Abstract
This study tested for horizontal transfer of plasmids among Buchnera aphidicola strains associated with ecologically and phylogenetically related aphid hosts (Uroleucon species). Phylogenetic congruence of Buchnera plasmid (trpEG and leuABC) and chromosomal (dnaN and trpB) genes supports strictly vertical long-term transmission of plasmids, which persist due to their contributions to host nutrition rather than capacity for infectious transfer. Synonymous divergences indicate elevated mutation on plasmids relative to chromosomal genes.
Bacterial genomes are characterized by remarkable plasticity that allows rapid genetic adaptations to environmental changes (reviewed in references 3, 33, 46). Plasmids, extrachromosomal DNA molecules that replicate autonomously, contribute to this plasticity by mediating lateral gene transfer among bacterial species and genera (15, 17, 21, 40, 57, 59, 65) and even between kingdoms (19, 24). In addition to their role in lateral gene transfer, plasmids also function in gene amplification and overexpression (46, 47). Just as chromosomal duplications are a common mechanism for increasing gene dosage in response to fluctuations in the environment (47, 54), amplification of loci on plasmids may be adaptive when selection favors increased gene dosages (12, 20).
In Buchnera aphidicola, the primary endosymbiont of aphids, genes for the biosynthesis of tryptophan (trpEG) and leucine (leuABCD) often occur on multicopy plasmids (pTrpEG and pLeu, respectively) (5, 6, 10, 31, 48, 49, 52, 63, 64). Comparative sequence analysis indicates that the ancestral location for both trpEG and leuABCD genes was the Buchnera chromosome, not an exogenous plasmid (7, 49, 63). This movement of chromosomal loci onto plasmids is considered a host-beneficial adaptation of Buchnera to overproduce these essential amino acids that are lacking in the hosts' diet of plant sap.
The role of horizontal transfer in the evolution of Buchnera biosynthetic plasmids remains unclear. In contrast to facultative symbionts such as Rhizobium and Vibrio, lateral gene transfer in Buchnera may be highly constrained since this obligate symbiont spends its entire life cycle within specialized host cells (bacteriocytes) (11, 43). In accordance with this hypothesis, several previous studies show phylogenetic congruence among chromosomal (trpB and 16S rRNA) and plasmid (trpEG and leuABCD) genes of Buchnera associated with the family Aphididae and suggest a lack of plasmid transfer in this symbiont group (5, 6, 10, 22, 48, 49, 51, 63, 64). However, recent work suggests horizontal transfer of the plasmid-encoded repA1 gene in Buchnera of Pemphigus spyrothecae (62).
Most previous studies were based on sampling Buchnera associated with different aphid genera and cannot address the issue of plasmid transfer among closely related strains, which may occur via biological vectors or acquisition of DNA from the environment (60). In order to maximize the chance of detecting gene transfer among related Buchnera lineages, we sampled Buchnera of Uroleucon, a recent radiation of aphids that specialize on Asteraceae and often share host plants, habitats, secondary endosymbionts, and parasitoids (42, 50). We compare phylogenies of chromosomal genes (dnaN and trpB) and plasmid-encoded genes (trpEG and leuABC) to test for plasmid transfer in this symbiont group.
Phylogeny reconstruction.
Collection data, aphid DNA extractions, and standard PCR conditions were described previously (42). The PCR was used to amplify three gene regions of Buchnera: dnaN (1,107 bp), leuABC (3,919 bp), and trpEG (1,767 bp) (primer sequences available upon request). DNA sequences were obtained as described previously (42) directly from PCR products or TA clones of PCR fragments. GenBank numbers for sequences obtained here and for previously published sequences are given in Table 1. Translated DNA sequences were aligned by using Megalign (DNAstar).
TABLE 1.
Aphid hosts of Buchnera lineages included in this study and GenBank accession numbers for gene regions sequences here (bold) and previously
| Aphid host | Abbreviation | GenBank accession no.
|
|||
|---|---|---|---|---|---|
| dnaN | trpB | leuABC | trpEG | ||
| Uroleucon rudbeckiaea | Urud | AF197882 | AF058439 | AF200469 | AF197464 |
| Uroleucon astronomus | Uast | AF197883 | AF058433 | AF197461 | |
| Uroleucon ambrosiaea | Uamb | AF197884 | AF058431 | AF197454 | AF197460 |
| Uroleucon aeneuma | Uaen | AF197885 | AF058432 | AF197455 | AF197459 |
| Uroleucon jaceae | Ujac | AF197886 | AF058440 | AF197463 | |
| Uroleucon solidaginis | Usol | AF197887 | AF058435 | AF197449 | |
| Uroleucon sonchia | Uson | AF197888 | AD001676 | AF197448 | AD001677 |
| Uroleucon obscurum | Uobs | AF197889 | AF058437 | AF197450 | |
| Uroleucon helianthicolaa | Uhel | AF197890 | AF058434 | AF197451 | AF197462 |
| Uroleucon ruralea | Urur | AF197891 | L81149 | AF200468, AF201382-3 | L81122 |
| Uroleucon caligatuma | Ucal | AF197892 | L81150 | AF197453 | L81124 |
| Uroleucon erigeronensea | Ue | AF197893 | L81151 | AF197452 | L81123 |
| Miscrosiphoniella ludovicianaea | Ml | AF197894 | AF058428 | AF197456 | AF197458 |
| Acryrthosiphon pisuma | Ap | AF197895 | L46355 | AF197457 | L43555 |
| Diuraphis noxia | Dn | AF041837 | L46769 | ||
| Schizaphis graminuma | Sg | AF008210 | Z19055 | AF041836 | Z21938 |
| Rhopalosiphum padia | Rp | AF197896 | L46358 | X71612 | L43551 |
Subset of taxa used for ML phylogeny estimations. Tests of phylogenetic congruence were performed with this subset of taxa, after pruning the outgroup taxa S. graminum and R. padi.
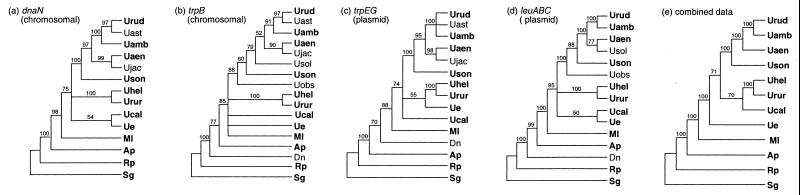
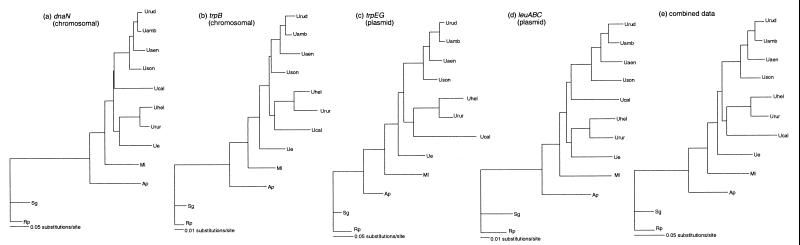
Genealogies of each of the four gene regions and for combined data were estimated by using maximum parsimony (MP) and maximum likelihood (ML) (Paup* 4 [56]). MP trees were estimated by heuristic searching, and confidence in nodes was assessed by bootstrapping (100 replications). MP trees estimated for the subset of taxa available for each locus (Fig. 1, taxa in bold) agree with relationships shown in the larger bootstrap trees for all available taxa (Fig. 1). These MP trees were generally very similar across genes. ML phylogenies were estimated for the subset of Buchnera lineages sequenced for each gene region after excluding third codon positions. ML parameters and topologies were alternatively estimated until there was no improvement in the likelihood score, according to the successive approximation method suggested by Swofford (55). The proportion of invariant sites and base frequencies were set to empirical levels, and substitution rates were allowed to vary among sites according to a gamma distribution (four site categories) under the Hasegawa-Kishino-Yano model of substitution. Phylogenies and the ML parameters alpha (the gamma shape parameter) and transition/transversion ratio were estimated separately for each region. Only two ML topologies were found: (i) that of leuABC and dnaN and (ii) that of trpB, trpEG, and combined data (Fig. 2).
FIG. 1.
Maximum parsimony-based phylogeny of four Buchnera gene regions: the chromosomal genes dnaN (a) and trpB (b), the plasmid gene regions trpEG (c) and leuABC (d), and combined data for the subset of taxa sequenced at each locus (e). Bootstrap values (100 replications) are given at nodes. Taxa common to each data set are given in bold. See Table 1 for abbreviations.
FIG. 2.
Maximum likelihood-based phylogenies of first and second codon positions of four Buchnera gene regions and combined data. Parameters were estimated separately for each locus (see the text). Branch lengths are proportional. Likelihood scores and ML parameters were estimated as follows: (a) dnaN, −Ln L = 3,731.411, ti/tv = 1.612 (k = 3.986), a = 0.492; (b) trpB, −Ln L = 1,492.105, ti/tv = 1.242 (k = 2.42), a = 0.177; (c) trpEG, −Ln L = 5,881.237, ti/tv = 1.09 (k = 2.283), a = 2.010; (d) leuABC, −Ln L = 9,962.228, ti/tv = 1.53 (k = 3.10), a = 0.198; (e) combined data, −Ln L = 21,296.363, ti/tv = 1.363 (k = 2.836), a = 0.239. See Table 1 for abbreviations.
MP and ML estimates give similar phylogenies for all gene regions. Notably, the MP and ML trees for combined data are identical. Slight discrepancies between MP and ML estimations result primarily from the placement of two taxa, Buchnera of Uroleucon erigeronense and Uroleucon caligatum. These discrepancies are only weakly supported, as seen in the low MP bootstrap values (Fig. 1) and short internal branches on ML trees (Fig. 2). The relationships at each gene generally agree with relationships among the Uroleucon hosts (14).
Phylogenetic congruence among loci.
Outgroup species (Rhopalosiphum padi and Schizaphis graminum) were excluded from tests of phylogenetic congruence to avoid biasing the outcome towards congruence. First, we tested the null hypothesis, using TREEMAP (44), that MP trees for each data set are no more congruent than expected by chance (i.e., randomly related). All pairs of MP trees were more similar than expected by chance (P < 0.001 for each comparison). However, disproving the null hypothesis of random relatedness provides only weak evidence for congruence, since gene transfer may not erase all traces of historical associations (see reference 14). We therefore tested the null hypothesis that different gene regions support the same topology. The Kishino-Hasegawa test evaluates whether a data set has a significantly better likelihood score across its own ML tree than across the alternative ML topology (28) (using Paup*4). Similarity in likelihood scores for both ML trees indicates that discrepancies between the two Buchnera ML phylogenies are not statistically significant for any gene region (Table 2).
TABLE 2.
Results of the Kishino-Hasegawa (KH) test, comparing the likelihood score of four datasets and combined data across the two alternative ML phylogenies
| Data seta | ML treeb | −Ln L | −Ln L difference | SD (difference) | Pc |
|---|---|---|---|---|---|
| Combined | A | 18,319.854 | (best) | ||
| B | 18,322.090 | 2.236 | 10.324 | 0.829 | |
| dnaA | A | 3,096.556 | 1.696 | 6.463 | 0.793 |
| B | 3,094.859 | (best) | |||
| trpB | A | 1,351.312 | (best) | ||
| B | 1,355.496 | 4.185 | 3.256 | 0.199 | |
| trpEG | A | 5,164.011 | (best) | ||
| B | 5,166.557 | 2.546 | 5.867 | 0.664 | |
| leuABC | A | 8,554.481 | 6.104 | 6.316 | 0.334 |
| B | 8,548.376 | (best) |
Data set mapped across ML trees.
ML tree across which data sets were mapped (A, ML tree for trpB, trpEG, and combined data; B, ML tree for dnaN and leuABC).
Probability that likelihood scores of a given data set are different across alternative ML trees.
This phylogenetic congruence of plasmid and chromosomal genes strongly supports a lack of plasmid transfer among Buchnera strains associated with aphid hosts that share habitats, host plants, and parasitoids and secondary endosymbionts (50). A recent study found congruence of gene genealogies in Buchnera of Uroleucon ambrosiae, suggesting strictly vertical transfer even within the same host species (22). These results support previous conclusions of congruence among Buchnera genealogies and contribute to the larger picture of vertical plasmid transmission across millions of years (5, 6, 10, 48, 49, 52, 63, 64). Our data suggest that the single proposed instance of plasmid transfer in Buchnera may represent a very rare event that occurred early in the evolution of the Pemphigidae (62). This plasmid stability in Buchnera contrasts with genome fluidity in most bacterial species, where plasmids mobilize ecologically important features such as pathogenic and symbiotic capacities (69) and antibiotic resistance (4, 37) and contribute to the mosaic-like genome structure of some bacterial genomes (26, 32, 34, 41). For example, in Escherichia coli, the close free-living relative of Buchnera, incongruence among genealogies of chromosomal and plasmid-encoded genes indicates several recombination and horizontal transfer events (35).
Selection for plasmid maintenance.
The maintenance of bacterial plasmids has been attributed to a combination of infectious transfer and selection for plasmid-encoded traits (38). Since the two biosynthetic plasmids of Buchnera experience little if any lateral transfer, they must be maintained solely by selection for plasmid-encoded traits. In endosymbionts, selection may occur within hosts (resulting from differential replication of different endosymbiont genotypes within an individual host) and between hosts (resulting from differential reproductive rates of hosts that contain different symbiont genotypes) (1, 45). At the level of within-host selection, plasmid amplification of biosynthetic genes in Buchnera is probably neutral or deleterious, since the overproduction of tryptophan and leucine and the replication of plasmids may be costly to individual Buchnera cells (36, 53). Any selection favoring plasmid maintenance in Buchnera must occur between aphids, which require symbiont biosynthetic functions for adequate nutrition. This impact of host-level selection may explain the prevalence of these two plasmids in Buchnera of the Aphididae, in which relatively rapid growth and high fecundity may increase physiological demands for amino acids (7). Host-level selection may also explain the parallel changes in level of amplification of trpEG and leuABCD in particular aphid species (58).
With the above reasoning, selection on bacterial cells will tend to favor plasmid loss while selection on aphid hosts will favor plasmid maintenance. Such conflict may be partially resolved by an attenuation of negative effects of plasmids on bacterial fitness (e.g., see reference 9). Based on the divergence times of aphids with plasmid-bearing Buchnera (about 50 to 70 million years for the family Aphididae) and the estimated generation time of Buchnera (about 50 doublings per year [13]), pTrpEG and pLeu have been vertically transmitted with the Buchnera chromosome for approximately 2.5 to 3.5 billion bacterial generations, over which time selection may have minimized deleterious effects of plasmids on bacterial fitness. One possible mechanism by which individual Buchnera cells may benefit from (or be “addicted” to) pTrpEG is through selection to preserve dnaA boxes borne by this plasmid (30). These sites may titrate DnaA, a protein that is also involved in initiation of chromosomal replication.
Elevated divergence at plasmid-borne genes.
Some plasmids may experience elevated rates of sequence evolution compared to chromosomal genes due to higher rates of adaptive fixation or higher mutation rates (18, 61). The latter might arise from more frequent recombination (27), greater densities of transposons (2), dependence on different, more error-prone polymerases (25, 29), or higher rates of transcription (16). The vertical transmission of Buchnera plasmids provides a rare opportunity to contrast rates of sequence divergence among completely linked, autonomously replicating DNA molecules. In many bacterial species, selection for codon usage at particular loci (adaptive codon bias) may lead to differences in rates of synonymous divergence among genes (50). However, since Buchnera lacks adaptive codon bias (66), synonymous substitution rates are expected to approximate neutral mutation rates. Under the hypothesis that different replicons have equal mutation rates, divergence at synonymous sites is expected to be similar for plasmid and chromosomal genes.
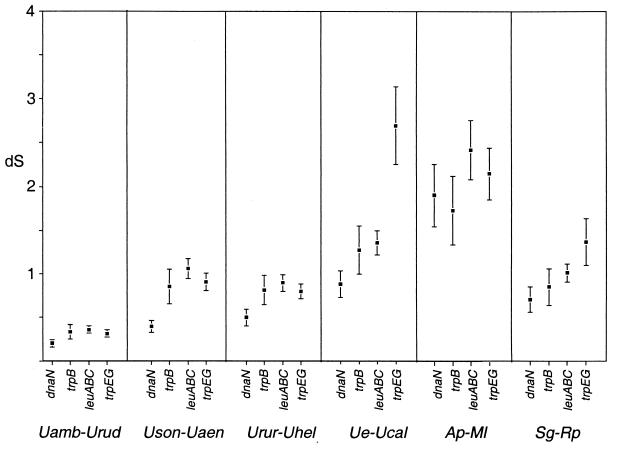
Synonymous divergences were estimated for each gene region across phylogenetically independent pairs of Buchnera. Synonymous divergences were estimated using the method of Li (39), adjusting for moderate levels of sequence divergence (using the program Molecular Evolutionary Analysis [E. Moriyama, Yale University]) and the maximum-likelihood-based method of Goldman and Yang (23) (codeML package of PAML [67]). Compared to other methods, this likelihood approach accounts for unequal base (codon) frequencies and biased transition/transversion ratios and provides a more realistic evolutionary model for DNA sequences with extreme base compositions (reviewed in reference 68). Assumptions of the likelihood method as implemented here include a constant base composition and a uniform rate of substitution across codons of a particular gene (the shape parameter alpha = infinity).
Discrepancies between Goldman and Yang's (23) and Li's (39) methods illustrate the effects of accounting for base composition when calculating sequence divergences (Table 3). However, both methods show higher divergences at trpEG and leuABC compared to those at dnaN and trpB for four of the six pairwise comparisons (Fig. 3; Table 3). Higher synonymous divergence at trpEG than at chromosomal genes agrees with previous studies based on smaller, more divergent data sets (13, 48) and suggests elevated neutral mutation rates on pTrpEG. In contrast with the elevated synonymous divergence at leuABC in our data set, previous studies based on fewer and more divergent taxa did not find elevated rates at leucine biosynthesis genes (5, 13). The Uroleucon sample used here consists of numerous recently diverged isolates, provides low standard errors for divergence estimates, and offers improved ability to compare divergence levels among loci. Overall, our analysis suggests that the mutation rates for both pTrpEG and pLeu may show a moderate increase over that of the chromosome, at least in Buchnera of Uroleucon. Mechanisms for higher mutation rates at plasmid loci may include the use of different DNA polymerases that vary in error rate (29) or higher levels of transcription, which may elevate mutation rates (8, 16).
TABLE 3.
Pairwise estimates of synonymous divergences at chromosomal (dnaN and trpB) and plasmid (leuABC and trpEg) genes for six phylogenetically independent pairs of Buchnera isolates, showing generally higher divergences at the plasmid-encoded loci for any given pairwise comparison
| Method and comparisona | Divergence (SE)
|
|||
|---|---|---|---|---|
| dnaN | trpB | leuABC | trpEG | |
| dS | ||||
| Uamb vs Urud | 0.201 (0.042) | 0.333 (0.081) | 0.360 (0.045) | 0.314 (0.040) |
| Uson vs Uaen | 0.393 (0.070) | 0.857 (0.199) | 1.063 (0.117) | 0.910 (0.099) |
| Urur vs Uhel | 0.499 (0.095) | 0.815 (0.171) | 0.897 (0.097) | 0.799 (0.082) |
| Ue vs Ucal | 0.878 (0.154) | 1.269 (0.277) | 1.350 (0.138) | 2.688 (0.444) |
| Ap vs Ml | 1.909 (0.358) | 1.735 (0.394) | 2.426 (0.336) | 2.153 (0.299) |
| Rp vs Sg | 0.710 (0.148) | 0.851 (0.211) | 1.012 (0.102) | 1.368 (0.283) |
| Ks | ||||
| Uamb vs Urud | 0.116 (0.029) | 0.137 (0.033) | 0.134 (0.014) | 0.190 (0.025) |
| Uson vs Uaen | 0.197 (0.038) | 0.301 (0.058) | 0.340 (0.027) | 0.561 (0.059) |
| Urur vs Uhel | 0.201 (0.034) | 0.356 (0.064) | 0.312 (0.028) | 0.512 (0.049) |
| Ue vs Ucal | 0.389 (0.065) | 0.442 (0.076) | 0.502 (0.038) | 1.202 (0.135) |
| Ap vs Ml | 0.679 (0.108) | 0.623 (0.110) | 0.663 (0.056) | 1.447 (0.271) |
| Rp vs Sg | 0.242 (0.042) | 0.295 (0.057) | 0.343 (0.027) | 0.604 (0.063) |
FIG. 3.
Phylogenetically independent pairwise estimates of synonymous divergence at Buchnera dnaN, trpB, leuABC, and trpEG genes, using a maximum likelihood-based estimation (67, 68). Error bars indicate the standard errors of individual pairwise estimates. See Table 1 for abbreviations.
Nucleotide sequence accession numbers.
GenBank numbers for sequences obtained in this study and for previously published sequences are given in Table 1.
Acknowledgments
This work was supported by a National Institutes of Health postdoctoral training grant in Molecular Insect Science (Center for Insect Science, University of Arizona) to J.J.W. and a National Science Foundation grant (DEB-9815413) to N.A.M.
We thank J. Sandström for obtaining most of the original Uroleucon samples and P. Baumann for DNA extractions. Three anonymous reviewers gave helpful comments.
REFERENCES
- 1.Anderson R, May R. Coevolution of hosts and parasites. Parasitology. 1982;85:411–426. doi: 10.1017/s0031182000055360. [DOI] [PubMed] [Google Scholar]
- 2.Arber W. Elements in microbial evolution. J Mol Evol. 1991;33:4–12. doi: 10.1007/BF02100190. [DOI] [PubMed] [Google Scholar]
- 3.Arber W. The generation of variation in bacterial genomes. J Mol Evol. 1995;40:7–12. [Google Scholar]
- 4.Baquero F, Blazquez J. Evolution of antibiotic resistance. Trends Ecol Evol. 1997;12:482–487. doi: 10.1016/s0169-5347(97)01223-8. [DOI] [PubMed] [Google Scholar]
- 5.Baumann L, Baumann P, Moran N A, Sandström J. Genetic characterization of plasmids containing genes encodoing enzymes of leucine biosynthesis in endosymbionts (Buchnera) of aphids. J Mol Evol. 1999;48:77–85. doi: 10.1007/pl00006447. [DOI] [PubMed] [Google Scholar]
- 6.Baumann L, Clark M A, Rouhbakhsh D, Baumann P, Moran N A, Voegtlin D J. Endosymbionts (Buchnera) of the aphid Uroleucon sonchi contain plasmids with trpEG and remnants of trpE pseudogenes. Curr Microbiol. 1997;35:18–21. [Google Scholar]
- 7.Baumann P, Baumann L, Clark M A, Thao M L. Buchnera aphidicola: the endosymbiont of aphids. ASM News. 1998;64:203–209. [Google Scholar]
- 8.Beletskii A, Bhagwat A S. Transcription-induced mutations: increase in C to T mutations in the nontranscribed strand during transcription in Escherichia coli. Proc Natl Acad Sci USA. 1996;93:13919–13924. doi: 10.1073/pnas.93.24.13919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouma J E, Lenski R. Evolution of a bacteria/plasmid association. Nature. 1988;335:351–352. doi: 10.1038/335351a0. [DOI] [PubMed] [Google Scholar]
- 10.Bracho A M, Martinez-Torres D, Moya A, Latorre A. Discovery and molecular characterization of a plasmid localized in Buchnera sp. bacterial endosymbiont of the aphid Rhopalosiphum padi. J Mol Evol. 1995;41:67–73. doi: 10.1007/BF00174042. [DOI] [PubMed] [Google Scholar]
- 11.Buchner P. Endosymbiosis of animals with plant microorganisms. New York, N.Y: Interscience Publishers, John Wiley and Sons, Inc.; 1965. [Google Scholar]
- 12.Castillo M, Flores M, Mavingui P, Martinez-Romero E, Palacios R, Hernandez G. Increase in alfalfa nodulation, nitrogen fixation, and plant growth by specific DNA amplification in Sinorhizobium meliloti. Appl Environ Microbiol. 1999;65:2716–2722. doi: 10.1128/aem.65.6.2716-2722.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark A M, Moran N A, Baumann P. Sequence evolution in bacterial endosymbionts having extreme base compositions. Mol Biol Evol. 1999;16:1586–1598. doi: 10.1093/oxfordjournals.molbev.a026071. [DOI] [PubMed] [Google Scholar]
- 14.Clark A M, Moran N A, Baumann P, Wernegreen J J. Cospeciation between bacterial endosymbionts (Buchnera) and a recent radiation of aphids (Uroleucon) and pitfalls of testing for phylogenetic congruence. Evolution. 2000;54:517–525. doi: 10.1111/j.0014-3820.2000.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 15.Dahlberg C, Bergstrom M, Andreasen M, Chistensen B, Molin S, Hermansson M. Interspecies bacterial conjugation by plasmids from marine environments visualized by gfp expression. Mol Biol Evol. 1998;15:385–390. [Google Scholar]
- 16.Datta A, Jinks-Robertson S. Association of increased spontaneous mutation rates with high levels of transcription in yeast. Science. 1995;268:1616–1619. doi: 10.1126/science.7777859. [DOI] [PubMed] [Google Scholar]
- 17.Davidson J. Genetic exchange between bacteria in the environment. Plasmid. 1999;42:73–91. doi: 10.1006/plas.1999.1421. [DOI] [PubMed] [Google Scholar]
- 18.Eberhard W G. Why do bacterial plasmids carry some genes and not others? Plasmid. 1989;21:167–174. doi: 10.1016/0147-619x(89)90040-1. [DOI] [PubMed] [Google Scholar]
- 19.Farrand S K. Conjugal transfer of Agrobacterium plasmids. In: Clewell D B, editor. Bacterial conjugation. New York, N.Y: Plenum Press; 1993. pp. 255–291. [Google Scholar]
- 20.Fernandez M, Margolles A, Suarez J, Mayo B. Duplication of the beta-galactosidase gene in some Lactobacillus plantarum strains. Int J Food Microbiol. 1999;48:113–123. doi: 10.1016/s0168-1605(99)00031-8. [DOI] [PubMed] [Google Scholar]
- 21.Firth N, Berg T, Skurray R. Evolution of conjugative plasmids from Gram-positive bacteria. Mol Microbiol. 1999;31:1598–1600. [PubMed] [Google Scholar]
- 22.Funk D J, Helbling L, Wernegreen J J, Moran N A. Evolutionary congruence among multiple symbiont genomes in an aphid species. 2000. Proc. R. Soc. Lond. Ser. B, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldman N, Yang Z. A codon-based model of nucleotide substitution for protein-coding sequences. Mol Biol Evol. 1994;11:725–736. doi: 10.1093/oxfordjournals.molbev.a040153. [DOI] [PubMed] [Google Scholar]
- 24.Heinemann J A, Sprague G F., Jr Bacterial conjugative plasmids mobilize DNA transfer between bacteria and yeast. Nature. 1989;340:205–209. doi: 10.1038/340205a0. [DOI] [PubMed] [Google Scholar]
- 25.Helinski D R, Toukdarian A E, Novick R P. Replication control and other stable maintenance mechanisms of plasmids. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. 2nd ed. Vol. 2. Washington, D.C.: ASM Press; 1996. pp. 2295–2324. [Google Scholar]
- 26.Holloway B W. Plasmids that mobilize bacterial chromosome. Plasmid. 1979;2:1–19. doi: 10.1016/0147-619x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- 27.Janniere L, Niaudef B, Erlich S D. Repeated DNA sequences recombine 1,000 times more frequently in a plasmid than in a chromosome of Bacillus subtilis. In: Helinski D, Cohen S, Clewell D, Jackson D, Hollaender A, editors. Plasmids in bacteria. New York, N.Y: Plenum Press; 1985. pp. 93–103. [DOI] [PubMed] [Google Scholar]
- 28.Kishino H, Hasegawa M. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in Hominoidea. J Mol Evol. 1989;29:170–179. doi: 10.1007/BF02100115. [DOI] [PubMed] [Google Scholar]
- 29.Kunkel T A. Biological asymmetries and the fidelity of eukaryotic DNA replication. Bioessays. 1992;14:303–308. doi: 10.1002/bies.950140503. [DOI] [PubMed] [Google Scholar]
- 30.Lai C-Y, Baumann P, Moran N. The endosymbiont (Buchnera sp.) of the aphid Diruaphis noxia contains plasmids consisting of trpEG and tandem repeats of trpEG pseudogenes. Appl Environ Microbiol. 1996;62:332–339. doi: 10.1128/aem.62.2.332-339.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai C-Y, Baumann L, Baumann P. Amplification of trpEG: adaptation of Buchnera aphidicola to an endosymbiotic association with aphids. Proc Natl Acad Sci USA. 1994;91:19–23. doi: 10.1073/pnas.91.9.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawrence J, Ochman H. Molecular archaeology of the Escherichia coli genome. Proc Natl Acad Sci USA. 1998;95:9413–9417. doi: 10.1073/pnas.95.16.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawrence J, Roth J. Genomic flux: genome evolution by gene loss and acquisition. In: Charlesbois R, editor. Organization of the prokaryotic genome. Washington, D.C.: ASM Press; 1999. pp. 263–289. [Google Scholar]
- 34.Lawrence J G. Gene transfer, speciation, and the evolution of bacterial genomes. Curr Opin Microbiol. 1999;2:519–523. doi: 10.1016/s1369-5274(99)00010-7. [DOI] [PubMed] [Google Scholar]
- 35.Lecointre G, Rachdi L, Darlu P, Denamur E. Escherichia coli molecular phylogeny using the incongruence length difference test. Mol Biol Evol. 1998;15:1685–1695. doi: 10.1093/oxfordjournals.molbev.a025895. [DOI] [PubMed] [Google Scholar]
- 36.Lenski R, Bouma J. Effects of segregation and selection on instability of plasmid pACYC184 in Escherichia coli B. J Bacteriol. 1987;169:5314–5316. doi: 10.1128/jb.169.11.5314-5316.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leski T, Gniadkowski M, Skoczynska A, Stefaniuk E, Trzcinski K, Hryniewicz W. Outbreak of mupirocin-resistant staphyolocci in a hospital in Warsaw, Poland, due to plasmid transmission and clonal spread of several strains. J Clin Microbiol. 1999;37:2781–2788. doi: 10.1128/jcm.37.9.2781-2788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin B R. The maintenance of plasmids and transposons in natural populations of bacteria. In: Levy S B, editor. Antibiotic resistance genes: ecology, transfer, and expression. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1986. pp. 57–70. [Google Scholar]
- 39.Li W H. Unbiased estimation of the rates of synonymous and nonsynonymous substitution. J Mol Evol. 1993;36:96–99. doi: 10.1007/BF02407308. [DOI] [PubMed] [Google Scholar]
- 40.Mazodier P, Davies J. Gene transfer between distantly related bacteria. Annu Rev Genet. 1991;25:147–171. doi: 10.1146/annurev.ge.25.120191.001051. [DOI] [PubMed] [Google Scholar]
- 41.Milkman R. Recombination and population structure in E. coli. Genetics. 1997;146:745–750. doi: 10.1093/genetics/146.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moran N A, Kaplan M E, Gelsey M J, Murphy T G, Scholes E A. Phylogenetics and evolution of the aphid genus Uroleucon based on mitochondrial and nuclear DNA sequences. Syst Entomol. 1999;24:85–93. [Google Scholar]
- 43.Moran N A, Telang A. Bacteriocyte-associated symbionts of insects. Bioscience. 1998;48:295–304. [Google Scholar]
- 44.Page R D M. Parallel phylogenies: reconstructing the history of host-parasite assemblages. Cladistics. 1994;10:155–173. [Google Scholar]
- 45.Rispe C, Moran N A. Accumulation of deleterious mutations in endosymbionts: Muller's ratchet with two levels of selection. Amer Nat. 2000;156:425–441. doi: 10.1086/303396. [DOI] [PubMed] [Google Scholar]
- 46.Romero D, Palacios R. Gene amplification and genomic plasticity in prokaryotes. Annu Rev Genet. 1997;31:91–111. doi: 10.1146/annurev.genet.31.1.91. [DOI] [PubMed] [Google Scholar]
- 47.Roth J, Benson N, Galitski T, Haak K, Lawrence J, Meisel L. Rearrangements of the bacterial chromosome: formation and applications. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular Biology. 2nd ed. Vol. 2. Washington D.C.: ASM Press; 1996. pp. 2256–2276. [Google Scholar]
- 48.Rouhbakhsh D, Clark M A, Baumann L, Moran N A, Baumann P. Evolution of the tryptophan biosynthetic pathway in Buchnera (aphid endosymbionts): studies of plasmid-associated trpEG within the genus Uroleucon. Mol Phylog Evol. 1997;8:167–176. doi: 10.1006/mpev.1997.0419. [DOI] [PubMed] [Google Scholar]
- 49.Rouhbakhsh D, Lai C-Y, von Dohlen C D, Clark M A, Baumann L, Baumann P, Moran N A, Voegtlin D J. The tryptophan biosynthetic pathway of aphid endosymbionts (Buchnera): genetics and evolution of plasmid-associated anthranilate synthase (trpEG) within the Aphididae. J Mol Evol. 1996;42:414–421. doi: 10.1007/BF02498635. [DOI] [PubMed] [Google Scholar]
- 50.Sandström J P, Russell J A, White J P, Moran N A. Independent origins and horizontal transfer of bacterial symbionts of aphids. 2000. Mol. Ecol., in press. [DOI] [PubMed] [Google Scholar]
- 51.Sharp P M. Determinants of DNA sequence divergence between Escherichia coli and Salmonella typhimurium—codon usage, map position, and concerted evolution. J Mol Evol. 1991;33:23–33. doi: 10.1007/BF02100192. [DOI] [PubMed] [Google Scholar]
- 52.Silva F, van Ham R, Sabater B, Latorre B. Structure and evolution of the leucine plasmids carried by the endosymbiont (Buchnera aphidicola) from aphids of the family Aphididae. FEMS Microbiol Lett. 1999;168:43–49. doi: 10.1111/j.1574-6968.1998.tb13253.x. [DOI] [PubMed] [Google Scholar]
- 53.Smith M, Bidochka M. Bacterial fitness and plasmid loss: the importance of culture conditions and plasmid size. Can J Microbiol. 1998;44:351–355. [PubMed] [Google Scholar]
- 54.Sonti S, Roth J. Role of gene duplications in the adaptation of Salmonella typhimurium to growth on limiting carbon sources. Genetics. 1989;123:19–28. doi: 10.1093/genetics/123.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swofford D, Olsen G, Waddell P, Hillis D. Phylogenetic inference. In: Hillis D, Moritz B M C, editors. Molecular systematics. Vol. 2. Sunderland, Mass: Sinauer; 1996. pp. 407–514. [Google Scholar]
- 56.Swofford D L. PAUP* 4.0. Beta version 4.0b2. Sunderland, Mass: Sinauer Associates; 1999. [Google Scholar]
- 57.Syvanen M. Horizontal gene transfer: evidence and possible consequences. Annu Rev Genet. 1994;28:237–261. doi: 10.1146/annurev.ge.28.120194.001321. [DOI] [PubMed] [Google Scholar]
- 58.Thao L M, Baumann L, Baumann P, Moran N A. Endosymbionts (Buchnera) from the aphids Schizaphis graminum and Diuraphis noxia have different copy numbers of the plasmid containing the leucine biosynthetic genes. Curr Microbiol. 1998;36:238–240. doi: 10.1007/s002849900301. [DOI] [PubMed] [Google Scholar]
- 59.Thomas C. Promiscuous plasmids of gram-negative bacteria. London, United Kingdom: Academic Press; 1989. [Google Scholar]
- 60.Trevors J. DNA in soil: adsorption, genetic transformation, molecular evolution and genetic microchip. Antonie Leeuwenhoek. 1996;70:1–10. doi: 10.1007/BF00393564. [DOI] [PubMed] [Google Scholar]
- 61.Valvano M, Wolf M, Crosa L, Crosa J. Chromosomal localization of aerobactin-mediated iron uptake genes commonly encoded by certain ColV plasmids. In: Helinski D, Cohen S, Clewell D, Jackson D, Hollaender A, editors. Plasmids in bacteria. New York, N.Y: Plenum Press; 1985. p. 913. [Google Scholar]
- 62.van Ham R C H J, Gonzalez-Candelas F, Silva F J, Sabater B, Moya A, Latorre A. Postsymbiotic plasmid acquisition and evolution of the repA1-replicon in Buchnera aphidicola. Proc Natl Acad Sci USA. 2000;97:10855–10860. doi: 10.1073/pnas.180310197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Ham R C H J, Moya A, Latorre A. Putative evolutionary origin of plasmids carrying the genes involved in leucine biosynthesis in Buchnera aphidicola. J Bacteriol. 1997;179:4768–4777. doi: 10.1128/jb.179.15.4768-4777.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Ham R C H J, Torres D M, Moya A, Latorre A. Plasmid encoded anthranilate synthase (TrpEG) in Buchnera aphidicola from aphids of the family Pemphigidae. Appl Environ Microbiol. 1999;65:117–125. doi: 10.1128/aem.65.1.117-125.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watanabe K, Sato M. Plasmid-mediated gene transfer between insect-resident bacteria, Enterobacter cloacae, and plant epiphytic bacteria, Erwinia herbicola, in guts of silkworm larvae. Curr Microbiol. 1998;37:352–355. doi: 10.1007/s002849900391. [DOI] [PubMed] [Google Scholar]
- 66.Wernegreen J J, Moran N A. Evidence for genetic drift in endosymbionts (Buchnera): analyses of protein-coding genes. Mol Biol Evol. 1999;16:83–97. doi: 10.1093/oxfordjournals.molbev.a026040. [DOI] [PubMed] [Google Scholar]
- 67.Yang Z. PAML, a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biol Sci. 1997;13:555–556. doi: 10.1093/bioinformatics/13.5.555. [DOI] [PubMed] [Google Scholar]
- 68.Yang, Z., and R. Nielsen. Estimating synonymous and nonsynonymous substitution rates under realistic evolutionary models. Mol. Biol. Evol. 17:32–43. [DOI] [PubMed]
- 69.Young J P W, Haukka K. Diversity and phylogeny of rhizobia. New Phytol. 1996;133:87–94. [Google Scholar]