Abstract
The ohr (organic hydroperoxide resistance) gene product of Pseudomonas aeruginosa was essential for optimal resistance to organic hydroperoxides (OHPs) but not to hydrogen peroxide or paraquat. A Δohr mutant was hypersusceptible to OHPs in disk inhibition assays and showed enhanced killing by OHPs in liquid culture. The ohr gene product was demonstrated to contribute to the decomposition of OHPs. Transcription of ohr was induced up to 15-fold upon exposure to OHPs, and this induction was independent of OxyR. Somewhat enhanced ohr-lacZ activity was detected in mutant strains affected in ohr, ahpC, and oxyR, and this phenotype correlated with hypersusceptibility to OHPs, suggesting overlapping or compensatory functions of the ohr and ahpC gene products. A single transcriptional start site for ohr was determined, and ohr transcripts were abundant in cells treated with a sublethal dose of OHPs but not in cells treated with paraquat. An 84-bp portion upstream of the ohr mRNA start site was sufficient for ohr induction by OHPs. Thus, the ohr gene appears to encode an antioxidant enzyme that is not part of the OxyR regulon yet is specifically induced by OHPs.
Aerobic respiration in Pseudomonas aeruginosa leads to the production of toxic metabolic byproducts, including the superoxide anion (O2⋅−), hydrogen peroxide (H2O2), the hydroxyl radical (HO⋅), and organic hydroperoxides (OHPs). Sublethal levels of O2⋅− and H2O2 are detoxified by two superoxide dismutases (Fe-SOD and Mn-SOD) and two catalases (KatA and KatB), respectively (1, 3–7, 10). Although much is known of systems that combat O2⋅− and H2O2 stress in P. aeruginosa and of regulators that facilitate such control (7, 10), little is known of those involved in resistance to OHPs. Bacterial OHP resistance is mediated by alkyl hydroperoxide reductases (Ahps) that detoxify the peroxide by reducing it to an alcohol (9, 21). In Salmonella enterica serovar Typhimurium, activity is mediated by a dimer composed of AhpC and AhpF subunits (14). Recently, a novel gene from Xanthomonas campestris restored wild-type resistance to t-butyl hydroperoxide (t-BHP) in an ahpCF mutant of Escherichia coli (11). This gene was designated ohr for OHP resistance and, strikingly, showed no significant homology to any known bacterial genes. In this study, we describe the ohr locus in P. aeruginosa, specifically focusing on its genetic regulation and role in OHP resistance.
Identification and cloning of a P. aeruginosa ohr homolog.
The amino acid sequence of the X. campestris Ohr protein was used to search the complete P. aeruginosa genome (http://www.pseudomonas.com). An ohr-like gene (Pa-ohr), composed of 426 bp encoding a predicted 14.5-kDa hydrophilic protein, was identified at bases 3203997 to 3204425. Pa-Ohr was 68% identical to the Ohr protein of X. campestris (GenBank accession no. AF036166) and 62% identical to an unknown protein from Acinetobacter calcoaceticus (GenBank accession no. Y09102) (data not shown). Using the available sequence information of the unfinished microbial genome projects, additional putative homologs of Ohr with at least 50% identity to Pa-Ohr were detected in several eubacteria, including Pseudomonas putida, Legionella pneumophila, Bacillus subtilis, Deinococcus radiodurans, Caulobacter crescentus, Shewanella putrefaciens, Vibrio cholerae, Enterococcus faecalis, and Staphylococcus aureus. Each of these proteins possessed two conserved cysteine residues that are postulated to participate in the proper function of Ohr (11).
Enhanced sensitivity of an ohr mutant to OHPs.
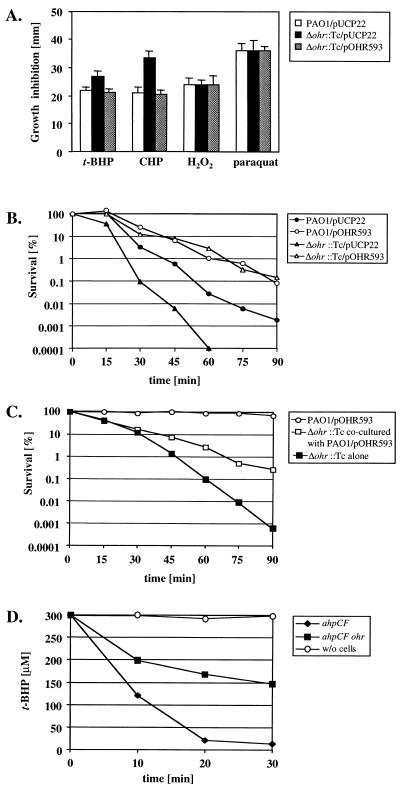
To determine the physiological role of Ohr in P. aeruginosa, a Δohr::Tc mutant was constructed from strain PAO1. Replacement of the ohr gene by a tetracycline resistance cassette was achieved through a biparental mating using E. coli SM10 harboring pEX100T-Δohr::Tc (Table 1), which allowed sucrose counterselection (16, 18). The deletion of the ohr locus ranged from 7 bp upstream of the ohr start codon to 80 bp downstream of the stop codon and was verified by Southern blotting (data not shown). Wild-type bacteria containing the control plasmid pUCP22 (23) and the Δohr::Tc mutant harboring either pUCP22 or the complementing plasmid pOHR593 (Table 1) were analyzed for their susceptibilities to oxidative stress-generating agents in disk inhibition assays as described before (13). The Δohr::Tc mutant was hypersusceptible to the organic peroxides t-BHP and cumene hydroperoxide (CHP) but not to H2O2 or paraquat, and this phenotype was fully complemented by plasmid pOHR593 containing the entire ohr gene (Fig. 1A). The susceptibilities to CHP of the Δohr::Tc mutant and wild-type bacteria were also compared in liquid medium containing 3 mM CHP. The corresponding kill curves indicated that Δohr::Tc mutant cells were hypersusceptible to CHP (Fig. 1B). Furthermore, wild-type or Δohr::Tc mutant cells containing plasmid pOHR593 were more resistant to killing by CHP than cells containing the pUCP22 vector control (Fig. 1B). The calculated rates during the exponential killing by CHP, expressed in logarithmic units per hour, were 4.8 (PAO1/pUCP22), 7.4 (PAO1 Δohr::Tc/pUCP22), and 2.5 (PAO1/pOHR593 and PAO1 Δohr::Tc/pOHR593). These data strongly suggest a protective role for the ohr gene product in the defense against oxidative stress specifically imposed by OHPs. This hypothesis is supported by the finding that an ohr overproducer strain (PAO1/pOHR593) partially protected a cocultured Δohr mutant from killing by 1 mM CHP (Fig. 1C). Furthermore, the contribution of the ohr gene product to the overall decomposition of OHP was assessed in ΔahpCF mutant background. The concentration of t-BHP diminished by >95% within 30 min when added to a culture of a ΔahpCF mutant but decreased only by 50% in a culture of Δohr ΔahpCF double mutant, indicating that the ohr gene product is involved in detoxifying OHPs (Fig. 1D). The Δohr mutation did not affect the susceptibility to H2O2 or paraquat. In fact, the total enzymatic activities of catalase and of SOD in Δohr::Tc mutant cells were identical to the levels measured in wild-type cells. Total catalase activities were determined as described elsewhere (3) and were 71 ± 12 U mg−1 (PAO1) and 72 ± 9 U mg−1 (Δohr::Tc). Total SOD activities were measured as previously described (6) and were 98 ± 3.5 U mg−1 (PAO1) and 95 ± 2.4 U mg−1 (Δohr::Tc).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Characteristicsa | Source or reference |
|---|---|---|
| E. coli | ||
| DH5α-MCR | F−lacZΔM15 recA1 hsdR17 supE44 Δ(lacZYA argF) | Bethesda Research Laboratories |
| SM10 | Kmr, mobilizer strain | 19 |
| P. aeruginosa | ||
| PAO1 | Prototrophic, wound isolate | 8 |
| PAO1 Δohr::Tc | Tcr, PAO1 harboring a 516-bp deletion of the ohr locus | This study |
| PAO1 ΔahpCF::Gm | Gmr, ΔahpCF::Gm mutant of PAO1 | 13 |
| PAO1 ΔoxyR::Gm | Gmr, ΔoxyR::Gm mutant of PAO1 | This study |
| PAO1 Δohr ΔahpCF | Gmr Tcr, Δohr ΔahpCF double mutant of PAO1 | This study |
| PAO1 ΔahpA ΔahpB | Gmr, Tcr, ΔahpA ΔahpB double mutant of PAO1 | This study |
| Plasmids | ||
| pCRII-2.1 | Apr Kmr TA cloning vector for PCR fragments | Invitrogen |
| pCRII-ohr-220 | pCRII-2.1 containing a 220-bp 5′ ohr portion generated with primers CCAACGATTTGTCTTGCGCAC and ACCTGTCTGATTTGTACGTTAAG; source of T7-expressed ohr riboprobe | This study |
| pCRII-omlA-444b | pCRII-2.1 containing a 444-bp omlA promoter fragment; source of T7-expressed omlA riboprobe | 12 |
| pEX100T | AproriT mob sacB | 18 |
| pEX100T-Δohr::Tc | pEX100T containing a Δohr::Tc cassette; used for deleting ohr from bases 203999 to 204505 | This study |
| pPZTC | Apr, pPZ30-based broad-host-range vector used for transcriptional lacZ fusions | 17 and this study |
| pOHR593 | pUCP22 containing the ohr gene and ohr promoter, from bases 203847 to 204439 | This study |
| pPZ-Pohr | pPZTC containing portions of the ohr promoter as indicated in Fig. 5; series of ohr-lacZ fusions | This study |
| pUCP22 | Apr, multicopy broad-host-range expression vector | 23 |
mob, mobilization site; oriT, origin of transfer (RK2); sacB, encodes a levansucrase; Apr, ampicillin resistance; Kmr, kanamycin resistance; Gmr, gentamicin resistance; Tcr, tetracycline resistance.
FIG. 1.
Susceptibility of a P. aeruginosa ohr mutant to oxidative stress agents. (A) Inhibition assay of PAO1/pUCP22, PAO1 Δohr::Tc/pUCP22, and PAO1 Δohr::Tc/pOHR593 growth around disks containing 10 μl of 1% (114 mM) t-BHP, 20% (1.25 M) CHP, 0.5% (145 mM) H2O2, or 0.5% (20 mM) paraquat. Normalized amounts of cells (0.2 A600 unit, roughly 6 × 108 cells) grown in M9–0.4% glucose medium were mixed with 3 ml of M9 top agarose and poured onto M9 agar. The zones of growth inhibition were measured after incubation for 16 h at 37°C. The error bars represent the standard deviations of quadruplicate assays. (B) Killing of P. aeruginosa by CHP. PAO1/pUCP22, PAO1/pOHR593, PAO1 Δohr::Tc/pUCP22, and PAO1 Δohr::Tc/pOHR593 were grown at 37°C to mid-exponential phase (A600 = 0.5) and then exposed to a sublethal concentration (300 μM) of CHP for 15 min, after which CHP was added to a final concentration of 3 mM. The number of viable cells was determined in serially diluted samples taken every 15 min postexposure. The experiment was performed separately four times, and one representative set of data is shown. (C) Killing of the ohr mutant in coculture with an ohr overproducer strain. Mid-log-phase PAO1/pOHR593 and Δohr::Tc cells were washed, and 0.2 A600 unit of each ml−1 (roughly 6 × 108 cells ml−1) were cocultured in fresh antibiotic-free M9 medium containing 1 mM CHP. Viable cells of the two strains in the mixed culture were determined by plating on agar containing carbenicillin (PAO1/pOHR593) or tetracycline (PAO1 Δohr::Tc). Also shown is the kill curve for Δohr::Tc cells in a monoculture at a comparable cell density (A600 = 0.4). (D) Decomposition of t-BHP. An ohr mutant in an ahpCF background and its parental strain were grown to stationary phase in M9 medium and then exposed to 300 μM t-BHP. Hydroperoxide concentrations were determined at 10-min intervals by mixing 100 μl of supernatant with 900 μl of a freshly prepared solution of 25 mM sulfuric acid, 100 μM xylenol orange, and 100 μM ferrous sulfate in methanol-water (9:1), allowing the color to develop for 15 min, and measuring the A560. A standard curve was previously established using known concentrations of t-BHP (data not shown).
Inducible expression of ohr.
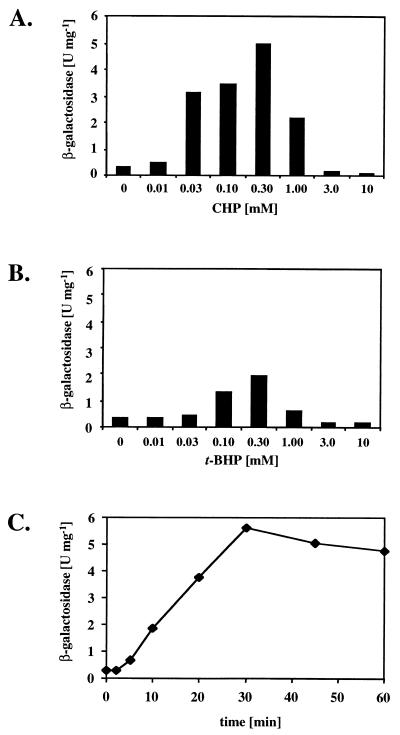
Expression of ohr was studied by using plasmid pPZ-Pohr-99 containing a transcriptional fusion of the ohr promoter to the promoterless lacZ reporter gene. β-Galactosidase activity was detectable at low levels (<0.5 U mg−1) in cell extracts from untreated PAO1/pPZ-Pohr-99 cultures. This basal ohr-lacZ expression was only marginally affected by the carbon source added to M9-based medium. The presence of 1% citrate resulted in a twofold increase, while 1% corn oil resulted in a twofold decrease compared to reporter activity measured in M9-1% glucose cultures (data not shown). Reporter activity increased up to 15-fold upon treatment of bacteria with 0.03 to 0.30 mM CHP (Fig. 2A). Up to fivefold greater β-galactosidase activity was detected in cells exposed to 0.10 or 0.30 mM t-BHP (Fig. 2B). Due to cell death, CHP or t-BHP concentrations equal to or higher than 1 mM did not result in such a response (data not shown). In contrast, the addition of H2O2 (1 mM) and the O2⋅− generating compound paraquat (0.35 mM), both of which lead to induction of other antioxidant genes in P. aeruginosa, such as katB, ahpB, and ahpCF, at these sublethal concentrations (13), or the presence of a high iron concentration [100 μM Fe(III)], did not result in higher ohr-lacZ expression (data not shown). Thus, ohr expression appeared to respond specifically to OHPs. The reason for the observed difference in maximal ohr induction upon exposure to either CHP or t-BHP is unclear. It may be a consequence of different uptake efficiencies or reactivities of these two compounds. In fact, CHP is poorly water soluble and therefore may readily bind to the cell surface, while the more water-soluble t-BHP remains dissolved in the medium. The expression of ohr in response to OHP exposure was further studied by analyzing extracts of PAO1/pPZ-Pohr-99 at various time points after the addition of 100 μM CHP to mid-exponential-phase cultures (A600 = 0.5). A roughly twofold increase in β-galactosidase activity was observed after 5 min. Then the response was linear until 30 min postexposure (Fig. 2C), with a calculated β-galactosidase production rate of 0.20 U mg−1 min−1. After 30 min, β-galactosidase activity reached a plateau, presumably due to a new steady-state level of ohr expression or due to complete detoxification of CHP, as shown above.
FIG. 2.
Response of ohr-lacZ expression to OHPs. (A) Induction by CHP. P. aeruginosa PAO1 harboring pPZ-Pohr-99 was grown aerobically in 50 ml of M9–0.4% glucose medium at 37°C and split into 2.5-ml subcultures at mid-exponential phase (A600 = 0.5), to which increasing concentrations of CHP ranging from 0 to 10 mM were added. The cells were shaken for 1 h, and the β-galactosidase reporter activities were determined as previously described (13). (B) Induction by t-BHP. Cells were grown as for panel A and exposed to increasing concentrations of t-BHP prior to analysis of β-galactosidase reporter activity. (C) Time response curve of ohr-lacZ expression. Mid-exponential-phase cultures (A600 = 0.5) of PAO1/pPZ-Pohr-99 in M9 medium at 37°C were exposed to 100 μM CHP, and β-galactosidase activities were determined in samples taken at the indicated time points. The values are the means of triplicate experiments.
Role of Ohr in compensating for an absence of Ahps.
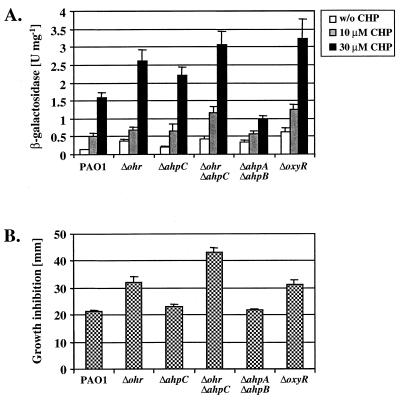
Since ohr expression was inducible by OHPs, Ohr may function similarly to Ahps. Therefore, expression of ohr was monitored using pPZ-Pohr-99 in various mutant strains affected in single or multiple ahp genes that were described earlier (13). Basal expression of ohr was threefold higher in the Δohr::Tc mutant than in wild-type cells, and induction by low concentrations of CHP (10 and 30 μM) also resulted in higher activities (Fig. 3A). In the ΔahpCF::Gm mutant, ohr expression was somewhat higher than in wild-type cells, and in the Δohr::Tc ΔahpCF::Gm double mutant, ohr was expressed at higher levels than in the single mutants, suggesting an additive effect (Fig. 3A). Similarly, ΔoxyR::Gm mutant cells, which are affected in the production of at least AhpB, AhpC-AhpF, and KatB-AnkB (13), expressed ohr at higher levels than wild-type cells. In contrast, a ΔahpA::Tc ΔahpB::Gm double mutant had a somewhat higher basal expression of ohr but did not respond to CHP to the extent observed in the other mutants. The susceptibilities of these mutant strains to CHP were determined using a disk inhibition assay as described above (Fig. 3B). Strikingly, the susceptibilities correlated well with the observed ohr-lacZ activities, with the Δohr::Tc ΔahpCF::Gm double mutant being the most susceptible. Taken together, the data suggest overlapping functions of Ohr and AhpC-AhpF. However, the AhpA, AhpB, AhpC-AhpF, and Ohr proteins of P. aeruginosa have not yet been characterized biochemically, especially regarding their potential for use of multiple substrates. Some of the observed ohr expression levels and the CHP susceptibilities of the mutant strains may be caused by compensatory induction of alternate antioxidant genes, as has been described for other bacteria (2, 15) and for P. aeruginosa (13). Specifically, mutants affected in either ohr, ahpC, or both exhibited higher ohr expression and induction levels, presumably due to higher oxidative stress in these backgrounds, while the ahpA ahpB double mutant showed lower ohr induction, since it may respond to this defect with a compensatory upregulation of ahpC-ahpF.
FIG. 3.
Role of ohr in mutant strains affected in Ahp reductase genes. (A) Expression of ohr-lacZ in various mutant strains. Cultures of PAO1, single mutants (Δohr::Tc, ΔahpCF::Gm, and ΔoxyR::Gm), and double mutants (Δohr::Tc ΔahpCF::Gm and ΔahpA::Tc ΔahpB::Gm) harboring pPZ-Pohr-99 were grown in 15 ml of M9 medium at 37°C and split into 2.5-ml subcultures at mid-exponential phase (A600 = 0.5), to which various concentrations (0, 10, and 30 μM) of CHP were added. The cells were harvested 30 min postexposure and β-galactosidase activities were determined. The error bars show the standard deviations of triplicate experiments. (B) Growth inhibition of mutant strains by CHP. The PAO1 wild type and the indicated single and double mutants were assayed for their susceptibilities to 20% (1.25 M) CHP in a disk inhibition assay as described for Fig. 1.
A genetic analysis of the ohr promoter.
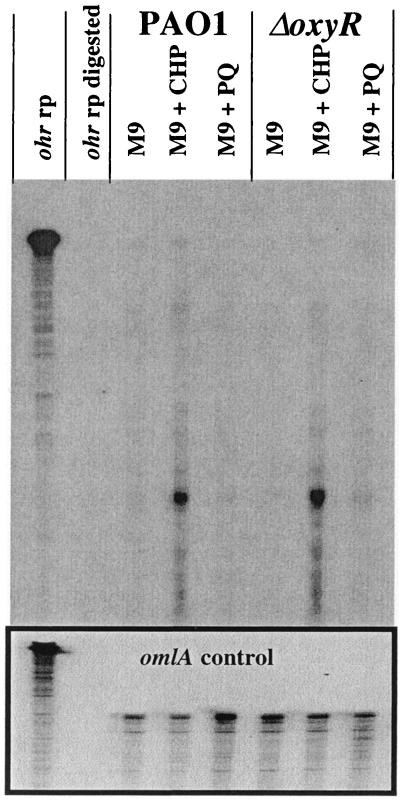
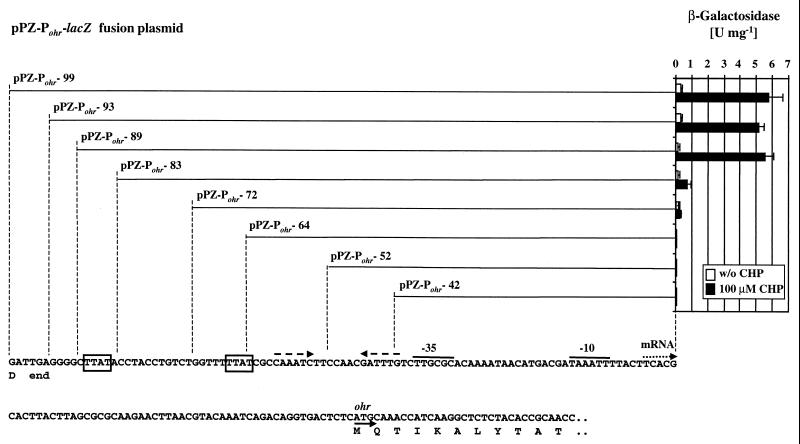
The transcriptional start site of ohr was mapped to a single T at bp −56 relative to the ATG translational start codon. Specific ohr transcripts were detected using an ohr riboprobe covering the 5′ end of the ohr transcript in RNase protection assays as described elsewhere (13). Total RNA of untreated wild-type cells contained barely detectable levels of ohr mRNA, while a dramatic increase in ohr mRNA was detected in cells that had been exposed to 100 μM CHP for 30 min (Fig. 4). The presence of 100 μM paraquat did not affect the level of ohr mRNA. An identical pattern of ohr expression was observed in ΔoxyR mutant cells. These findings indicate that ohr induction occurred at the transcriptional level and was independent of OxyR, which is in agreement with the results obtained using ohr-lacZ reporter fusions. To determine whether other known regulators of the P. aeruginosa oxidative stress machinery were involved in ohr regulation, ohr-lacZ expression was also measured in mutant strains affected in soxR, rpoS, and rhlR lasR. In these cases, induction by 100 or 300 μM CHP was not affected (data not shown). Therefore, ohr gene expression appeared to be upregulated by a novel mechanism involving a yet-to-be-identified positive or negative factor responding specifically to OHPs. As a first step toward the identification of such a regulatory factor, the ohr promoter was analyzed using a series of pPZ-Pohr-lacZ fusions containing systematically shorter upstream sequences (Fig. 5). A sequence of at least 84 bp upstream of the mapped transcriptional start site was required for full induction of the ohr promoter (pPZ-Pohr-89). A 6-bp-shorter promoter fragment (pPZ-Pohr-83) resulted in normal basal expression but responded only marginally to the presence of CHP. Constructs containing only 59 bp or less of upstream sequence did not result in any detectable ohr-lacZ expression, although they contained the −35 and −10 promoter elements (Fig. 5). These results demonstrate that the 84-bp sequence located upstream of the mRNA start site was essential for the stress response. This sequence contained inverted repeats (CAAATC-N7-GATTTG) with the potential to form a stem-loop structure (ΔG0 = −3.9 kcal mol−1) and contained two short direct repeats (TTAT) spaced 21 bp apart. Deletion of the upstream TTAT element resulted in the loss of inducibility (Fig. 5). The spacing of the two TTAT motifs suggested a location on the same face of the DNA, spaced two turns apart, and may represent the target site of a positive activator of ohr. Similar cis-acting regulatory elements have been demonstrated to play a crucial role in antioxidant gene activation by the well-characterized OxyR protein (13, 20, 22). We therefore postulate the existence of a trans-acting regulatory protein involved in ohr regulation, and future approaches to identify such a regulator could include the screening of a mutant library for the lack of ohr-lacZ induction or affinity purification of a putative regulator on its immobilized target sequence. Such studies are currently under way.
FIG. 4.
RNase protection assay of ohr mRNA. A riboprobe specific for the 5′ portion of ohr (ohr rp) was generated by runoff in vitro transcription from the T7 promoter on pCRII-ohr-220 and used to detect ohr transcripts. RNA was isolated from wild-type cells and from ΔoxyR::Gm mutant cells grown aerobically to mid-exponential phase in M9 medium at 37°C. Parallel cultures were treated with 100 μM CHP or 100 μM paraquat (PQ) as indicated. Normalized samples (20 μg) of total RNA were used. Aliquots of the same RNA batches were subjected to control hybridizations to a riboprobe specific for the constitutively expressed omlA gene (12), as shown in the boxed part at the bottom.
FIG. 5.
Analysis of the ohr promoter. A series of pPZ-Pohr-lacZ fusions containing the indicated portions of the ohr promoter and upstream sequence was constructed. Their reporter activities were measured in triplicate cultures of wild-type cells in the absence or presence of 100 μM CHP. Beside the indicated −35 and −10 promoter elements and the mapped mRNA start site, elements potentially involved in ohr regulation are shown, including two TTAT motifs (boxed) and a stem-loop structure (dashed arrows). Also shown are the translational start of ohr and the end of the coding sequence of the unrelated upstream gene.
Acknowledgments
This work was supported by a grant from the National Institute of Allergy and Infectious Diseases (AI-15490) to M.L.V. and was supported in part by a Public Health Service grant (AI-40541) to D.J.H. and a Cystic Fibrosis Grant (HASSET97PO) to D.J.H.
REFERENCES
- 1.Brown S M, Howell M L, Vasil M L, Anderson A J, Hassett D J. Cloning and characterization of the katB gene of Pseudomonas aeruginosa encoding a hydrogen peroxide-inducible catalase: purification of KatB, cellular localization, and demonstration that it is essential for optimal resistance to hydrogen peroxide. J Bacteriol. 1995;177:6536–6544. doi: 10.1128/jb.177.22.6536-6544.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bsat N, Chen L, Helmann J D. Mutation of the Bacillus subtilis alkyl hydroperoxide reductase (ahpCF) operon reveals compensatory interactions among hydrogen peroxide stress genes. J Bacteriol. 1996;178:6579–6586. doi: 10.1128/jb.178.22.6579-6586.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hassett D J, Alsabbagh E, Parvatiyar K, Howell M L, Wilmott R W, Ochsner U A. A protease-resistant catalase, KatA, released upon cell lysis during stationary phase is essential for aerobic survival of a Pseudomonas aeruginosa oxyR mutant at low cell densities. J Bacteriol. 2000;182:4557–4563. doi: 10.1128/jb.182.16.4557-4563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hassett D J, Howell M L, Ochsner U A, Vasil M L, Johnson Z, Dean G E. An operon containing fumC and sodA encoding fumarase C and manganese superoxide dismutase is controlled by the ferric uptake regulator in Pseudomonas aeruginosa: fur mutants produce elevated alginate levels. J Bacteriol. 1997;179:1452–1459. doi: 10.1128/jb.179.5.1452-1459.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassett D J, Ma J F, Elkins J G, McDermott T R, Ochsner U A, West S E, Huang C T, Fredericks J, Burnett S, Stewart P S, McFeters G, Passador L, Iglewski B H. Quorum sensing in Pseudomonas aeruginosa controls expression of catalase and superoxide dismutase genes and mediates biofilm susceptibility to hydrogen peroxide. Mol Microbiol. 1999;34:1082–1093. doi: 10.1046/j.1365-2958.1999.01672.x. [DOI] [PubMed] [Google Scholar]
- 6.Hassett D J, Schweizer H P, Ohman D E. Pseudomonas aeruginosa sodA and sodB mutants defective in manganese- and iron-cofactored superoxide dismutase activity demonstrate the importance of the iron-cofactored form in aerobic metabolism. J Bacteriol. 1995;177:6330–6337. doi: 10.1128/jb.177.22.6330-6337.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassett D J, Woodruff W A, Wozniak D J, Vasil M L, Cohen M S, Ohman D E. Cloning and characterization of the Pseudomonas aeruginosa sodA and sodB genes encoding manganese- and iron-cofactored superoxide dismutase: demonstration of increased manganese superoxide dismutase activity in alginate-producing bacteria. J Bacteriol. 1993;175:7658–7665. doi: 10.1128/jb.175.23.7658-7665.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holloway B W. Genetics of Pseudomonas. Bacteriol Rev. 1969;33:419–443. doi: 10.1128/br.33.3.419-443.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobson F S, Morgan R W, Christman M F, Ames B N. An alkyl hydroperoxide reductase from Salmonella typhimurium involved in the defense of DNA against oxidative damage. Purification and properties. J Biol Chem. 1989;264:1488–1496. [PubMed] [Google Scholar]
- 10.Ma J-F, Ochsner U A, Klotz M G, Nanayakkara V K, Howell M L, Johnson Z, Posey J E, Vasil M L, Monaco J J, Hassett D J. Bacterioferritin A modulates catalase A (KatA) activity and resistance to hydrogen peroxide in Pseudomonas aeruginosa. J Bacteriol. 1999;181:3730–3742. doi: 10.1128/jb.181.12.3730-3742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mongkolsuk S, Praituan W, Loprasert S, Fuangthong M, Chamnongpol S. Identification and characterization of a new organic hydroperoxide resistance (ohr) gene with a novel pattern of oxidative stress regulation from Xanthomonas campestris pv. phaseoli. J Bacteriol. 1998;180:2636–2643. doi: 10.1128/jb.180.10.2636-2643.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ochsner U A, Vasil A I, Johnson Z, Vasil M L. Pseudomonas aeruginosa fur overlaps with a gene encoding a novel outer membrane lipoprotein, OmlA. J Bacteriol. 1999;181:1099–1109. doi: 10.1128/jb.181.4.1099-1109.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ochsner U A, Vasil M L, Alsabbagh E, Parvatiyar K, Hassett D J. Role of the Pseudomonas aeruginosa oxyR-recG operon in oxidative stress defense and DNA repair: OxyR-dependent regulation of katB-ankB, ahpB, and ahpC-ahpF. J Bacteriol. 2000;182:4533–4544. doi: 10.1128/jb.182.16.4533-4544.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poole L B, Ellis H R. Flavin-dependent alkyl hydroperoxide reductase from Salmonella typhimurium. 1. Purification and enzymatic activities of overexpressed AhpF and AhpC proteins. Biochemistry. 1996;35:56–64. doi: 10.1021/bi951887s. [DOI] [PubMed] [Google Scholar]
- 15.Rosner J L, Storz G. Effects of peroxides on susceptibilities of Escherichia coli and Mycobacterium smegmatis to isoniazid. Antimicrob Agents Chemother. 1994;38:1829–1833. doi: 10.1128/aac.38.8.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schweizer H P. Allelic exchange in Pseudomonas aeruginosa using novel ColE1-type vectors and a family of cassettes containing a portable oriT and the counter-selectable Bacillus subtilis sacB marker. Mol Microbiol. 1992;6:1195–1204. doi: 10.1111/j.1365-2958.1992.tb01558.x. [DOI] [PubMed] [Google Scholar]
- 17.Schweizer H P. Improved broad-host-range lac-based plasmid vectors for the isolation and characterization of protein fusions in Pseudomonas aeruginosa. Gene. 1991;103:87–92. doi: 10.1016/0378-1119(91)90396-s. [DOI] [PubMed] [Google Scholar]
- 18.Schweizer H P, Hoang T T. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene. 1995;158:15–22. doi: 10.1016/0378-1119(95)00055-b. [DOI] [PubMed] [Google Scholar]
- 19.Simon R, Priefer U, Puehller A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 20.Storz G, Imlay J A. Oxidative stress. Curr Opin Microbiol. 1999;2:188–194. doi: 10.1016/s1369-5274(99)80033-2. [DOI] [PubMed] [Google Scholar]
- 21.Storz G, Jacobson F S, Tartaglia L A, Morgan R W, Silveira L A, Ames B N. An alkyl hydroperoxide reductase induced by oxidative stress in Salmonella typhimurium and Escherichia coli: genetic characterization and cloning of ahp. J Bacteriol. 1989;171:2049–2055. doi: 10.1128/jb.171.4.2049-2055.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toledano M B, Kullik I, Trinh F, Baird P T, Schneider T D, Storz G. Redox-dependent shift of OxyR-DNA contacts along an extended DNA-binding site: a mechanism for differential promoter selection. Cell. 1994;78:897–909. doi: 10.1016/s0092-8674(94)90702-1. [DOI] [PubMed] [Google Scholar]
- 23.West S E, Schweizer H P, Dall C, Sample A K, Runyen-Janecky L J. Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene. 1994;148:81–86. doi: 10.1016/0378-1119(94)90237-2. [DOI] [PubMed] [Google Scholar]