Abstract
The outer membrane of the thermophilic bacterium Thermus thermophilus was isolated using sucrose step gradient centrifugation. Its detergent extracts contained an ion-permeable channel with an extremely high single-channel conductance of 20 nS in 1 M KCl. The channel protein was purified by preparative sodium dodecyl sulfate (SDS)-polyacylamide gel electrophoresis. It has a high molecular mass of 185 kDa, and its channel-forming ability resists boiling in SDS for 10 min.
Porins are membrane proteins that form channels for small hydrophilic solutes in the outer membrane of gram-negative bacteria (1, 26, 27) and in the cell wall of the mycolata (20, 34, 35). Porins mediate the passive diffusion of ions and small hydrophilic nutrient molecules across the outer membrane or the cell wall according to their molecular masses (exclusion limit of about 600 Da in Escherichia coli) or according to kind of the substrate when the porins are substrate specific (specific porins), such as LamB for carbohydrates (5, 22) or Tsx for nucleosides (4). Specific porins have binding sites for the diffusion of specific classes of nutrients across the cell wall (24). Porins of the outer membrane of gram-negative bacteria consist of three identical subunits, which are stable against denaturing conditions due to their predominating β-barrel structure and embedding in the membrane (25). Some of the outer membrane porins from different organisms have been crystallized (12, 31, 37). According to their three-dimensional structure, each of the three monomers contains a channel with a diameter of between 0.8 and 1.4 nm. These porins have a single-channel conductance of between 150 pS and 3.5 nS in 1 M KCl and are only moderately ion selective (1).
Enteric gram-negative bacteria such as E. coli and Salmonella enterica serovar Typhimurium have been well characterized for the presence of porins with molecular masses of between 30 and 60 kDa in the outer membrane (1). Comparatively little is known about the porins of other gram-negative bacteria, such as the thermophilic Thermus thermophilus. Here, besides interesting studies of S-layer proteins (10, 13, 14, 28) and the composition of the peptidoglycan (29), little is known about the structure of the outer membrane and about the existence of porins. In this study, we present the identification and purification of the first outer membrane porin of the thermophilic gram-negative bacterium T. thermophilus.
T. thermophilus strain HB8 was obtained from the Deutsche Stammsammlung. It was grown in batch cultures at 70°C using a New Brunswick shaker at 120 rpm for about 1 to 2 days until the cells reached the stationary phase. The growth medium was composed of either standard medium (8) or Luria-Bertani medium. The cells were harvested by centrifugation (10,000 rpm for 10 min in a Beckman J2-21M/E centrifuge [rotor JA20]) and washed twice in 50 mM Tris-HCl (pH 8.0). About 5 g of cells (wet weight) was suspended in 50 ml of 50 mM Tris-HCl (pH 8.0) and kept on ice. The cells were disrupted with a Branson sonifier (8). Unbroken cells were removed by centrifugation at 12,500 × g for 10 min at 4°C. The cell envelopes (cytoplasmic membrane, murein, and outer membrane) were obtained by centrifugation of the supernatant at 170,000 × g for 60 min (Beckman Omega 90 XL ultracentrifuge, rotor 70.1 Ti) at 4°C. The pellet was resuspended in 3 ml of 50 mM Tris-HCl (pH 8.0) and centrifuged again under the same conditions for 30 min. The final pellet was resuspended in 0.5 ml of 50 mM Tris-HCl (pH 8) and applied to a step gradient of 30% (3 ml), 40% (4 ml), 50% (2 ml), 55% (2 ml), and 65% (1 ml) sucrose. The gradient was centrifuged at 110,000 × g for 16 h in a Beckman Optima 90 XL ultracentrifuge (rotor SW40Ti) at 4°C.
Eight fractions of the gradient (F1 to F8, from top to bottom) were collected, each with a volume of about 1.5 ml, and analyzed for protein content by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and for the presence of pore-forming activity by reconstitution experiments in lipid bilayer membranes. Fractions F3, F4, F5, and F6 were either yellow or orange and contained the inner membrane. Fraction F7 contained two white bands. The highest pore-forming activity in the lipid bilayer assay was measured for fraction F7. This fraction contained only a few protein bands. One predominant one had a very high molecular mass of about 180 kDa. Some minor channel-forming activity was found in fractions F3 to F6, presumably due to contamination of the inner membrane with outer membrane, but it was significantly less than in F7. Fractions F1, F2, and F8 exhibited no channel-forming activity.
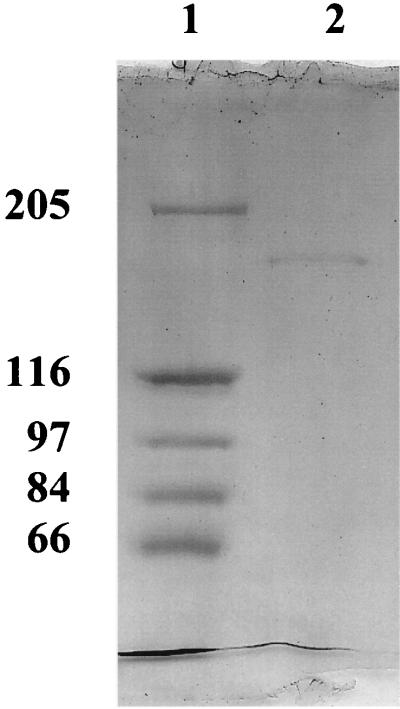
The classical method, treatment of the cell envelope with 2% SDS and isolation of the murein together with the murein-associated proteins (23), failed because all inner and outer membrane components became soluble and no proteins remained associated with the murein. As a consequence, we used a fractionated extraction of the cell envelope with increasing concentrations of LDAO. The cell envelope was resuspended in 10 mM Tris-HCl (pH 8) and increasing concentrations of LDAO (0.01, 0.1, 02, 0.4, and finally 0.6%) supplemented with 10 mM CaCl2, followed always by centrifugation at 170,000 × g for 30 min (Beckman Omega 90 XL ultracentrifuge, rotor 70.1 Ti) at 20°C. Each supernatant was inspected for channel-forming activity using the lipid bilayer assay and for its protein composition by SDS-PAGE stained with Coomassie brilliant blue or with silver (18). The highest channel-forming activity was found in the supernatant of the step with 0.6% LDAO and 10 mM CaCl2. This fraction also contained a high-molecular-mass band as a prominent protein (Fig. 1). In further experiments, we subjected the 0.6% LDAO fraction to preparative SDS-PAGE (7% gels, similar to Fig. 1) and eluted six different regions of the gel with 1% Genapol–10 mM Tris (pH 8) overnight at 4°C. The eluted proteins of the SDS-PAGE gel showed no channel-forming activity for molecular mass regions below 120 kDa. However, high activity was found for protein eluted from the 185-kDa band. The 185-kDa fractions were collected and electrophoresed again. The 185-kDa protein was found to be essentially free of contaminant protein, as Fig. 2 clearly demonstrates. It is the outer membrane porin of T. thermophilus. We investigated its biochemical properties and found it to be extremely stable. Even heating to 100°C for more than 10 min in 1% SDS and treatment with organic solvents did not affect its channel-forming activity. Furthermore, its molecular mass did not change as the result of such treatments. The results presented here indicate that the outer membrane porin of T. thermophilus is indeed extremely stable.
FIG. 1.
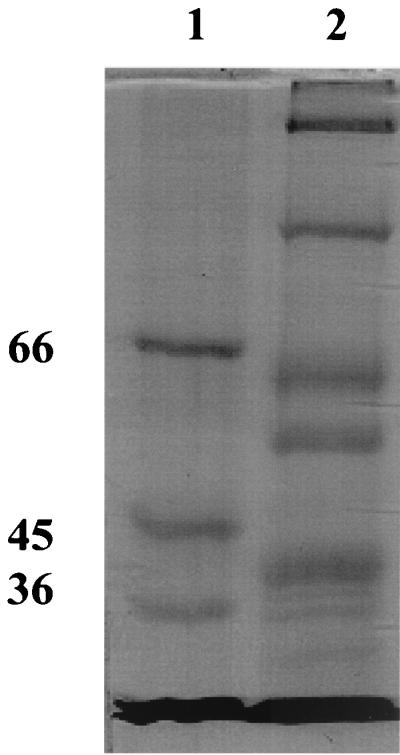
SDS–7% PAGE of the supernatant of cell envelopes treated with 10 mM Tris-HCl, 10 mM CaCl2 (pH 8), and 0.6% LDAO. Lane 1, molecular mass markers (66, 45, and 36 kDa). Lane 2, 50 μg of protein of the supernatant was solubilized at 30°C for 10 min in 15 μl of sample buffer. The gel was stained with Coomassie brilliant blue.
FIG. 2.
SDS–5% PAGE of the 185-kDa protein of the outer membrane of T. thermophilus obtained by elution from preparative SDS-polyacrylamide gels. Lane 1, high-molecular-mass markers (205, 116, 97, 84, and 66 kDa). Lane 2, 5 μg of the pure protein solubilized at 100°C for 10 min in 7 μl of sample buffer. The gel was stained with Coomassie brilliant blue.
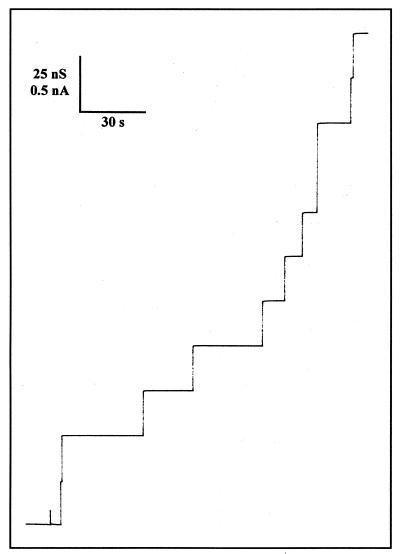
The channel formation mediated by the 185-kDa porin was further analyzed in single-channel experiments. Small amounts of the porin protein (about 5 ng/ml) were added to the aqueous phase on one or both sides of the membrane. After a delay of 1 to 2 min, probably caused by slow aqueous diffusion of the protein, the current increased in a stepwise fashion similar to that observed previously for gram-negative bacterial porins (2). Figure 3 demonstrates that the channels had an extremely high single-channel conductance compared to that of the enteric bacteria (1, 2). The channels had a long lifetime, because only on-steps were observed within at least several minutes. Interestingly, the single-channel distribution was fairly homogeneous, and more than 80% of the channels were localized within the conductance range between 17.5 and 22.5 nS.
FIG. 3.
Single-channel recording of the 185-kDa porin from the outer membrane of T. thermophilus. Channel-forming activity was measured with lipid bilayer membranes made of a 1% (wt/vol) solution of diphytanoyl phosphatidylcholine (Avanti Polar Lipids, Alabaster, Ala.) in n-decane (2). The aqueous phase contained 1 M KCl and 5 ng of protein per ml. The applied membrane potential was 20 mV; T = 20°C. The single-channel recordings were performed using Ag/AgCl electrodes (with salt bridges) connected in series to a voltage source and a Keithley 427 current amplifier. The amplified signal was monitored on a digital oscilloscope and recorded on a strip chart or tape recorder.
The giant pore from the T. thermophilus outer membrane was only moderately cation selective. This can be derived from single-channel experiments in which KCl was replaced by LiCl or potassium acetate (KAc), i.e., the mobile ions K+ and Cl− were replaced by the less-mobile ions Li+ and acetate− (Table 1). The single-channel conductance in 1 M LiCl and 1 M KAc decreased on average by a factor of about 2 compared with the conductance in 1 M KCl. This result indicated that the channel conductance followed the bulk aqueous conductivity of these salt solutions because they are about half that in 1 M KCl. Furthermore, we observed a linear dependence of the single-channel conductance as a function of the bulk aqueous conductivity (Table 1). This is of course expected when we assume that the outer membrane porin of T. thermophilus is wide and water-filled, as is suggested by its huge single-channel conductance in 1 M KCl.
TABLE 1.
Average single-channel conductance (G) of the T. thermophilus porin as a function of different salt solutionsa
| Salt | Concn (M) | Vm (mV) | G (nS) |
|---|---|---|---|
| LiCl | 1.0 | 20 | 10.0 |
| KCl | 0.01 | 20 | 0.45 |
| 0.03 | 20 | 1.0 | |
| 0.1 | 20 | 2.5 | |
| 0.3 | 20 | 7.0 | |
| 1.0 | 20 | 20 | |
| 3.0 | 20 | 50 | |
| KAc (pH 7) | 1.0 | 20 | 12.5 |
The membranes were formed from 1% diphytanoyl phosphatidylcholine dissolved in n-decane; T = 20°C. The pH of the aqueous salt solutions was around 6 unless otherwise indicated. G is given as the mean of at least 100 single steps similar to those shown in Fig. 3.
The results of the single-channel experiments agree with zero-current membrane potential measurements in the presence of salt gradients (3). After incorporation of a large number of channels in membranes bathed in 50 mM KCl, 10-fold salt gradients were established across the membranes by addition of small amounts of concentrated KCl solution to one side of the membrane. For KCl and KAc, the more diluted side of the membrane became slightly positive, which indicated preferential movement of potassium through the channel, i.e., the cell wall porin is weakly cation selective, as suggested by the single-channel recordings. When we used LiCl, the more dilute side became slightly negative. The zero-current membrane potential for the salts mentioned above had values of −3.4 mV (LiCl), 12 mV (KCl), and 26 mV (KAc). Analysis of these potentials using the Goldman-Hodgkin-Katz equation (3) suggested that the outer membrane channel has only a small selectivity, because the ratios of the permeability Pcation and Panion were 0.85 (LiCl), 1.8 (KCl), and 3.7 (KAc), which indicates that the outer membrane pore of T. thermophilus is indeed a general diffusion pore.
The outer membrane porin of T. thermophilus was purified to homogeneity using preparative SDS-PAGE, and its properties were studied in lipid bilayer membranes. The protein has an extremely large molecular mass (185 kDa) compared with the outer membrane porins of gram-negative bacteria investigated to date, which normally range between 30 kDa for the general diffusion porin of Paracoccus denitrificans (30) and 58 kDa for the sucrose-specific ScrY of enteric bacteria (32). Only the monomeric outer membrane receptors such as FhuA (11) and BtuB12 (33) have higher molecular masses (around 60 to 80 kDa) than the porins. The gated channels formed by the receptors are wider and contain 22 β-strands (9, 15, 21), in contrast to the trimeric P. denitrificans and ScrY channels, where the three monomers in a trimer are formed by 16 and 18 β-strands, respectively (17, 19). The outer membrane porin of T. thermophilus also has another interesting feature, its high resistance to SDS and heat, because we did not find any decrease in its channel-forming activity when it was heated for a long time in sample buffer or when the protein was precipitated with organic solvent. This means that the channel-forming complex has a much higher stability when heated than other gram-negative bacterial porins, particularly porins from enteric bacteria, which dissociate under these conditions and become inactivated. Another interesting feature of the 185-kDa outer membrane porin of T. thermophilus is its extremely high single-channel conductance compared with that of other gram-negative bacterial porins, which range between 10 pS for the nucleoside-specific Tsx (4) and 3.5 nS for the general diffusion porin of Rhodobacter capsulatus (6) under otherwise identical conditions.
Earlier investigations into the structure of the outer membrane of T. thermophilus have already suggested that it has a normal membrane-like structure because it undergoes thermotropic phase transitions dependent on the growth temperature (36). In other investigations, it has been demonstrated that calcium plays a major role in the stability of proteins from the cell envelope of T. thermophilus (7). In our study, we showed that the proteins of the outer membrane have quite different properties from those of enteric bacteria, because we could not find any peptidoglycan-associated proteins, and the wash of the cell envelope with SDS and isolation of the peptidoglycan-associated protein (23) failed. The S-layer protein of T. thermophilus HB8, which has a molecular mass of about 100 kDa, has been studied in some detail in recent years (13, 14, 16, 28) and is probably already lost upon washing either with the Tris-HCl buffer or with the first wash with 0.01% LDAO, because we did not find the 100-kDa band in the outer membrane fraction of the sucrose density centrifugation or in the supernatants of the different LDAO steps. Although it has been claimed that the S-layer protein may form porin-like structures (10), it seems clear that it is not responsible for the permeability properties of the outer membrane, because the 100-kDa protein is not an integral outer membrane protein, although it contains a domain that interacts with the peptidoglycan layer (28).
Acknowledgments
This study was supported by grants from the Deutsche Forschungsgemeinschaft (project Be 865/10-1) and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Benz R. Solute uptake through bacterial outer membranes. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier Science B.V.; 1994. pp. 397–423. [Google Scholar]
- 2.Benz R, Janko K, Boos W, Läuger P. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1978;511:305–319. doi: 10.1016/0005-2736(78)90269-9. [DOI] [PubMed] [Google Scholar]
- 3.Benz R, Schmid A, Hancock R E W. Ion selectivity of gram-negative bacterial porins. J Bacteriol. 1985;162:722–727. doi: 10.1128/jb.162.2.722-727.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benz R, Schmid A, Maier C, Bremer E. Characterization of the nucleoside-specific binding site inside the Tsx channel of Escherichia coli outer membrane: reconstitution experiments with lipid bilayer membranes. Eur J Biochem. 1988;176:699–705. doi: 10.1111/j.1432-1033.1988.tb14333.x. [DOI] [PubMed] [Google Scholar]
- 5.Benz R, Schmid A, Vos-Scheperkeuter G H. Mechanism of sugar transport through the sugar-specific LamB channel of Escherichia coli outer membrane. J Membrane Biol. 1987;100:21–29. doi: 10.1007/BF02209137. [DOI] [PubMed] [Google Scholar]
- 6.Benz R, Woitzik D, Flammann H T, Weckesser J. Pore forming activity of the major outer membrane protein of Rhodobacter capsulatus in lipid bilayer membranes. Arch Microbiol. 1987;148:226–230. [Google Scholar]
- 7.Berenguer J, Faraldo M L M, de Pedro M A. Ca2+-stabilized oligomeric protein complexes are major components of the cell envelope of Thermus thermophilus HB8. J Bacteriol. 1988;170:2441–2447. doi: 10.1128/jb.170.6.2441-2447.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boeck B, Schinzel R. Growth dependence of α-glucan phosphorylase activity in Thermus thermophilus. Res Microbiol. 1998;149:171–176. doi: 10.1016/s0923-2508(98)80077-6. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan S K, Smith B S, Venkatramani L, Xia D, Esser L, Palnitkar M, Chakraborty R, van der Helm D, Deisenhofer J. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat Struct Biol. 1999;6:56–63. doi: 10.1038/4931. [DOI] [PubMed] [Google Scholar]
- 10.Caston J R, Berenguer J, de Pedro M A, Carrascosa J L. S-layer protein from Thermus thermophilus HB8 assembles into porin-like structures. Mol Microbiol. 1993;9:65–75. doi: 10.1111/j.1365-2958.1993.tb01669.x. [DOI] [PubMed] [Google Scholar]
- 11.Coulton J W. The ferrichrome-iron receptor of Escherichia coli K-12: antigenicity of the FhuA protein. Biochim Biophys Acta. 1982;717:154–162. doi: 10.1016/0304-4165(82)90393-2. [DOI] [PubMed] [Google Scholar]
- 12.Cowan S W, Schirmer T, Rummel G, Steiert M, Ghosh R, Pauptit R A, Jansonius J N, Rosenbusch J P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992;358:727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- 13.Faraldo M L M, de Pedro M A, Berenguer J. Cloning and expression in Escherichia coli of the structural gene coding for the monomeric protein of Thermus thermophilus HB8. J Bacteriol. 1991;173:5346–5351. doi: 10.1128/jb.173.17.5346-5351.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faraldo M M, de Pedro M A, Berenguer J. Sequence of the S-layer gene of Thermus thermophilus HB8 and functionality of its promoter in Escherichia coli. J Bacteriol. 1992;174:7458–7462. doi: 10.1128/jb.174.22.7458-7462.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson A D, Hofmann E, Coulton J W, Diederichs K, Welte W. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science. 1998;282:2215–2220. doi: 10.1126/science.282.5397.2215. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Herrero L A, Olabarria G, Caston J R, Lasa I, Berenguer J. Horizontal transference of S-layer genes within Thermus thermophilus. J Bacteriol. 1995;177:5460–5466. doi: 10.1128/jb.177.19.5460-5466.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forst D, Welte W, Wacker T, Diederichs K. Structure of the sucrose-specific porin ScrY from Salmonella typhimurium and its complex with sucrose. Nat Struct Biol. 1998;5:37–46. doi: 10.1038/nsb0198-37. [DOI] [PubMed] [Google Scholar]
- 18.Gross H J, Baier H, Blum H. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–99. [Google Scholar]
- 19.Hirsch A, Breed J, Saxena K, Richter O M, Ludwig B, Diederichs K, Welte W. The structure of porin from Paracoccus denitrificans at 3.1 Å resolution. FEBS Lett. 1997;404:208–210. doi: 10.1016/s0014-5793(97)00131-2. [DOI] [PubMed] [Google Scholar]
- 20.Lichtinger T, Reiss G, Benz R. Biochemical identification and biophysical characterization of a channel-forming protein from Rhodococcus erythropolis. J Bacteriol. 2000;182:764–770. doi: 10.1128/jb.182.3.764-770.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Locher K P, Rees B, Koebnik R, Mitschler A, Moulinier L, Rosenbusch J P, Moras D. Transmembrane signaling across the ligand-gated FhuA receptor: crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell. 1998;95:771–778. doi: 10.1016/s0092-8674(00)81700-6. [DOI] [PubMed] [Google Scholar]
- 22.Luckey M, Nikaido H. Specificity of diffusion channels produced by λ-phage receptor protein of Escherichia coli. Proc Natl Acad Sci USA. 1980;77:165–171. doi: 10.1073/pnas.77.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikaido H. Proteins forming large channels from bacterial and mitochondrial outer membranes: porins and phage lambda receptor protein. Methods Enzymol. 1983;97:85–100. doi: 10.1016/0076-6879(83)97122-7. [DOI] [PubMed] [Google Scholar]
- 24.Nikaido H. Porins and specific channels of bacterial outer membranes. Mol Microbiol. 1992;6:435–442. doi: 10.1111/j.1365-2958.1992.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 25.Nikaido H. Porins and specific diffusion channels in bacterial outer membranes. J Biol (Bronx NY) 1994;269:3905–3908. [PubMed] [Google Scholar]
- 26.Nikaido H, Rosenberg E Y. Effect of solute size on diffusion rates through the transmembrane pores of the outer membrane of Escherichia coli. J Gen Physiol. 1981;77:121–135. doi: 10.1085/jgp.77.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olabarria G, Carrascosa J L, de Pedro M A, Berenguer J. A conserved motif in S-layer proteins is involved in peptidoglycan binding in Thermus thermophilus. J Bacteriol. 1996;178:4765–4772. doi: 10.1128/jb.178.16.4765-4772.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quintela J C, Pittenauer E, Altmaier G, Aran V, de Pedro M A. Structure of peptidoglycan from Thermus thermophilus HB8. J Bacteriol. 1995;177:4947–4962. doi: 10.1128/jb.177.17.4947-4962.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saxena K, Richter O-M H, Ludwig B, Benz R. Molecular cloning and functional characterization of the Paracoccus denitrificans porin. Eur J Biochem. 1997;245:300–306. doi: 10.1111/j.1432-1033.1997.00300.x. [DOI] [PubMed] [Google Scholar]
- 31.Schirmer T, Keller T A, Wang Y F, Rosenbusch J P. Structural basis for sugar translocation through maltoporin channels at 3.1 Å resolution. Science. 1995;267:512–514. doi: 10.1126/science.7824948. [DOI] [PubMed] [Google Scholar]
- 32.Schülein K, Schmid K, Benz R. The sugar specific outer membrane channel ScrY contains functional characteristics of general diffusion pores and substrate-specific porins. Mol Microbiol. 1991;5:2233–2241. doi: 10.1111/j.1365-2958.1991.tb02153.x. [DOI] [PubMed] [Google Scholar]
- 33.Taylor R, Burgner J W, Clifton J, Cramer W A. Purification and characterization of monomeric Escherichia coli vitamin B12 receptor with high affinity for colicin E3. J Biol Chem. 1998;273:31113–31118. doi: 10.1074/jbc.273.47.31113. [DOI] [PubMed] [Google Scholar]
- 34.Trias J, Benz R. Characterization of the channel formed by the mycobacterial porin of Mycobacterium chelonae in lipid-bilayer membranes: demonstration of voltage dependent regulation and the presence of negative point charges at the channel mouth. J Biol Chem. 1993;268:6234–6240. [PubMed] [Google Scholar]
- 35.Trias J, Jarlier V, Benz R. Porins in the cell wall of mycobacteria. Science. 1992;258:1479–1481. doi: 10.1126/science.1279810. [DOI] [PubMed] [Google Scholar]
- 36.Wakayama N, Oshima T. Membrane properties of an extreme thermophile. I. Detection of the phase transition and its dependence on growth temperature. J Biochem (Tokyo) 1978;83:1687–1692. doi: 10.1093/oxfordjournals.jbchem.a132081. [DOI] [PubMed] [Google Scholar]
- 37.Weiss M S, Kreusch A, Schiltz E, Nestel U, Welte W, Weckesser J, Schulz G E. The structure of porin from Rhodobacter capsulatus at 1.8 Å resolution. FEBS Lett. 1991;280:379–382. doi: 10.1016/0014-5793(91)80336-2. [DOI] [PubMed] [Google Scholar]