ABSTRACT
Background
Serum globulin is a major component of total protein and can be elevated in inflammatory disease states. While inflammation is common in hemodialysis patients and associated with mortality and morbidity, the association between serum globulin and mortality has never been examined in hemodialysis patients.
Methods
In a retrospective cohort of 104 164 incident hemodialysis patients treated by a large dialysis organization from 2007 to 2011, we explored the association between baseline serum globulin, albumin: globulin (A:G) ratio and serum protein levels and all-cause, cardiovascular and infection-related mortality with adjustments for demographic variables and laboratory markers of malnutrition and inflammation using Cox proportional hazards models.
Results
Patients with a globulin concentration >3.8 g/dL had a higher all-cause and infection-related mortality risk {hazard ratio [HR] 1.11 [95% confidence interval (CI) 1.06–1.16] and HR 1.28 [95% CI 1.09–1.51], respectively} in the fully adjusted model when compared with the reference group of 3.0– <3.2 g/dL. In addition, patients with an A:G ratio <0.75 had a 45% higher all-cause mortality hazard [HR 1.45 (95% CI 1.38–1.52)] and patients with total serum protein <5.5 g/dL had a 34% higher risk of death [1.34 (95% CI 1.27–1.42)] when compared with the reference (A:G ratio 1.05– <1.15 and total serum protein 6.5– <7 g/dL).
Conclusions
Among incident hemodialysis patients, a higher globulin level was associated with a higher mortality risk independent of other markers of malnutrition and inflammation, including albumin. A lower A:G ratio and serum protein was also associated with a higher mortality hazard. The mechanisms that contribute to elevated serum globulin should be further explored.
Keywords: albumin-to-globulin ratio, globulin, hemodialysis, mortality, protein
KEY LEARNING POINTS.
What is already known about this subject?
In chronic kidney disease (CKD), hypoalbuminemia has long been associated with a higher mortality risk while less is known about the other component of total serum protein, serum globulin.
Higher serum globulin, also known as gamma gap, has previously been shown to be associated with a higher mortality risk in nondialysis CKD patients.
What this study adds?
Among incident hemodialysis patients, higher globulin concentration, lower A:G ratio and lower total protein concentration are associated with higher all-cause, cardiovascular and infection-related mortality.
The association between higher globulin and mortality was notably found to be independent of serum albumin.
What impact this may have on practice or policy?
Our findings may be useful in establishing thresholds for gamma gap and A:G ratio values to determine the need for further testing and monitoring in patients starting hemodialysis.
INTRODUCTION
While the association between low serum albumin and increased mortality risk has been well described in patients with chronic kidney disease (CKD) on hemodialysis, the same cannot be said of serum globulin, the other major component of serum total protein [1–4]. Serum globulin, also known as gamma gap or protein gap, is typically calculated as the difference between total protein and albumin and thus includes all non-albumin proteins, including globulin, fibrinogen, C-reactive protein, interleukins, leukotrienes and other regulatory and prothrombotic proteins [5]. Serum globulin values may be elevated in conditions such as infection, inflammation and liver and connective tissue diseases, while a decrease in serum globulin may be caused by malnutrition and nephrotic syndrome due to decreased synthesis and protein loss through the kidney, respectively [4, 6]. Serum protein electrophoresis (SPEP) may be conducted to quantify the fractional components of globulin in patients with abnormal serum protein levels, which may be helpful in reaching a diagnosis in a number of underlying plasma cell disorders, including multiple myeloma, Waldenstrom's macroglobulinemia and primary amyloidosis [7, 8].
In a large cohort of 7.7 million life insurance applicants ranging from 20 to 89 years old, Fulks et al. [9] showed an increase in mortality risk with serum globulin >3.2 g/dL, with values ≥3.5 g/dL associated with at least a 25% higher mortality risk. Similarly, Juraschek et al. [10] analyzed data that included 12 260 participants of the National Health and Nutrition Examination Survey (NHANES) and found that an increasing gamma gap (per 1 g/dL) was associated with a higher all-cause death risk and a higher risk for mortality due to pulmonary causes, but not for cardiovascular (CV) mortality.
In CKD patients, chronic inflammation is common and may be caused by factors including uremic toxins, dialysis-related factors and/or oxidative stress [11, 12]. Because inflammation is associated with adverse outcomes in this population [13–16], we sought to determine if higher serum globulin is associated with greater mortality outcomes in incident hemodialysis patients. Recent studies have also presented the albumin: globulin (A:G) ratio as a prognosticator for mortality risk in CKD patients [5, 17], so we further examined the A:G ratio and total serum protein concentration in relation to mortality outcomes.
MATERIALS AND METHODS
Study population and data source
We examined the data from a total of 208 820 patients with end-stage renal disease (ESRD) who initiated hemodialysis therapy from 1 January 2007 to 31 December 2011 in a large dialysis care organization in the USA. This cohort has been previously described [18]. We excluded 46 156 patients who had <60 days of total treatment during their total follow-up time, 29 502 for receiving treatment with a modality other than in-center hemodialysis, 21 145 for not having treatment data in the first patient quarter (91 days from patient first dialysis date) and 7853 for not having globulin and A:G ratio data in the first patient quarter. The final analytical cohort consisted of 104 164 incident hemodialysis patients (Supplementary data, Figure S1). In additional analyses, two patients were excluded for missing total protein concentration in the first patient quarter, resulting in a secondary analytical cohort of 104 162 incident hemodialysis patients.
All data were obtained from the electronic records database of a large dialysis organization (LDO), including information on race/ethnicity (self-categorized), primary insurance and the presence of diabetes. International Classification of Diseases, Ninth Revision codes were used to determine presence of the following conditions with a look back period of one year prior to dialysis initiation: alcohol dependence, congestive heart failure, chronic obstructive pulmonary disease, cerebrovascular disease, human immuno deficiency virus (HIV), history of cancer, history of hypertension, atherosclerotic heart disease, liver disease, other cardiac disease, diabetes mellitus, dyslipidemia and drug dependence.
Blood samples were drawn using standardized techniques in all dialysis clinics and were transported to a central laboratory in Deland, Florida, USA, typically within 24 hours. All laboratory values were measured using automated and standardized methods. To minimize variability, variables with repeated measures within each patient quarter were averaged to obtain a single quarterly mean value. Measurements taken during the first patient quarter after dialysis initiation were used as baseline values. Body mass index (BMI) was calculated using average post dialysis body weight over the first quarter.
The study was approved by the institutional review committees of the University of California, Irvine. The study was exempt from informed written consent due to its nonintrusive nature and anonymity of patients.
Exposure and outcome ascertainment
Globulin was the primary exposure of interest and was extracted from the laboratory dataset. Globulin is typically calculated as the total protein minus the serum albumin level. We divided serum globulin concentrations into nine a priori categories (<2.4, 2.4–<2.6, 2.6–<2.8, 2.8–<3.0, 3.0–<3.2, 3.2–<3.4, 3.4–<3.6, 3.6–<3.8 and ≥3.8 g/dL). In further analyses, the A:G ratio and total protein were used as secondary exposures of interest. The A:G ratio was divided into 10 a priori selected groups (<0.75, 0.75–<0.85, 0.85–<0.95, 0.95–<1.05, 1.05–<1.15, 1.15–<1.25, 1.25–<1.35, 1.35–<1.45, 1.45–<1.55 and ≥1.55), while total protein was divided into 6 a priori selected groups (<5.5, 5.5–<6.0, 6.0–<6.5, 6.5–<7.0, 7.0–<7.5 and ≥7.5 g/dL). Reference values for the exposures of interest were selected based on median values: serum globulin 3.0–<3.2 g/dL, A:G ratio 1.05–<1.15 and total protein 6.5–<7 g/dL.
The outcomes of interest were all-cause mortality, CV mortality and infection-related mortality, which were ascertained from death records from the LDO database.
Statistical analysis
Data were summarized using proportions, means [± standard deviation (SD)] or median [interquartile range (IQR)] as appropriate and multiple linear regression models were fitted to construct Pearson's and partial correlations. We evaluated the association between all-cause mortality, CV mortality, and infection-related mortality with globulin, A:G ratio and total protein, respectively, using Cox proportional hazards regression models. Patients were considered at-risk until death or censoring for renal transplantation, transfer to another dialysis clinic or the end of the study period (31 December 2011). The proportionality assumption was checked for all proportional hazards regression models.
For sensitivity analysis, we examined associations of serum globulin, A:G ratio and total protein with all-cause mortality using restricted cubic splines with four knots at the 5th, 35th, 65th and 95th percentiles.
Additionally, percentile rank score analysis was performed with baseline serum globulin and serum albumin as exposure to assess the influences of concordant and discordant changes in values [19]. Patients were ranked with respect to baseline serum globulin and serum albumin from the 0th to 100th percentiles and the difference between percentiles of serum globulin minus serum albumin were determined, resulting in a percentile rank score value between −100 to +100.
For each analysis, three sequential models of adjustment were used: an unadjusted model that included mortality as the outcome; a case-mix adjusted model that included age, gender, race/ethnicity (African American and other self-categorized black, Caucasian, Asian, Hispanic and other), primary insurance (Medicare, Medicaid and other), the 13 different comorbid conditions, dialysis access type and dialysis adequacy as indicated by Kt/V; and malnutrition-inflammation complex syndrome (MICS)-adjusted models that included all of the covariates in the case-mix model as well as surrogates of nutritional status and inflammation including 15 laboratory variables: serum albumin, serum creatinine, serum total iron-binding capacity (TIBC), serum ferritin, serum phosphorus, serum calcium, serum bicarbonate, peripheral white blood cell (WBC) count, potassium, hemoglobin, iron saturation, alkaline phosphatase (ALP), aspartate aminotransferase (AST), intact parathyroid hormone and normalized protein catabolic rate (nPCR) as an indicator of daily protein intake, also known as the normalized protein nitrogen appearance (nPNA). Additionally, the MICS model included post dialysis session weight, systolic blood pressure and diastolic blood pressure. There were two exceptions: analysis with A:G ratio and total protein, as exposure did not include serum albumin in the MICS model, and percentile rank score analysis with serum globulin and albumin, as exposure did not include serum albumin in the MICS model.
Subgroup analyses were conducted to examine associations of mortality with higher globulin (≥3.2 g/dL) versus reference lower globulin (<3.2 g/dL) across strata of a priori selected subgroups. We further explored the effect modification of serum albumin on high globulin levels (serum globulin ≥3.2 g/dL) and all-cause mortality association with adjustment for demographics and biomarkers of malnutrition and inflammation by using restricted cubic spline models.
Missing covariate data (<1% for demographic variables and <5% for laboratory variables) were imputed by mean. Analyses were completed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and Stata version 13.1 (StataCorp, College Station, TX, USA).
RESULTS
Baseline demographic, clinical and laboratory characteristics
Our final study cohort consisted of 104 164 patients, for which the mean age was 63 ± 15 years, 44% were female, 31% were African American, 58% were diabetic and 36% had congestive heart failure (Table 1). The mean globulin was 3.1 ± 0.6 g/dL, mean A:G ratio was 1.2 ± 0.3 and mean total protein was 6.6 ± 0.7 g/dL.
Table 1.
Demographic and clinical characteristics of 104 164 hemodialysis patients stratified by nine a priori selected groups of baseline serum globulin
| Globulin (g/dL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Total | <2.4 | 2.4– <2.6 | 2.6– <2.8 | 2.8– <3.0 | 3.0– <3.2 | 3.2– <3.4 | 3.4– <3.6 | 3.6– <3.8 | ≥3.8 | P-value |
| Patients, n (%) | 104 164 | 11 371 (11) | 11 283 (11) | 14 206 (14) | 15 090 (14) | 14 778 (14) | 11 120 (11) | 8818 (8) | 5898 (6) | 11 600 (11) | |
| Age (years) | 63 ± 15 | 65 ± 17 | 64 ± 16 | 64 ± 16 | 63 ± 15 | 63 ± 15 | 62 ± 14 | 62 ± 14 | 61 ± 14 | 60 ± 14 | <0.001 |
| Female (%) | 44 | 41 | 41 | 43 | 44 | 45 | 45 | 45 | 46 | 43 | <0.001 |
| Diabetes (%) | 58 | 45 | 52 | 56 | 59 | 62 | 63 | 64 | 65 | 62 | <0.001 |
| Race (%) | |||||||||||
| Caucasian | 47 | 75 | 64 | 55 | 48 | 41 | 37 | 33 | 31 | 27 | <0.001 |
| African American | 31 | 11 | 17 | 22 | 28 | 33 | 38 | 44 | 46 | 54 | <0.001 |
| Asians | 3 | 3 | 3 | 3 | 4 | 4 | 4 | 3 | 4 | 3 | <0.001 |
| Others | 4 | 2 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | <0.001 |
| Ethnicity (%) | |||||||||||
| Hispanics | 15 | 9 | 14 | 16 | 17 | 17 | 18 | 16 | 15 | 13 | <0.001 |
| Insurance (%) | 0.0785 | ||||||||||
| Medicare | 53 | 55 | 55 | 55 | 53 | 53 | 52 | 52 | 53 | 51 | |
| Medicaid | 7 | 5 | 5 | 6 | 7 | 7 | 8 | 9 | 9 | 10 | |
| Others | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 39 | 38 | |
| Access type (%) | |||||||||||
| CVC | 78 | 69 | 71 | 73 | 76 | 79 | 82 | 83 | 84 | 86 | <0.001 |
| AV fistula | 15 | 24 | 21 | 19 | 16 | 13 | 11 | 10 | 9 | 7 | <0.001 |
| AV graft | 4 | 5 | 5 | 5 | 5 | 4 | 4 | 4 | 3 | 3 | <0.001 |
| AV other | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0 | 0.2 | 0 | <0.001 |
| Unknown | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 4 | 4 | <0.001 |
| Comorbidities (%) | |||||||||||
| ASHD | 14 | 14 | 14 | 14 | 14 | 15 | 14 | 14 | 14 | 14 | 0.54 |
| CHF | 36 | 33 | 34 | 35 | 37 | 37 | 38 | 39 | 38 | 38 | <0.001 |
| Other CVD | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 16 | 0.19 |
| CBVD | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0.1 |
| Hypertension | 51 | 50 | 51 | 51 | 52 | 52 | 52 | 51 | 51 | 51 | 0.93 |
| COPD | 5 | 6 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 0.02 |
| Cancer | 2 | 4 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | <0.001 |
| HIV | 0.5 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.4 | 0.5 | 0.7 | 2.3 | <0.001 |
| Dyslipidemia | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 24 | 0.064 |
| Liver disease | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 3 | <0.001 |
| Alcohol | 0.2 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 | 0.4 | <0.0001 |
| Substance abuse | 0.2 | 0.1 | 0.2 | 0.1 | 0.2 | 0.2 | 0.4 | 0.3 | 0.3 | 0.5 | <0.001 |
| Serum laboratory values | |||||||||||
| Albumin (g/dL) | 3.5 ± 0.5 | 3.62 ± 0.48 | 3.62 ± 0.46 | 3.6 ± 0.45 | 3.57 ± 0.45 | 3.53 ± 0.45 | 3.48 ± 0.44 | 3.43 ± 0.45 | 3.37 ± 0.46 | 3.18 ± 0.49 | <0.001 |
| A:G ratio | 1.2 ± 0.3 | 1.6 ± 0.3 | 1.4 ± 0.2 | 1.3 ± 0.2 | 1.2 ± 0.2 | 1.1 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.7 ± 0.2 | <0.001 |
| ALP (IU/L) | 87 (69–115) | 77 (62–98) | 80 (64–103) | 83 (66–107) | 85 (68–110) | 87 (69–113) | 90 (72–119) | 93 (74–125) | 97 (76–131) | 103 (78–145) | <0.001 |
| AST (IU/L) | 22 ± 14 | 20 ± 13 | 20 ± 11 | 20 ± 13 | 20 ± 13 | 21 ± 14 | 22 ± 12 | 23 ± 15 | 23 ± 15 | 27 ± 19 | <0.001 |
| Bicarbonate (mEq/L) | 24 ± 2.7 | 24 ± 3 | 23 ± 3 | 24 ± 3 | 24 ± 3 | 24 ± 3 | 24 ± 3 | 24 ± 2.6 | 24 ± 3 | 24 ± 3 | <0.001 |
| Calcium (mg/dL) | 9.1 ± 0.6 | 9 ± 0.6 | 9 ± 0.6 | 9 ± 0.5 | 9.1 ± 0.5 | 9.1 ± 0.6 | 9.1 ± 0.5 | 9.1 ± 0.6 | 9.2 ± 0.6 | 9.2 ± 0.6 | <0.001 |
| Creatinine (g/dL) | 5.9 ± 2.4 | 5.6 ± 2.3 | 5.8 ± 2.4 | 5.8 ± 2.3 | 5.9 ± 2.4 | 5.9 ± 2.3 | 5.9 ± 2.4 | 5.9 ± 2.4 | 5.8 ± 2.3 | 5.9 ± 2.4 | <0.001 |
| Ferritin (ng/mL), median (IQR) | 284 (165–488) | 273 (161–479) | 261 (154–441) | 258 (153–438) | 269 (158–450) | 278 (159–467) | 287 (170–484) | 298 (173–511) | 316 (183–537) | 373 (208–660) | <0.001 |
| Hemoglobin (g/dL) | 11.1 ± 1.2 | 11.1 ± 1.1 | 11.2 ± 1.1 | 11.2 ± 1.1 | 11.2 ± 1.1 | 11.1 ± 1.1 | 11.1 ± 1.1 | 11.1 ± 1.2 | 11 ± 1.2 | 10.7 ± 1.3 | <0.001 |
| iPTH (pg/mL), median (IQR) | 313 (196–485) | 293 (187–449) | 307 (195–468) | 316 (200–488) | 321 (208–491) | 324 (207–503) | 328 (203–506) | 320 (198–501) | 314 (193–488) | 289 (171–465) | 0.173 |
| ISAT (%) | 23 ± 9 | 25 ± 10 | 24 ± 9 | 23 ± 8 | 23 ± 8 | 22 ± 8 | 22 ± 9 | 23 ± 9 | 23 ± 9 | 24 ± 11 | <0.001 |
| KRU (mL/min/1.73 m2), median (IQR) | 3 (2–5) | 3 (2–6) | 3 (2–6) | 3 (2–6) | 3 (2–6) | 3 (2–5) | 3 (2–5) | 3 (2–5) | 3 (2–5) | 3 (1–5) | <0.001 |
| LDL (IU/L) | 80 ± 36 | 79 ± 36 | 79 ± 35 | 81 ± 36 | 81 ± 36 | 81 ± 35 | 81 ± 36 | 81 ± 36 | 80 ± 35 | 75 ± 34 | <0.001 |
| nPCR (g/kg/day) | 0.79 ± 0.22 | 0.82 ± 0.23 | 0.81 ± 0.22 | 0.8 ± 0.22 | 0.8 ± 0.21 | 0.8 ± 0.22 | 0.78 ± 0.21 | 0.78 ± 0.21 | 0.76 ± 0.21 | 0.74 ± 0.21 | <0.001 |
| Phosphorus (mg/dL) | 4.9 ± 1.1 | 4.8 ± 1.2 | 4.9 ± 1.2 | 4.9 ± 1.1 | 5 ± 1.1 | 5 ± 1.1 | 4.9 ± 1.1 | 4.9 ± 1.1 | 4.9 ± 1.1 | 4.9 ± 1.1 | 0.002 |
| Potassium (mEq/L) | 4.4 ± 0.5 | 4.4 ± 0.5 | 4.4 ± 0.5 | 4.4 ± 0.5 | 4.4 ± 0.5 | 4.4 ± 0.5 | 4.4 ± 0.5 | 4.4 ± 0.5 | 4.4 ± 0.5 | 4.4 ± 0.5 | <0.001 |
| Sodium (mEq/L) | 138 ± 3 | 139 ± 3 | 138 ± 3 | 138 ± 3 | 138 ± 3 | 138 ± 3 | 138 ± 3 | 138 ± 3 | 137 ± 3 | 137 ± 3 | <0.001 |
| TIBC (mg/dL) | 225 ± 49 | 228 ± 47 | 232 ± 47 | 231 ± 47 | 230 ± 48 | 227 ± 48 | 223 ± 49 | 221 ± 49 | 217 ± 50 | 205 ± 52 | <0.001 |
| Total protein (g/dL) | 6.6 ± 0.7 | 5.8 ± 0.5 | 6.1 ± 0.5 | 6.3 ± 0.5 | 6.5 ± 0.5 | 6.6 ± 0.5 | 6.8 ± 0.4 | 6.9 ± 0.5 | 7.0 ± 0.5 | 7.5 ± 0.6 | <0.001 |
| WBC count (×103/mm3) | 7.8 ± 2.7 | 7.5 ± 3.3 | 7.6 ± 2.5 | 7.7 ± 2.5 | 7.8 ± 2.5 | 7.8 ± 2.3 | 8 ± 2.6 | 8 ± 2.5 | 8 ± 2.6 | 8 ± 3 | <0.001 |
| BMI (kg/m2) | 28 ± 7 | 27 ± 6 | 28 ± 7 | 28 ± 7 | 28 ± 7 | 29 ± 7 | 29 ± 8 | 29 ± 8 | 29 ± 8 | 28 ± 8 | <0.001 |
| Post-HD weight (kg) | 80 ± 22 | 76 ± 19 | 78 ± 21 | 79 ± 21 | 80 ± 22 | 81 ± 23 | 82 ± 23 | 83 ± 24 | 82 ± 24 | 82 ± 25 | <0.001 |
| Blood pressure (mmHg) | |||||||||||
| Post-HD systolic | 144 ± 18 | 143 ± 18 | 144 ± 18 | 144 ± 18 | 145 ± 18 | 145 ± 18 | 145 ± 18 | 144 ± 19 | 144 ± 18 | 142 ± 19 | 0.0003 |
| Post-HD diastolic | 76 ± 11 | 74 ± 11 | 75 ± 11 | 75 ± 11 | 76 ± 11 | 76 ± 11 | 76 ± 11 | 76 ± 11 | 76 ± 11 | 76 ± 11 | <0.001 |
| spKt/v | 1.47 ± 0.32 | 1.51 ± 0.32 | 1.5 ± 0.32 | 1.49 ± 0.32 | 1.47 ± 0.31 | 1.47 ± 0.32 | 1.45 ± 0.32 | 1.43 ± 0.31 | 1.43 ± 0.32 | 1.4 ± 0.31 | <0.001 |
CVC, central venous catheter; AV, arteriovenous; ASHD, atherosclerotic heart disease; CHF, congestive heart failure; CVD, cardiovascular disease; CBVD, cerebrovascular disease; COPD, chronic obstructive pulmonary disease; iPTH, intact parathyroid hormone; ISAT, iron saturation; KRU, residual urea clearance; LDL, low-density lipoprotein; TIBC, total iron-binding capacity; BMI, body mass index; HD, hemodialysis; spKt/V, single-pooled Kt/V.
Values are presented as mean ± SD unless stated otherwise. Percentage may not add up to 100% due to rounding.
Association between baseline serum globulin and mortality outcomes
Patients in our cohort had a median follow-up time of 484 days (IQR 227–897) and 26 750 deaths (26%) occurred during a maximum follow-up time of 5 years. Using a serum globulin of 3.0–<3.2 g/dL as the reference group, we observed a J-shaped association between serum globulin and all-cause mortality in an unadjusted model (Figure 1A; Supplementary data, Table S1A). While higher globulin was associated with higher mortality, patients with a baseline globulin ≤2.4 g/dL had a 16% higher risk of death {hazard ratio [HR] 1.16 [95% confidence interval (CI) 1.10–1.22]} compared with patients with globulin levels in the referent group. After adjustment for demographics and comorbidities, this mortality association for lower globulin was reversed and lower serum globulin levels were associated with an 8%, 14%, 9% and 8% lower mortality risk for serum globulin groups ≤2.4, 2.4–<2.6, 2.6–<2.8 and 2.8–<3.0 g/dL, respectively. At higher levels of globulin (≥3.8 g/dL) we observed a 58% higher risk of all-cause mortality [HR 1.58 (95% CI 1.51–1.66)] that remained significant after full adjustment [HR 1.11 (95% CI 1.06–1.16)].
Figure 1:
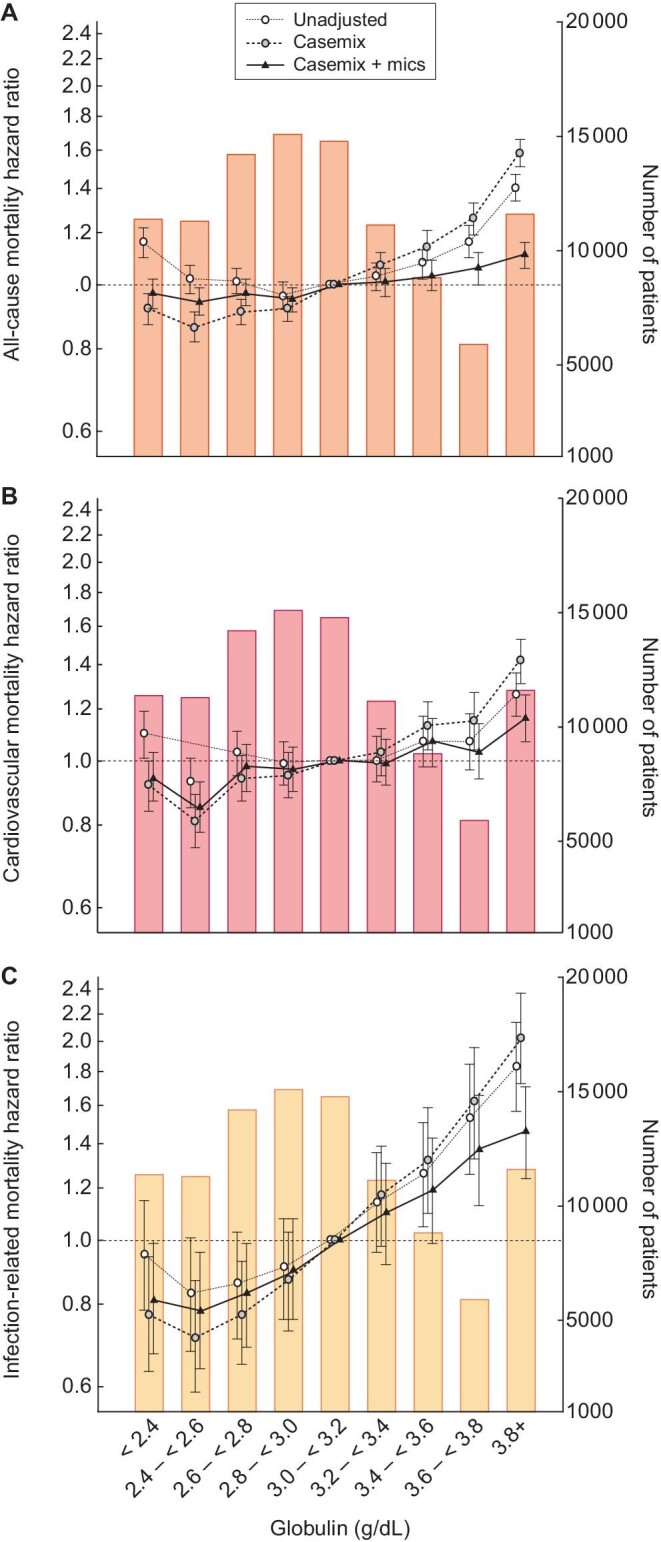
Associations of serum globulin (g/dL) with (A) all-cause, (B) cardiovascular and (C) infection-related mortality with hierarchical adjustments in 104 164 incident hemodialysis patients.
Furthermore, higher serum globulin levels trended toward a higher CV mortality hazard in both the unadjusted and case-mix-adjusted model (reference 3.0–<3.2 g/dL) (Figure 1B; Supplementary data, Table S1B). This association was most pronounced in patients with baseline serum globulin ≥3.8 g/dL in the case-mix model [HR 1.42 (95% CI 1.31–1.53)], as well as in the full adjustment model [HR 1.16 (95% CI 1.07–1.26)].
Finally, the serum globulin and infection-related mortality followed a reversed J-shaped pattern in the unadjusted and case-mix-adjusted model (Figure 1C). The association was mitigated for higher globulin groups after MICS adjustment. Patients with serum globulin of 2.4–<2.6 g/dL consistently had the lowest infection-related mortality hazard [HR 0.71 (95% CI 0.59–0.87) and HR 0.78 (95% CI 0.64–0.96) for the case-mix and MICS-adjusted model, respectively] (Supplementary data, Table S1C).
For the 32 452 African American patients included in our cohort, 6840 deaths occurred during a median follow-up of 557 days (IQR 264–981), while among 48 608 Caucasian patients 15 571 deaths occurred during a median follow-up of 412 days (IQR 195–791). In African Americans, higher serum globulin levels were associated with higher mortality when compared with the reference group (3.0–<3.2 g/dL) in the unadjusted and case-mix-adjusted model. Notably, African Americans with baseline serum globulin ≥3.8 g/dL had a 61% higher mortality hazard in the case-mix-adjusted model [HR 1.61 (95% CI 1.48–1.74)]. This association was mitigated in the fully adjusted model, but remained significant [HR 1.15 (95% CI 1.06–1.25)] (Supplementary data, Table S2A, Figure S2A). In Caucasian patients, the serum globulin–mortality association followed a linear shape. In the case-mix-adjusted model, Caucasian patients with baseline serum globulin concentrations ≥3.2 g/dL had a higher mortality risk compared with the reference group (3.0–<3.2 g/dL), with the highest risk for patients with serum globulin ≥3.8 g/dL [HR 1.43 (95% CI 1.33–1.53)]. This association was no longer seen after adjusting for variables of malnutrition and inflammation (Supplementary data, Table S2B, Figure S2B).
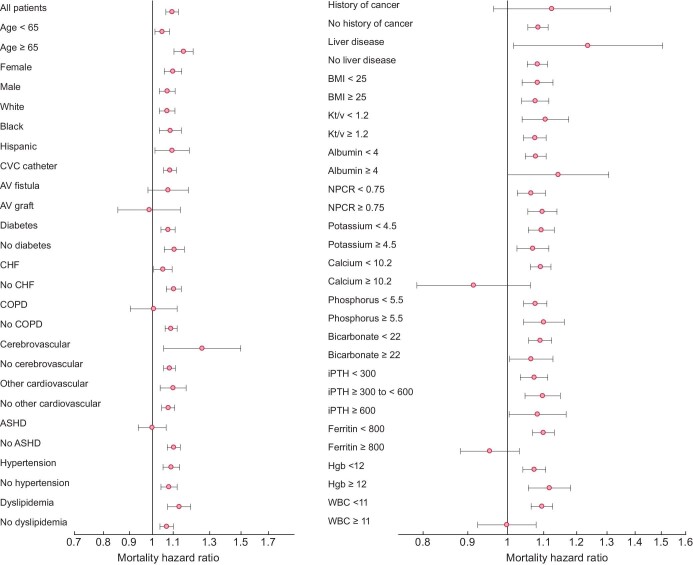
In subgroup analysis, the fully adjusted mortality HRs were above unity across almost all examined subgroups, which indicates a higher mortality risk with higher globulin levels (≥3.2 g/dL) compared with the reference group (globulin concentration <3.2 g/dL) (Figure 2). For all-cause mortality, significant interactions between globulin ≥3.2 g/dL and subgroups were observed for age, diabetes, congestive heart failure, chronic obstructive pulmonary disease, atherosclerotic heart disease, serum calcium, ferritin and WBC count (Supplementary data, Table S3).
Figure 2:
Subgroup analyses examining the association of high serum globulin (≥3.2 g/dL) with all-cause mortality in the fully adjusted model among 104 164 hemodialysis patients. ASHD, atherosclerotic heart disease; COPD, chronic obstructive pulmonary disease; CVC, central venous catheter; AV, arteriovenous; CHF, congestive heart failure; CVD, cardiovascular disease; CBVD, cerebrovascular disease; HGB, hemoglobin; iPTH, intact parathyroid hormone; ISAT, iron saturation; KRU, residual urea clearance; LDL, low-density lipoprotein; NPCR, normalized protein catabolic rate; TIBC, total iron-binding capacity; WBC, white blood cell count; BMI, body mass index; HD, hemodialysis; spKt/V, single-pooled Kt/V.
Age in years; SBP and DBP in mmHg; weight in kg; albumin, hemoglobin and creatinine in g/dL; calcium, phosphorus and TIBC in mg/dL; ferritin in ng/mL; iPTH in pg/mL; bicarbonate and potassium in mEq/L; ALP and AST in IU/L; ISAT in %; WBC in ×103/mm3.
We also found that serum albumin did not substantially alter the globulin–mortality association in patients with serum globulin ≥3.2 g/dL. Lower and higher serum albumin enhanced the mortality risk associated with higher serum globulin (Figure 3).
Figure 3:
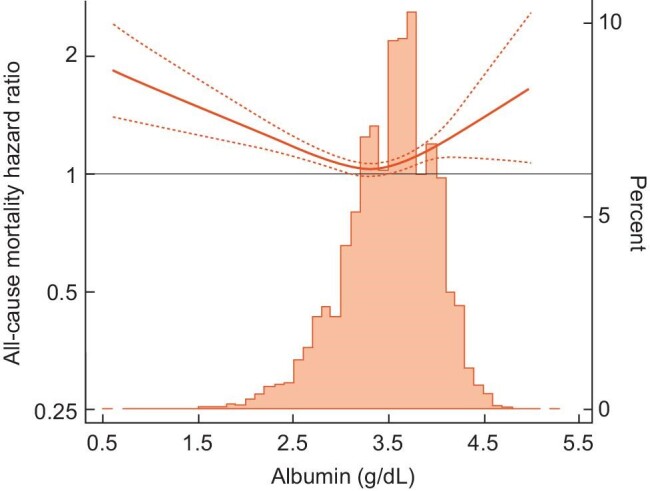
Effect modification of serum albumin on the association of high globulin (≥3.2 g/dL) and all-cause mortality association splined over continuous serum albumin in 37 436 incident hemodialysis patients.
Association between baseline serum A:G ratio and mortality outcomes
The A:G ratio–mortality association followed a reverse linear pattern across all three models for all-cause and CV mortality (Supplementary data, Figure S3A and S3B). Compared with the reference group (A:G ratio 1.05–<1.15), lower A:G ratio groups were associated with higher all-cause mortality risk, while higher A:G ratio groups were associated with lower all-cause mortality risk. Patients with an A:G ratio <0.75 had a 45% higher mortality risk [HR 1.45 (95% CI 1.38–1.52)] and patients with an A:G ratio ≥1.55 had a 30% lower mortality risk [HR 0.70 (95% CI 0.66–0.74)] after full adjustment (Supplementary data, Figure S3A, Table S4A).
Likewise, in the fully adjusted model, patients with an A:G ratio <0.75 had a 28% higher CV mortality risk [HR 1.28 (95% CI 1.17–1.39)] when compared with the reference group. In contrast, an A:G ratio ≥1.15 was associated with lower risk, with the greatest benefit for patients with an A:G ratio ≥1.55 [HR 0.69 (95% CI 0.63–0.76)] (Supplementary data, Figure S3B, Table S4B).
The association between A:G ratio and infection-related mortality followed a reverse linear pattern, where patients with an A:G ratio <1.15 had a higher risk for infection-related mortality. Notably, a 71% higher mortality risk was found in patients with an A:G ratio <0.75 [HR 1.71 (95% CI 1.44–2.03)] when compared with the reference group in the fully adjusted model (Supplementary data, Figure S3C, Table S4C).
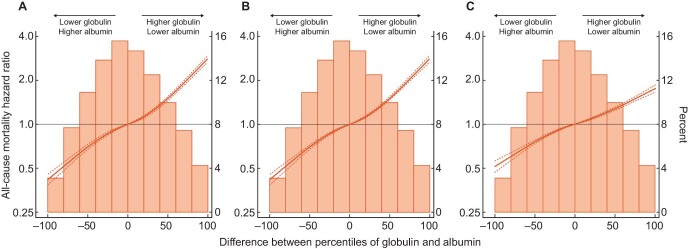
Percentile rank score analysis showed that the combination of a lower serum globulin level with a higher serum albumin level was protective, while a higher serum globulin but lower serum albumin level increased the death risk across all three models (Figure 4A–C).
Figure 4:
Mortality association of baseline globulin and albumin in 104 164 incident hemodialysis patients. (A–C) The sum of differences between percentiles of baseline serum globulin and albumin in (A) unadjusted, (B) case-mix and (C) case-mix and MICS-adjusted models.
Each patient received a percentile score between −100 and +100 according to the baseline serum globulin and serum albumin level. Differences of the scores created a percentile rank score between −100 and +100.
Association between baseline serum total protein and mortality outcomes
The association between serum total protein and mortality outcomes followed a reversed J-shaped pattern, with higher mortality in patients with lower baseline serum total protein in the unadjusted model (reference 6.5–<7.0 g/dL) (Supplementary data, Figure S4). In the fully adjusted model, patients with serum total protein <5.5 g/dL had a 34% higher all-cause mortality risk [HR 1.34 (95% CI 1.27–1.42)], while patients with serum total protein ≥7.5 g/dL had a 10% higher risk [HR 1.10 (95% CI 1.05–1.16)] (Supplementary data, Figure S4A, Table S5A). The association between serum total protein and higher CV mortality was mitigated after adjustment for patient demographics and comorbidities, remaining significant only for patients with baseline serum total protein <6.5 g/dL. Patients with serum total protein <5.5, 5.5–<6 and 6–<6.5 g/dL had a 22%, 21% and 7% higher CV mortality risk, respectively, compared with the reference group in the fully adjusted model (Supplementary data, Figure S4B, Table S5B). Lastly, in the fully adjusted model, serum total protein <5.5 and ≥7.5 g/dL was associated with a higher infection-related mortality hazard [HR 1.37 (95% CI 1.13–1.65) and HR 1.32 (95% CI 1.11–1.59), respectively] (Supplementary data, Figure S4C, Table S5C).
Association between time-varying serum globulin and mortality outcomes
We further explored the relationship of serum globulin and all-cause mortality using time-varying models (Supplementary data, Table S6). Similar to the baseline results, a J-shaped association was found across all levels of adjustment. In the unadjusted model, time-varying globulin ≥3.8 g/dL was associated with a 2-fold higher all-cause mortality risk [HR 2.41 (95% CI 2.30–2.52)] compared with the reference (globulin 3.0–<3.2). After adjustment for time-varying MICS covariates, this association remained consistent, where higher time-varying globulin (≥3.2 g/dL) was associated with a 26%, 47%, 67% and 161% higher all-cause mortality risk for time-varying globulin groups 3.2–<3.4, 3.4–<3.6, 3.6–<3.8, and ≥3.8 g/dL, respectively, compared with our reference group.
DISCUSSION
In the present study we examined the association between serum globulin, A:G ratio and serum total protein each with all-cause mortality in a cohort of ESRD patients. In 104 154 incident hemodialysis patients, higher serum globulin (≥3.8 g/dL) was associated with a higher all-cause and infection-related mortality risk in a model adjusted for patients’ demographics, comorbidities and other variables of malnutrition and inflammation. Moreover, a lower A:G ratio and lower total serum protein were also associated with higher all-cause, CV and infection-related mortality.
A recent Chinese study showed that the gamma gap was an independent predictor for 4-year all-cause mortality in individuals ≥90 years of age, and ageing, similar to ESRD, is linked to low-grade inflammation [20, 21]. Indeed, an increase in globulins can be found in conditions associated with inflammation such as infections, malignancy, hematological disorders and connective tissue disease [6, 7, 22]. Infection and infection-related complications are major causes of morbidity and mortality in hemodialysis patients, in which vascular access site, dialysate back-leak, impaired humoral and cellular immunity and non sterile dialysate are all risk factors for infection in this population [23, 24]. We found that incident hemodialysis patients with higher serum globulin tended to have a higher all-cause, CV and infection-related mortality risk in the model adjusted for patients’ demographics and comorbidities. The association was mitigated after further adjustment for variables of malnutrition and inflammation; however, the globulin–mortality relationship persisted for all-cause mortality in patients with serum globulin ≥3.8 g/dL and for infection-related mortality in patients with serum globulin ≥3.6 g/dL.
A higher serum globulin level is most commonly due to an increase in the immunoglobulin fraction [4]. Immunoglobulins are crucial in fighting infections and may also be involved in the pathophysiology of different disease states. For instance, immunoglobulins have been shown to contribute to the formation of atheromatous lesions in CV disease [25, 26]. Notably, in our subgroup analysis, we found that patients with atherosclerotic heart disease and serum globulin ≥3.2 g/dL were at greater mortality risk. Moreover, it has been suggested that globulin may be a marker of severity in chronic obstructive pulmonary disease (COPD) patients, correlating with the number of readmissions after 6 months of hospitalization and forced expiratory volume in 1 sec [27]. Similarly, we also found that COPD patients with serum globulin ≥3.2 g/dL tended to have a higher all-cause mortality risk compared with non-COPD patients. Finally, our subgroup analysis revealed that patients with a higher WBC count or ferritin and serum globulin concentrations ≥3.2 g/dL had a higher all-cause mortality risk than patients with a lower inflammatory milieu.
In our cohort, patients grouped into higher globulin baseline groups also tended to have lower serum albumin concentrations, a trend that is characteristic for many inflammatory states (e.g. late phase of acute inflammation, chronic inflammation or chronic active inflammation) [22]. Thus a lower A:G ratio can be driven by low albumin, high globulin or a combination of both, and in this way the measure combines two strong predictors of mortality that is independent of fluid status.
In recent years, the prognostic value of the A:G ratio has been investigated not only in a number of clinical contexts, predominantly for prognosis in cancer patients [28–36], but also in small cohorts of CKD patients [5, 17, 37]. Our findings that a lower A:G ratio is associated with higher all-cause, CV and infection-related mortality in incident hemodialysis patients is consistent with the reverse linear associations between the A:G ratio and all-cause and CV mortality reported in all three of these previous studies, though their cohorts consisted of patients on peritoneal dialysis [5, 37] or an undefined dialysis modality (hemodialysis versus peritoneal dialysis) [17]. These findings were further corroborated with percentile rank score analysis, which showed that patients with lower serum albumin and higher globulin had a higher risk for all-cause mortality.
Furthermore, we observed racial/ethnic disparities in the globulin–mortality association. In our cohort, African Americans tended to have higher globulin concentrations than Caucasian patients, which has been previously observed [38, 39]. Interestingly, in Caucasian patients, the higher globulin–mortality association was abolished after adjustment for other markers of malnutrition and inflammation, whereas it persisted in African Americans with serum globulin ≥3.8 g/dL. This racial/ethnic disparity appears consistent with a study that found that African Americans had higher mortality rates after adjustment for markers of the malnutrition–inflammation complex and postulated that more favorable nutritional and inflammatory profiles among African Americans may potentially explain the survival disparities between racial/ethnic groups [40]. The mechanism remains to be elucidated but may be related to the increased incidence of autoimmune disease or blood dyscrasias in African Americans that affect serum globulin levels [41–43].
Strengths of this study include a large and diverse sample size, thorough adjustment for common markers of malnutrition and inflammation and refined categories of serum globulin, A:G ratio and serum total protein. Nevertheless, several limitations of this study should be mentioned. We are inclined to believe that the association between globulin and mortality is independent of the etiology of increased globulin given that the association remained after adjustment for multiple comorbid conditions, although further studies are needed to confirm our findings and elucidate the underlying pathophysiology between increased globulin levels and mortality. In addition, our cohort included many patients with catheters, which are associated with higher mortality and also a cause of inflammation, but we adjusted for catheter use in an attempt to limit confounding. However, there remains the possibility of residual confounding. We are unable to pinpoint a specific mechanism driving the serum globulin–mortality association, in part due to lack of data on the fractional components of serum globulin as well as other confounders such as C-reactive protein, fibrinogen, markers of oxidative stress and dietary intake and information on surgical procedures or infectious status that were unavailable for analysis. Moreover, we cannot provide additional information on medication data, including antibiotic treatment. Despite these limitations, our findings may be useful in establishing thresholds for gamma gap and A:G ratio values to determine the need for further testing and monitoring in patients starting hemodialysis.
In conclusion, we demonstrated that higher globulin concentrations, lower A:G ratio and lower total protein concentration are associated with higher all-cause, CV and infection-related mortality in incident hemodialysis patients. Moreover, the association between higher globulin and mortality was found to be independent of serum albumin. Future prospective studies are needed that analyze the fractional components of serum globulin in order to elucidate the driving mechanisms for our findings and to guide therapeutic strategies for better long-term clinical outcomes in CKD patients.
Supplementary Material
ACKNOWLEDGEMENTS
K.K.Z. is supported by the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases) grants K24-DK091419 and also by philanthropic grants from Harold Simmons, Louis Chang, Joseph Lee and AVEO. The authors thank DaVita Clinical Research for providing the clinical data for this study.
Contributor Information
Alex Y Pai, Harold Simmons Center for Kidney Disease Research and Epidemiology, Division of Nephrology and Hypertension, University of California Irvine Medical Center, Orange, CA, USA.
John Sy, Harold Simmons Center for Kidney Disease Research and Epidemiology, Division of Nephrology and Hypertension, University of California Irvine Medical Center, Orange, CA, USA; Nephrology Section, Tibor Rubin VA Medical Center, Long Beach, CA, USA.
Joseph Kim, Harold Simmons Center for Kidney Disease Research and Epidemiology, Division of Nephrology and Hypertension, University of California Irvine Medical Center, Orange, CA, USA.
Carola-Ellen Kleine, Harold Simmons Center for Kidney Disease Research and Epidemiology, Division of Nephrology and Hypertension, University of California Irvine Medical Center, Orange, CA, USA.
Jessica Edward, Harold Simmons Center for Kidney Disease Research and Epidemiology, Division of Nephrology and Hypertension, University of California Irvine Medical Center, Orange, CA, USA.
Jui-Ting Hsiung, Harold Simmons Center for Kidney Disease Research and Epidemiology, Division of Nephrology and Hypertension, University of California Irvine Medical Center, Orange, CA, USA.
Csaba P Kovesdy, Division of Nephrology, University of Tennessee Health Science Center, Memphis VA Medical Center, Memphis, TN, USA.
Kamyar Kalantar-Zadeh, Harold Simmons Center for Kidney Disease Research and Epidemiology, Division of Nephrology and Hypertension, University of California Irvine Medical Center, Orange, CA, USA; Nephrology Section, Tibor Rubin VA Medical Center, Long Beach, CA, USA.
Elani Streja, Harold Simmons Center for Kidney Disease Research and Epidemiology, Division of Nephrology and Hypertension, University of California Irvine Medical Center, Orange, CA, USA; Nephrology Section, Tibor Rubin VA Medical Center, Long Beach, CA, USA.
AUTHORS’ CONTRIBUTIONS
K.K.Z and E.S. were responsible for the research idea, study design, data acquisition, supervision and mentorship. E.S., C.E.K., J.S., C.P.K., and A.P. were responsible for the data analysis/interpretation, C.E.K., J.K., J.E., J.T.H. and E.S. were responsible for the statistical analysis. Each author contributed important intellectual content during manuscript drafting.
FUNDING
There is no funding to report.
CONFLICT OF INTEREST STATEMENT
C.P.K. received honoraria from Abbott, Akebia, Astra-Zeneca, Bayer, Cara Therapeutics, CSL Behring, Rockwell and Vifor. K.K.Z. has received honoraria and/or support from Abbott, AbbVie, Alexion, Amgen, American Society of Nephrology, Astra Zeneca, Aveo, Chugai, DaVita, Fresenius, Genetech, Haymarket Media, Hospira, Kabi, Keryx, National Institutes of Health, National Kidney Foundation, Relypsa, Resverlogix, Sanofi, Shire Vifor, and ZS Pharma. ES has received honoraria or support from Edwards LifeSciences and Astra-Zeneca.
REFERENCES
- 1. Pifer TB, McCullough KP, Port FKet al. Mortality risk in hemodialysis patients and changes in nutritional indicators: DOPPS. Kidney Int 2002; 62: 2238–2245 [DOI] [PubMed] [Google Scholar]
- 2. Herselman M, Esau N, Kruger JMet al. Relationship between serum protein and mortality in adults on long-term hemodialysis: exhaustive review and meta-analysis. Nutrition 2010; 26: 10–32 [DOI] [PubMed] [Google Scholar]
- 3. Bergstrom J, Lindholm B, Lacson E Jret al. What are the causes and consequences of the chronic inflammatory state in chronic dialysis patients? Semin Dial 2000; 13: 163–164 [DOI] [PubMed] [Google Scholar]
- 4. Busher JT. Serum albumin and globulin. In: Walker, HK, Hall WD, Hurst JW (eds). Clinical Methods: The History, Physical, and Laboratory Examinations. Boston: Butterworths, 1990. [PubMed] [Google Scholar]
- 5. Peng F, Sun L, Chen Tet al. Albumin–globulin ratio and mortality in patients on peritoneal dialysis: a retrospective study. BMC Nephrol 2020; 21: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dispenzieri A, Gertz MA, Therneau TMet al. Retrospective cohort study of 148 patients with polyclonal gammopathy. Mayo Clin Proc 2001; 76: 476–487 [DOI] [PubMed] [Google Scholar]
- 7. O'Connell TX, Horita TJ, Kasravi B. Understanding and interpreting serum protein electrophoresis. Am Fam Physician 2005; 71: 105–112 [PubMed] [Google Scholar]
- 8. Munshi NC. Plasma cell disorders: an historical perspective. Hematology Am Soc Hematol Educ Program 2008; doi: 10.1182/asheducation-2008.1.297 [DOI] [PubMed] [Google Scholar]
- 9. Fulks M, Stout RL, Dolan VF. Serum globulin predicts all-cause mortality for life insurance applicants. J Insur Med 2014; 44: 93–98 [PubMed] [Google Scholar]
- 10. Juraschek SP, Moliterno AR, Checkley Wet al. The gamma gap and all-cause mortality. PLoS One 2015; 10: e0143494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalantar-Zadeh K, Ikizler TA, Block Get al. Malnutrition–inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis 2003; 42: 864–881 [DOI] [PubMed] [Google Scholar]
- 12. Locatelli F, Canaud B, Eckardt KUet al. Oxidative stress in end-stage renal disease: an emerging threat to patient outcome. Nephrol Dial Transplant 2003; 18: 1272–1280 [DOI] [PubMed] [Google Scholar]
- 13. Noh H, Lee SW, Kang SWet al. Serum C-reactive protein: a predictor of mortality in continuous ambulatory peritoneal dialysis patients. Perit Dial Int 1998; 18: 387–394 [PubMed] [Google Scholar]
- 14. Yeun JY, Levine RA, Mantadilok Vet al. C-reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 2000; 35: 469–476 [DOI] [PubMed] [Google Scholar]
- 15. Zimmermann J, Herrlinger S, Pruy Aet al. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int 1999; 55: 648–658 [DOI] [PubMed] [Google Scholar]
- 16. Selim G, Stojceva-Taneva O, Zafirovska Ket al. Inflammation predicts all-cause and cardiovascular mortality in haemodialysis patients. Prilozi 2006; 27: 133–144 [PubMed] [Google Scholar]
- 17. Wu PP, Hsieh YP, Kor CTet al. Association between albumin–globulin ratio and mortality in patients with chronic kidney disease. J Clin Med 2019; 8: 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuttykrishnan S, Kalantar-Zadeh K, Arah OAet al. Predictors of treatment with dialysis modalities in observational studies for comparative effectiveness research. Nephrol Dial Transplant 2015; 30: 1208–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kalantar-Zadeh K, Streja E, Molnar MZet al. Mortality prediction by surrogates of body composition: an examination of the obesity paradox in hemodialysis patients using composite ranking score analysis. Am J Epidemiol 2012; 175: 793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang M, Xie L, Liu Xet al. The gamma gap predicts 4-year all-cause mortality among nonagenarians and centenarians. Sci Rep 2018; 8: 1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Akchurin OM, Kaskel F. Update on inflammation in chronic kidney disease. Blood Purif 2015; 39: 84–92 [DOI] [PubMed] [Google Scholar]
- 22. Vavricka SR, Burri E, Beglinger Cet al. Serum protein electrophoresis: an underused but very useful test. Digestion 2009; 79: 203–210 [DOI] [PubMed] [Google Scholar]
- 23. Vanholder R, Ringoir S, Dhondt Aet al. Phagocytosis in uremic and hemodialysis patients: a prospective and cross sectional study. Kidney Int 1991; 39: 320–327 [DOI] [PubMed] [Google Scholar]
- 24. Kaysen GA. The microinflammatory state in uremia: causes and potential consequences. J Am Soc Nephrol 2001; 12: 1549–1557 [DOI] [PubMed] [Google Scholar]
- 25. Sims FH, Gavin JB, Edgar Set al. Diffusion of gamma globulin into the arterial wall identifies localized entry of lipid and cells in atherosclerosis. Coron Artery Dis 2001; 12: 21–30 [DOI] [PubMed] [Google Scholar]
- 26. Tsiantoulas D, Diehl CJ, Witztum JLet al. B cells and humoral immunity in atherosclerosis. Circ Res 2014; 114: 1743–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boixeda R, Capdevila JA, Vicente Vet al. Gamma globulin fraction of the proteinogram and chronic obstructive pulmonary disease exacerbations. Med Clin (Barc) 2017; 149: 107–113 [DOI] [PubMed] [Google Scholar]
- 28. Fujikawa H, Toiyama Y, Inoue Yet al. Prognostic impact of preoperative albumin-to-globulin ratio in patients with colon cancer undergoing surgery with curative intent. Anticancer Res 2017; 37: 1335–1342 [DOI] [PubMed] [Google Scholar]
- 29. Liu J, Dai Y, Zhou Fet al. The prognostic role of preoperative serum albumin/globulin ratio in patients with bladder urothelial carcinoma undergoing radical cystectomy. Urol Oncol 2016; 34: 484.e1–484. e8. [DOI] [PubMed] [Google Scholar]
- 30. Oki S, Toiyama Y, Okugawa Yet al. Clinical burden of preoperative albumin–globulin ratio in esophageal cancer patients. Am J Surg 2017; 214: 891–898 [DOI] [PubMed] [Google Scholar]
- 31. Guo HW, Yuan TZ, Chen JXet al. Prognostic value of pretreatment albumin/globulin ratio in digestive system cancers: a meta-analysis. PLoS One 2018; 13: e0189839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang J, Liu X, Yang Zet al. The pretreatment albumin to globulin ratio, a validated biomarker, predicts prognosis in hepatocellular carcinoma. J BUON 2016; 21: 925–934 [PubMed] [Google Scholar]
- 33. Park S, Park S, Lee SHet al. Pretreatment albumin-to-globulin ratio as a predictive marker for tyrosine kinase inhibitor in non-small cell lung cancer. Cancer Biomark 2016; 16: 425–433 [DOI] [PubMed] [Google Scholar]
- 34. He X, Guo S, Chen Det al. Preoperative albumin to globulin ratio (AGR) as prognostic factor in renal cell carcinoma. J Cancer 2017; 8: 258–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen Z, Shao Y, Yao Het al. Preoperative albumin to globulin ratio predicts survival in clear cell renal cell carcinoma patients. Oncotarget 2017; 8: 48291–48302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Azab BN, Bhatt VR, Vonfrolio Set al. Value of the pretreatment albumin to globulin ratio in predicting long-term mortality in breast cancer patients. Am J Surg 2013; 206: 764–770 [DOI] [PubMed] [Google Scholar]
- 37. Tsai CC, Hsieh YP, Tsai SMet al. Superiority of albumin–globulin ratio over albumin to predict mortality in patients undergoing peritoneal dialysis. Sci Rep 2020; 10: 19764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Holmes EG, Stanier MW, Semambo YBet al. An investigation of serum proteins of Africans in Uganda. Trans R Soc Trop Med Hyg 1951; 45: 371–382 [DOI] [PubMed] [Google Scholar]
- 39. Keltz H, Comstock GW.. Serum globulin levels in whites and Negroes. N Engl J Med 1959; 260: 1268–1271 [DOI] [PubMed] [Google Scholar]
- 40. Streja E, Kovesdy CP, Molnar MZet al. Role of nutritional status and inflammation in higher survival of African American and Hispanic hemodialysis patients. Am J Kidney Dis 2011; 57: 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roberts DH, Little TW. An auto-immune response associated with porcine enzootic pneumonia. Vet Rec 1970; 86: 328. [DOI] [PubMed] [Google Scholar]
- 42. Kirtane K, Lee SJ. Racial and ethnic disparities in hematologic malignancies. Blood 2017; 130: 1699–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beutler E, West C. Hematologic differences between African-Americans and whites: the roles of iron deficiency and alpha-thalassemia on hemoglobin levels and mean corpuscular volume. Blood 2005; 106: 740–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.