ABSTRACT
Background
Restless legs syndrome (RLS) is common among patients with end-stage kidney disease (ESKD) and is associated with poor outcomes. Several recently published studies had focused on pharmacological and non-pharmacological treatments of RLS, but an updated meta-analysis has not been conducted.
Methods
The study population was adult ESKD patients on dialysis with RLS. Randomized controlled trials (RCTs) were selected. The primary outcome was reduction in RLS severity. The secondary outcomes were improvement in sleep quality and treatment-related adverse events. Frequentist standard network meta-analysis (NMA) and additive component NMA were performed. The evidence certainty was assessed using the Confidence in NMA (CINeMA) framework.
Results
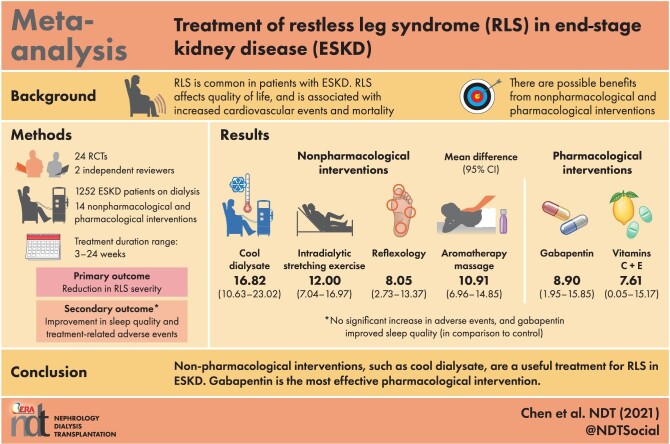
A total of 24 RCTs with 1252 participants were enrolled and 14 interventions were compared. Cool dialysate produced the largest RLS severity score reduction {mean difference [MD] 16.82 [95% confidence interval (CI) 10.635–23.02]} and a high level of confidence. Other potential non-pharmacological interventions include intradialytic stretching exercise [MD 12.00 (95% CI 7.04–16.97)] and aromatherapy massage [MD 10.91 (95% CI 6.96–14.85)], but all with limited confidence of evidence. Among the pharmacological interventions, gabapentin was the most effective [MD 8.95 (95% CI 1.95–15.85)], which also improved sleep quality [standardized MD 2.00 (95% CI 0.47–3.53)]. No statically significant adverse events were detected.
Conclusions
The NMA supports that cool dialysate is appropriate to treat patients with ESKD and RLS. Gabapentin is the most effective pharmacological intervention and also might improve sleep quality. Further parallel RCTs with sufficient sample sizes are required to evaluate these potential interventions and long-term effects.
Keywords: cool dialysate, end-stage kidney disease, gabapentin, restless legs syndrome
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
Several different pharmacological or non-pharmacological interventions were studied and applied in end-stage kidney disease (ESKD) patients with restless legs syndrome (RLS). Aromatherapy massage, cool dialysate and different types of intradialytic exercise were demonstrated to be a benefit in recent trials.
What this study adds?
We demonstrated that cool dialysate might be the most effective intervention for RLS treatment in ESKD. Gabapentin is the most effective pharmacological intervention and could also improve sleep quality. Other potentially effective interventions include aromatherapy massage, intradialytic stretching exercises and reflexology.
What impact this may have on practice or policy?
Non-pharmacological interventions like cool dialysate could be applied to ESKD patients with RLS. Considering other potential benefits of cool dialysate, it is appropriate to be applied in ESKD patients with RLS. A combination of effective pharmacological and non-pharmacological interventions could be considered as well.
INTRODUCTION
Restless legs syndrome (RLS) is a neuromuscular disorder characterized by uncomfortable sensations in the extremities accompanied by an urge to move those limbs. These uncomfortable sensations usually occur at night or at rest and can be relieved by movement. The prevalence of RLS in end-stage kidney disease (ESKD) patients ranges from 6.6% to 70% [1]. RLS is not only associated with a negative effect on quality of life, but is also associated with increased cardiovascular events and mortality [2–4].
Factors reported to be associated with RLS include peripheral neuropathy, dopaminergic system dysfunction and iron deficiency in specific cerebral areas [5]. Haung et al. [6] concluded that gabapentin is the most effective treatment for RLS in patients with ESKD. However, several randomized control trials (RCTs) have been undertaken since then. Furthermore, several recent trials have reported possible benefits from non-pharmacological interventions, including cool dialysate and aromatherapy massage [7–12]. Therefore we aimed to conduct an updated systematic review and component network meta-analysis (NMA) that included new RCTs and non-pharmacological interventions to compare the treatment efficacy and acceptability of these interventions used in the treatment of adult patients with ESKD and RLS.
MATERIALS AND METHODS
Literature search strategy
We performed this NMA in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for NMA (Supplementary data, Table S1) [13]. We registered our study protocol in PROSPERO (CRD42021252543).
Two independent reviewers (J.-J.C. and T.H.L.) searched for studies published prior to 1 May 2021 in the databases of PubMed, MEDLINE, the Cochrane Library and Embase. Search strategies targeted published clinical trials that compared the efficacy of different interventions in treating RLS among adult patients on dialysis. The detailed search strategy and results of that search process are provided in Supplementary data, Table S2. Review articles and meta-analyses were not included in our analysis, but their references were screened for relevant studies. A gray literature search was also conducted in Google Scholar and ResearchGate using the following keywords: ‘restless legs syndrome’ and ‘dialysis’ OR ‘end stage renal disease’. We also examined unpublished or ongoing clinical trials.
Study eligibility criteria
The titles and abstracts of studies returned by the search were independently examined by two reviewers (J.-J.C. and T.H.L.) and the articles were excluded upon initial screening if their titles or abstracts indicated that they were clearly irrelevant to the objective of the current study. Full texts of relevant articles were reviewed to determine whether the studies were eligible to be included in the NMA. A study was included for analysis in the present study if it enrolled adults with ESKD who were under dialysis; allocated patients to at least two different intervention arms to compare the efficacy of interventions; reported outcomes on changes in RLS severity, sleep quality, treatment-related adverse events or quality of life and was an RCT. A third reviewer (G.K.) was consulted to reach an agreement through consensus in the case of any disagreement regarding a given study's eligibility.
Crossover-design trials and single-arm trials were excluded. The study population focus was on patients with ESKD and RLS, thus those studies that examined patients with idiopathic RLS or RLS preceding ESKD were excluded.
Data extraction and outcomes
Two investigators (J.-J.C. and T.H.L.) independently classified the therapies and extracted the study parameters. The primary outcome was the treatment efficacy of RLS severity. In most studies, the severity of RLS was evaluated according to the International RLS (IRLS) rating scale, which comprises an overall score that ranges from 0 to 40 [14]. If a study used more than one rating scale to assess RLS severity, we accorded priority to the IRLS scale. Secondary outcomes, including sleep quality and quality of life, were also extracted. The patients’ subjective sleep quality levels were assessed by using the Pittsburgh Sleep Quality Index (PSQI) and the Epworth Sleepiness Scale (ESS). The PSQI is based on a 4-point Likert scale with total scores ranging from 0 to 21 [15]. The ESS consists of eight situations with a total score ranging from 0 to 24 [16]. To assess patient quality of life we used the 36-item Short Form Survey (SF-36) [17]. Another secondary outcome was treatment acceptability, which was evaluated by the extent of treatment-related adverse events (i.e. unfavorable or unintended signs and symptoms associated with interventions) recorded in the enrolled RCTs.
Data synthesis and analysis
As for the evaluation of the effects of treatments on RLS severity, we extracted the mean difference (MD) with 95% confidence interval (CI) or standard deviation (SD) of the MD associated with each intervention arm from baseline to posttreatment stage; this was done to pool continuous outcomes. To evaluate the effects of the different treatments on sleep quality (according to the various scales), we extracted the standardized MD (SMD) with the standard error of the SMD. For treatment-related adverse events, odds ratios (ORs) were used to pool binary outcomes. Heterogeneity was examined by I2. A two-tailed P-value <0.05 was considered statistically significant. The frequentist NMA and additive component NMA with random-effects model was performed using the statistical package netmeta in R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria) [18]. All pairwise comparisons were summarized in a league table. In the frequentist standard NMA, a P-score, ranging between 0 and 1, was used to rank treatments compared within an NMA for a particular outcome (treatment effects on RLS severity, sleep quality and treatment-related adverse events) [19]. P-score has a similar interpretation to its Bayesian counterpart, surface under the cumulative ranking curve (SUCRA); a treatment with a higher P-score has a higher rank than a treatment with a lower P-score. The inconsistency was evaluated by the design-by-treatment interaction model and node-splitting model [20]. Small-study effect bias was assessed by the Egger test and a funnel plot.
Risk-of-bias and quality assessments
The study quality was assessed by two investigators (J.-J.C. and T.H.L.) using the revised Cochrane risk-of-bias tool for randomized trials (RoB 2) [21] and any disagreements among the two investigators were resolved through consensus with another author (G.K.). We also assessed the confidence of evidence by using the confidence in NMA (CINeMA) framework [22].
Component NMA
Additive component NMA was conducted. Component NMA, a variant of the standard NMA, allows for decomposition of complex interventions and estimates the treatment effects of each component from composite interventions. We examined whether the effects of components are additive, i.e. the effect of a component combined with another component is equal, or is greater (synergistic) or smaller (antagonistic) than the sum of the two components by comparing the results of the standard NMA with that of the additive component NMA. In component NMA, we used the mvrnorm function in the R package MASS for running 1000 stimulations, based on the variance–covariance matrix of the component effect estimates, to obtain ranking probabilities of components.
RESULTS
Study characteristics
The flow and search strategy are detailed in Supplementary data, Figure S1 and Table S2. Through the electronic database search and after removing the duplicate articles, a total of 1047 potentially eligible studies were screened according to the abstract and the titles. After screening, 36 full-text articles were further assessed for eligibility. Finally, 24 RCTs were included (18 two-arm studies, 5 three-arm studies and 1 four-arm study) [7–12, 23–40]. A total of 1252 participants were enrolled: these participants had been randomly allocated to 1 of 14 intervention or control groups (dopamine agonist, n = 118; gabapentin, n = 86; iron, n = 27; vitamin C, n = 30; vitamin E, n = 15; vitamin C and vitamin E, n = 15; cool dialysate, n = 52; intradialytic stretching exercise, n = 86; intradialytic aerobic exercise, n = 64; intradialytic aerobic exercise and dopamine agonist, n = 7; aromatherapy massage, n = 186; reflexology, n = 65; acupoint therapy, n = 64; neuromuscular electrical stimulation, n = 30; control group, n = 395). The characteristics of the selected RCTs are summarized in Table 1.
Table 1.
Characteristics of the included studies
| Study | N | Average age (years) | RRT modality | Average RRT vintage (months) | Treatment detail (arm) | Treatment duration (weeks) | Study design | Risk of bias | Outcome evaluation |
|---|---|---|---|---|---|---|---|---|---|
| Fauzi and Triaswati, 2021 [7] | 38 | 45.6 | HD (100%) | NR | Intradialytic stretching exercise | 8 | RCT, non-blind | High risk | RLS-3 scale, PSQI scale |
| Control | |||||||||
| Ajorpaz et al., 2019 [23] | 90 | 56.7 | HD (100%) | NR | Glycerin oil message (aromatherapy massage) | 4 | RCT, double-blind | Low risk | IRLS score |
| Lavender oil message (aromatherapy massage) | |||||||||
| Control | |||||||||
| Ali et al., 2020 [8] | 26 | 45.5 | HD (100%) | 62.54 | Levodopa, 110 mg QD | 4 | RCT | Some concerns | IRLS score, PSQI scale |
| Gabapentin, 200 mg TIW | |||||||||
| Aliabadi et al., 2020 [9] | 40 | 54.5 | HD (100%) | NR | Intradialytic stretching exerciseCold dialysate, 35.5°C | 6 | RCT, double-blind | Low risk | IRLS score |
| Aliasgharpour et al., 2016 [24] | 33 | NR | HD (100%) | NR | Intradialytic stretching exerciseControl | 8 | RCT | Some concerns | IRLS score |
| Dauvilliers et al., 2016 [25] | 25 | 55.0 | HD (100%) | 39.6 | Rotigotine, 1-3 mg QDPlacebo | 5* | RCT, double-blind | High risk | PLMI ratio, PLM during sleep with arousals/hour TST, IRLS score, CGI-1, RLS-6, RLS-QoL28, SF-36 |
| Deng et al., 2017 [26] | 32 | 63.9 | HD (100%) | 35.34 | Iron sucrose, 100 mg TIWPlacebo | NR | RCT, non-blind, placebo-controlled | High risk | IRLS score |
| Gao et al., 2016 [27] | 69 | 45.0 | HD (100%) | NR | Moxibustion (acupoint therapy) | 16 | RCT | Low risk | IRLS score, PSQI scale |
| Control | |||||||||
| Ghasemi et al., 2018 [28] | 70 | 50.5 | HD (100%) | 58.92 | ReflexologyPlacebo | 4 | RCT, single-blind | Low risk | IRLS score |
| Giannaki et al., 2013 [47] | 29 | 56.3 | HD (100%) | 46.22 | Intradialytic aerobic exerciseRopinirole, 0.25 mg QDPlacebo | 24 | RCT, partial double-blind | High risk | Sleep diary, ESS, Zung depression scale, IRLS score, SF-36, SF-36 PCS score |
| Giannaki et al., 2015 [30] | 13 | 55.1 | HD (100%) | 45.97 | Intradialytic aerobic exercise +Ropinirole, 0.25 mg QD | 24 | RCT, double-blind | Low risk | IRLS score |
| Intradialytic aerobic exercise +Placebo | |||||||||
| Hajian et al., 2020 [11] | 60 | 64.0 | HD (100%) | 46.20 | Pramipexole, 0.18 ng QDGabapentin, 100 mg QD | 4 | RCT | High risk | IRLS score |
| Hashemi et al., 2015 [31] | 59 | 59.3 | HD (100%) | 45.60 | Lavender oil massage (aromatherapy massage) | 3 | RCT, single-blind | High risk | IRLS score |
| Control | |||||||||
| Kashani et al., 2019 [32] | 63 | 55.9 | HD (100%) | 35.92 | Cool dialysate, 35.5°CControl dialysate, 37°C | 4 | RCT, triple-blind | Low risk | IRLS score |
| Mohammadi et al., 2018 [33] | 60 | 56.7 | HD (100%) | 36.88 | Near-infrared light to acupoints (acupoint therapy) | 4 | RCT, single-blind | High risk | IRLS score |
| Sham therapy | |||||||||
| Mortazavi et al., 2013 [34] | 26 | 41.5 | HD (100%) | NR | Intradialytic aerobic exerciseControl | 16 | RCT | High risk | RLSQ questionnaire, SF-36 |
| Nasiri et al., 2019 [35] | 55 | 52.0 | HD (100%) | NR | Olive oil message (aromatherapy massage) | 3 | RCT, double-blind | Low risk | IRLS score |
| Placebo massage | |||||||||
| Oshvandi et al., 2021 [10] | 105 | 51.9 | HD (100%) | >12 months | Lavender oil message (aromatherapy massage) | 3 | RCT, double-blind | Low risk | IRLS score, PSQI scale |
| Orange oil message (aromatherapy massage) | |||||||||
| Control | |||||||||
| Rafie et al., 2016 [36] | 44 | 57.4 | HD (100%) | 31.3 | Pramipexole, 0.18 mg QD | 8 | RCT, double-blind | Low risk | IRLS score |
| Vitamin C, 250 mg QD | |||||||||
| Placebo | |||||||||
| Razazian et al., 2015 [37] | 82 | 55.3 | HD (100%) | 34.18 | Gabapentin, 200 mg TIWLevodopa, 110 mg QD | 4 | RCT, double-blind | Low risk | IRLS score, PSQI scale |
| Sagheb et al., 2012 [38] | 60 | 52.7 | HD (100%) | 15.92 | Vitamin C, 200 mg QD | 8 | RCT, double-blind | Low risk | IRLS score |
| Vitamin E, 400 mg QD | |||||||||
| Vitamin C, 200 mg QD + vitamin E, 400 mg QD | |||||||||
| Placebo | |||||||||
| Shahgholian et al., 2016 [39] | 90 | 55.5 | HD (100%) | 35.34 | ReflexologyIntradialytic stretching exercisesControl | 4 | RCT | High risk | IRLS score, RLS severity questionnaires |
| Sloand et al., 2004 [40] | 23 | 55.2 | HD (88%), PD (12%) | 35.40 | Iron dextran, 1000 mg TIWPlacebo | 4 | RCT, double-blind | Low risk | RLS score |
| Youssef et al., 2019 [12] | 60 | 44.7 | HD (100%) | 23.82 | Neuromuscular electrical stimulation | 12 | RCT | High risk | STS-5, STS-60, IRLS score |
| Intradialytic aerobic exercise | RCT, non-blind | RLS-3 scale, PSQI scale |
Treatment duration including 3 weeks of treatment dosage titration and 2 weeks of maintenance.
HD, hemodialysis; N, number; PD, peritoneal dialysis; PSQI, Pittsburgh Sleep Quality Index; RLS-6, RLS 6-item questionnaire; RLS-QoL28, RLS quality-of-life questionnaire; RRT, renal replacement therapy; TST, total sleep time.
The included RCTs had sample sizes ranging from 13 to 105 participants per trial. The RLS diagnoses followed the International Restless Legs Syndrome Study Group criteria in all selected studies. More than half of the participants (53.6%) were women and the average age of participants was 53.7 years. Most participants received haemodialysis, except for those in one study where 12% of participants received peritoneal dialysis [40]. The durations of interventions were 3–24 weeks. Treatment-related adverse events were extracted. Nausea, vomiting or allergic reaction were reported in patients receiving dopamine agonist treatment [36, 37]. Somnolence and lethargy were reported in patients receiving gabapentin treatment [37]. Nausea and dyspepsia were observed in patients taking vitamin C, vitamin E or a combination of the two [38]. One patient withdrew from the cool dialysate intervention group due to chills [9]. No critical adverse events were reported in the enrolled RCTs.
Figure 1 illustrates the network plot with 15 interventions (15 nodes) and 21 direct comparative arms to compare the treatment efficacy in reducing RLS symptom severity from 21 selected studies. Supplementary data, Figure S2A and B illustrate the network plots for efficacy of treatments of sleep quality and for treatment-related adverse events, respectively.
FIGURE 1:
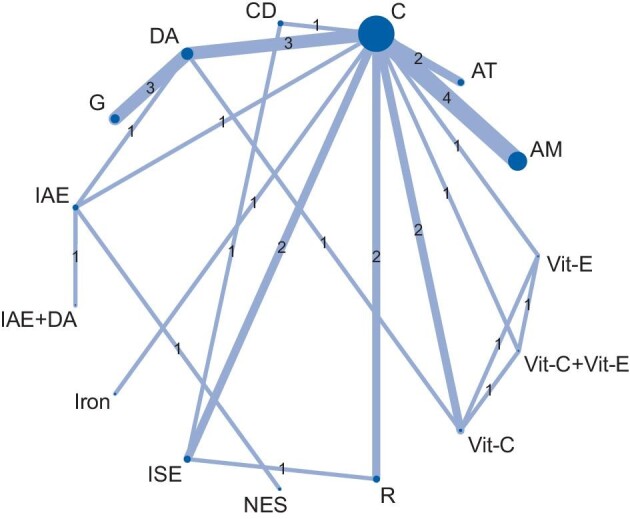
Network plot of eligible comparisons among interventions for RLS symptom relief. The size of each node indicates the number of randomized allocated participants and the width of each line indicates the number of trials of each comparison. AM, aromatherapy massage; AT, acupoint therapy; C, control; CD, cool dialysate; DA, dopamine agonist; G, gabapentin; IAE, intradialytic aerobic exercise; ISE, intradialytic stretching exercise; NES, neuromuscular electrical stimulation; Vit-C, vitamin C; Vit-E, vitamin E; R, reflexology.
NMA outcomes
RLS severity
The pooled treatment results of 14 interventions are summarized in Figure 2A and Supplementary data, Table S3. Cool dialysate achieved the greatest reduction in RLS severity compared with the control group [MD 16.82 (95% CI 10.63–23.02)]. Intradialytic stretching exercises exhibited the second highest efficacy in reducing RLS severity [MD 12.00 (95% CI 7.04–16.97)]. Two non-pharmacological interventions also showed potential benefit in RLS treatment: aromatherapy massage [MD 10.91 (95% CI 6.96–14.85)] and reflexology [MD 8.05 (95% CI 2.73–13.27)]. Among the pharmacological interventions, gabapentin was the most effective [MD 8.95 (95% CI 1.95–15.85)]. Considerable heterogeneity across studies was noted (I2 = 90.8%). The funnel plot indicated no small-study bias and the P-value of the Egger test was 0.72 (Supplementary data, Figure S3A). The result from the current NMA mostly came from indirect evidence (Supplementary data, Figure S4A). The full design-by-treatment interaction model revealed incoherence among various study designs with Q = 22.19, P = 0.001. The node-splitting model revealed potential loop incoherence from six comparisons, but these results were not statistically significant (Supplementary data, Figure S5A and Supplementary Document).
FIGURE 2:
Forest plot of (A) NMA of RLS symptom relief and of (B) component NMA of RLS symptom relief.
Sleep quality
The results of the present NMA suggested that gabapentin and acupoint therapy improved the sleep quality of patients with ESKD and RLS compared with the control group (Figure 3A and Supplementary data, Table S4). Among these interventions, gabapentin achieved the greatest improvement in sleep quality compared with the control group n [SMD 2.00 (95% CI 0.47–3.53)]. Exercise, including intradialytic aerobic exercise and intradialytic stretching exercise, was not associated with a significant improvement in sleep quality compared with the control group [SMD −0.10 (95% CI −0.84–0.64)]. The heterogeneity was low (I2 = 0%) and the inconsistencies within and between groups were not significant. The funnel plot was symmetrical (Supplementary data, Figure S3B). The full design-by-treatment interaction model revealed no significant incoherence from different study designs, with Q = 0.01 and P = 0.94. More than half of the results of the pairwise comparison came from indirect evidence (Supplementary data, Figure S4B). The node no-splitting model revealed no loop incoherence (Supplementary data, Figure S5B).
FIGURE 3:
Forest plot of (A) NMA of sleep quality and (B) treatment-related adverse events.
Adverse events
Reflexology, intradialytic stretching exercises, vitamin C, vitamin E, cool dialysate, dopamine agonist and gabapentin were all associated with increased adverse events compared with the control, but not significantly so (Figure 3B and Supplementary data, Table S5). The Egger test and comparison-adjusted funnel plots revealed no small-study bias (Supplementary data, Figure S3C). The full design-by-treatment interaction model revealed no incoherence, with Q = 0.99 and P = 0.99. The result from the current NMA mostly came from indirect evidence (Supplementary data, Figure S4C). The node-splitting model revealed no loop incoherence from direct and indirect evidence (Supplementary data, Figure S5C).
Component NMA
We explored the individual treatment effects of different components of included composite interventions by using component NMA. As indicated in Supplementary data, Figure S6, the standard NMA and additive component NMA did not significantly differ (P = 0.83). The result demonstrated that a combination of two interventions might be additive rather than synergistic or antagonistic. The component NMA indicated that cool dialysate might still be the most potent component for RLS severity relief [MD 16.82 (95% CI 10.55–23.08); Figure 2B]. Regarding RLS treatment efficacy, the best and worst interventions in component NMA were cool dialysate and neuromuscular electrical stimulation, respectively (Supplementary data, Table S6 and Figure S7).
Assessing risk of bias and confidence of NMA
The results of the risk-of-bias assessment are illustrated in the Supplementary data, Figure S8 and summarized in the Supplementary Document. The degree of confidence in the evidence was assessed using the CINeMA framework. All enrolled studies were considered to warrant no concern regarding reporting bias and indirectness. To evaluate imprecision and heterogeneity, the IRLS score was defined to indicate clinical importance if MD was >3, in accordance with the definition used in previous studies [41]. Overall, compared with the controls, the treatment effect of cool dialysate was verified with high confidence (Supplementary Document and Supplementary data, Table S7). The treatment results of 14 interventions, their rankings by P-score and the associated levels of confidence in the evidence are summarized in Table 2. Figure 4 summarizes the results of our NMA by the scatter plot of the P-score of RLS intervention efficacy and acceptability.
Table 2.
Summary of treatment effects, treatment-related adverse events, the rank by P-score and the level of confidence from 14 interventions
| RLS severity | Sleep quality | Adverse events | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P-score | P-score | P-scores | Confidence | |||||||
| Intervention | MD (95% CI) | (%) | Rank | SMD (95% CI) | (%) | Rank | OR (95% CI) | (%) | Rank | of evidenceb |
| Cool dialysate | 16.82 (10.63–23.02) | 97.24 | 1 | – | – | – | 2.25 (0.14–35.58) | 41.30 | 9 | High |
| ISE | 12.00 (7.04–16.97) | 81.46 | 2 | −0.10 (−0.84–0.64) | 0.1418 | 4a | 1.27 (0.18–8.72) | 28.98 | 12 | Low |
| Aromatherapy massage | 10.91 (6.96–14.85) | 76.22 | 3 | – | – | – | 0.71 (0.10–5.10) | 67.57 | 1 | Low |
| Gabapentin | 8.90 (1.95–15.85) | 63.06 | 4 | 2.00 (0.47–3.53) | 0.9426 | 1 | 4.00 (0.29–54.52) | 28.98 | 13 | Very low |
| Reflexology | 8.05 (2.73–13.37) | 56.37 | 5 | – | – | – | 1.07 (0.08–15.04) | 57.26 | 7 | Very low |
| Vitamin C | 7.73 (2.14–13.32) | 54.62 | 6 | – | – | – | 1.66 (0.15–18.03) | 49.59 | 8 | Moderate |
| Vitamin C + vitamin E | 7.61 (0.05–15.17) | 53.82 | 7 | – | – | – | 5.35 (0.41–69.49) | 22.59 | 14 | Low |
| Vitamin E | 7.41 (−0.28–15.10) | 52.50 | 8 | – | – | – | 3.54 (0.25–49.78) | 32.07 | 11 | Moderate |
| Dopamine agonist | 7.14 (2.01–12.27) | 49.86 | 9 | 0.52 (−0.80–1.85) | 0.4400 | 3 | 2.78 (0.44–17.73) | 35.14 | 10 | Very low |
| Iron | 6.57 (−1.00–14.14) | 47.43 | 10 | – | – | – | 0.69 (0.04–11.70) | 65.74 | 2 | Very low |
| IAE + dopamine agonist | 5.13 (−9.91–20.16) | 42.66 | 11 | – | – | – | 0.92 (0.01–117.00) | 57.32 | 6 | Low |
| IAE | 3.83 (−4.87–12.53) | 32.93 | 12 | −0.10 (−0.84–0.64) | 0.1418 | 4a | 0,92 (0.06–13.22) | 60.73 | 3 | Very low |
| Acupoint therapy | 1.61 (−3.67–6.88) | 18.83 | 13 | 1.34 (0.62–2.06) | 0.7684 | 2 | 1.00 (0.06–16.26) | 58.38 | 4 | Low |
| NES | −0.97 (−12.42–10.47) | 12.99 | 14 | – | – | – | 0.92 (0.01–107.97) | 57.41 | 5 | Very low |
Intradialytic stretching exercise and intradialytic aerobic exercise were merged as one group when analysing sleep quality.
P-scores (as percentages) and rank for each intervention were obtained from standard network meta-analysis.
bConfidence of evidence was assessed according to the results of NMA regarding RLS severity treatment effect by CINeMA.
IAE, intradialytic aerobic exercise; ISE, intradialytic stretching exercise; NES, neuromuscular electrical stimulation.
FIGURE 4:
Scatter plot of P-score of efficacy and acceptability in the indicated treatments for ESRD patients with RLS. x-axis: efficacy; y-axis: acceptability. AM, aromatherapy massage; AT, acupoint therapy; C, control; CD, cool dialysate; DA, dopamine agonist; G, gabapentin; IAE, intradialytic aerobic exercise; ISE, intradialytic stretching exercise; NES, neuromuscular electrical stimulation; Vit-C, vitamin C; Vit-E, vitamin E; R, reflexology.
DISCUSSION
The present study revealed several points worth summarizing. First, cool dialysate and gabapentin were the most potent treatments for reducing RLS severity among the non-pharmacological and pharmacological interventions, respectively. Second, gabapentin and acupoint therapy may improve patients’ sleep quality. Third, although several interventions produced treatment-related adverse events, none of those effects was statistically significant when comparisons were made with the control group.
Some experts have suggested attempting non-pharmacological interventions first in response to problems of sleep quality among patients with ESKD [2]. Among the non-pharmacological interventions investigated by the current meta-analysis, cool (35.5°C) dialysate had positive effects in reducing RLS severity compared with dialysate of standard temperature (37°C) [2, 9]. The efficacy of cool dialysate might originate from its capacity to reduce sensory receptor function and lower impulse input frequencies to nerve terminals while lowering body temperature [42]. Apart from reducing RLS severity, cool dialysate has been demonstrated to reduce intradialytic hypotension, fatigue, brain ischaemia [43, 44] and cardiovascular mortality [45]. With no associated critical adverse events and only symptoms of chills during dialysis, cool dialysate represents a reasonable choice of a non-pharmacological approach for patients with RLS on dialysis. Intradialytic exercise has demonstrated mixed results in previous studies [46, 47]. In the current study, we analysed two types of intradialytic exercise separately regarding their effects on relieving RLS severity. Intradialytic stretching was the second most effective non-pharmacological intervention for reducing RLS severity; the mechanism underlying this reduction is potentially associated with elevated cardiac output, dilation of blood vessels and increased lower limb circulation [7, 48]. Several types of oil massage were investigated to identify their capacities to reduce RLS severity and all of them were merged into the aromatherapy massage group in the present study. Gupta et al. [49] observed that 76.9% of patients with RLS preferred to massage their legs to alleviate unpleasant sensations. Our study revealed that aromatherapy massage might play a role in reducing RLS severity. However, among the included studies, only the study of Nasiri et al. [35] compared the effects of aromatherapy massage with those of a placebo massage (in which paraffin was used). Because the evidence was limited, distinguishing whether these treatment effects were the result of massage therapy generally or oil-based aromatherapy massage is difficult.
In the present study, gabapentin was the most effective pharmacological intervention in reducing RLS symptoms. The gabapentin dosage in the included studies was 100–300 mg after dialysis sessions [8, 11, 37]. Although the treatment dosage was within the recommended range, gabapentin-related adverse events and patient dropouts were still reported [37]. Dopaminergic dysfunction has been implicated in the pathogenesis of RLS, and L-dopa therapy has proven its efficacy in previous studies [50]. In the current study, dopamine agonists reduced RLS severity when comparisons were made with a control group.
Only Giannaki et al. [30] addressed the effects of a combination of pharmacological (dopamine agonist) and non-pharmacological (intradialytic aerobic exercise) treatments. No antagonist effect was detected according to the current component NMA, therefore this combination might be a reasonable approach.
In addition to reductions in RLS severity and improvements in sleep quality, we had planned to evaluate the enhancements in patients’ quality of life associated with various interventions. However, only three RCTs—those of Dauvilliers et al. [25], Giannaki et al. [29] and Mortazavi et al. [34]—reported outcomes about patients’ quality of life. No newer RCTs investigating quality of life have emerged, thus the conclusion on improving patient quality of life is expected to be the same as that in a previous report [6].
Our study has several strengths. First, we conducted a component NMA to evaluate the treatment effect of each component in composite interventions. Second, we performed an updated NMA including several non-pharmacological interventions that had not been reviewed before. Third, we demonstrated that different types of intradialytic exercise induce different effects in the reduction of RLS severity. Fourth, we assessed the degree of confidence in the evidence associated with our work using CINeMA.
The present study had several limitations. First, we grouped different dopamine agonists into one group and aromatherapy massage treatments with different oils into one group when comparing their effects on reducing RLS severity. However, the treatment response might be different. Second, to enroll the maximum number of participants, three studies exhibiting different RLS severity scores were excluded [7, 34, 40]. Third, the short duration of interventions in enrolled studies limits the validity of extrapolations to the long-term outcomes of the interventions. Fourth, most of the enrolled studies were based on haemodialysis patients, with only one study with mixed haemodialysis patients and peritoneal dialysis patients [40]. This mixed population could be a source of heterogeneity. Furthermore, the protocols of non-pharmacological interventions used in enrolled studies were based on haemodialysis sessions. Therefore, the effectiveness of current mentioned non-pharmacological interventions and their clinical applications in peritoneal dialysis patients need further discussion. Fourth, most of the comparisons came from indirect evidence. Moreover, most of the comparisons were considered to have low to very low confidence of evidence according to CiNeMA. The reason for low confidence is that most of the comparisons were judged as having major concerns regarding imprecision and incoherence.
CONCLUSION
Cool dialysate was concluded with high confidence to be the most effective treatment in reducing RLS severity and gabapentin was the most potent pharmacological treatment. Because cool dialysate is effective and has other potential benefits, it is appropriate to apply it to treat patients with ESKD and RLS. Other potential interventions include aromatherapy massage, intradialytic stretching exercises and reflexology, but all have limited confidence of evidence. More effectively designed parallel RCTs with sufficient sample sizes are required to evaluate these potential interventions and their long-term outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank the Institute of Epidemiology and Preventive Medicine, College of Public Health, National Taiwan University, for its statistical support. This study was supported by grants from Chang Gung Memorial Hospital, Taiwan (CMRPG5K0141) and C.-H.C. was supported by the Ministry of Science and Technology (109-2314-B-182A-124).
Contributor Information
Jia-Jin Chen, Department of Nephrology, Chang Gung Memorial Hospital, Linkou, Taiwan.
Tao Han Lee, Department of Nephrology, Chang Gung Memorial Hospital, Linkou, Taiwan.
Yu-Kang Tu, Institute of Epidemiology and Preventive Medicine, College of Public Health, National Taiwan University, Taipei, Taiwan.
George Kuo, Department of Nephrology, Chang Gung Memorial Hospital, Linkou, Taiwan; Department of Nephrology, Kidney Research Center, Chang Gung Memorial Hospital, Taiwan.
Huang-Yu Yang, Department of Nephrology, Chang Gung Memorial Hospital, Linkou, Taiwan; Department of Nephrology, Kidney Research Center, Chang Gung Memorial Hospital, Taiwan.
Chieh-Li Yen, Department of Nephrology, Chang Gung Memorial Hospital, Linkou, Taiwan.
Pei-Chun Fan, Department of Nephrology, Chang Gung Memorial Hospital, Linkou, Taiwan; Department of Nephrology, Kidney Research Center, Chang Gung Memorial Hospital, Taiwan.
Chih-Hsiang Chang, Department of Nephrology, Chang Gung Memorial Hospital, Linkou, Taiwan; Department of Nephrology, Kidney Research Center, Chang Gung Memorial Hospital, Taiwan.
AUTHORS’ CONTRIBUTIONS
C.-H.C., T.H.L. and J.-J.C. participated in conceptualization (create ideas and overarching research goal). T.H.L., J.-J.C. and G.K. participated in methodology, validation and writing the original draft. J.-J.C. carried out the software programing and supporting algorithms. G.K. and J.-J.C. carried out the formal analysis. T.H.L., J.-J.C. and G.K. carried out the investigation and data curation. P.-C.F. and H.-Y.Y. were responsible for resources and visualization. C.-L.Y. and H.-Y.Y. reviewed and edited the manuscript. C.-H.C. and Y.-K. were responsible for the supervision and project administration. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Lin CH, Wu VC, Li WYet al. Restless legs syndrome in end-stage renal disease: a multicenter study in Taiwan. Eur J Neurol 2013; 20: 1025–1031 [DOI] [PubMed] [Google Scholar]
- 2. Scherer JS, Combs SA, Brennan F.. Sleep disorders, restless legs syndrome, and uremic pruritus: diagnosis and treatment of common symptoms in dialysis patients. Am J Kidney Dis 2017; 69: 117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lin XW, Zhang JF, Qiu MYet al. Restless legs syndrome in end stage renal disease patients undergoing hemodialysis. BMC Neurol 2019; 19: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Winkelman JW, Chertow GM, Lazarus JM. Restless legs syndrome in end-stage renal disease. Am J Kidney Dis 1996; 28: 372–378 [DOI] [PubMed] [Google Scholar]
- 5. Massey TH, Robertson NP.. Restless legs syndrome: causes and consequences. J Neurol 2020; 267: 575–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang CW, Lee MJ, Wang LJet al. Comparative efficacy and acceptability of treatments for restless legs syndrome in end-stage renal disease: a systematic review and network meta-analysis. Nephrol Dial Transplant 2020; 35: 1609–1618 [DOI] [PubMed] [Google Scholar]
- 7. Fauzi A, Triaswati R.. The effect of intradialytic stretching training on restless legs syndrome and sleep quality in hemodialysis patients. Korean J Adult Nurs 2021; 33: 37–43 [Google Scholar]
- 8. Ali M, Iram H, Nasim Fet al. Comparison of the efficacy of gabapentin versus levodopa-C for the treatment of restless legs syndrome in end-stage renal disease on hemodialysis patients. Cureus 2020; 12: e12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zirak Aliabadi A, Mirhosseini Z, Rastaghi Set al. Comparison of the effect of cold dialysate versus stretching exercises on severity of restless legs syndrome in patients undergoing hemodialysis: a randomized controlled trial. Evidence Based Care 2020; 10: 15–22 [Google Scholar]
- 10. Oshvandi K, Mirzajani Letomi F, Soltanian ARet al. The effects of foot massage on hemodialysis patients' sleep quality and restless leg syndrome: a comparison of lavender and sweet orange essential oil topical application. J Complement Integr Med 2021; doi: 10.1515/jcim-2020-0121 [DOI] [PubMed] [Google Scholar]
- 11. Hajian S, Rajabpoor Nikfam MR, Esmayeilzad Z. Comparison of the effects of pramipexole and gabapentin on the treatment of restless leg syndrome in end-stage chronic renal failure patients undergoing hemodialysis. J Nephropathol 2020; 9: e25 [Google Scholar]
- 12. Youssef MK. Efficacy of neuromuscular electric stimulation versus aerobic exercise on uraemic restless legs syndrome. Int Ther Rehabil 2019; 26: 1–12 [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff Jet al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–269, W264 [DOI] [PubMed] [Google Scholar]
- 14. Geraint M. Withdrawal of 0.6% glauline (metipranolol). Br J Ophthalmol 1991; 75: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buysse DJ, Reynolds CF 3rd, Monk THet al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28: 193–213 [DOI] [PubMed] [Google Scholar]
- 16. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14: 540–545 [DOI] [PubMed] [Google Scholar]
- 17. Kalantar-Zadeh K, Kopple JD, Block Get al. Association among SF36 quality of life measures and nutrition, hospitalization, and mortality in hemodialysis. J Am Soc Nephrol 2001; 12: 2797–2806 [DOI] [PubMed] [Google Scholar]
- 18. Balduzzi S, Rucker G, Schwarzer G.. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019; 22: 153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rucker G, Schwarzer G.. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Method 2015; 15: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higgins JP, Jackson D, Barrett JKet al. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 2012; 3: 98–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sterne JAC, Savović J, Page MJet al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 22. Nikolakopoulou A, Higgins JPT, Papakonstantinou Tet al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med 2020; 17: e1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mirbagher Ajorpaz N, Rahemi Z, Aghajani Met al. Effects of glycerin oil and lavender oil massages on hemodialysis patients' restless legs syndrome. J Bodyw Mov Ther 2020; 24: 88–92 [DOI] [PubMed] [Google Scholar]
- 24. Aliasgharpour M, Abbasi Z, Pedram Razi Set al. The effect of stretching exercises on severity of restless legs syndrome in patients on hemodialysis. Asian J Sports Med 2016; 7: e31001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dauvilliers Y, Benes H, Partinen Met al. Rotigotine in hemodialysis-associated restless legs syndrome: a randomized controlled trial. Am J Kidney Dis 2016; 68: 434–443 [DOI] [PubMed] [Google Scholar]
- 26. Deng Y, Wu J, Jia Q.. Efficacy of intravenous iron sucrose in hemodialysis patients with restless legs syndrome (RLS): a randomized, placebo-controlled study. Med Sci Monit 2017; 23: 1254–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gao Y, Zhao J, Guo C.. Effect of governor moxibustion for restless legs syndrome of maintenance hemodialysis:a randomized controlled trial. Zhongguo Zhen Jiu 2016; 36: 597–600 [DOI] [PubMed] [Google Scholar]
- 28. Ghasemi M, Rejeh N, Heravi-Karimooi Met al. The effectiveness of foot reflexology in the severity of restless legs syndrome in female patients undergoing dialysis: a randomized controlled trial. Crit Care Nurs J 2018; 11: e68945 [Google Scholar]
- 29. Giannaki CD, Sakkas GK, Karatzaferi Cet al. Effect of exercise training and dopamine agonists in patients with uremic restless legs syndrome: a six-month randomized, partially double-blind, placebo-controlled comparative study. BMC Nephrol 2013; 14: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Giannaki CD, Sakkas GK, Karatzaferi Cet al. Combination of exercise training and dopamine agonists in patients with RLS on dialysis: a randomized, double-blind placebo-controlled study. ASAIO J 2015; 61: 738–741 [DOI] [PubMed] [Google Scholar]
- 31. Hashemi SH, Hajbagheri A, Aghajani M.. The effect of massage with lavender oil on restless leg syndrome in hemodialysis patients: a randomized controlled trial. Nurs Midwifery Stud 2015; 4: e29617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kashani E, Mirhosseini Z, Rastaghi Set al. The effect of the cool dialysate on the restless leg syndrome in hemodialysis patients: randomized triple-blind clinical trial. Iran J Nurs Midwifery Res 2019; 24: 200–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mohammadi MM, Raygani AAV, Ghobadi Aet al. Effect of near-infrared light therapy based on acupoints on the severity of restless legs syndrome in patients undergoing hemodialysis: a single-blind, randomized controlled trial. Clin Med Res 2018; 16: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mortazavi M, Vahdatpour B, Ghasempour Aet al. Aerobic exercise improves signs of restless leg syndrome in end stage renal disease patients suffering chronic hemodialysis. Sci World J 2013; 2013: 628142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nasiri M, Abbasi M, Khosroabadi ZYet al. Short-term effects of massage with olive oil on the severity of uremic restless legs syndrome: a double-blind placebo-controlled trial. Complement Ther Med 2019; 44: 261–268 [DOI] [PubMed] [Google Scholar]
- 36. Rafie S, Jafari M, Azizi Met al. Restless legs syndrome in hemodialysis patients. Saudi J Kidney Dis Transpl 2016; 27: 326–330 [DOI] [PubMed] [Google Scholar]
- 37. Razazian N, Azimi H, Heidarnejadian Jet al. Gabapentin versus levodopa-C for the treatment of restless legs syndrome in hemodialysis patients: a randomized clinical trial. Saudi J Kidney Dis Transpl 2015; 26: 271–278 [DOI] [PubMed] [Google Scholar]
- 38. Sagheb MM, Dormanesh B, Fallahzadeh MKet al. Efficacy of vitamins C, E, and their combination for treatment of restless legs syndrome in hemodialysis patients: a randomized, double-blind, placebo-controlled trial. Sleep Med 2012; 13: 542–545 [DOI] [PubMed] [Google Scholar]
- 39. Shahgholian N, Jazi SK, Karimian Jet al. The effects of two methods of reflexology and stretching exercises on the severity of restless leg syndrome among hemodialysis patients. Iran J Nurs Midwifery Res 2016; 21: 219–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sloand JA, Shelly MA, Feigin Aet al. A double-blind, placebo-controlled trial of intravenous iron dextran therapy in patients with ESRD and restless legs syndrome. Am J Kidney Dis 2004; 43: 663–670 [DOI] [PubMed] [Google Scholar]
- 41. Allen RP. Minimal clinically significant change for the International Restless Legs Syndrome Study Group rating scale in clinical trials is a score of 3. Sleep Med 2013; 14: 1229. [DOI] [PubMed] [Google Scholar]
- 42. Palmieri-Smith RM, Leonard-Frye JL, Garrison CJet al. Peripheral joint cooling increases spinal reflex excitability and serum norepinephrine. Int J Neurosci 2007; 117: 229–242 [DOI] [PubMed] [Google Scholar]
- 43. Larkin JW, Reviriego-Mendoza MM, Usvyat LAet al. To cool, or too cool: is reducing dialysate temperature the optimal approach to preventing intradialytic hypotension? Semin Dial 2017; 30: 501–508 [DOI] [PubMed] [Google Scholar]
- 44. Eldehni MT, Odudu A, McIntyre CW.. Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol 2015; 26: 957–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dasgupta I, Thomas GN, Clarke Jet al. Associations between hemodialysis facility practices to manage fluid volume and intradialytic hypotension and patient outcomes. Clin J Am Soc Nephrol 2019; 14: 385–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Song YY, Hu RJ, Diao YSet al. Effects of exercise training on restless legs syndrome, depression, sleep quality, and fatigue among hemodialysis patients: a systematic review and meta-analysis. J Pain Symptom Manage 2018; 55: 1184–1195 [DOI] [PubMed] [Google Scholar]
- 47. Giannaki CD, Hadjigeorgiou GM, Karatzaferi C. et al. A single-blind randomized controlled trial to evaluate the effect of 6 months of progressive aerobic exercise training in patients with uraemic restless legs syndrome. Nephrol Dial Transplant 2013; 28: 2834–2840 [DOI] [PubMed] [Google Scholar]
- 48. van Vilsteren MC, de Greef MH, Huisman RM.. The effects of a low-to-moderate intensity pre-conditioning exercise programme linked with exercise counselling for sedentary haemodialysis patients in The Netherlands: results of a randomized clinical trial. Nephrol Dial Transplant 2005; 20: 141–146 [DOI] [PubMed] [Google Scholar]
- 49. Gupta R, Goel D, Ahmed Set al. What patients do to counteract the symptoms of Willis-Ekbom disease (RLS/WED): effect of gender and severity of illness. Ann Indian Acad Neurol 2014; 17: 405–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Trenkwalder C, Stiasny K, Pollmacher Tet al. L-dopa therapy of uremic and idiopathic restless legs syndrome: a double-blind, crossover trial. Sleep 1995; 18: 681–688 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.