Abstract
A pyrophosphate-dependent phosphofructokinase (PPi-PFK) and an ATP-dependent phosphofructokinase (ATP-PFK) from Thermotoga maritima have been cloned and characterized. The PPi-PFK is unique in that the Km and Vmax values indicate that polyphosphate is the preferred substrate over pyrophosphate; the enzyme in reality is a polyphosphate-dependent PFK. The ATP-PFK was not significantly affected by common allosteric effectors (e.g., phosphoenolpyruvate) but was strongly inhibited by PPi and polyphosphate. The results suggest that the control of the Embden-Meyerhof pathway in this organism is likely to be modulated by pyrophosphate and/or polyphosphate.
The Embden-Meyerhof (EM), or glycolytic, pathway is nearly ubiquitous in all life forms, and enzymes of the reaction sequence are highly conserved. One of the key and definitive enzymes of the pathway is phosphofructokinase (PFK). In the majority of organisms, ATP is the phosphoryl donor for the enzyme and the reaction is a nonreversible step in the pathway. Due to its position, PFK is usually allosterically regulated by intracellular metabolites, e.g., phosphoenolpyruvate (PEP), GDP, and/or ADP (27). PFK subtypes utilizing pyrophosphate (PPi) as the phosphoryl donor, where the reaction becomes more reversible and the enzyme is generally not subject to allosteric control mechanisms, have also been described (16, 18, 25).
Thermotoga maritima is a non-spore-forming, rod-shaped hyperthermophilic bacterium with an optimum growth temperature of 80°C and is phylogenetically classified in the order Thermotogales. The phylogeny of the small-subunit rRNA shows that this organism represents one of the deepest and most slowly evolving lineages of bacteria (12). T. maritima ferments various carbohydrates, including monosaccharides and polysaccharides, primarily via the EM pathway, and ATP-dependent PFK (ATP-PFK) activity in cell extracts has been reported (23, 24). The genome sequence of this organism indicated the presence of another PFK gene, and sequence comparison showed homology to PPi-dependent PFK (PPi-PFK) enzymes (17). If both genes code for functional enzymes, then Thermotoga would represent the unusual situation of an organism possessing two distinct PFK activities. Because of its phylogenetic position, the occurrence and origin of these genes are of importance with respect to the origins of the EM pathway. This paper describes the cloning, expression, and characterization of these enzymes, both of which exhibit unusual features.
T. maritima strain 3109 was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH and grown in the medium described by Huber et al. (12). Escherichia coli DH5α and expression plasmid pProEX HTb were obtained from Life Technologies. E. coli was grown at 30°C with vigorous aeration (200 rpm) in Luria-Bertani broth supplemented with ampicillin (100 μg ml−1) when appropriate. The PFK assay was conducted essentially as described by Ding et al. (6). Preparation of genomic DNA and the alkaline lysis and cesium chloride gradient methods for large scale plasmid DNA purification followed standard procedures (21).
Construction of the PPi-PFK and ATP-PFK expression clones.
The open reading frames representing the full-length sequences of the PPi- and ATP-PFK genes were amplified directly from genomic DNA from T. maritima. Primer design was based on the nucleotide sequences of the 5′and 3′ ends of the putative PFK genes (17). For the PPi-PFK gene, the forward primer, corresponding to the N terminus, contained an upstream SfoI site (in bold) and 5′-end spacer (5′-GGAA GGC GCC ATG GCT GAA AGA TTG GGG ATA CTC G-3′), and the reverse primer, corresponding to the C terminus, contained a flanking HindIII site (in bold) and a 5′-end spacer (5′-GCTA AAG CTT TAT GGA AGC TCT GTC GTA TGC CAG-3′). The primers for the ATP-PFK gene also contained SfoI and HindIII sites, and their sequences were 5′-GGCT GGC GCC ATG AAG AAG ATA GCA GTA TAC-3′ and 5′-CCA TAA GCT TTA TGA AAG CAT ATG TGC TAT TTC-3′ for forward and reverse primers, respectively. AmpliTaq Gold DNA polymerase was used for PCR (Perkin Elmer). Both PCR products for the two genes (pfp and pfk) were of the sizes predicted from their nucleotide sequences, approximately 1,200 and 950 bp, respectively (17). These products were sequenced to confirm their identity (1) and then cloned into the expression vector after restriction digestion with SfoI and HindIII, followed by ligation with T4 DNA ligase using standard protocols (21). The ligation mixture containing restriction enzyme-digested plasmid and PCR product was used to transform E. coli strain DH5α by electroporation, according to the manufacturer's instructions (Gene Pulser; Bio-Rad). Screening of the clones for those with inserts was carried out through alkaline lysis miniprep plasmid isolation (21) followed by restriction enzyme analysis.
Expression, purification, and characterization of the recombinant PPi- and ATP-PFKs.
Flask cultures of the recombinant E. coli clones were grown at 30°C in 700 ml of Luria-Bertani broth plus 100 μg of ampicillin ml−1 and were induced with 1 mM isopropyl-β-d-thiogalactoside when the culture optical density at 600 nm reached approximately 0.6. After 5 h of induction, the cells were harvested by centrifugation and sonicated, and the cell lysate was incubated for 40 min at 80°C. Further purification of the enzymes from the supernatant was performed using a 3.0-ml column of nickel nitrilotriacetic acid resin and elution following the manufacturer's instructions (Life Technologies). Single bands were obtained for each of the nickel nitrilotriacetic acid resin-purified proteins on denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, indicating a high degree of purity. The estimated molecular weights for the PPi- and ATP-PFK proteins from SDS-PAGE were approximately 48,000 and 38,000, respectively (Table 1), which is in close agreement with the molecular masses derived from the amino acid sequences (including the N-terminal histidine tag, which is approximately 2 kDa). The conceptual translation masses of the full-length open reading frames of the pfp and pfk genes are 46,403 Da and 34,447 Da for the PPi- and ATP-PFKs, respectively. The recombinant PPi-PFK had a molecular mass of 96 kDa as determined by its elution during gel filtration chromatography, which suggests that the active molecule exists as a homodimer. In contrast, the molecular mass of the ATP-PFK was 200 kDa, and thus a homotetramer is the most probable quaternary structure.
TABLE 1.
Properties of the cloned T. maritima PPi- and ATP-PFKs
| Property | PPi-PFKa | ATP-PFKb |
|---|---|---|
| pH optima | ||
| Forward reaction | 5.6–5.8 | 7.2–8.0 |
| Reverse reaction | 5.6–6.8 | NDc |
| MgCl2 optimum (mM) | 0.5–7.0 | 1.0–10.0 |
| Sp act (U mg−1) | 203 | 432 |
| Thermostability (half-life at 90°C)d | >5 h | >5 h |
| Apparent molecular mass (kDa) | ||
| SDS-PAGE | 48 | 38 |
| Gel filtration | 97 | 200 |
| Phosphoryl donors (%)e | Poly-P (157), PPPi (123), PPi (100), ATP (0), ADP (0) | ATP (100), GTP (42), UTP (14), CTP (13), TTP (10), PPi (0), ADP (0) |
| Cation specificity (%)f | Mg2+ (100) > Co2+ (49) > Mn2+ (40) > Ni2+ (38) | Mg2+ (100) > Mn2+ (90) > Fe2+ (34) |
| Sensitivity to cations (% of control activity)g | ||
| 1.0 μM Cu2+ | 57 | 72 |
| 1.0 μM Zn2+ | 56 | 72 |
Experimental conditions were 1.0 mM PPi, 3 mM F-6-P, 5 mM MgCl2, 0.2 mM NADH, 175 mM KCl, and 30 mM bis-Tris (pH 5.8) at 50°C.
Experimental conditions were 0.5 mM ATP, 3 mM F-6-P, 5 mM MgCl2, 0.2 mM NADH, 175 mM KCl, and 30 mM Tris (pH 7.8) at 50°C.
Not detected.
175 mM KCl, 0.02% Triton X-100, 0.05 mM dithiothreitol, 3 mM MgCl2, and 50 mM phosphate buffer (pH 7.0).
0.1 mM phosphoryl donors. 3 mM F-6-P, 5 mM MgCl2, 0.2 mM NADH, 175 mM KCl, and 30 mM bis-Tris (pH 5.8) at 50°C for PPi-PFK; 30 mM Tris (pH 7.8) at 50°C for ATP-PFK.
0.1 mM cations, 3 mM F-6-P, 5 mM MgCl2, 0.2 mM NADH, 175 mM KCl, and 30 mM bis-Tris (pH 5.8) for PPi-PFK; 30 mM Tris (pH 7.8) at 50°C for ATP-PFK.
Cu2+ or Zn2 added in standard assay.
Both recombinant proteins showed enzyme activity specific for their expected phosphoryl donors; thus, the PPi-PFK was active with PPi but had no activity with ATP, and vice versa for the ATP-PFK. Thermotoga is thus confirmed as the only prokaryote reported which possesses two different functional phosphoryl donor subtypes of PFK. As expected, the enzymes were extremely thermostable, with half-lives for both being greater than 5 h at 90°C in phosphate buffer.
PFKs generally have a requirement for a low concentration (<100 mM) of either sodium or potassium ions for optimal activity (5, 20, 27). For the Thermotoga enzymes, the requirement for potassium ions and the stimulation of activity by their presence was more pronounced. The optimum concentration of K+ for both enzymes was 175 mM, and activity for the ATP- and PPi-PFKs fell 50 and 60%, respectively, when KCl was omitted from the reaction mixture. It is possible that this requirement reflects the marine environment from which the organism was isolated. Magnesium ions were required for optimal activities of both enzymes with Co2+, Mn2+, and Ni2+ being able to substitute for Mg2+ with the PPi-PFK and Mn2+ and Fe2+ being able to substitute for Mg2+ with the ATP-PFK (Table 1). Both Thermotoga enzymes were extremely sensitive to Cu2+ and Zn2+ (Table 1). This sensitivity was also found within the Dictyoglomus thermophilum native and recombinant PPi-PFKs (6, 7) and the archaeal Desulfurococcus amylolyticus ATP-PFK (5).
The Thermotoga PPi-PFK was unique in that it exhibited higher activity with tripolyphosphate (PPPi) and polyphosphate (poly-P) as phosphoryl donors than with PPi as the donor, and the apparent Km and Vmax values (Table 2) indicate that the Thermotoga PPi-PFK functions as a poly-P-dependent PFK. This is the first report of a PFK with such characteristics. The PPi-PFK catalyzes a typically reversible reaction, but with the Thermotoga enzyme the pH optima for the forward and reverse reactions are unusually close; pH 5.6 to 5.8 for the forward reaction and pH 5.6 to 6.8 for the reverse reaction (Table 1). In general, other PPi-PFKs have a pH difference of up to one unit between the forward and reverse reactions. Similar to other PPi-PFKs, the Thermotoga PPi-PFK exhibited essentially no response to traditional allosteric effectors, and presumably the reaction direction and rate (due only to the PPi-PFK) are dictated simply by the concentrations of intracellular metabolites and the level of the enzyme.
TABLE 2.
Kinetics of Thermotoga PPi-PFK
| Phosphoryl donor | Km (mM) | Vmax (U mg−1) | Vmax/Km |
|---|---|---|---|
| PPi | 0.067 | 203 | 3 × 103 |
| PPPi | 0.010 | 249 | 2.5 × 104 |
| Poly-P | 0.0038 | 319 | 8.4 × 104 |
The Thermotoga ATP-PFK displayed the highest activity with ATP as the phosphoryl donor (Table 3) but had significant activity when this was replaced by GTP, UTP, CTP, and TTP. No activity was detected with either PPi, PPPi, poly-P, or ADP as the phosphoryl donor (Table 1). The pH optimum for the ATP-PFK was between 7.2 and 8.0 (which is more likely to reflect the intracellular pH of the organism). The ATP-PFK showed no significant response to the common allosteric regulators. Thus, activity was only slightly inhibited by citrate at 1.0 mM, and PEP concentrations up to 5 mM did not affect the normal hyperbolic kinetic curve for fructose 6-phosphate (F-6-P). The allosteric response of the ATP-PFKs from E. coli and Bacillus stearothermophilus is potentially controlled by a glutamic acid residue at position 187 (E187) via the binding of PEP (2, 8, 22). Sequence alignment shows that the Thermotoga ATP-PFK also possesses an equivalent E187 residue, but the biochemical properties from this characterization suggest that PEP is not vital for regulating the Thermotoga enzyme and thus probably does not regulate glycolysis in this organism (7). ADP had opposing effects on ATP-PFK activity, as the enzyme was slightly activated at a low concentration of ADP (129% at 0.05 mM) and partially inhibited at higher concentrations (70% at 1.0 mM), but the magnitude of these effects does not reflect allosteric control.
TABLE 3.
Apparent ATP-PFK Km values for ATP, F-6-P, and GTP
| Phosphoryl donor | Km (mM) | Vmax (U mg−1) | Vmax/Km |
|---|---|---|---|
| ATP | 0.009 | 432 | 4.8 × 104 |
| F-6-P | 0.437 | 464 | 1.0 × 103 |
| GTP | 1.36 | 294 | 2.1 × 102 |
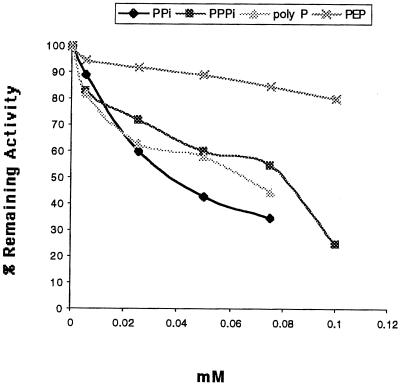
Surprisingly, the Thermotoga ATP-PFK activity was strongly inhibited by PPi, PPPi, or poly-P (n = 15 ± 3) at concentrations of less than 0.10 mM and under conditions in which chelation effects on available Mg2+ could be excluded (Table 4; Fig. 1). In particular, activity was strongly inhibited by both PPi and poly-P at concentrations reported to be common in bacteria (10 to 100 μM) (14). Interestingly, the inhibition of ATP-PFK activity by PPi could be partially alleviated by the presence of nucleotide diphosphates, i.e., ADP, GDP, or TDP (Table 5). This type of allosteric control has not previously been reported, and it seems that PPi and/or poly-P might replace, either partially or fully, the function of PEP and other potential modulators within this organism. Although nonallosteric ATP-PFKs have been identified in other organisms, including D. amylolyticus (5, 9), Trypanosoma brucei (15), and Lactobacillus bulgaricus (4), the responses of these enzymes to PPi and poly-P have not been investigated.
TABLE 4.
Effects of PPi, PPPi, poly-P, citrate, and PEP on the apparent Km for F-6-P of the ATP-PFK
| Effector | Km (mM) | Vmax (U mg−1) | Vmax/Km |
|---|---|---|---|
| 0.01 mM PPi | 0.436 | 148 | 3.4 × 102 |
| 0.025 mM PPi | 0.749 | 136 | 1.8 × 102 |
| 0.05 mM PPPi | 0.983 | 256 | 2.6 × 102 |
| 0.05 mM poly-P | 1.376 | 278 | 2.0 × 102 |
| 1.0 mM citrate | 0.0765 | 224 | 2.9 × 102 |
| 5.0 mM PEP | 0.315 | 260 | 8.2 ×102 |
FIG. 1.
Effects of PPi, PPPi, poly-P, and PEP on Thermotoga ATP-PFK activity.
TABLE 5.
Effects of some compounds on the PPi inhibition on Thermotoga ATP-PFKa
| Effector | % Activity |
|---|---|
| None | 100 |
| 0.1 mM PPi | 20 |
| 0.1 mM PPi and 0.1 mM ADP | 57 |
| 0.1 mM PPi and 0.1 mM AMP | 22 |
| 0.1 mM PPi, 0.1 mM ADP, and 0.1 mM AMP | 57 |
| 0.1 mM PPi and 0.1 mM TDP | 69 |
| 0.1 mM PPi and 0.1 mM GDP | 68 |
| 0.1 mM PPi and 0.1 mM CDP | 27 |
| 0.1 mM PPi and 0.1 mM UDP | 47 |
Experimental conditions were 0.5 mM F-6-P, 0.25 mM ATP, 5 mM MgCl2, 0.2 mM NADH, 175 mM KCl, and 30 mM Tris (pH 7.8) at 50°C.
Role of poly-P.
Both Thermotoga enzymes have unique properties related to poly-P: it is a preferred substrate for the PPi-PFK and an allosteric regulator for the ATP-PFK. Poly-P is a component of volcanic condensates and deep-oceanic hydrothermal vents, and it is ubiquitously distributed in all living organisms (13) and possibly played a role in the prebiotic evolution of metabolism (3, 29). Significantly, poly-P has been used as an alternate phosphoryl and/or energy source to ATP for other enzymes involved with glucose metabolism. For example, poly-P-dependent glucokinase activity has been observed in Mycobacterium tuberculosis (11) and Propionibacterium freudenreichii (26, 28), and a poly-P-fructokinase has been found in Mycobacterium phlei (28). The poly-P-glucokinase from P. freudenreichii was particularly responsive to phosphoester chain length, with the apparent Km declining from 4.3 μM to 0.2 nM for polymer lengths of 30 and 724 residues, respectively (28). The PPi-PFK from Thermotoga demonstrated a similar, though less pronounced, effect, with a decline in Km values from 67 to 3.8 μM as phosphoester chain length increased from 2 to 18. In contrast, the PPi-PFKs from D. thermophilum and Spirochaeta thermophila favor the pyrophosphate substrate (7, 20).
The results presented here indicate that the control of the EM pathway in Thermotoga may be mediated by a quite different mechanism than that conventionally found, where the activity of ATP-PFK is allosterically controlled by either PEP, ADP, AMP, F-2,6-P2, citrate, succinate, or a combination of these. For glycolysis to proceed utilizing the ATP-PFK, the PPi and poly-P concentrations would have to remain low (<100 μM). If poly-P accumulated and/or the pH fell, then the ATP-PFK would be inhibited and the PPi-PFK activity would predominate. Poly-P is regarded as ubiquitous in all tested organisms (13, 14) and is present at concentrations above that needed to inhibit the ATP-PFK. Interestingly, no gene encoding a poly-P kinase has been identified in the genome of Thermotoga, though in other organisms other enzymes have also been implicated in the synthesis of poly-P, e.g., adenylate kinase in Acinetobacter johnsonii (19) and an acetate kinase in E. coli (10). Possibly, the PPi-PFK could produce poly-P by means of the reverse reaction at intracellular pH values between 6.0 and 7.0. The presence of ATP-PFK activity in cell extracts of Thermotoga has been reported (23, 24). We found both PPi-PFK and ATP-PFK activities in cell extracts if the assay pH was adjusted to the optimum for each enzyme (results not shown), so the enzymes appear to be expressed simultaneously. The intracellular concentration of PPi and poly-P and the internal pH of Thermotoga are unknown, but it will be important to determine these if the control of glycolysis in Thermotoga is to be understood. In summary, Thermotoga appears to be unique in that it contains the genes for two distinct PFKs and both genes can express functional enzymes. Both enzymes have unique properties, in particular, their responses to PPi and poly-P, and it is likely that these metabolites may play a central role in the control of glucose metabolism in this organism.
Acknowledgments
We thank the Royal Society Marsden Science Foundation and the University of Waikato for its financial support during the course of this study.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers W, Lipman D L. Basic local alignment search tool. J Mol Biol. 1997;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Auzat I, Le Bras G, Branny P, Torre F D L, Theunissen B, Garel J-R. The role of Glu 187 in the regulation of phosphofructokinase by phosphoenolpyruvate. J Mol Biol. 1994;235:68–72. doi: 10.1016/s0022-2836(05)80014-2. [DOI] [PubMed] [Google Scholar]
- 3.Baltscheffsky H. Energy conversion leading to the origin and early evolution of life: did inorganic pyrophosphate precede adenosine triphosphate? In: Baltscheffsky H, editor. Origin and evolution of biological energy conversion. New York, N.Y: VCH Publishers; 1996. pp. 1–9. [Google Scholar]
- 4.Branny P, De La Torre F, Garel J-R. Cloning, sequencing, and expression in Escherichia coli of the gene coding for phosphofructokinase in Lactobacillus bulgaricus. J Bacteriol. 1993;175:5344–5349. doi: 10.1128/jb.175.17.5344-5349.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding Y-H R. Phosphofructokinases from extremely thermophilic microorganisms. Ph.D. thesis. Hamilton, New Zealand: The University of Waikato; 2000. [Google Scholar]
- 6.Ding Y-H R, Ronimus R S, Morgan H W. Purification and properties of the pyrophosphate-dependent phosphofructokinase from Dictyoglomus thermophilum Rt46 B.1. Extremophiles. 1999;3:131–137. doi: 10.1007/s007920050108. [DOI] [PubMed] [Google Scholar]
- 7.Ding Y-H R, Ronimus R S, Morgan H W. Sequencing, cloning, and high-level expression of the pfp gene, encoding a PPi-dependent phosphofructokinase from the extremely thermophilic eubacterium Dictyoglomus thermophilum. J Bacteriol. 2000;182:4661–4666. doi: 10.1128/jb.182.16.4661-4666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans P R, Hudson P J. Structure and control of phosphofructokinase from Bacillus stearothermophilus. Nature. 1979;279:500–504. doi: 10.1038/279500a0. [DOI] [PubMed] [Google Scholar]
- 9.Hansen T, Schönheit P. Purification and properties of the first-identified, archaeal, ATP-dependent 6-phosphofructokinase, an extremely thermophilic non-allosteric enzyme, from the hyperthermophilic Desulfurococcus amylolyticus. Arch Microbiol. 2000;173:103–109. doi: 10.1007/s002039900114. [DOI] [PubMed] [Google Scholar]
- 10.Hardoyo, Yamada K, Muramatsu A, Anbe Y, Kato J, Ohtake H. Molecular genetics of polyphosphate accumulation in Escherichia coli. In: Torriani-Gorini A, Yagil E, Silver S, editors. Phosphate in microorganisms: cellular and molecular biology. Washington, D.C.: ASM Press; 1994. pp. 209–214. [Google Scholar]
- 11.Hsieh P-C, Shenoy B C, Jentoft J E, Phillips N F B. Purification of polyphosphate and ATP glucose phosphotransferase from Mycobacterium tuberculosis H37Ra: evidence that poly(P) and ATP glucokinase activities are catalyzed by the same enzyme. Protein Expr Purif. 1993;4:76–84. doi: 10.1006/prep.1993.1012. [DOI] [PubMed] [Google Scholar]
- 12.Huber R, Langworthy T A, Konig H, Thomm M, Woese C R, Sleytr U B, Stetter K O. Thermotoga maritima sp. nov. represents a new genus of unique extremely thermophilic eubacteria growing up to 90oC. Arch Microbiol. 1986;144:324–333. [Google Scholar]
- 13.Kornberg A. Inorganic polyphosphate: toward making a forgotten polymer unforgettable. J Bacteriol. 1995;177:491–496. doi: 10.1128/jb.177.3.491-496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulaev I S. The biochemistry of inorganic polyphosphates. New York, N.Y: John Wiley & Sons, Inc.; 1979. [Google Scholar]
- 15.Michels P A M, Chevalier N, Opperdoes F R, Rider M H, Rigden D J. The glycosomal ATP-dependent phosphofructokinase of Trypanosoma brucei must have evolved from an ancestral pyrophosphate-dependent enzyme. Eur J Biochem. 1997;250:698–704. doi: 10.1111/j.1432-1033.1997.00698.x. [DOI] [PubMed] [Google Scholar]
- 16.Morgan H W, Ronimus R S. Pyrophosphate-dependent phosphofructokinase in thermophilic and non-thermophilic microorganisms. In: Adams M W, Wiegel J W, editors. Thermophiles: the key to molecular evolution and the origin of life. London, United Kingdom: Taylor and Francis; 1998. pp. 269–278. [Google Scholar]
- 17.Nelson K E, Clayton R A, Gill S R, Gwinn M L, Dodson G R, Haft D D, Hickey K, Peterson J D, Nelson W C, Ketchum K A, McDonald L, Utterback T R, Malek J A, Linher K D, Garrett M M, Steward A M, Cotton M D, Pratt M S, Phillips C A, Richardson D, Heidelberg J, Sutton G G, Fleischmann R D, Eisen J A, White O, Salzberg S L, Smith H O, Venter J C, Fraser C M. Evidence for lateral gene transfer between archaea and bacteria from genome sequence of Thermotoga maritima. Nature. 1999;399:323–329. doi: 10.1038/20601. [DOI] [PubMed] [Google Scholar]
- 18.Reeves R E, South D J, Blytt H J, Warren L G. Pyrophosphate:d-fructose 6-phosphate 1-phosphotransferase. J Biol Chem. 1974;249:7737–7741. [PubMed] [Google Scholar]
- 19.Resnick S M, Zehnder A J. In vitro ATP regeneration from polyphosphate and AMP by polyphosphate:AMP phosphotransferase and adenylate kinase from Acinetobacter johnsonii 210A. Appl Environ Microbiol. 2000;66:2045–2051. doi: 10.1128/aem.66.5.2045-2051.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ronimus R S, Morgan H M, Ding Y-H R. Phosphofructokinase activities within the order Spirochaetales and the characterization of the pyrophosphate-dependent phosphofructokinase from Spirochaeta thermophila. Arch Microbiol. 1999;172:401–406. doi: 10.1007/s002030050777. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 22.Schirmer T, Evans P R. Structural basis of the allosteric behaviour of phosphofructokinase. Nature. 1990;343:140–145. doi: 10.1038/343140a0. [DOI] [PubMed] [Google Scholar]
- 23.Schröder C, Selig M, Schönheit P. Glucose fermentation to acetate, CO2, and H2 in the anaerobic hyperthermophilic eubacterium Thermotoga maritima: involvement of the Embden-Meyerhof pathway. Arch Microbiol. 1994;161:460–470. [Google Scholar]
- 24.Selig M, Xavier K B, Santos H, Schönheit P. Comparative analysis of Embden-Meyerhof and Entner-Doudoroff glycolytic pathways in the hyperthermophilic archaea and the bacterium Thermotoga. Arch Microbiol. 1997;167:217–232. doi: 10.1007/BF03356097. [DOI] [PubMed] [Google Scholar]
- 25.Siebers B, Klenk H P, Hensel R. PPi-dependent phosphofructokinase from Thermoproteus tenax, an archaeal descendant of an ancient line in phosphofructokinase evolution. J Bacteriol. 1998;180:2137–2143. doi: 10.1128/jb.180.8.2137-2143.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uryson S O, Kulaev I S. The presence of polyphosphate glucokinase in bacteria. Dokl Akad Nauk SSSR. 1968;183:957–959. [PubMed] [Google Scholar]
- 27.Uyeda K. Phosphofructokinase. Adv Enzymol. 1979;48:193–241. doi: 10.1002/9780470122938.ch4. [DOI] [PubMed] [Google Scholar]
- 28.Wood H G, Clark J E. Biological aspects of inorganic polyphosphates. Annu Rev Biochem. 1988;57:235–260. doi: 10.1146/annurev.bi.57.070188.001315. [DOI] [PubMed] [Google Scholar]
- 29.Yamagata Y, Watanabe H, Saitoh M, Namba T. Volcanic production of polyphosphates and its relevance to prebiotic evolution. Nature. 1991;352:516–519. doi: 10.1038/352516a0. [DOI] [PubMed] [Google Scholar]