Abstract
Fibroblast growth factor 23 (FGF23) is a circulating hormone derived from the bone whose release is controlled by many factors and exerts a multitude of systemic actions. There are congenital and acquired disorders of increased and decreased FGF23 levels. In chronic kidney disease (CKD), elevations of FGF23 levels can be 1000-fold above the upper physiological limit. It is still debated whether this high FGF23 in CKD is a biomarker or causally related to morbidity and mortality. Data from human association studies support pathogenicity, while experimental data are less robust. Knowledge of the biology and pathobiology of FGF23 has generated a plethora of means to reduce FGF23 bioactivity at many levels that will be useful for therapeutic translations. This article summarizes these approaches and addresses several critical questions that still need to be answered.
Keywords: chronic renal failure, CKD, FGF23, Klotho, mineral metabolism
INTRODUCTION
Fibroblast growth factor 23 (FGF23) is a circulating glycoprotein hormone produced by osteoblasts and osteocytes whose physiological function was originally deemed to be dedicated to phosphate (P) homeostasis. FGF23 decreases P reabsorption in the renal proximal tubules [1] and suppresses vitamin D synthesis, resulting in a decreased intestinal uptake of P [1]. In addition, FGF23 controls parathyroid hormone (PTH) secretion, also resulting in lowering of P levels [1]. When P loads or levels are high, FGF23 is upregulated to maintain normal P balance and prevent P overload and toxicity. Many other factors can increase FGF23 synthesis, including calcium levels, active vitamin D, PTH, leptin, catecholamines, mineralocorticoids, volume depletion, lithium, iron deficiency, high-fat diet, Klotho deficiency and inflammation; these are summarized in detail in excellent recent reviews [2–4]. Theoretically, manipulation of one or more of these upstream regulators is a potential means of controlling FGF23 levels.
DERANGEMENTS OF FGF23 LEVELS
Disturbances of FGF23 levels disrupt P homeostasis, with dire consequences. In conditions of primary FGF23 excess, hypophosphataemia and total body P depletion occur, impairing bone mineralization and resulting in growth retardation and bone deformity in children and bone pain and muscle weakness in adults [5]. Several congenital as well as acquired disturbances cause a primary increase in FGF23 levels that lacks definitive therapy (Table 1) [5].
Table 1.
Diseases causing an increase in FGF23
| Congenital | Acquired |
|---|---|
|
Autosomal dominant hypophosphataemic rickets Autosomal recessive hypophosphataemic rickets XLH Fibrous dysplasia McCune–Albright syndrome |
Tumour-induced osteomalacia CKD |
Far more common than primary increases in FGF23 are secondary high FGF23 states, universally seen in chronic kidney disease (CKD) in 11–15% of the world population [6]. In early CKD, high FGF23 may be adaptive to maintain P balance. In later stages of CKD, high FGF23 is conceivably maladaptive and can contribute to extrarenal morbidities [7]. The pathologic effects of FGF23 have been proposed to be mediated in part by FGF receptor 1 (FGFR1)/Klotho-independent signalling via the FGFR4–phospholipase C (PLC)γ–calcineurin–nuclear factor of activated T cells (NFAT) cascade [6]. This review will focus mostly on treating the secondary high FGF23 states such as CKD.
RATIONALE FOR TREATING HIGH FGF23 BIOACTIVITY IN CKD
There is a strong epidemiologic association between FGF23 level and undesirable clinical events or mortality [1]. Although the association of FGF23 with adverse outcomes in humans is well documented, the experimental data supporting causality are less robust. The critical question in CKD is whether FGF23 is a biomarker or causal of undesired sequelae.
In the heart, data implicating a pathologic effect are more abundant. In CKD patients, FGF23 levels are associated with left ventricular hypertrophy (LVH) [8]. In vitro cell culture and in vivo animal experiments both suggest that FGF23 can induce hypertrophy via direct FGFR4-mediated signalling on cardiomyocytes [8, 9]. In contrast, data on LVH in X-linked hypophosphataemia (XLH; an X-linked dominant form of rickets) patients are inconsistent and sparse [10]. This has been attributed to the lower FGF23 levels, absence of uraemic toxins and the fact that XLH patients are P-depleted rather than phosphotoxic. Not all studies of XLH have shown a relationship between FGF23 and LVH [11, 12]. Interestingly, increased cardiac afterload, myocardial infarction, cardiac hypertrophy and heart failure in humans are also associated with high FGF23 [13, 14], raising a possibility that FGF23 may be upregulated following cardiovascular disease.
Associations between FGF23 with atherosclerosis and vascular dysfunction have been found in CKD patients [15, 16]. Preclinical data are not definitive. In a rat model of CKD and in cultured vascular smooth muscle cells, FGF23 induced calcification, but oddly only in the presence of Klotho [17]. There is also a paradoxical protective effect of FGF23 on vascular calcification after upregulation with active vitamin D [18].
FGF23 might affect the liver by increasing both interleukin-6 (IL-6) and C-reactive protein production via FGFR4 [19]. Also, it is associated with the risk of inflammation-related mortality in haemodialysis patients [20]. Activation of FGFR2 by FGF23 may inhibit selectin and chemokine-triggered β2 integrin activation in neutrophils [21], thus impairing the host immune response [21]. An alternative view is that high FGF23 is a consequence of inflammation rather than a cause, since FGF23 secretion in osteocytes is upregulated in inflammation [22].
If causality between FGF23 and adverse outcomes in CKD is not yet established, why should one even consider anti-FGF23 therapy? There are reasons to devote effort to this front. First, since the correlative clinical data and more definitive preclinical data are both quite strong, devising strategies to block its action is quite appropriate. Second, one should have an armamentarium of therapies at one’s disposal when further data are eventually secured supporting causality. Not all modalities work, so one should be equipped with multiple strategies. In addition, there are likely opportunities for synergistic therapies. Third, and most importantly, the only way to confirm causality in human CKD is by intervention trials, which mandates the availability of effective control of FGF23 bioactivity. The journey of FGF23 from origin to target organ along with the multiple locales of current strategies at our disposal to reduce its bioactivity are summarized in Figure 1. Seldom has one encountered an example of an endocrine disorder with such diverse multilevel corrective measures.
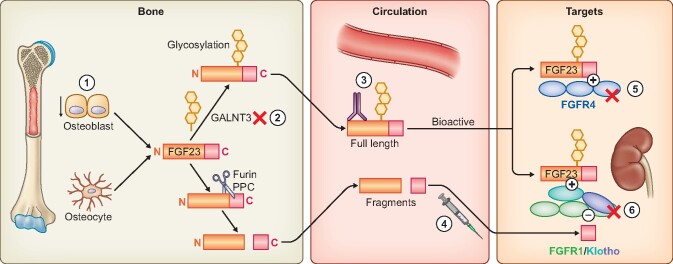
FIGURE 1.
Production and action of endocrine FGF23 and strategies to lower FGF23 bioactivity. 1. Reduce production. Achieved by reduction of P intake and absorption (see Figure 2) and pharmacologically by calcimimetics. 2. Inhibit glycosylation of newly synthesized FGF23 by small molecules. Glycosylation of FGF23 by GALNT3 (UDP-N-acetyl-alpha-D-galactosamine: polypeptide N-acetylgalactosaminyltransferase 3) protects FGF23 from cleavage by furin proprotein convertase (PPC). Inhibition of GALNT3 results in accelerated FGF23 cleavage and clearance. 3. Neutralizing antibodies to circulating FGF23 reduce circulating active FGF23 4. Exogenous C-term fragment of FGF23 competitively blocks full-length FGF23 on-target action on the FGFR1–Klotho coreceptors (see Figure 3). 5. FGFR4 inhibitors block the off-target effects of FGF23 on FGFR4. 6. Pan-FGFR inhibitors block FGFR1–Klotho coreceptor complex and the other FGFRs.
LOWERING FGF23 PRODUCTION
The first approach is to reduce FGF23 production from the bone using physiologic and pharmacologic means. This approach is presumably the most beneficial way to reduce FGF23 levels in patients with early CKD, since it does not interfere with the remaining renal phosphaturic activity of FGF23, thus preventing phosphotoxicity.
Controlling P load and levels
Dietary manipulation
Since P is a physiologic stimulus for increasing FGF23, a simple way to reduce FGF23 levels in patients with CKD is by lowering their P load and body fluid levels. Even for this, multiple approaches can be used—limiting P or protein intake, modifying the type of P ingested, using P binders or blocking intestinal transcellular and paracellular P absorption (Figure 2).
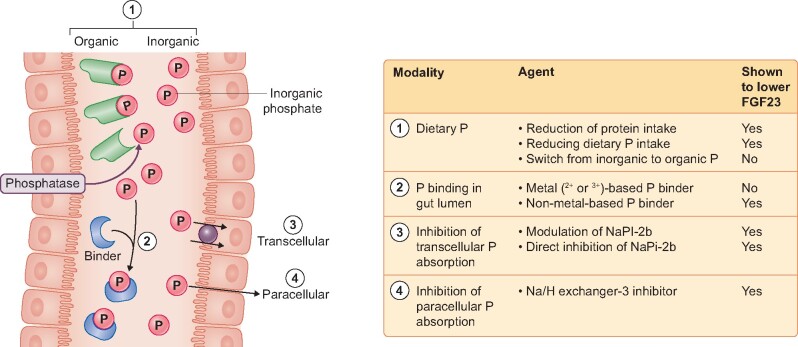
FIGURE 2.
Lowering FGF23 by decreasing P load. P is ingested as organic or inorganic (additive to natural diet) forms. P is released by luminal phosphatases and absorbed via paracellular and transcellular routes. 1. Dietary restriction of total P intake or P modification, switching from high to low bioavailable P (from inorganic to organic P). 2. Luminal P binders can be divalent metal (Ca2+, Mg2+ and Fe2+), trivalent metal (Al3+ and La3+) and anion exchange polymers. 3. Inhibition of transcellular transport by direct or indirect inhibition of NaPi-2b. 4. Blocking paracellular transport is achieved by lumen-restricted inhibition of apical NHE3 with secondary effects on paracellular permeability.
After 1 week, patients with CKD receiving a very low-protein diet (0.3 g/kg bodyweight per day) had 33% lower FGF23 serum levels compared to patients on low-protein diet (0.6 g/kg body weight/day) [23]. In a controlled trial, FGF23 is responsive to a reduction in dietary P intake in CKD patients [24].
Inorganic P is much more bioavailable than organic P, which in turn is more bioavailable than plant-based P. A plant-based phosphorus-equivalent diet led to marginally lower urinary P and plasma FGF23 [25].
Luminal sequestration
P binders lower the luminal P available for absorption from the intestine (Figure 2) and have been our mainstream therapy in P control in CKD. They are limited by competition with the highly efficient P uptake mechanisms in the intestinal lumen. No effect was observed using calcium-based P binders, possibly because the absorbed calcium stimulates FGF23 synthesis [26]. In contrast, non-calcium-based P binders lower plasma FGF23 [26]. Ferric citrate is felt to be most potent, with a 40% lowering of FGF23 levels [24]. For sevelamer, some studies found a large effect while others did not, and lanthanum is only able to lower FGF23 levels in combination with dietary P restriction [24].
Transport inhibitors
Transcellular intestinal P absorption is mediated by the sodium-dependent phosphate transport protein 2b (NaPi-2b). Many compounds are capable of inhibiting NaPi-2b and some even NaPi-2a in the kidney, where reduced intestinal absorption can be theoretically complemented by increased phosphaturia. A catalogue of this approach and candidate compounds were summarized in Sorribas et al. [27]. Compounds harbouring P moieties such as phosphono-formate, -acetate and -proprionate, phosphinylphosphonates and phloretin-derivatives are potential inhibitors of NaPi-2b. However, their actions are not specific to NaPi-2b, systemic toxicity exists and thus have not explored for clinical use at this juncture. The tubulotoxicity of phosphonoformic acid as an antiviral agent is well known.
It has been known for a long time that nicotinamide, nicotinamide adenine dinucleotide and nicotinic acid can inhibit NaPi-2b [28]. In a randomized clinical trial of nicotinamide in patients with Stage 3 CKD, an 11% decrease in FGF23 concentration was found after 24 weeks [29]. Contrary to these findings, a recent and longer randomized trial in CKD patients did not find an effect on FGF23 levels [30]. An ideal agent will be direct, specific and a non-absorbable inhibitor of NaPi-2b, which exists and is in the pharmaceutical pipeline.
Targeting the transcellular absorption of P is handicapped by the significant amount of paracellular absorption. The non-reabsorbable inhibitor of the luminal sodium–hydrogen exchanger 3 (NHE3) is able to inhibit this paracellular P reabsorbtion [27]. Originally targeted to block sodium absorption from the intestine, an unexpected/unintended side effect turned out to be inhibition of paracellular P reabsorption, presumably mediated by inside-out signalling from cell acidification [31]. After P-binder washout in haemodialysis patients, serum FGF23 increased, but the NHE3 inhibitor tenapanor significantly decreased serum FGF23 [32].
Calcimimetics
Calcimimetics are small organic molecules that allosterically bind to the calcium-sensing receptor distinct from the physiological ligand (calcium ion; Ca2+) and increase sensitivity to Ca2+, thereby lowering the setpoint for calcium responsiveness [33]. By doing so, calcimimetics decrease serum PTH levels [33]. These compounds are used to reduce secondary hyperparathyroidism in CKD.
Cinacalcet is approved for the treatment of secondary hyperparathyroidism. In a prospective randomized controlled trial, it lowered FGF23 levels significantly compared with vitamin D [34]. Still, it is unclear whether calcimimetics lower FGF23 via its effects on serum P, calcium and PTH or it has an independent direct effect on FGF23 synthesis.
Etelcalcetide is a second-generation calcimimetic with a pharmacokinetic profile that renders intravenous administration possible after haemodialysis to improve adherence [31]. It is more effective than cinacalcet in lowering PTH concentrations, with similar safety and tolerability [35]. Interestingly, etelcalcetide produces a more pronounced reduction in FGF23 levels compared with cinacalcet and can potentially be harnessed as an FGF23-lowering agent.
INCREASING FGF23 CLEARANCE
Polypeptide N-acetylgalactosaminyltransferase 3 (ppGalNAc-T3) inhibitor
There are physiologic conditions where increased FGF23 production is matched by contemporaneous FGF23 clearance, leaving the bioactivity unchanged [1]. FGF23 undergoes O-glycosylation at several sites (Figure 1, #2). O-glycosylation at Thr178 is performed by the enzyme ppGalNAc-T3, which acts specifically on FGF23 as a substrate. O-glycosylation protects FGF23 from cleavage by the furin protease [36]. After this, FGF23 is secreted to exert its systemic biologic effects. However, if FGF23 is not O-glycosylated, it is readily inactivated by cleavage into inactive fragments (Figure 1). In addition, the C-terminal tail is able to competitively block signalling via the FGFR1–Klotho complex [37]. Proof-of-concept is provided by two experiments of nature—autosomal dominant hypophosphataemic rickets and familial tumoural calcinosis, where decreased and increased FGF23 clearance, respectively, leads to P wasting or accumulation [2].
Song and Linstedt [38] identified a small molecule, T3Inh-1, that inhibits the activity of ppGalNAc-T3 and O-glycosylation of FGF23 at Thr178, increasing the ratio of cleaved to intact FGF23 in the blood. Given the significant effect the inhibitor was shown to have in vivo on this ratio in the blood, this might be a potent approach for treating secondary high FGF23 in CKD or primary FGF23 excess. In CKD, using this inhibitor might only be beneficial for patients with end-stage renal disease (ESRD), since in early CKD it can interfere with the phosphaturic activity of FGF23 and possibly cause phosphotoxicity; once again highlighting the importance of sorting out whether FGF23 is a friend or foe in different CKD stages. Data on the use of this inhibitor in CKD models are not yet available.
Dialysis
Epidemiological evidence shows that FGF23 levels are associated with mortality in patients with CKD on haemodialysis [1]. Longitudinal analysis of FGF23 levels in haemodialysis patients shows that FGF23 levels remain stable [39]. In contrast, haemodiafiltration is able to lower FGF23 levels, presumably due to better middle molecule clearance [39]. Unexpectedly, this lowering of FGF23 levels using haemodiafiltration does not associate with reduced mortality [39].
One possible explanation is survival bias in prevalent dialysis studies, where patients with a relatively good condition remain and the effect of FGF23 on mortality is diminished. Studies in patients initiating dialysis may show a larger effect of lowering FGF23. Therefore longitudinal studies in both prevalent and incident dialysis on the effect of reducing FGF23 levels on mortality should be performed.
NEUTRALIZING FGF23
In addition to manipulating the generation and clearance of FGF23, another approach is to directly neutralize it using an anti-FGF23 antibody (Figure 1, #3). However, it is difficult to control the degree of inhibition, which can result in either inadequate or too much neutralization of FGF23. In experimental CKD, complete abrogation of FGF23 causes hyperphosphataemia, diffuse vascular calcification and death [40].
While this approach is logical for primary FGF23 excess, as it targets the underlying pathophysiology, secondary high FGF23 such as in CKD is quite a different matter. In rats with early-stage CKD, an anti-FGF23 antibody enhanced proximal tubule reabsorption of P compatible with antagonism of the phosphaturic effects of FGF23 [41]. In later stages of CKD, a monoclonal antibody that completely neutralized FGF23 led to high mortality, possibly due to P retention [40].
The use of FGF23-neutralizing antibodies in CKD is not uniformly beneficial and may even be harmful. The neutralization in the aforementioned study was so powerful that serum FGF23 was undetectable. Therefore this study was not a model of FGF23 ‘control’, but more like FGF23 ‘annihilation’ [42]. These findings prompted important questions regarding the beneficial versus harmful effects of neutralizing FGF23. The outcome may be different in early CKD when high FGF23 is adaptive versus late CKD when high FGF23 is maladaptive. In ESRD, the phosphaturic function of FGF23 may be severely restricted, so very small changes in P levels occur when neutralizing antibodies are used. Finally, there may be a discreet optimal degree of FGF23 lowering, and it is not a situation of ‘the lower the better’.
The monoclonal anti-FGF23 antibody burosumab increased P reabsorption, normalized P levels, increased 1,25-dihydroxy vitamin D levels in patients with XLH [43] and was safe over 24 weeks [43]. There are not yet any data on the use of burosumab in human CKD.
INHIBITING FGF23 SIGNALLING
Pan-FGFR inhibitors
To date, there are no FGFR1/Klotho-specific inhibitory small molecules, but pan-FGFR blockers are available. These molecules target ATP binding sites of tyrosine kinase domains from all FGFR subtypes. At first glance, this type of pan-inhibition does not look enticing. None of these have been tested in patients with CKD. In rodent CKD, however, these inhibitors attenuated and even reversed FGF23-induced LVH [8, 44]. It is unclear whether these inhibitors are beneficial or harmful in early CKD, as they impair the phosphaturia of FGF23. In ESRD, they have less impact on the phosphaturic activity of FGF23 since renal function is exceedingly low. In addition, by blocking FGFR1, they can impair upregulation of FGF23 expression in osteocytes as reported in a rat model of CKD [45]. Lastly, by blocking FGFR4 (see below) they can attenuate the pathologic signalling of FGF23 at these high levels.
In testimony to the ability to block FGF23 bioactivity, a study in Hyp and Dmp1-null mice representing XLH and autosomal recessive hypophosphataemic rickets, respectively, showed that administration of a pan-FGF23 inhibitor normalized P levels [46]. Since these pan-FGFR inhibitors also target FGFR2 and FGFR3, the critical studies should be directed at examining systemic off-target effects.
Specific FGFR1–Klotho blocking peptide
A more specific approach to lower the bioactivity of FGF23 is to use FGFR1–Klotho complex blockers. FGF23 is cleaved by subtilisin-like pro-protein convertases, producing two different fragments (Figure 1, #4) [32]. Interestingly, the C-terminal fragment binds to where the C-terminus of full-length FGF23 binds the FGFR1–Klotho complex but does not activate signalling, and hence functions as a competitive inhibitor of FGF23 [37]. This binding site is on parts of both the KL1 and KL2 domains of the Klotho protein (Figure 3) [47].
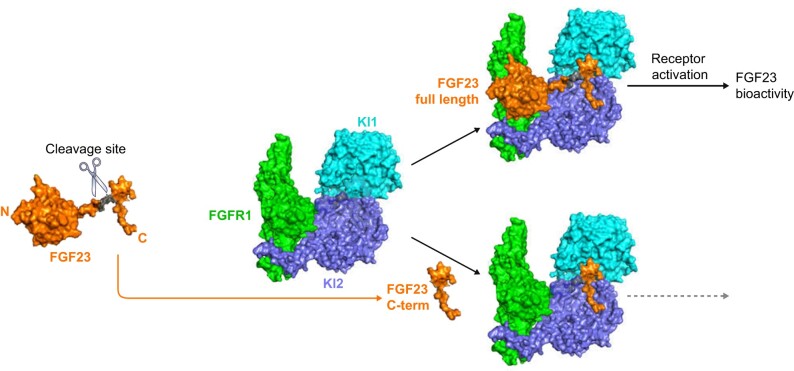
FIGURE 3.
Structural basis of the antagonistic action of FGF23 C-terminus. FGF23 is naturally cleaved by the furin PPC (left). FGF23 engages the FGFR1–Klotho coreceptor complex and activates signalling. The natural C-terminal tail of FGF23 (C-term) or shorter minimal binding regions of the C-term binds to the Kl1 and Kl2 domains on Klotho but not FGFR1 and does not activate FGF23 signalling and bioactivity. Molar excess of C-term functions as a specific blocker of FGF23 action on FGFR1–Klotho. Images derived from Chen et al. [42].
In cultured cells, the C-terminal tail of FGF23 specifically inhibited the FGF23-induced tyrosine phosphorylation of FRS2a in a dose-dependent fashion [37]. In renal proximal tubule cells, it antagonized the inhibition of P uptake by FGF23 [37]. Lastly, in vivo experiments in hyp mice (model of XLH) showed that the C-terminal tail of FGF23 reduces phosphaturia and the downregulation of NaPi-2a and NaPi-2c cotransporters [37]. Together, this suggests that by binding Klotho within the FGFR1–Klotho complex, the FGF23 C-terminal tail can block the action of intact FGF23. Interestingly, the half-life of the FGF23 C-terminal tail was lengthened by generating an FGF23 C-tail-Fc fusion molecule and the chimera still inhibits FGF23-induced tyrosine phosphorylation of FRS2a [48].
Although these inhibitors can be effective in treating patients with primary FGF23 increases, they are likely not beneficial for patients with CKD due to interference with the phosphaturic effects of FGF23. Moreover, FGFR4-mediated signalling is not inhibited, meaning that pathological extrarenal effects persist.
FGFR4 blockers
Klotho-independent effects of FGF23 are proposed to be mediated by FGFR4 via the PLCγ–calcineurin–NFAT cascade [9, 19]. This pathway is possibly involved in LVH [9] and hepatocyte-induced cytokine production [19]. Therefore inhibition of the FGFR4 receptor can potentially be a therapeutic strategy at multiple levels. Importantly, by targeting FGFR4, this inhibitor blocks pathologic activity of FGF23 while maintaining the canonical phosphaturic activity of FGF23 via the FGFR1–Klotho complex. Therefore this may be an effective approach for patients with early CKD. This inhibitor is not beneficial for patients with primary FGF23 increase diseases, since pathology via FGFR4 is less evident and they do not eliminate hypophosphataemia.
In a 5/6 nephrectomy rat model of CKD, blocking FGFR4 with an anti-FGFR4 antibody attenuated FGF23-induced LVH [9]. In addition, anti-FGFR4 antibody reduced the hepatic and serum levels of IL-6 and CRP [19]. This effect may be beneficial since inflammatory molecules are associated with a risk of morbidity and mortality in patients with CKD [49]. Overall, this suggests that FGFR4 blockade can potentially reduce FGF23-related pathology in patients with CKD.
FGFRs have an important role in cancer by promoting proliferation, metastasis and cell survival [50], which has driven the development of FGFR4-specific blockade using neutralizing antibodies, antisense oligonucleotides and small molecule inhibitors [50]. The advances in this field and safety data can be applied to the treatment of CKD patients with excess FGF23.
OTHER FGF23–LOWERING STRATEGIES
Klotho
In haemodialysis patients, low circulating Klotho is associated with increased cardiovascular events [51]. In animal models of CKD and uraemic cardiomyopathy, FGF23 levels are positively correlated with cardiac hypertrophy and fibrosis; however, this relationship is only evident in animals with low Klotho, suggesting that Klotho deficiency is a required coconspirator for the negative actions of FGF23 [52]. There is an interaction between FGF23-induced LVH and soluble Klotho [53]. In vivo and in vitro co-administration of FGF23 and Klotho prevented FGF23-mediated FGF23/FGFR4/PLCγ signalling [53]. Thus Klotho may reduce FGF23-induced LVH in patients with high FGF23 as well as other FGFR4-mediated effects of FGF23, although its mechanism of action is not fully elucidated.
Vitamin D
In a 5/6 nephrectomy CKD model, calcitriol suppressed cardiac expression of FGFR4 and calcineurin/NFAT activation [54]. Together with renin–angiotensin system inhibition, calcitriol reduced LVH in cultured cardiomyocytes [54]. Paricalcitol, a vitamin D receptor (VDR) agonist, improves LVH in rat and human CKD [55]. To prevent the upregulation of FGF23 after administration of a VDR agonist, blocking FGFR signalling could be beneficial. In line with this, co-administration of PD173074, a pan-FGFR inhibitor, enhanced the reduction of LVH in the rat [55]. Importantly, the effect of paricalcitol on LVH was dependent on the levels of FGF23. Higher levels of FGF23 require higher doses of paricalcitol to counter hypertrophy. The clinical implication is that dosing of paricalcitol should be adjusted to the patient’s baseline FGF23 level. The same study showed a non-significant trend towards higher P levels in patients using PD173074, possibly due to its anti-phosphaturic effects. Whether the beneficial effects of co-administration of PD173074 and VDR agonists on LVH outweigh the possible comorbidities following the increased P levels is yet to be determined.
Renin–angiotensin–aldosterone system (RAAS)
In experimental and clinical studies, angiotensin-converting enzyme (ACE) inhibition increases Klotho [56]. Since Klotho reduces FGF23-mediated FGF23/FGFR4/PLCγ signalling, RAAS blockade might prevent FGF23-induced pathologic effects [49]. In a mouse model of progressive renal disease and diabetes, ACE inhibition was able to lower systemic and renal FGF23 expression [57]. However, human studies have found no changes in FGF23 levels [56].
Local RAAS can have paracrine effects on cardiomyocytes. A mouse CKD model showed that local RAAS activation associates with cardiac fibrosis [58]. In vitro stimulation of cardiac myocytes and fibroblasts with FGF23 upregulates RAAS-associated genes along with fibrosis and LVH [58]. Another study postulated that cardiac RAAS activation induces local FGF23 production [59].
Thus cardiac RAAS and FGF23 may synergistically worsen LVH and fibrosis. Inhibition of RAAS may attenuate the induction of LVH and cardiac fibrosis directly or indirectly via downregulation of FGF23. However, there are insufficient data thus far to use RAAS inhibition as a way to lower FGF23 bioactivity.
CONCLUSIONS
In diseases with a primary FGF23 increase such as XLH, the situation is relatively simple. Lowering the bioactivity of FGF23 arrests P wasting, which, in conjunction with other potential effects, ameliorates the phenotype. A more central question in a much more common but complex disease is how to deal with the very high FGF23 in CKD. In early CKD, lowering FGF23 bioactivity may be harmful by exacerbating phosphotoxicity. Approaches in these patients should focus more on targeting the P load and levels. In patients with ESRD, many more approaches are available. No matter what agents in our armamentarium are being contemplated to block FGF23 in CKD, studies in XLH patients should be informative to help design studies in CKD patients.
Many pivotal questions need to be posed and addressed in the therapeutic reduction of FGF23 bioactivity in CKD:
Do the very high levels of FGF23 in CKD have a pathobiologic role or is it simply a biomarker for undesirable outcomes? Can it be both?
If FGF23 is functionally important, is it adaptive or maladaptive for CKD progression and extrarenal complications?
Can this role be dynamic such that it is adaptive for early CKD but maladaptive for late CKD? How does one identify when does the role switch?
What should be the therapeutic goals? Are there target FGF23 levels that one attempts to lower it to and how can these be identified? Is there an ‘optimal’ FGF23 level or is it also a moving target depending on the CKD stage and comorbidities? Could the optimal level be different in individual patients so the target needs to be tailored?
Should one monitor FGF23 levels during therapy or is it better to track other parameters reflecting the downstream effects of FGF23?
If one tries to lower or block FGF23, what is the best approach or approaches? Is there a role for a combination regimen using more than one method of reducing FGF23 bioactivity—should one target FGF23 itself, FGFR1–Klotho, FGFR4 or a combination?
Is there individual uniqueness to the need for combination therapy—should the ‘cocktail’ be customized?
Many of these questions need to be answered in human CKD with intervention trials supplementing the preclinical studies. The array of modalities to block FGF23 bioactivity will enable the above questions to be answered, but it will take a lot of preclinical studies and well-designed clinical interventional trials.
ACKNOWLEDGEMENTS
We are grateful for the secretarial assistance from Yesenia Aguirre.
Contributor Information
Devin Verbueken, Department of Physiology, Radboud Institute for Molecular Life Sciences, Radboud University Medical Center, Nijmegen, The Netherlands.
Orson W Moe, Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX, USA; Charles and Jane Pak Center of Mineral Metabolism and Clinical Research, Dallas, TX, USA; Department of Physiology, University of Texas Southwestern Medical Center, Dallas, TX, USA.
FUNDING
The authors are supported by the National Institutes of Health (R01-DK081423, R01-DK115703, R01-DK091392 and R01-DK092461), the O’Brien Kidney Research Centre (P30 DK-079328) and the Charles Pak Foundation (Endowed Professor Collaborative Support and Innovative Research Grant).
CONFLICT OF INTEREST STATEMENT
None declared. The authors declare that this article has not been published previously in whole or part.
REFERENCES
- 1. Musgrove J, Wolf M.. Regulation and effects of FGF23 in chronic kidney disease. Annu Rev Physiol 2020; 82: 365–390 [DOI] [PubMed] [Google Scholar]
- 2. Edmonston D, Wolf M.. FGF23 at the crossroads of phosphate, iron economy and erythropoiesis. Nat Rev Nephrol 2020; 16: 7–19 [DOI] [PubMed] [Google Scholar]
- 3. Vervloet M. Renal and extrarenal effects of fibroblast growth factor 23. Nat Rev Nephrol 2019; 15: 109–120 [DOI] [PubMed] [Google Scholar]
- 4. Agoro R, Ni P, Noonan ML, White KE.. Osteocytic FGF23 and its kidney function. Front Endocrinol 2020; 11: 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saito T, Fukumoto S.. Fibroblast growth factor 23 (FGF23) and disorders of phosphate metabolism. Int J Pediatr Endocrinol 2009; 2009: 496514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hill NR, Fatoba ST, Oke JL. et al. Global prevalence of chronic kidney disease – a systematic review and meta-analysis. PLoS One 2016; 11: e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wahl P, Wolf M.. FGF23 in chronic kidney disease. Adv Exp Med Biol 2012; 728: 107–125 [DOI] [PubMed] [Google Scholar]
- 8. Faul C, Amaral AP, Oskouei B. et al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011; 121: 4393–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grabner A, Amaral AP, Schramm K. et al. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab 2015; 22: 1020–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takashi Y, Kinoshita Y, Hori M. et al. Patients with FGF23-related hypophosphatemic rickets/osteomalacia do not present with left ventricular hypertrophy. Endocr Res 2017; 42: 132–137 [DOI] [PubMed] [Google Scholar]
- 11. Liu ES, Thoonen R, Petit E. et al. Increased circulating FGF23 does not lead to cardiac hypertrophy in the male hyp mouse model of XLH. Endocrinology 2018; 159: 2165–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pastor-Arroyo EM, Gehring N, Krudewig C. et al. The elevation of circulating fibroblast growth factor 23 without kidney disease does not increase cardiovascular disease risk. Kidney Int 2018; 94: 49–59 [DOI] [PubMed] [Google Scholar]
- 13. Andrukhova O, Slavic S, Odörfer KI, Erben RG.. Experimental myocardial infarction upregulates circulating fibroblast growth factor-23. J Bone Miner Res 2015; 30: 1831–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsui I, Oka T, Kusunoki Y. et al. Cardiac hypertrophy elevates serum levels of fibroblast growth factor 23. Kidney Int 2018; 94: 60–71 [DOI] [PubMed] [Google Scholar]
- 15. Mirza MA, Hansen T, Johansson L. et al. Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol Dial Transplant 2009; 24: 3125–3131 [DOI] [PubMed] [Google Scholar]
- 16. Yilmaz MI, Sonmez A, Saglam M. et al. FGF-23 and vascular dysfunction in patients with stage 3 and 4 chronic kidney disease. Kidney Int 2010; 78: 679–685 [DOI] [PubMed] [Google Scholar]
- 17. Jimbo R, Kawakami-Mori F, Mu S. et al. Fibroblast growth factor 23 accelerates phosphate-induced vascular calcification in the absence of Klotho deficiency. Kidney Int 2014; 85: 1103–1111 [DOI] [PubMed] [Google Scholar]
- 18. Lim K, Lu TS, Molostvov G. et al. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation 2012; 125: 2243–2255 [DOI] [PubMed] [Google Scholar]
- 19. Singh S, Grabner A, Yanucil C. et al. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int 2016; 90: 985–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chonchol M, Greene T, Zhang Y. et al. Low vitamin D and high fibroblast growth factor 23 serum levels associate with infectious and cardiac deaths in the HEMO study. J Am Soc Nephrol 2016; 27: 227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rossaint J, Oehmichen J, Van Aken H. et al. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest 2016; 126: 962–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ito N, Wijenayaka AR, Prideaux M. et al. Regulation of FGF23 expression in IDG-SW3 osteocytes and human bone by pro-inflammatory stimuli. Mol Cell Endocrinol 2015; 399: 208–218 [DOI] [PubMed] [Google Scholar]
- 23. Di Iorio B, Di Micco L, Torraca S. et al. Acute effects of very-low-protein diet on FGF23 levels: a randomized study. Clin J Am Soc Nephrol 2012; 7: 581–587 [DOI] [PubMed] [Google Scholar]
- 24. Sigrist M, Tang M, Beaulieu M. et al. Responsiveness of FGF-23 and mineral metabolism to altered dietary phosphate intake in chronic kidney disease (CKD): results of a randomized trial. Nephrol Dial Transplant 2013; 28: 161–169 [DOI] [PubMed] [Google Scholar]
- 25. Moorthi RN, Armstrong CL, Janda K. et al. The effect of a diet containing 70% protein from plants on mineral metabolism and musculoskeletal health in chronic kidney disease. Am J Nephrol 2014; 40: 582–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Isakova T, Ix JH, Sprague SM. et al. Rationale and approaches to phosphate and fibroblast growth factor 23 reduction in CKD. J Am Soc Nephrol 2015; 26: 2328–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sorribas V, Guillén N, Sosa C.. Substrates and inhibitors of phosphate transporters: from experimental tools to pathophysiological relevance. Pflugers Arch Eur J Physiol 2019; 471: 53–65 [DOI] [PubMed] [Google Scholar]
- 28. Kempson SA, Colon-Otero G, Ou SY. et al. Possible role of nicotinamide adenine dinucleotide as an intracellular regulator of renal transport of phosphate in the rat. J Clin Invest 1981; 67: 1347–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rao M, Steffes M, Bostom A. et al. Effect of niacin on FGF23 concentration in chronic kidney disease. Am J Nephrol 2014; 39: 484–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ix JH, Isakova T, Larive B. et al. Effects of nicotinamide and lanthanum carbonate on serum phosphate and fibroblast growth factor-23 in CKD: the COMBINE trial. J Am Soc Nephrol 2019; 30: 1096–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. King AJ, Siegel M, He Y. et al. Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability. Sci Transl Med 2018; 10: eaam6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Block GA, Rosenbaum DP, Leonsson-Zachrisson M. et al. Effect of tenapanor on serum phosphate in patients receiving hemodialysis. J Am Soc Nephrol 2017; 28: 1933–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harrington PE, Fotsch C.. Calcium sensing receptor activators: calcimimetics. Curr Med Chem 2007; 14: 3027–3034 [DOI] [PubMed] [Google Scholar]
- 34. Kim HJ, Kim H, Shin N. et al. Cinacalcet lowering of serum fibroblast growth factor-23 concentration may be independent from serum Ca, P, PTH and dose of active vitamin D in peritoneal dialysis patients: a randomized controlled study. BMC Nephrol 2013; 14: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Block GA, Bushinsky DA, Cheng S. et al. Effect of etelcalcetide vs cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: a randomized clinical trial. JAMA 2017; 317: 156–164 [DOI] [PubMed] [Google Scholar]
- 36. Kato K, Jeanneau C, Tarp MA. et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis: secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem 2006; 281: 18370–18377 [DOI] [PubMed] [Google Scholar]
- 37. Goetz R, Nakada Y, Hu MC. et al. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc Natl Acad Sci USA 2010; 107: 407–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Song L, Linstedt AD.. Inhibitor of ppGalNAc-T3-mediated O-glycosylation blocks cancer cell invasiveness and lowers FGF23 levels. eLife 2017; 6: e24051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bouma-de Krijger A, de Roij van Zuijdewijn CLM, Nubé MJ. et al. Change in FGF23 concentration over time and its association with all-cause mortality in patients treated with haemodialysis or haemodiafiltration. Clin Kidney J 2020; 14: 891–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shalhoub V, Shatzen EM, Ward SC. et al. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest 2012; 122: 2543–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hasegawa H, Nagano N, Urakawa I. et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 2010; 78: 975–980 [DOI] [PubMed] [Google Scholar]
- 42. Moe OW. Fibroblast growth factor 23: friend or foe in uremia? J Clin Invest 2012; 122: 2354–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Insogna KL, Briot K, Imel EA. et al. A randomized, double-blind, placebo-controlled, phase 3 trial evaluating the efficacy of burosumab, an anti-FGF23 antibody, in adults with X-linked hypophosphatemia: week 24 primary analysis. J Bone Miner Res 2018; 33: 1383–1393 [DOI] [PubMed] [Google Scholar]
- 44. Di Marco GS, Reuter S, Kentrup D. et al. Treatment of established left ventricular hypertrophy with fibroblast growth factor receptor blockade in an animal model of CKD. Nephrol Dial Transplant 2014; 29: 2028–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hassan A, Durlacher K, Silver J. et al. The fibroblast growth factor receptor mediates the increased FGF23 expression in acute and chronic uremia. Am J Physiol Renal Physiol 2016; 310: F217–F221 [DOI] [PubMed] [Google Scholar]
- 46. Wöhrle S, Henninger C, Bonny O. et al. Pharmacological inhibition of fibroblast growth factor (FGF) receptor signaling ameliorates FGF23-mediated hypophosphatemic rickets. J Bone Miner Res 2013; 28: 899–911 [DOI] [PubMed] [Google Scholar]
- 47. Chen G, Liu Y, Goetz R. et al. αKlotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature 2018; 553: 461–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Johnson K, Levine K, Sergi J. et al. Therapeutic effects of FGF23 c-tail Fc in a murine preclinical model of X-linked hypophosphatemia via the selective modulation of phosphate reabsorption. J Bone Miner Res 2017; 32: 2062–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nowak KL, Chonchol M.. Does inflammation affect outcomes in dialysis patients? Semin Dial 2018; 31: 388–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lang L, Teng Y.. Fibroblast growth factor receptor 4 targeting in cancer: new insights into mechanisms and therapeutic strategies. Cells 2019; 8: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Memmos E, Sarafidis P, Pateinakis P. et al. Soluble Klotho is associated with mortality and cardiovascular events in hemodialysis. BMC Nephrol 2019; 20: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hu MC, Shi M, Cho HJ. et al. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol 2015; 26: 1290–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Han X, Cai C, Xiao Z, Quarles LD.. FGF23 induced left ventricular hypertrophy mediated by FGFR4 signaling in the myocardium is attenuated by soluble Klotho in mice. J Mol Cell Cardiol 2020; 138: 66–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leifheit-Nestler M, Grabner A, Hermann L. et al. Vitamin D treatment attenuates cardiac FGF23/FGFR4 signaling and hypertrophy in uremic rats. Nephrol Dial Transplant 2017; 32: 1493–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Czaya B, Seeherunvong W, Singh S. et al. Cardioprotective effects of paricalcitol alone and in combination with FGF23 receptor inhibition in chronic renal failure: experimental and clinical studies. Am J Hypertens 2019; 32: 34–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. de Seigneux S, Martin PY.. Phosphate and FGF23 in the renoprotective benefit of RAAS inhibition. Pharmacol Res 2016; 106: 87–91 [DOI] [PubMed] [Google Scholar]
- 57. Zanchi C, Locatelli M, Benigni A. et al. Renal expression of FGF23 in progressive renal disease of diabetes and the effect of ACE inhibitor. PLoS One 2013; 8: e70775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Böckmann I, Lischka J, Richter B. et al. FGF23-mediated activation of local RAAS promotes cardiac hypertrophy and fibrosis. Int J Mol Sci 2019; 20: 4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Leifheit-Nestler M, Kirchhoff F, Nespor J. et al. Fibroblast growth factor 23 is induced by an activated renin-angiotensin-aldosterone system in cardiac myocytes and promotes the pro-fibrotic crosstalk between cardiac myocytes and fibroblasts. Nephrol Dial Transplant 2018; 33: 1722–1734 [DOI] [PubMed] [Google Scholar]