Abstract
Background
Few study has investigated how paraspinal muscle endurance deteriorates in lumbar spinal stenosis (LSS) patients. In addition, little information is available on the relationship between clinical outcomes and the endurance of paraspinal muscles.
Objective
To explore the correlation between paraspinal extensor muscle endurance, quality of life (QOL) and sagittal spinopelvic alignment. Besides, we attempted to propose a paraspinal extensor muscle endurance test (PEMET) classification for identifying the severity of clinical symptoms and sagittal imbalance in LSS patients.
Methods
171 hospitalized LSS patients and 100 healthy controls from the community were prospectively enrolled in this study. The paraspinal extensor endurance test was performed at baseline according to Ito test. The LSS patients were stratified into three groups based on the performance time of endurance test: grade I (<10s); grade II (10–60s); and grade III (>60s). Clinical measures of QOL included the visual analog scale scores (VAS) for back pain and leg pain and the Oswestry Disability Index (ODI). Sagittal alignment was analysed by standing posteroanterior and lateral whole spine X-ray in LSS patients.
Results
The LSS group had a significantly shorter performance time of the endurance test than the control group. The paraspinal muscle endurance significantly correlated with VAS-back, VAS-leg, ODI, pelvic tilt, lumbar lordosis and sagittal vertical axis (SVA; all p < 0.05). In binary logistic regression, the performance time of the endurance test was an independent factor of both poor functional status (ODI >40; p = 0.005, OR = 0.985) and global sagittal imbalance (SVA >50 mm; p = 0.019, OR = 0.985). Based on PEMET classification, moving from the grade III group to the grade I group, there was progressive worsening in VAS-back and ODI (all adjusted p < 0.05). Moreover, the grade I group had significantly greater VAS- leg, less LL and greater SVA than the other two groups (all adjusted p < 0.05).
Conclusion
Paraspinal muscle endurance was associated with QOL and sagittal spinopelvic alignment in LSS patients. A PEMET classification system has been constructed and has shown a correlation with QOL and sagittal imbalance.
Translational potential statement
The PEMET classification system proposed in this study could be available for identifying the severity of clinical symptoms and sagittal imbalance during preoperative evaluation in LSS patients.
Keywords: Classification system, Lumbar spinal stenosis, Paraspinal muscle endurance, Quality of life, Sagittal imbalance
1. Introduction
Degenerative lumbar spinal stenosis (LSS), a common spinal disease, can induce lumbosacral pain and intermittent claudication, and severely decrease quality of life (QOL) [1,2]. Besides, sagittal imbalance also occurs in LSS patients, which can also compromise patients’ QOL [3,4].
Previous studies have reported that patients with LSS might have impaired muscle function [5,6]. Evaluating the endurance of paraspinal muscles can directly reflect muscle function with a greater discriminative validity than contractile force [7,8]. Ito et al. have developed a test for evaluating isometric endurance of paraspinal muscles [9]. With high reproducibility and safety, Ito test may be more applicable to clinical practice. Several studies have investigated the endurance of trunk extensor muscles in healthy population and patients with non-specific low back pain [[9], [10], [11]]. However, how paraspinal muscle endurance deteriorates in LSS has not been investigated.
Although the importance of the paraspinal muscles in degenerative lumbar pathologies and spinal alignment is well recognized, little information is available on the role of paraspinal muscle endurance [[12], [13], [14]]. Several studies have explored that the degeneration of paraspinal muscles morphology would influence the clinical outcomes adversely in LSS patients [15,16]. In addition, patients with small mass and high fatty degeneration of paraspinal muscles were inclined to have a poor sagittal alignment [[17], [18], [19]]. However, no study has investigated the relationship between clinical outcomes and the endurance of paraspinal muscles which might reflect muscle function more directly.
We hypothesized that the patients with poor paraspinal muscle endurance would be associated with poor QOL and sagittal imbalance. The purpose of this study was to (I) compare the paraspinal muscle endurance between LSS patients and asymptomatic controls (II) investigate the correlation between paraspinal muscle endurance, QOL and sagittal spinopelvic alignment in preoperative LSS patients (III) develop a preliminary paraspinal extensor muscle endurance test (PEMET) classification and compare the clinical outcomes between the different groups based on the classification in LSS patients.
2. Materials and methods
2.1. Study population
This is a cross-sectional study performed within the framework of a prospective cohort. We prospectively recruited hospitalized LSS patients between February 2020 and October 2021. We also recruited healthy controls from the community. All the participants have performed the physical examination and magnetic resonance imaging (MRI). Inclusion criteria for LSS patients included (1) age ≥45 years, (2) diagnosed LSS through a combination of clinical history, physical examination and radiological changes showing spinal canal stenosis on MRI (the distance of the antero-posterior diameter of spinal canal less than 10 mm or cross sectional area of the dural sac less than 100 mm2 according to previous study [20]), (3) underwent paraspinal muscle endurance test and radiographic assessments before surgery. The inclusion criteria for healthy controls were: (1) age ≥45 years; (2) without spinal canal stenosis on MRI; (3) without chronic low back pain (LBP) and neurogenic claudication [[21], [22], [23]]. Exclusion criteria for all subjects were (1) patients with spondylolisthesis (> grade 1), (2) acute or severe chronic back pain of spinal stenosis that could interfere with the evaluation of endurance (3) other serious diseases impacting the evaluation of endurance, (4) occurred an severely increasing pain or numbness in the legs after the endurance test, (5) patients with previous spinal surgery, bone tumor, ankylosing spondylitis, diffuse idiopathic skeletal hyperostosis, rheumatoid arthritis, tuberculosis, or secondary osteoporosis. Finally, 171 LSS patients (101 females; mean age, 63.29 years) and 100 healthy controls (53 females; mean age, 61.56 years) were included in this study. All participants received a participant information sheet and gave their written informed consent. This study was approved by Peking University Third Hospital Medical Science Research Ethics Committee (M2019400). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
2.2. Data collection
Age, sex, body mass index (BMI), smoking, comorbidities, duration of symptom and number of stenotic levels and QOL were collected. The modified 5-item frailty index (mFI-5) is a concise comorbidity-based risk stratification tool that was calculated based on the presence of the 5 co-morbidities (e.g., diabetes mellitus and chronic obstructive pulmonary disease) [24]. The mFI-5 score was calculated based on the sum of each of the 5 co-morbidities. LSS patient-reported outcomes, including the visual analog scale (VAS) for back pain and leg pain and the Oswestry Disability Index (ODI) scores ranging from 0 to 100, with the highest score indicating the worst disability, were evaluated for QOL at baseline [25]. Poor functional status was defined as ODI scores >40 according to previous study [26].
2.3. Sagittal alignment evaluation
The radiographic assessments were analysed by standing posteroanterior and lateral whole spine X-ray at baseline in LSS patients. The parameters including pelvic tilt (PT), sacral slope (SS), pelvic incidence (PI), lumbar lordosis (LL), sagittal vertical axis (SVA) and Cobb angle were measured by two experienced orthopedic surgeons who were not otherwise involved in this study, and the average of their results was recorded. We defined global sagittal imbalance as SVA >50 mm [17].
2.4. Paraspinal muscle endurance test
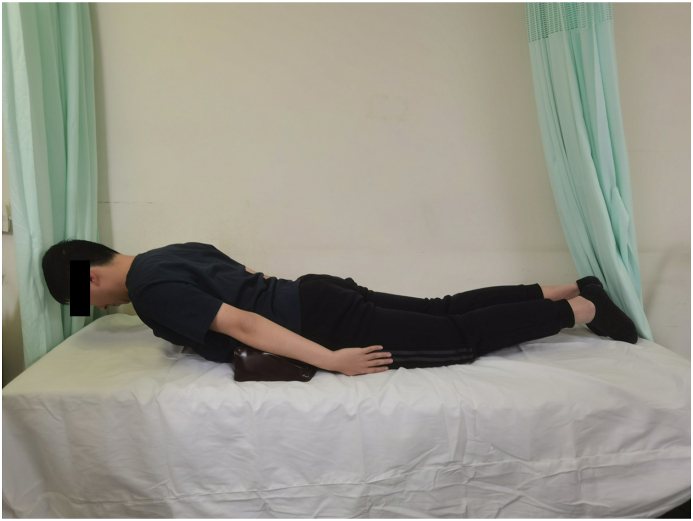
The paraspinal muscle endurance test was performed according to Ito test in previous studies [9,27]. Previous studies have demonstrated that Ito test had a less LL during test and might not induce pain and result in less spinal loading than conventional methods [8,9]. The participants were in a prone starting position on an examination table with a 10-cm-high pad placed under the lower abdomen to decrease LL. The participants were instructed to raise their upper body to an individual adjusted endpoint (by ∼15°) with the arms kept parallel to the body axis, the cervical spine held in a neutral position and both feet on the examination table throughout the entire test (Fig. 1).
Figure 1.
The illustration of the endurance test for measuring paraspinal extensor muscle endurance.
The test would cease until they felt voluntary fatigue for a maximum of 5 min. Task failure was determined by a drop in the angle of trunk of greater than 10° at any point [28]. The test started with a trial for recognition, followed by a 5 min rest for baseline data and then two recorded trials with 5 min rest in between. The result of the test was identified as the best record of two trials.
In our study, all the enrolled participants were performed a pain assessment before and after endurance test in order to verify the safety. Each participant had a VAS score ≤6 before test for reducing the impact of pain on the test according to previous studies [9,27]. We also placed the pad under the lower abdomen to decrease canal narrowing due to extension. The safety and validity of test was considered to be acceptable since none of healthy controls reported a increasing pain after test. In the LSS group, no patients reported a severely increasing pain or numbness in the legs.
2.5. Statistical analysis
Continuous variables were expressed as means and standard deviations for normally distributed data. Categorical variables were represented as absolute numbers. Unpaired t test and Chi-square test were used for the comparison of clinical characteristics and the endurance test between the LSS patients and healthy controls. The correlations between the performance time of endurance test, QOL and sagittal alignment were evaluated in the LSS patients. Multiple linear regression analyses were also used to determine the aforesaid relationship (age, BMI, duration of symptom, mFI-5, and the number of stenotic levels were included in each model). Moreover, binary logistic regression models by enter method were used to identify the independent risk factors of poor functional status (ODI scores >40) and sagittal imbalance (SVA >50 mm). Age, gender, BMI, mFI-5, smoking, number of stenotic levels and the endurance test were included in the regression models. Furthermore, LSS patients were stratified into three groups on the basis of the performance time of the endurance test: grade I, poor muscle function, the performance time <10 s; grade II, fair muscle function, the performance time was 10–60 s; and grade III, good muscle function, the performance time >60 s. These groups were compared in terms of clinical characteristics, QOL and sagittal parameters using analysis of variance (ANOVA) tests with post hoc comparisons and Chi-square test with Bonferroni correction. Statistical significance was set at P value < 0.05. All statistical analyses were performed using SPSS 22.0 (IBM Corp).
3. Results
There was no significant difference of age and sex between the LSS group and the control group (both p > 0.05; Table 1). The LSS group had a significantly shorter performance time of endurance test than the control group (p < 0.001; Table 1). Besides, BMI, VAS-back and mFI-5 were higher in the LSS patients than in the healthy controls (all p < 0.05; Table 1).
Table 1.
Comparisons of clinical characteristics and endurance test between LSS and healthy controls.
| LSS (n = 171) | Control (n = 100) | p | |
|---|---|---|---|
| Age (years) | 63.29 ± 8.10 | 61.56 ± 6.1 | 0.094 |
| Sex (male/female) | 70/101 | 47/53 | 0.374 |
| BMI (kg/m2) | 25.48 ± 3.32 | 24.42 ± 2.8 | 0.012 |
| VAS-back | 4.85 ± 2.1 | 1.18 ± 1.77 | <0.001 |
| mFI-5 | 1.02 ± 1.08 | 0.39 ± 0.57 | <0.001 |
| Smoking | 17 | 14 | 0.328 |
| Duration of symptom (mo) | 89.25 ± 100.96 | — | |
| Number of stenotic levels | 2.11 ± 1.13 | — | |
| Endurance test (s) | 38.18 ± 37.24 | 134.23 ± 80.2 | <0.001 |
LSS, lumbar spinal stenosis; BMI, body mass index; VAS, visual analog scale; mFI-5, modified 5-item frailty index
The performance time of endurance test in the LSS patients were shorter than in the healthy controls in terms of different percentiles (Table 2). Approximately one-quarter of the patients could not hold the position for more than 10 s. In addition, only one in four LSS patients could hold the position for more than 60 s, whereas 80% of the healthy controls could do that.
Table 2.
The distribution of the performance time of endurance test in LSS patients and healthy controls.
| Percentile valuesa | 10% | 20% | 25% | 30% | 40% | 50% | 60% | 70% | 75% | 80% |
|---|---|---|---|---|---|---|---|---|---|---|
| Test of LSS patients (s) | 0 | 0 | 10 | 17 | 24 | 31 | 38 | 50 | 56 | 60 |
| Test of healthy controls (s) | 43 | 62 | 71 | 79 | 85 | 112 | 147 | 174 | 184 | 206 |
LSS, lumbar spinal stenosis
From poor function to good function.
Correlation analyses showed that the performance time of endurance test had a negative correlation with VAS-back, VAS-leg and ODI (r = −0.46, p < 0.001; r = −0.192, p = 0.012; r = −0.357, p < 0.001, respectively; Table 3). We also found a positive relation of paraspinal muscle endurance with LL (r = 0.212, p = 0.007; Table 3). In addition, the endurance test had a significant correlation with SVA and PT (r = −0.308, p < 0.001; r = −0.164, p = 0.038, respectively; Table 3). In multiple linear regression analysis, paraspinal muscle endurance remained significant correlations with VAS-back, ODI and SVA (β coefficient = −8.631 (−11.15, −6.111), p < 0.001; β coefficient = −0.686 (−1.06, −0.312), p < 0.001; β coefficient = −0.252 (−0.39, −0.113), p < 0.001, respectively; Table 4).
Table 3.
Correlations between the endurance test, symptoms and functional status and sagittal parameters in the LSS patients.
| VAS-back | VAS-leg | ODI | PT | SS | PI | LL | SVA | Cobb | |
|---|---|---|---|---|---|---|---|---|---|
| r | −0.46 | −0.192 | −0.357 | −0.164 | 0.065 | −0.069 | 0.212 | −0.308 | −0.141 |
| p | <0.001 | 0.012 | <0.001 | 0.038 | 0.413 | 0.388 | 0.007 | <0.001 | 0.076 |
VAS, visual analog scale; ODI, Oswestry Disability Index; PT, pelvic tilt; SS, sacral slope; PI, pelvic incidence; LL, lumbar lordosis; SVA, sagittal vertical axis
Table 4.
Multiple linear regression analysis of the relationship between the endurance test and clinical outcomes.
| Variable | β coefficient | Standardized β coefficient | P | |
|---|---|---|---|---|
| Model 1 | VAS-back | −8.631 (-11.15, −6.111) | −0.484 | <0.001 |
| Model 2 | VAS-leg | −1.614 (-4.372, 1.143) | −0.094 | 0.249 |
| Model 3 | ODI | −0.686 (-1.06, −0.312) | −0.285 | <0.001 |
| Model 4 | PT | −0.569 (-1.238, 0.1) | −0.142 | 0.095 |
| Model 5 | LL | 0.357 (-0.03, 0.744) | 0.153 | 0.07 |
| Model 6 | SVA | −0.252 (-0.39, −0.113) | −0.288 | <0.001 |
Age, BMI, duration of symptom, mFI-5, and the number of stenotic levels were included in each of the six models.
VAS, visual analog scale; ODI, Oswestry Disability Index; PT, pelvic tilt; LL, lumbar lordosis; SVA, sagittal vertical axis
Moreover, binary logistic regression revealed that the performance time of endurance test was an independent factor of poor functional status (p = 0.006, OR = 0.985; Table 5). Moreover, we found that the performance time of endurance test was also an independent factor of global sagittal imbalance (p = 0.007, OR = 0.981; Table 6).
Table 5.
Independent risk factors of poor functional status (ODI scores > 40) identified by logistic regression.
| Variable | Odds Ratio (95% CI) | P |
|---|---|---|
| Sex | 0.489 (0.231, 1.033) | 0.061 |
| Age | 1.044 (0.995, 1.096) | 0.076 |
| BMI | 0.923 (0.828, 1.029) | 0.151 |
| mFI-5 | 1.209 (0.843, 1.736) | 0.303 |
| Smoking | 1.327 (0.358, 4.921) | 0.673 |
| Duration of symptom | 0.997 (0.994, 1.001) | 0.163 |
| Number of stenotic levels | 1.058 (0.775, 1.443) | 0.724 |
| Endurance test | 0.985 (0.974, 0.996) | 0.006 |
BMI, body mass index; mFI-5, modified 5-item frailty index
Table 6.
Independent risk factors of sagittal imbalance (SVA > 50 mm) identified by logistic regression.
| Variable | Odds Ratio (95% CI) | P |
|---|---|---|
| Sex | 0.522 (0.236, 1.157) | 0.11 |
| Age | 1.034 (0.98, 1.091) | 0.227 |
| BMI | 1.207 (1.063, 1.372) | 0.004 |
| mFI-5 | 0.923 (0.636, 1.34) | 0.673 |
| Smoking | 2.666 (0.787, 9.031) | 0.115 |
| Duration of symptom | 1.001 (0.997, 1.005) | 0.661 |
| Number of stenotic levels | 0.825 (0.585, 1.165) | 0.275 |
| Endurance test | 0.981 (0.968, 0.995) | 0.007 |
BMI, body mass index; mFI-5, modified 5-item frailty index
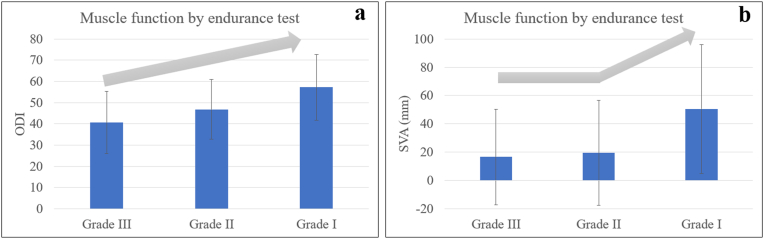
The classification by increased muscle endurance revealed that there were 41, 93 and 37 subjects in the groups with a performance time of <10, 10 to 60 and > 60 s, respectively. In PEMET classification, moving from the grade III group to the grade I group, there was a progressive worsening in VAS-back and ODI (all adjusted p < 0.05; Table 7 and Table 8; Fig. 2a). Moreover, the grade I group had significantly greater VAS- leg, less LL and greater SVA than the other two groups (all adjusted P < 0.05; Table 7, Table 8; Fig. 2b).
Table 7.
Comparison of clinical characteristics, quality of life and sagittal parameters for the three LSS groups according to PEMET classification.
| Grade I (<10s) | Grade II (10–60s) | Grade III (≥60s) | P | |
|---|---|---|---|---|
| Number of participants | 41 | 93 | 37 | |
| Endurance test (s) | 0.88 ± 2.48 | 33.39 ± 13.35 | 91.57 ± 38.88 | <0.001 |
| Age (year) | 63.68 ± 8.58 | 62.57 ± 8.04 | 64.65 ± 7.8 | 0.396 |
| Sex (male/female) | 16/25 | 41/52 | 13/24 | 0.619 |
| BMI (kg/cm2) | 27 ± 3.5 | 25.3 ± 3.43 | 25.04 ± 3.16 | 0.194 |
| mFI-5 | 1 ± 0.87 | 1.16 ± 1.18 | 0.7 ± 0.97 | 0.09 |
| Smoking (yes) | 4 | 9 | 4 | 0.98 |
| Duration of symptom (mo) | 98.83 ± 91.55 | 87.8 ± 102.09 | 82.39 ± 109.72 | 0.758 |
| Number of stenotic levels | 2.31 ± 1.44 | 2.06 ± 0.97 | 2.03 ± 1.11 | 0.456 |
| VAS-back | 5.63 ± 2.05 | 4.6 ± 1.85 | 3.11 ± 2 | <0.001 |
| VAS-leg | 5.76 ± 1.67 | 4.81 ± 2.17 | 4.41 ± 2.37 | 0.013 |
| ODI | 57.22 ± 15.49 | 46.57 ± 14.07 | 40.81 ± 14.74 | <0.001 |
| ODI ≥40 (yes) | 36 (87.8%) | 55 (59.1%) | 17 (45.9%) | <0.001 |
| PT | 25.23 ± 10.51 | 22.27 ± 8.42 | 21.34 ± 9.77 | 0.15 |
| SS | 29.27 ± 11.42 | 30.92 ± 9.13 | 30.58 ± 10.05 | 0.682 |
| PI | 54.49 ± 13.56 | 53.19 ± 11.25 | 51.92 ± 14.01 | 0.68 |
| LL | 32.23 ± 16.31 | 40.69 ± 15.17 | 41.26 ± 14.27 | 0.01 |
| SVA | 51.69 ± 45.86 | 18.35 ± 37.67 | 17.55 ± 33.63 | <0.001 |
| SVA ≥50 mm (yes) | 21 (51.2%) | 22 (23.7%) | 6 (16.2%) | 0.001 |
LSS, lumbar spinal stenosis; BMI, body mass index; mFI-5, modified 5-item frailty index; VAS, visual analog scale; ODI, Oswestry Disability Index; PT, pelvic tilt; SS, sacral slope; PI, pelvic incidence; LL, lumbar lordosis; SVA, sagittal vertical axis
Table 8.
Post hoc tests of clinical outcomes between the three groups.
| Grade I and II | Grade I and III | Grade II and III | |
|---|---|---|---|
| Endurance test | <0.001 | <0.001 | <0.001 |
| VAS-back | 0.005 | <0.001 | <0.001 |
| VAS-leg | 0.018 | 0.005 | 0.33 |
| ODI | <0.001 | <0.001 | 0.043 |
| LL | 0.004 | 0.013 | 0.856 |
| SVA | <0.001 | <0.001 | 0.92 |
VAS, visual analog scale; ODI, Oswestry Disability Index; LL, lumbar lordosis; SVA, sagittal vertical axis
Figure 2.
The mean ODI and SVA for groups by increasing severity of the paraspinal muscle endurance.
4. Discussion
The degeneration of paraspinal muscles, a common phenomenon in elderly patients, is implicated in multiple degenerative lumbar pathologies [[12], [13], [14]]. Whereas few studies have investigated the paraspinal muscle endurance in LSS patients. In our study, the LSS group had a poorer muscle endurance than the control group. Furthermore, the paraspinal muscle function of the top 20% of the LSS patients was approximately equivalent to that of the bottom 20% of the healthy controls. Leinonen et al. found that paraspinal muscle fatigue was more obvious in LSS patients than in healthy people, whereas isoinertial back endurance time was similar to that of healthy controls [6]. The discrepant result might be due to the different endurance tests since Ito test is isometric test and requires a greater back extension that might be strenuous for patients and could better distinguish between LSS patients and healthy controls [8]. Besides, LSS patients had a smaller muscularity and higher fat infiltration of paraspinal muscles when compared with healthy controls in Jiang et al.’s study, which could also illustrate that LSS patients had severer paraspinal muscle degeneration [29]. Park et al. also reported that LSS patients were inclined to occur sarcopenia, indicating that LSS patients had a decline not only in whole-body muscle function but in the back area [30].
Previous studies have demonstrated that evaluating the endurance of paraspinal muscles can directly reflect the muscle function with a greater discriminative validity [7,8]. However, little information is available on the role of paraspinal endurance on clinical outcomes in LSS patients. In the present study, paraspinal muscle endurance had a negative correlation with VAS-back, VAS-leg and ODI. Furthermore, a poor endurance was an independent factor of poor functional status. Previous studies also reported that paraspinal muscle morphometry like atrophy and fatty infiltration was associated with poor QOL in LSS patients [[31], [32], [33]]. However, a previous study reported that paraspinal muscle atrophy was not correlated with clinical outcomes after lumbar surgery [34]. In Battié et al.’s study, contrary to expectation, greater multifidus cross-sectional area was found ipsilateral to the pathology at the level of herniation in patients with unilateral symptoms of radiculopathy [35]. These inconsonant results might reflect a relatively unreliable marker of paraspinal muscle morphology on account of unstandardized imaging measurement and indirect reflection of muscle function. Moreover, Park et al. have reported that isometric extension strength had a weak correlation with ODI in univariate analysis [36]. This might indicate that the evaluation of muscle endurance was more applicable than maximal extension strength for predicting QOL. We hypothesized that the pain of spinal stenosis caused patients to reduce functional exercise of back muscles to some extent, potentially contributing to decreased functional and health status [25]. Lee et al. found that the strength of paraspinal muscles might improve pain and function after spinal fusion surgery [37]. They recommended exercise therapy not only on generalized muscle but also on localized back muscle would be potentially helpful and necessary for improving QOL.
Paraspinal muscles are supposed to provide dynamic stability to the lumbar spine [38]. However, the relationship between the endurance of paraspinal muscles and sagittal alignment has not been explored. We found that paraspinal muscle endurance had a significant correlation with SVA and was also an independent factor of global sagittal imbalance. Multiple studies have focused on the morphology and reported that a small cross-sectional area and high fat infiltration of paraspinal muscles were associated with increased SVA [17,18,39,40]. Park et al. have also reported a poor muscle function of whole body including impaired hand grip strength and gait velocity in patients with increased SVA [17]. As paraspinal muscles play an essential role in the maintenance of postural balance, the patients with poor extensor endurance might be prone to a more forward leaning posture contributing to global sagittal imbalance [17,25].
In addition, we also found a positive relation of paraspinal muscle endurance test with LL. Several studies have reported accordant results that the strength and morphology of back extensor muscles were associated with LL [18,39,41]. However, the current study can not establish the nature of the causal relationship between spine curvature and muscle function. One plausible hypothesis is that different lumbar curvature requires a variety of stabilizing forces and paraspinal muscle forces will change correspondingly with the change of LL [42]. Some modelling studies and imaging studies have demonstrated that greater muscle function is required to provide stability in lumbar spines that have larger curvatures [18,42,43]. In patients with LSS, the narrowing of the spinal canal forces patients to bend forward thus decreasing LL and eventually leading to muscle atrophy and poor muscle function. Another possible mechanism is that spine should conform to a shape which can be successfully stabilized by the available muscle forces [42]. The patients with poor paraspinal muscle endurance might tend to maintain a reduced LL.
Moreover, the endurance test had a negative correlation with PT in our study. Previous studies have also revealed a correlation between degeneration of paraspinal muscle morphology and pelvis retroversion [44,45]. With progressive back extensor musculature fatigue, patients would adopt pelvis retroversion more to maintain an upright posture [39,46].
Of note, in our study, a preliminary PEMET classification for LSS patients was constructed. We used 10 s and 60 s that were approximatively two quartiles of the performance time in the LSS patients as the dividing values of the classification. We revealed that there was progressive worsening in QOL and undesired sagittal alignment when moving from the grade III group to the grade I group. Both 10 s and 60 s of the performance time were cut-off values for QOL while only 10 s was cut-off value for sagittal imbalance. These might be interpreted by the findings that the LSS patients who held the position for more than 60 s were more like healthy controls with better functional status. For global sagittal imbalance, our findings suggested that the LSS patients with endurance test less than 10 s might not be able to maintain the postural balance. Bae et al. found that sagittal decompensation correlated with poor paraspinal muscle quality and they thought that degenerated muscles were causes of initial decompensation of decompensated sagittal deformity [45,47]. Combined with our study, the endurance test less than 10 s might be an indicator for patients who have decompensation of sagittal imbalance. Pre- or at least postsurgical physical exercise regimen might be applicable for these patients to improve sagittal imbalance to some extent. Our PEMET classification system might be applied into clinical practice for distinguishing the degree of paraspinal muscle endurance and predicting patients’ QOL and sagittal imbalance during preoperative evaluation.
We recognize limitations in the present study. First, the causal relationship between poor paraspinal muscle endurance, undesired QOL and sagittal imbalance could not be confirmed, because this study reflects only cross-sectional relationship. Second, the endurance test of paraspinal muscles could be affected by pain in LSS patients. Whereas we have ruled out the patients with acute or severe chronic pain of spinal stenosis to reduce the impact of pain. The instant VAS of all the patients before test were limited in six scores according to previous study [9]. Moreover, the sample size of the three groups according to PEMET classification was relatively small, which might produce bias. The clinical value of the novel classification of muscle endurance should be verified by further prognosis studies.
In conclusion, the LSS group had a poorer paraspinal muscle endurance than the control group. Paraspinal muscle endurance had a significant correlation with QOL and sagittal spinopelvic alignment. Furthermore, we demonstrated that poor endurance was an independent factor of both undesired functional status and increased SVA. It is speculated that strengthening the back extensor muscle in an appropriate way in LSS patients is beneficial for enhancing QOL and maintaining a desired sagittal spinal curve. Moreover, we proposed a PEMET classification for LSS patients constructed by paraspinal muscle endurance that has shown correlations with QOL and sagittal alignment. This classification system might be available for identifying the severity of clinical symptoms and sagittal imbalance, which needs further work to refine.
Funding/support
This work was supported by the Clinical Cohort Construction Program of Peking University Third Hospital, China National Key Research and Development Program (2018YFB1307700).
Declaration of competing interest
The authors declare no conflict of interests.
Acknowledgments
Not Applicable.
References
- 1.Siebert E., Prüss H., Klingebiel R., Failli V., Einhäupl K.M., Schwab J.M. Lumbar spinal stenosis: syndrome, diagnostics and treatment. Nat Rev Neurol. 2009;5:392–403. doi: 10.1038/nrneurol.2009.90. [DOI] [PubMed] [Google Scholar]
- 2.Kalichman L., Cole R., Kim D.H., Li L., Suri P., Guermazi A., et al. Spinal stenosis prevalence and association with symptoms: the Framingham Study. Spine J. 2009;9:545–550. doi: 10.1016/j.spinee.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrokhi M.R., Haghnegahdar A., Rezaee H., Sharifi Rad M.R. Spinal sagittal balance and spinopelvic parameters in patients with degenerative lumbar spinal stenosis; a comparative study. Clin Neurol Neurosurg. 2016;151:136–141. doi: 10.1016/j.clineuro.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Shin E.K., Kim C.H., Chung C.K., Choi Y., Yim D., Jung W., et al. Sagittal imbalance in patients with lumbar spinal stenosis and outcomes after simple decompression surgery. Spine J. 2017;17:175–182. doi: 10.1016/j.spinee.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 5.Wang J., Ullah S., Solano M.A., Overley S.C., Bumpass D.B., Mannen E.M. Changes in kinematics, kinetics, and muscle activity in patients with lumbar spinal stenosis during gait: systematic review. Spine J. 2021;22:157–167. doi: 10.1016/j.spinee.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Leinonen V., Määttä S., Taimela S., Herno A., Kankaanpää M., Partanen J., et al. Paraspinal muscle denervation, paradoxically good lumbar endurance, and an abnormal flexion-extension cycle in lumbar spinal stenosis. Spine (Phila Pa 1976. 2003;28:324–331. doi: 10.1097/01.BRS.0000048495.81763.8C. [DOI] [PubMed] [Google Scholar]
- 7.Biering-Sørensen F. Physical measurements as risk indicators for low-back trouble over a one-year period. Spine (Phila Pa 1976. 1984;9:106–119. doi: 10.1097/00007632-198403000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Demoulin C., Vanderthommen M., Duysens C., Crielaard J.M. Spinal muscle evaluation using the Sorensen test: a critical appraisal of the literature. Joint Bone Spine. 2006;73:43–50. doi: 10.1016/j.jbspin.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Ito T., Shirado O., Suzuki H., Takahashi M., Kaneda K., Strax T.E. Lumbar trunk muscle endurance testing: an inexpensive alternative to a machine for evaluation. Arch Phys Med Rehabil. 1996;77:75–79. doi: 10.1016/s0003-9993(96)90224-5. [DOI] [PubMed] [Google Scholar]
- 10.Müller R., Strässle K., Wirth B. Isometric back muscle endurance: an EMG study on the criterion validity of the Ito test. J Electromyogr Kinesiol. 2010;20:845–850. doi: 10.1016/j.jelekin.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Holmström E., Moritz U., Andersson M. Trunk muscle strength and back muscle endurance in construction workers with and without low back disorders. Scand J Rehabil Med. 1992;24:3–10. [PubMed] [Google Scholar]
- 12.Beneck G.J., Kulig K. Multifidus atrophy is localized and bilateral in active persons with chronic unilateral low back pain. Arch Phys Med Rehabil. 2012;93:300–306. doi: 10.1016/j.apmr.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Belavy D.L., Armbrecht G., Richardson C.A., Felsenberg D., Hides J.A. Muscle atrophy and changes in spinal morphology is the lumbar spine vulnerable after prolonged bed-rest? Spine (Phila Pa 1976. 2011;36:137–145. doi: 10.1097/BRS.0b013e3181cc93e8. [DOI] [PubMed] [Google Scholar]
- 14.Kalichman L., Carmeli E., Been E. The association between imaging parameters of the paraspinal muscles, spinal degeneration, and low back pain. BioMed Res Int. 2017;2017 doi: 10.1155/2017/2562957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barker K.L., Shamley D.R., Jackson D. Changes in the cross-sectional area of multifidus and psoas in patients with unilateral back pain: the relationship to pain and disability. Spine (Phila Pa 1976. 2004;29:E515–E519. doi: 10.1097/01.brs.0000144405.11661.eb. [DOI] [PubMed] [Google Scholar]
- 16.Fortin M., Lazary A., Varga P.P., Battie M.C. Association between paraspinal muscle morphology, clinical symptoms and functional status in patients with lumbar spinal stenosis. Eur Spine J. 2017;26:2543–2551. doi: 10.1007/s00586-017-5228-y. [DOI] [PubMed] [Google Scholar]
- 17.Park J.S., Park Y.S., Kim J., Hur J., Choe D.H. Sarcopenia and fatty degeneration of paraspinal muscle associated with increased sagittal vertical axis in the elderly: a cross-sectional study in 71 female patients. Eur Spine J. 2020;29:1353–1361. doi: 10.1007/s00586-020-06416-5. [DOI] [PubMed] [Google Scholar]
- 18.Xia W., Fu H., Zhu Z., Liu C., Wang K., Xu S., et al. Association between back muscle degeneration and spinal-pelvic parameters in patients with degenerative spinal kyphosis. BMC Muscoskel Disord. 2019;20:454. doi: 10.1186/s12891-019-2837-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim W.J., Shin H.M., Lee J.S., Song D.G., Lee J.W., Chang S.H., et al. Sarcopenia and back muscle degeneration as risk factors for degenerative adult spinal deformity with sagittal imbalance and degenerative spinal disease: a comparative study. World Neurosurg. 2021;148:e547–e555. doi: 10.1016/j.wneu.2021.01.053. [DOI] [PubMed] [Google Scholar]
- 20.Steurer J., Roner S., Gnannt R., Hodler J. Quantitative radiologic criteria for the diagnosis of lumbar spinal stenosis: a systematic literature review. BMC Muscoskel Disord. 2011;12:175. doi: 10.1186/1471-2474-12-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danneels L.A., Vanderstraeten G.G., Cambier D.C., Witvrouw E.E., De Cuyper H.J. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur Spine J. 2000;9:266–272. doi: 10.1007/s005860000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sions J.M., Elliott J.M., Pohlig R.T., Hicks G.E. Trunk muscle characteristics of the multifidi, erector spinae, psoas, and quadratus lumborum in older adults with and without chronic low back pain. J Orthop Sports Phys Ther. 2017;47:173–179. doi: 10.2519/jospt.2017.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen R.K., Jensen T.S., Koes B., Hartvigsen J. Prevalence of lumbar spinal stenosis in general and clinical populations: a systematic review and meta-analysis. Eur Spine J. 2020;29:2143–2163. doi: 10.1007/s00586-020-06339-1. [DOI] [PubMed] [Google Scholar]
- 24.Weaver D.J., Malik A.T., Jain N., Yu E., Kim J., Khan S.N. The modified 5-item frailty index: a concise and useful tool for assessing the impact of frailty on postoperative morbidity following elective posterior lumbar fusions. World Neurosurg. 2019 doi: 10.1016/j.wneu.2018.12.168. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Shen F., Kim H.J., Jeon S.W., Chang B.S., Lee C.K., Yeom J.S. Influence of handgrip strength and paraspinal muscles' volume on clinical outcomes in the patients with each sagittal imbalance and lumbar spinal stenosis. Global Spine J. 2021 doi: 10.1177/21925682211001871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan D.J., Protopsaltis T.S., Ames C.P., Hostin R., Klineberg E., Mundis G.M., et al. T1 pelvic angle (TPA) effectively evaluates sagittal deformity and assesses radiographical surgical outcomes longitudinally. Spine. 2014;39:1203–1210. doi: 10.1097/BRS.0000000000000382. [DOI] [PubMed] [Google Scholar]
- 27.Ekström L., Zhang Q., Abrahamson J., Beck J., Johansson C., Westin O., et al. A model for evaluation of the electric activity and oxygenation in the erector spinae muscle during isometric loading adapted for spine patients. J Orthop Surg Res. 2020;15:155. doi: 10.1186/s13018-020-01652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanderson A., Martinez-Valdes E., Heneghan N.R., Murillo C., Rushton A., Falla D. Variation in the spatial distribution of erector spinae activity during a lumbar endurance task in people with low back pain. J Anat. 2019;234:532–542. doi: 10.1111/joa.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang J., Wang H., Wang L., Zhang B., Guo Q., Yuan W., et al. Multifidus degeneration, a new risk factor for lumbar spinal stenosis: a case-control study. World Neurosurg. 2017;99:226–231. doi: 10.1016/j.wneu.2016.11.142. [DOI] [PubMed] [Google Scholar]
- 30.Park S., Kim H.J., Ko B.G., Chung J.W., Kim S.H., Park S.H., et al. The prevalence and impact of sarcopenia on degenerative lumbar spinal stenosis. Bone Joint Lett J. 2016;98-b:1093–1098. doi: 10.1302/0301-620X.98B8.37623. [DOI] [PubMed] [Google Scholar]
- 31.Fortin M., Lazáry À., Varga P.P., Battié M.C. Association between paraspinal muscle morphology, clinical symptoms and functional status in patients with lumbar spinal stenosis. Eur Spine J. 2017;26:2543–2551. doi: 10.1007/s00586-017-5228-y. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y.-Y., Pao J.-L., Liaw C.-K., Hsu W.-L., Yang R.-S. Image changes of paraspinal muscles and clinical correlations in patients with unilateral lumbar spinal stenosis. Eur Spine J. 2014;23:999–1006. doi: 10.1007/s00586-013-3148-z. [DOI] [PubMed] [Google Scholar]
- 33.Wang W., Sun Z., Li W., Chen Z. The effect of paraspinal muscle on functional status and recovery in patients with lumbar spinal stenosis. J Orthop Surg Res. 2020;15:235. doi: 10.1186/s13018-020-01751-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han G., Zou D., Li X., Zhang S., Li Z., Zhou S., et al. Can fat infiltration in the multifidus muscle be a predictor of postoperative symptoms and complications in patients undergoing lumbar fusion for degenerative lumbar spinal stenosis? A case-control study. J Orthop Surg Res. 2022;17:289. doi: 10.1186/s13018-022-03186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Battie M.C., Niemelainen R., Gibbons L.E., Dhillon S. Is level- and side-specific multifidus asymmetry a marker for lumbar disc pathology? Spine J. 2012;12:932–939. doi: 10.1016/j.spinee.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 36.Park W.H., Lee C.S., Kang K.C., Seo Y.G. Characteristics of back muscle strength in patients with scheduled for lumbar fusion surgery due to symptomatic lumbar degenerative diseases. Asian Spine J. 2014;8:659–666. doi: 10.4184/asj.2014.8.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee C.-S., Kang K.-C., Chung S.-S., Park W.-H., Shin W.-J., Seo Y.-G. How does back muscle strength change after posterior lumbar interbody fusion? J Neurosurg Spine. 2017;26:163–170. doi: 10.3171/2016.7.SPINE151132. [DOI] [PubMed] [Google Scholar]
- 38.Diebo B.G., Shah N.V., Boachie-Adjei O., Zhu F., Rothenfluh D.A., Paulino C.B., et al. Adult spinal deformity. Lancet. 2019;394:160–172. doi: 10.1016/S0140-6736(19)31125-0. [DOI] [PubMed] [Google Scholar]
- 39.Banno T., Yamato Y., Hasegawa T., Kobayashi S., Togawa D., Oe S., et al. Assessment of the cross-sectional areas of the psoas major and multifidus muscles in patients with adult spinal deformity: a case-control study. Clin Spine Surg. 2017;30:E968–e973. doi: 10.1097/BSD.0000000000000480. [DOI] [PubMed] [Google Scholar]
- 40.Han G., Wang W., Zhou S., Li W., Zhang B., Sun Z., et al. Paraspinal muscle degeneration as an independent risk for loss of local alignment in degenerative lumbar scoliosis patients after corrective surgery. Global Spine J. 2021 doi: 10.1177/21925682211022284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hongo M., Miyakoshi N., Shimada Y., Sinaki M. Association of spinal curve deformity and back extensor strength in elderly women with osteoporosis in Japan and the United States. Osteoporos Int. 2012;23:1029–1034. doi: 10.1007/s00198-011-1624-z. [DOI] [PubMed] [Google Scholar]
- 42.Meakin J.R., Fulford J., Seymour R., Welsman J.R., Knapp K.M. The relationship between sagittal curvature and extensor muscle volume in the lumbar spine. J Anat. 2013;222:608–614. doi: 10.1111/joa.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meakin J.R., Aspden R.M. Modeling the effect of variation in sagittal curvature on the force required to produce a follower load in the lumbar spine. J Mech Med Biol. 2012;12 [Google Scholar]
- 44.Menezes-Reis R., Bonugli G.P., Salmon C.E.G., Mazoroski D., Herrero C., Nogueira-Barbosa M.H. Relationship of spinal alignment with muscular volume and fat infiltration of lumbar trunk muscles. PLoS One. 2018;13 doi: 10.1371/journal.pone.0200198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bae J., Sathe A., Lee S.M., Theologis A.A., Deviren V., Lee S.H. Correlation of paraspinal muscle mass with decompensation of sagittal adult spinal deformity after setting of fatigue post 10-minute walk. Neurospine. 2021;18:495–503. doi: 10.14245/ns.2142510.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheung J.P.Y. The importance of sagittal balance in adult scoliosis surgery. Ann Transl Med. 2020;8:35. doi: 10.21037/atm.2019.10.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bae J., Theologis A.A., Jang J.S., Lee S.H., Deviren V. Impact of fatigue on maintenance of upright posture: dynamic assessment of sagittal spinal deformity parameters after walking 10 minutes. Spine (Phila Pa 1976. 2017;42:733–739. doi: 10.1097/BRS.0000000000001898. [DOI] [PubMed] [Google Scholar]