Abstract
Venous thromboembolism (VTE) is a coagulopathic disease that may appear with deep vein thrombosis (DVT), pulmonary embolism (PE), or both and is responsible for increased mortality and morbidity in children. We report a case of PE in a male teenager obese boy in the setting of a thrombophilic genetic disorder, infective condition, and immobility. Our experience underlines as PE in childhood is a multifactorial disease in which clinical risk factors and inherited thrombophilia contribute to the development. It is crucial to identify one or more risk factors leading to the most appropriate diagnostic workup.
Keywords: adolescent, pulmonary embolism, thrombophilic genetic disorder, venous thromboembolism
Introduction
Venous thromboembolism (VTE) is a coagulopathic disease that may appear with deep vein thrombosis (DVT), pulmonary embolism (PE), or both and is responsible for increased mortality and morbidity in children. Although it is considered rare compared to adults, its incidence has been increasing in the last decades due to improved diagnosis and survival of critically ill patients. First described in 1861 (1), PE occurs in about 8.6–57/100,000 in hospitalized children and 0.14–0.9/100,000 in those not hospitalized (2,3), with a peak in infants younger than one year and a second peak during adolescence (4,5). The risk of mortality can reach 26% (6,7). The wide range of incidence may depend on the often clinically silent nature of PE. It also seems that these numbers are underestimated, as evidenced by the incidence of 0.05 - 4.2% documented by autopsy (3). PE is a multifactorial disease with heritable and environmental risk factors stratified for age, often coexistent. 80% to 96% of children with PE have at least one risk factor (8,9), two or more in 81-88% (10,11) associated with an increased risk of recurrence of 79% in 4.80 years (9). Because PE clinical diagnosis is difficult, laboratory and imaging tests have a pivotal function to confirm the diagnosis. Guidelines for managing TE events in pediatric patients are extrapolated from the adult guidelines (8). Treatment includes supportive care, anticoagulants, thrombectomy.
We report a case of PE in a male teenager obese boy in the setting of a thrombophilic genetic disorder, infective condition, and immobility.
Case report
A. H. is a 13-year-old teenager of Moroccan origin with severe obesity, hospitalized in June 2019 for cough and fever, lasting a few days before. The first chest X-ray showed pulmonary thickening in the right basal field with ipsilateral pleural effusion. Dual broad-spectrum antibiotic therapy with cephalosporin and macrolide was initiated. After 12 hours, the patient required oxygen administration through high flows due to the onset of respiratory distress. Ciprofloxacin was associated after the isolation of Haemophilus influenzae from the culture test on bronchial sputum. Despite an initial clinical improvement with defervescence and reduction of inflammation indexes, oxygen dependence persisted. After a week of hospitalization, the boy suddenly showed the onset of chest pain and worsening dyspnea. Therefore, a chest CT scan was performed, with confirmation in both lung bases of multiple irregular triangular thickenings with a peripheral implant base (of which the largest at right with preserved air bronchogram), associated with some minor thickenings the right costophrenic breakthrough in the absence of air bronchogram. Broncho lavage was performed, which confirmed the presence of Haemophilus influenzae. Antibiotic therapy was further modified, added carbapenem and second level tests were performed to exclude other lung pathologies like tuberculosis and cystic fibrosis. Despite the therapeutic changes, the clinical situation did not improve. The patient required high flow nasal cannula (HFNC) ventilation.
Alteration of the inflammation indexes and neutrophilic leukocytosis were still persistent. The patient reported sudden pain in his left thigh in the following days, associated with hyperemia and edema. The echo color-doppler of the lower limb vessels showed massive DVT, involving the entire deep venous circle of the left lower limb, from the twin veins to the saphenous-femoral ostium. Hence, anticoagulant therapy was started immediately with enoxaparin and elastic compression. Total body CT scan documented a picture of diffuse pulmonary embolism (Figure 1) and thrombosis extended to the common, internal, and external left iliac vein and to the left femoral vein with normal echocardiography that allowed to classify low-risk PE based on the PESI score. Considering the young age and the apparent absence of predisposing conditions, thrombophilic screening was carried out, showing multiple genetic disorders associated with thrombophilia. Patients presented heterozygosity for factor II G20210A and factor V Leiden and homozygosity for the gene MTHFR on the hereditary side, borderline anticardiolipin IgM in the absence of LAC, and antibodies to β2glycoprotein 1 on the acquired side. Family history was negative for thromboembolic events. He started therapy with warfarin, and heparin was suspended after gaining the INR safe range. The inflammation indexes have normalized, and the white blood cell count has complete remission of critical aspects. The boy was discharged in good clinical conditions with an indication to continue the oral anticoagulant therapy and to start regular follow-up at the thromboembolic disease institute of Policlinico San Matteo in Pavia, Italy. After three months, echo color-doppler of the lower limb vessels did not show thrombosis, but only the outcome of progress injury at left femoral vein and popliteal vein. The parents provided written informed consent.
Figure 1.
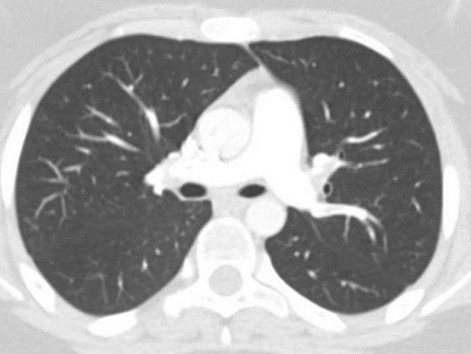
Total body CT scan documented diffuse pulmonary embolism.
Discussion
Clinical presentation of PE overlaps with many common pediatric diseases (8); thus, the diagnosis in children is complicated and often performed late, with an average of one week after the first symptoms. Although PE was thought to occur in the setting of infection, known comorbidities or other risk factors are frequently associated, in contrast to the adults, in which up to 30% of PE is idiopathic (5). Raffini et al. described two distinct variants of PE in children: in situ pulmonary artery thrombosis (ISPAT) and classic thromboembolic PE. ISPAT usually occurs in younger children with congenital heart disease or anomalies of pulmonary arteries (12). Classic thromboembolic PE is correlated with underlying risk factors. The most important is the presence of central venous catheter (CVC) for hospitalized children (12,13) and the use of the oral contraceptive pill or hormonal supplementation for those non-hospitalized (14,15). Other risk factors reported in infants and neonates are septicemia, dehydration, peripartum asphyxia, inherited thrombophilia. In older children and adolescents > 12 years, known risk factors include obesity (47-52%), autoimmune disease (19%), cancer (8%), immobility (22%), CVC (27%), infection (27%), surgery (27%), anatomical abnormality (20%), tobacco smoke (6%), hormonal therapy for contraception, gender transition (6-22%), trauma (3%), inherited thrombophilia (19%), other medical conditions (11%), dehydration, systemic lupus erythematosus, and prolonged total parenteral nutrition (13,16). The etiology of thromboembolic events is based on Virchow’s triad: venous stasis, injury to the vessel wall, and enhanced coagulability (17) (Table 1). The last two are the most critical factors in children, while stasis remains the main reason in adults. Pediatric patients with PE show DVT in 60% to 72.1% (8,18,19). Our patient was hospitalized for more than 15 days, had a documented condition of hypercoagulability, was obese, and showed Haemophilus Influenzae pneumonia. 35% of patients with a thrombotic event have either a congenital or an acquired prothrombotic disorder (19, 20). In adolescents, this number increases to 52% (2). In particular, the heterozygous association of the factor V and factor II mutation is described in the literature as a rare condition but linked to a higher risk of VTE. The percentages of heterozygous mutations of factor II and factor V are 4-8% and 20-25% in subjects with VTE. Despite this, no evidence supports thrombophilic screening in the general pediatric population. Tests may be considered in first-degree relatives of individuals with a personal history of early-onset VTE.
Table 1.
Virchow’s triad
Damage to the endothelium
|
Change in laminar flow
|
Thrombophilia
|
Inherited conditions
|
Obesity is a condition often associated with thromboembolism when compared to non-obese subjects, 35% vs. 17%, and a common risk factor in 43% of patients with recurrent thromboembolism (10). Two controlled studies reported a 2- to 3-fold increased risk of VTE in obese children compared with normal-weight children (21, 22). Sundbøll et al. described a correlation between obesity in childhood and a high risk of PE later in age, probably due to a life-long sedentary lifestyle that facilitates low-flow conditions and venous thrombus formation (23). Diagnosis of PE is complex. Clinical prediction rules for PE used for adults, such as the Well’s clinical probability score and the Geneva score (24), have not been validated yet for children due to specific innate protective mechanisms documented in children as a decreased capacity to generate thrombin related to decreased plasma concentrations of prothrombin, an enhanced capacity to inhibit thrombin reflecting increased plasma concentrations of alpha-2 macroglobulin and differences in platelet/vessel-wall interaction (14,15). Therefore, the classic triad of PE symptoms, consisting of pleuritic chest pain (32%), shortness of breath (57%), and hemostasis or demonstrating symptoms of DVT at presentation (28%) (10) can be incomplete or absent. This can lead to a possible diagnosis missing, significantly if an embolism obstructs less than 50% of pulmonary circulation (19,2) or mimic other pulmonary diseases like pneumonia, atelectasis, and thoracic tumors (3). Given the generally excellent cardiopulmonary reserve in pediatric patients, even large PE can be compensated, presenting only subtle clinical signs and symptoms. Hennelly et al. in a retrospective study combining two cohorts, found nine significant variables contributing to the diagnosis: age‐adjusted tachycardia, tachypnea, hypoxia, unilateral limb swelling, trauma/surgery requiring hospitalization in previous four weeks, prior thromboembolism (protein C, protein S or antithrombin deficiency), cancer, anemia, and leukocytosis (25). In this area, diagnostic tests play a crucial function. The D-dimer analysis has low diagnostic utility when adapted in children, being normal in 15% to 40% of children (7, 8). Hypoxia, hypocapnia, and respiratory alkalosis in blood gas analysis may be suggestive of PE even if not observed in all patients (26). ECG is non-specific and can reveal sinus tachycardia, right axis deviation, right bundle branch deviation, and ST-T segment abnormalities. The S1Q3T3 pattern in ECG may suggest the diagnosis of PE, but it is neither sensitive nor specific (24).
Imaging evaluation has a primary role in PE diagnosis. Using two or more risk factors as the clinical threshold for obtaining a diagnostic CT scan, the sensitivity for PE detection reaches 89%, and the specificity is 94%. The current criterion standard for PE diagnosis is computed tomography pulmonary angiogram (CTPA), but this carries the risk of radiation-induced malignancy (25,27). Conventional chest radiography is often the first imaging test performed and is abnormal in 88% of cases. An ultrasound exam is controversial, and it can be used to identify the thrombus source. Diagnostic tests for evaluation of PE can be divided into those needed for definitive diagnosis of PE (Table 2), tests that may aid in diagnosing the severity of PE (i.e., risk prediction and, thus, may help in decision making of management of PE) and various tests that should be performed prior to anticoagulant therapy of PE (28).
Table 2.
Diagnostic tests for PE.
| Investigations for diagnosis of PE | Laboratory: D-dimer |
| Imaging: Pulmonary angiography, Ventilation/Perfusion scan, CT-Pulmonary Magnetic resonance imaging/magnetic, Resonance pulmonary angiography. | |
| Investigations for severity assessment | Clinical examinations: signs of respiratory distress, sweating |
| POC: pulse oximetry, ABG, ECG | |
| Laboratory: troponin, BNP | |
| Imaging: echocardiogram | |
| Investigations to guide management | Laboratory: pregnancy test, CBC, CMP, PT/INR, PTT, fibrinogen, plasminogen |
| Investigations of underlying causes | Laboratory: thrombophilia screening |
| Congenital: protein C, protein S, ATIII, prothrombin, factor VIII, vWF, homocysteine, lipoprotein (a), factor V, R506Q, prothrombin G20210A, MTHFR C677T | |
| Acquired: ANA, ACLA IgG or IgM, Circulating | |
| Anticoagulant | |
| Imaging | Ultrasound of extremities |
Due to the lack of management guidelines in childhood, PE treatment has been extrapolated from adults (7, 29), which means that each medical center should arrange its multidisciplinary approach. Generally, hemodynamic status and underlying diseases should dictate the choice of treatment. Normally standard anticoagulant treatment is expected with either unfractionated or low molecular weight heparin as in our case presentation. Thrombolysis, ultrasound-assisted catheter-directed thrombolysis (USAT), or surgical or catheter embolectomy is reserved for patients at high risk of rapid hemodynamic deterioration, submassive PE, or who failed to achieve the optimal outcome (30-33). There are no studies regarding the optimal duration of treatment. Follow-up is necessary to establish resolution, progression, or recurrence and monitor potential long-term complications, such as pulmonary hypertension and chronic PE (34).
Conclusion
PE is a rare, potentially fatal disease that must be suspected in the presence of multiple risk factors, even in pediatric patients with subtle symptoms and signs. Non-specific symptoms may delay diagnosis in children for their intrinsic characteristics. Future additional studies stratifying by age are needed to improve the knowledge and understand this disease process and improve the care in this population in terms of diagnosis and treatment. Prediction models for the diagnosis of PE, such as the Wells criteria and Geneva score, validated for the adults, are greatly needed for the pediatric population. Current practices are based on adult literature. Our case underlines as PE in childhood is a multifactorial disease in which underlying clinical risk factors and inherited thrombophilia contribute to the development. Because diagnosis based on symptoms is difficult, it is essential to identify one or more risk factors that can lead to the most appropriate diagnostic workup.
Therefore, there is a clear need to carry out clinical trials in the pediatric population to clarify a rare pathology in children and more common in adults.
Conflict of interest:
Each author declares that they do not have commercial associations that might pose a conflict of interest in connection with the submitted article
References
- Stevenson GF, Stevenson FL. Pulmonary embolism in childhood. J Pediatr. 1949;34:62–9. doi: 10.1016/s0022-3476(49)80200-9. [DOI] [PubMed] [Google Scholar]
- Biss TT, Brandão LR, Kahr WH, et al. Clinical features and outcome of pulmonary embolism in children. Br J Haematol. 2008;142:808–18. doi: 10.1111/j.1365-2141.2008.07243.x. [DOI] [PubMed] [Google Scholar]
- Dijk FN, Curtin J, Lord D, et al. Pulmonary embolism in children. Paediatr Respir Rev. 2012;13(2):112–22. doi: 10.1016/j.prrv.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Jones RH, Sabiston DC. Pulmonary embolism in childhood. Monogr Surg Sci. 1966;3(1):35–51. [PubMed] [Google Scholar]
- Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC) Eur Heart J. 2008;29(18):2276–315. doi: 10.1093/eurheartj/ehn310. [DOI] [PubMed] [Google Scholar]
- Agha BS, Sturm JJ, Simon HK, et al. Pulmonary embolism in the pediatric emergency department. Pediatrics. 2013;132:663–7. doi: 10.1542/peds.2013-0126. [DOI] [PubMed] [Google Scholar]
- Biss TT. Pulmonary embolism in childhood: how can we be sure not to miss it? Arch Dis Child. 2018;103:814–6. doi: 10.1136/archdischild-2017-314428. [DOI] [PubMed] [Google Scholar]
- Rajpurkar M, Biss TT, Amankwah EK, et al. Pulmonary embolism and in situ pulmonary artery thrombosis in paediatrics. A systematic review. Thromb Haemost. 2017;117:1199–207. doi: 10.1160/TH16-07-0529. [DOI] [PubMed] [Google Scholar]
- Andrew M, David M, Adams M, et al. Venous thromboembolic complications (VTE) in children: first analyses of the Canadian Registry of VTE. Blood. 1994;83(5):1251–7. [PubMed] [Google Scholar]
- Ishola T, Kirk SE, Guffey D, et al. Risk factors and comorbidities in adolescent thromboembolism are different than those in younger children. Thrombosis Research. 2016;141:178–82. doi: 10.1016/j.thromres.2016.03.021. [DOI] [PubMed] [Google Scholar]
- Lee EY, Neuman MI, Lee NJ, et al. Pulmonary embolism detected by pulmonary MDCT angiography in older children and young adults: risk factor assessment. AJR. 2012;198:1431–1437. doi: 10.2214/AJR.11.8005. [DOI] [PubMed] [Google Scholar]
- Raffini L, Huang Y-S, Witmer C, et al. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124(4):1001–8. doi: 10.1542/peds.2009-0768. [DOI] [PubMed] [Google Scholar]
- Stein PD, Kayali F, Olson RE. Incidence of venous thromboembolism in infants and children: data from the National Hospital Discharge Survey. J Pediatr. 2004;145(4):563–5. doi: 10.1016/j.jpeds.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Wang CY, Ignjatovic V, Francis P, et al. Risk factors and clinical features of acute pulmonary embolism in children from the community. Thromb Res. 2016;138:86–90. doi: 10.1016/j.thromres.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Hancock HS, Wang M, Gist KM, et al. Cardiac findings and long-term thromboembolic outcomes following pulmonary embolism in children: a combined retrospective-prospective inception cohort study. Cardiol Young. 2013;23(3):344–52. doi: 10.1017/S1047951112001126. [DOI] [PubMed] [Google Scholar]
- Fan EM, Gordner C, Luty J. Venous Thromboembolism in a Transgender Adolescent on Testosterone Therapy: A Case Report and Literature Review. J Pediatr Hematol Oncol. 2020;42(5):e352–4. doi: 10.1097/MPH.0000000000001755. [DOI] [PubMed] [Google Scholar]
- Riedel M. Acute pulmonary embolism 1: pathophysiology, clinical presentation, and diagnosis. Heart. 2001;85:229–40. doi: 10.1136/heart.85.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery JL. Pulmonary embolism in children. Arch Dis Child. 1962;37(196):591–5. doi: 10.1136/adc.37.196.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ommen CH, Heyboer H, Groothoff JW, et al. Persistent tachypnea in children: keep pulmonary embolism in mind. J Pediatr Hematol Oncol. 1998;20(6):570–3. [PubMed] [Google Scholar]
- Young G, Albisetti M, Bonduel M, et al. Impact of inherited thrombophilia on venous thromboembolism in children: a systematic review and meta-analysis of observational studies. Circulation. 2008;118(13):1373–82. doi: 10.1161/CIRCULATIONAHA.108.789008. [DOI] [PubMed] [Google Scholar]
- Stokes S, Breheny P, Radulescu A, et al. Impact of obesity on the risk of venous thromboembolism in an inpatient pediatric population. Pediatr Hematol Oncol. 2014;31:475–80. doi: 10.3109/08880018.2014.886315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorson EE, Ervin SE, Russell TB, et al. Association of obesity and pediatric venous thromboembolism. Hosp Pediatr. 2016;6:22–26. doi: 10.1542/hpeds.2015-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundb⊘ll J, Angquist L, Kasper A, et al. Changes in Childhood Body-Mass Index and Risk of Venous Thromboembolism in Adulthood. J Am Heart Assoc. 2019;8(6):e011407. doi: 10.1161/JAHA.118.011407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354(22):2317–27. doi: 10.1056/NEJMoa052367. [DOI] [PubMed] [Google Scholar]
- Hennelly KE, Baskin MN, Monuteuax MC, et al. Detection of pulmonary embolism in high‐risk children. J Pediatr. 2016;178:214–8. doi: 10.1016/j.jpeds.2016.07.046. [DOI] [PubMed] [Google Scholar]
- Rodger MA, Carrier M, Jones GN, et al. Diagnostic value of arterial blood gas measurement in suspected pulmonary embolism. Am J Respir Crit Care Med. 2000;162:2105–8. doi: 10.1164/ajrccm.162.6.2004204. [DOI] [PubMed] [Google Scholar]
- Kanis J, Pike J, Hall CL, et al. Clinical characteristics of children evaluated for suspected pulmonary embolism with D-dimer testing. Arch Dis Child. 2018;103:835–40. doi: 10.1136/archdischild-2017-313317. [DOI] [PubMed] [Google Scholar]
- Zaidi AU, Hutchins KK, Rajpurkar M. Pulmonary Embolism in Children. Front Pediatr. 2017;5:170. doi: 10.3389/fped.2017.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Paredes N, Chan AK. Antithrombotic therapy in children with venous thromboembolism. Hamostaseologie. 2009;29:80–7. [PubMed] [Google Scholar]
- Monagle P, Barnes C, Ignjatovic V, et al. Developmental haemostasis: impact for clinical haemostasis laboratories. Thromb Haemost. 2006;95:362–72. doi: 10.1160/TH05-01-0047. [DOI] [PubMed] [Google Scholar]
- Law C, Raffini L. A guide to the use of anticoagulant drugs in children. Paediatr Drugs. 2015;17:105–114. doi: 10.1007/s40272-015-0120-x. [DOI] [PubMed] [Google Scholar]
- Samuel ZG, Henri B. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379:1835e–46. doi: 10.1016/S0140-6736(11)61904-1. [DOI] [PubMed] [Google Scholar]
- Kajy M, Blank N, Alraies C, et al. Treatment of a Child With Submassive Pulmonary Embolism Associated With Hereditary Spherocytosis Using Ultrasound-Assisted Catheter-Directed Thrombolysis. Ochsner J. 2019;19(3):264–70. doi: 10.31486/toj.18.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pengo V, Lensing AWA, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257–64. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]


