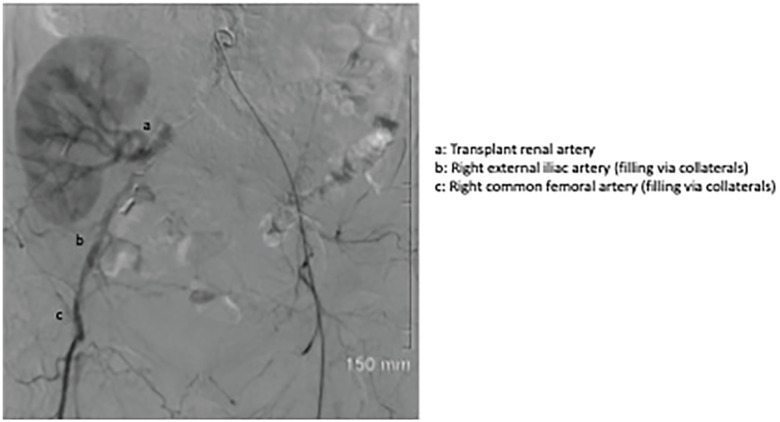
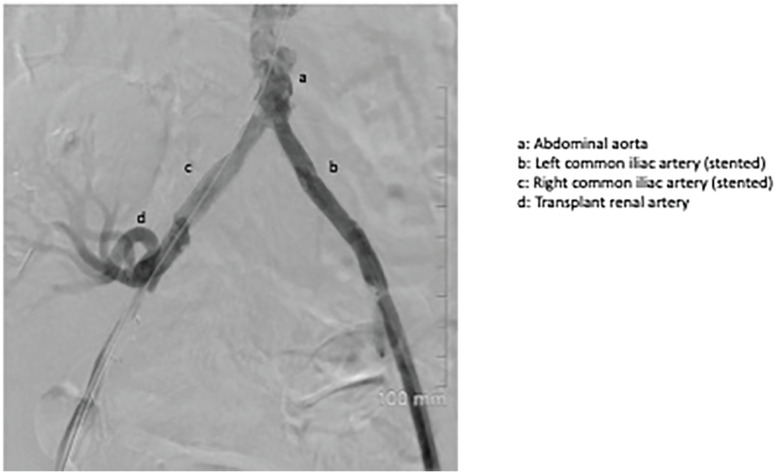
Bilateral common femoral arteries were accessed percutaneously. Initial attempts at retrograde recanalization of the right iliac system were unsuccessful. Subintimal right iliac recanalization was eventually successful via an antegrade approach from the contralateral access site. Two 7-mm balloon-mounted covered stents were deployed simultaneously in a kissing fashion at the aortic bifurcation. The right-sided stent was extended with an 8-mm uncovered stent into the right external iliac artery proximal to the transplant renal artery anastomosis. Post deployment angiogram demonstrated a satisfactory result with new antegrade flow in the right iliac system perfusing the transplant kidney (Figures 3 and 4).
Figure 3.
Pre-deployment angiogram showing occluded right iliac system with retrograde collateral flow to transplant kidney.
Figure 4.
Post-deployment angiogram: Image shows new antegrade flow in the right iliac system perfusing the transplant kidney, post stent deployment.
Follow-up and Outcomes
Post angioplasty and stenting, the patient’s blood pressure decreased significantly with improvement in graft function. The patient was subsequently followed in the transplant clinic, and 6 months after discharge from hospital, her graft function was stable (serum creatinine 76 µmol/L, with eGFR 66 mL/min per 1.73 m2 by CKD-EPI) (Figure 5). Blood pressure was well controlled (135/85 mmHg) on metoprolol 50 mg twice daily, amlodipine 5 mg daily, and spironolactone 12.5 mg daily 6 months post procedure.
Figure 5.
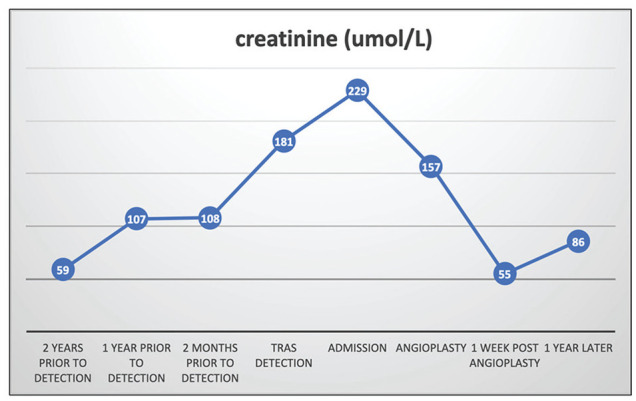
Time course of serum Cr from pre-detection of proximal TRAS to 1 year post-angioplasty.
Discussion
Hypertension is relatively common in patients with kidney transplantation, with a prevalence of 80% to 85%. Resistant hypertension (defined as blood pressure above target despite use of 3 or more antihypertensives) may affect 7% of transplant recipients. 4 TRAS is a well-known potentially reversible cause of post-transplant renovascular hypertension and renal allograft function. The incidence reported in the literature varies from 1% to 23%. However, stenosis of the iliac segment proximal to the transplant renal artery (proximal TRAS) causing dysfunction of the transplant kidney is relatively uncommon. The incidence of proximal TRAS has been reported to be 2.4%. 5
Factors associated with proximal TRAS include recipient age >65 years, diabetes mellitus, chronic smoking, atherosclerotic disease, and cytomegalovirus infection. Chronic treatment with corticosteroids and calcineurin inhibitors may accelerate atherosclerosis of native recipient vessels. 6
Clinical presentation may be similar to TRAS with resistant hypertension, flash pulmonary edema, or increase in serum creatinine with use of angiotensin-converting enzyme (ACE) inhibitors. Stenosis proximal to the graft reduces renal perfusion and represents the pathophysiology of “one-kidney one-clip” Goldblatt model of hypertension with renal hypoperfusion, which leads to activation of the renin-angiotensin-aldosterone system, resulting in retention of sodium and subsequently volume expansion, and sustained hypertension. 7
Various imaging techniques may be utilized for diagnosis of renovascular hypertension in patients post kidney transplant. Color Doppler ultrasonography is an easily accessible, relatively inexpensive noninvasive screening procedure for diagnosis of stenoses of transplant renal artery. Sensitivity may vary from 58% to 100% and specificity from 87% to 100%. 8 However, the results are dependent on operator skill and experience. Magnetic resonance (MR) angiography is a superior diagnostic tool for diagnosis of stenoses of the transplant renal artery. However, administration of gadolinium has been linked to nephrogenic systemic fibrosis especially in patients with moderate to severe renal impairment with eGFR <30 mL/min. 9 Conventional angiography remains the procedure of choice for definitive diagnosis of TRAS or proximal TRAS while simultaneously allowing treatment by percutaneous transluminal angioplasty (PTA) with or without stent placement. Limitations include invasiveness of the procedure with risk of atheroembolism and contrast nephropathy in patients with underlying moderate to severe renal impairment.
This clinical condition may be managed with angioplasty (with or without stenting) and surgery. Percutaneous balloon angioplasty may be technically successful in up to 80% of cases. 10 In our case, the interventional radiologist decided to proceed with PTA with stenting given the comparable patency rates to surgical revascularization, while avoiding the risks of surgery. 11 Given that the patient had significant atherosclerotic burden, an extensive endovascular procedure was performed to restore and maintain adequate perfusion to the transplant kidney. Surgical revascularization would be considered if it was deemed that the atherosclerotic burden was not amenable to endovascular recanalization.
Similar cases with proximal renal artery stenosis that have been successfully managed with angioplasty and stenting have been described in the literature. 12 In our patient, there was clinical suspicion of renal hypoperfusion 15 years post kidney transplant in view of uncontrolled blood pressure requiring 8 antihypertensive medications, associated with graft dysfunction. Renal Doppler ultrasound showed no evidence of TRAS, but there was reversal of flow in the right external iliac artery. Angiography showed complete occlusion of the right common and proximal external iliac arteries. Remarkably, the right transplant kidney was perfused in a retrograde fashion via the left iliac system and aorta. The patient’s blood pressure and graft function significantly improved after restoration of blood flow to her right iliac system.
Although the term “proximal TRAS” is being used to describe the feeding vessel stenosis to the renal artery, we would like to propose a new term FAS-PTRA (Feeding Artery Stenosis–Proximal to Transplant Renal Artery) to avoid incorrect messaging by the term “proximal TRAS”—as if the stenosis is in the proximal part of the renal artery of the transplant kidney.
In summary, our case highlights the importance of work-up for renovascular hypertension in a kidney transplant recipient with resistant hypertension and graft dysfunction. An extreme example of proximal TRAS with complete occlusion of the proximal iliac system, subsequent subintimal recanalization with angioplasty/stenting with marked and sustained improvement in blood pressure and graft function post procedure represent unique features of this case.
Footnotes
Ethics Approval and Consent to Participate: Not applicable.
Consent for Publication: The patient described in this educational case report provided informed consent for publication of the details of her medical history and diagnostic images.
Availability of Data and Materials: Not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Mohit Madken  https://orcid.org/0000-0002-2975-0142
https://orcid.org/0000-0002-2975-0142
References
- 1. Audard V, Matignon M, Hemery F, et al. Risk factors and long-term outcome of transplant renal artery stenosis in adult recipients after treatment by percutaneous transluminal angioplasty. Am J Transplant. 2006;6(1):95-99. [DOI] [PubMed] [Google Scholar]
- 2. Bruno S, Remuzzi G, Ruggenenti P. Transplant renal artery stenosis. J Am Soc Nephrol. 2004;15(1):134-141. [DOI] [PubMed] [Google Scholar]
- 3. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gago Fraile M, Fernandez Fresnedo G, Gómez-Alamillo C, de Castro SS, Arias M. Clinical and epidemiological characteristics of refractory hypertension in renal transplant patients. Transplant Proc. 2009;41(6):2132-2133. [DOI] [PubMed] [Google Scholar]
- 5. Voiculescu A, Hollenbeck M, Plum J, et al. Iliac artery stenosis proximal to a kidney transplant: clinical findings, duplex-sonographic criteria, treatment, and outcome. Transplantation. 2003;76(2):332-339. [DOI] [PubMed] [Google Scholar]
- 6. First MR, Neylan JF, Rocher LL, Tejani A. Hypertension after renal transplantation. J Am Soc Nephrol. 1994;4(8):S30. [DOI] [PubMed] [Google Scholar]
- 7. Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental hypertension. J Exp Med. 1934;59(3):347-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Neill WC, Baumgarten DA. Ultrasonography in renal transplantation. Am J Kidney Dis. 2002;39(4):663-678. [DOI] [PubMed] [Google Scholar]
- 9. Schieda N, Blaichman JI, Costa AF, et al. Gadolinium-based contrast agents in kidney disease: comprehensive review and clinical practice guideline issued by the Canadian association of radiologists. Can Assoc Radiol J. 2018;69(2):136-150. [DOI] [PubMed] [Google Scholar]
- 10. Sankari BR, Geisinger M, Zelch M, Brouhard B, Cunningham R, Novick AC. Post-transplant renal artery stenosis: impact of therapy on long-term kidney function and blood pressure control. J Urol. 1996;155(6):1860-1864. [DOI] [PubMed] [Google Scholar]
- 11. Aslam S, Salifu MO, Ghali H, Markell MS, Friedman EA. Common iliac artery stenosis presenting as renal allograft dysfunction in two diabetic recipients. Transplantation. 2001;71(6):814-817. [DOI] [PubMed] [Google Scholar]
- 12. Pappas P, Zavos G, Kaza S, et al. Angioplasty and stenting of arterial stenosis affecting renal transplant function. Transplant Proc. 2008;40(5):1391-1396. [DOI] [PubMed] [Google Scholar]