Highlights
-
•
Apical surface heparan sulfate proteoglycans (HSPG) have unique endothelial roles.
-
•
Inflammation and shear stress derangement can alter HSPG synthesis and sulfation.
-
•
Reduced HSPG expression impacts mechanisms that regulate endothelial cell homeostasis.
-
•
Changes to endothelial glycocalyx HSPG sulfation are vast and contextually dependent.
-
•
Targeting endothelial HSPG biosynthesis and sulfation are promising therapeutic strategies.
Keywords: Vascular endothelium, Glycocalyx, Heparan sulfate proteoglycan, Synthesis, Sulfation
Abbreviations: ACE2, Angiotensin-converting enzyme 2; CLP, cecal ligation and puncture; COVID-19, Coronavirus disease 2019; eGCX, Endothelial glycocalyx; eNOS, Endothelial nitric oxide synthase; EXT, Exostosin; EXTL, Exostosin-like glycosyltransferase; FFP, Fresh frozen plasma; FGF, Fibroblast growth factor; FGFR1, Fibroblast growth factor receptor 1; GAG, Glycosaminoglycan; Gal, Galactose; GlcNAc, N-actetyl glucosamine; GlcA, Glucuronic acid; GPC, Glypican; HLMVEC, Human lung microvascular endothelial cell; HS, Heparan sulfate; HS2ST, Heparan sulfate 2-O-sulfotransferase; HS3ST, Heparan sulfate 3-O-sulfotransferase; HS6ST, Heparan sulfate 6-O-sulfotransferase; HSPG, Heparan sulfate proteoglycan; HUVEC, Human umbilical vein endothelial cell; LPS, lipopolysaccharide; NDST, N-deacetylase/N-sulfotransferase; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SDC, Syndecan; Sulf, Endosulfatase; TNFα, Tumor necrosis factor alpha; UA, Hexuronic acid; VEGF, Vascular endothelial growth factor; Xyl, Xylose; XYLT, Xylosyltransferase
Abstract
The glycocalyx attached to the apical surface of vascular endothelial cells is a rich network of proteoglycans, glycosaminoglycans, and glycoproteins with instrumental roles in vascular homeostasis. Given their molecular complexity and ability to interact with the intra- and extracellular environment, heparan sulfate proteoglycans uniquely contribute to the glycocalyx’s role in regulating endothelial permeability, mechanosignaling, and ligand recognition by cognate cell surface receptors. Much attention has recently been devoted to the enzymatic shedding of heparan sulfate proteoglycans from the endothelial glycocalyx and its impact on vascular function. However, other molecular modifications to heparan sulfate proteoglycans are possible and may have equal or complementary clinical significance. In this narrative review, we focus on putative mechanisms driving non-proteolytic changes in heparan sulfate proteoglycan expression and alterations in the sulfation of heparan sulfate side chains within the endothelial glycocalyx. We then discuss how these specific changes to the endothelial glycocalyx impact endothelial cell function and highlight therapeutic strategies to target or potentially reverse these pathologic changes.
Introduction
The endothelial glycocalyx (eGCX) is a highly dynamic, gel-like coating anchored to the apical surface of vascular endothelial cells with myriad functions in maintaining blood vessel homeostasis. The delicate eGCX is comprised of heparan sulfate (HS) proteoglycans (HSPG), hyaluronan, and sialic acid-capped glycoproteins that contribute uniquely to the regulation of vascular permeability, immune cell trafficking, hemostasis, and vascular tone [1], [2]. HSPGs, in particular, have critical roles in homeostatic endothelial cell signaling given their unique structures and interactions with both the intra- and extracellular environments [3]. Therefore, it is not surprising that pathological modifications to eGCX HSPGs promote endothelial dysfunction and vascular disease.
Research into the structure and function of HSPGs in the context of normal endothelial cell biology burgeoned in the 1980s and 1990s as summarized in reviews by Rosenberg et al. [4] and Weinbaum et al. [5]. Over the last 20 years, advances in our understanding of vascular biology have brought about greater clarity around the roles of eGCX-specific HSPGs in cardiovascular homeostasis and how HSPG modifications impact endothelial function. The purpose of this narrative review is to provide a modern perspective on the role of endothelial apical surface HSPGs in vascular disease by highlighting the latest research into mechanisms that alter the synthesis and sulfation status of eGCX HSPGs and the untoward effects of these alterations on endothelial functions. In addition, practical and theoretical treatment strategies to improve HSPG integrity are discussed and remaining knowledge gaps identified to help inform future research efforts.
Overview of eGCX HSPG structure, synthesis, and function
The major HSPGs identified within the eGCX are syndecans (SDC) (subtypes 1–4) and glypican (GPC) (subtype 1 only) [4], [6], [7], [8]. Membrane-spanning SDCs are primarily differentiated from GPCs by their single-pass, highly conserved transmembrane domains and their cytoplasmic endodomains that anchor to cytoskeletal proteins, allowing direct interaction with signaling molecules such as protein kinase C, synectin/syntenin, and calcium/calmodulin-dependent serine protein kinase [4], [9]. GPCs, on the other hand, are anchored by glycosylphosphatidylinositol linkages to lipid microdomains in the cell membrane, including caveolae [10], [11]. GPCs indirectly participate in intracellular signaling through affiliation with lipid domain- or caveolae-linked effector molecules such as Src family kinases, endothelial nitric oxide synthase (eNOS), and G protein-coupled receptors [12], [13]. SDCs are also distinct from GPCs in the location of glycosaminoglycan (GAG) linkages (e.g., heparan sulfate (HS), chondroitin sulfate) within the proteoglycan ectodomain. SDCs express HS attachment sites (potentially 2–5 chains per proteoglycan) near the amino-terminus distal to the cell membrane with some SDC subfamilies (e.g., SDC-1 and −3) expressing chondroitin sulfate side chains near the carboxyl-terminus proximal to the cell membrane [3]. GPCs express only HS chains with 2–3 putative GAG attachments near the ectodomain carboxyl-terminus [14], [15].
Endothelial cell synthesis of HSPGs begins in the rough endoplasmic reticulum with the formation of the core proteoglycan [16]. A xylose (Xyl) molecule is then added to an available serine residue within the proteoglycan HS attachment region by xylosyltransferase (XYLT) in either the endoplasmic reticulum or Golgi [17]. Next, within the Golgi two galactose (Gal) molecules and a d-glucuronic acid (GlcA) molecule are sequentially attached to xylose to form a Xyl-Gal-Gal-GlcA tetrasaccharide linkage [18]. The exostosin (EXT)-like family of glycosyltransferases (EXTL) then transfer an N-acetyl glucosamine (GlcNAc) residue to the linkage tetrasaccharide, committing the molecule to become HS [19]. Then, multiple repeating disaccharide sequences of a hexuronic acid (UA) linked to GlcNAc are attached by sequential [UA(1 → 4)GlcNAc] linkages predominantly by EXT1 with support by EXT2 (generally estimated to be 50–150 disaccharide repeats at an approximate length of 40–125 nm per HS chain) [14], [18], [19], [20]. The hexuronic acid residue within each disaccharide unit typically begins as GlcA; however, during HS chain lengthening, GlcA may undergo epimerization by d-glucuronyl C5-epimerase to form l-iduronic acid. The unbranched HS chain may undergo further modification by sulfation of varying disaccharide units at the N-, 3-O or 6-O positions on GlcNAc or the 2-O position on the hexuronic acid (Fig. 1). While not all disaccharide units undergo the same pattern of sulfation, addition of sulfate residues typically begins with N-deacetylation and N-sulfation of GlcNAc by N-deacetylase/N-sulfotransferase (NDST). Next, 2-O sulfation of the hexuronic acid may occur by HS 2-O-sulfotransferase (HS2ST). Then, HS 3-O-sulfotransferase (HS3ST) and HS 6-O-sulfotransferase (HS6ST) may attach sulfate residues to GlcNAc at the 3-O and 6-O positions, respectively. There are four isoforms of NDST, one isoform of HS2ST, seven isoforms of HS3ST, and three isoforms of HS6ST that have been identified in humans [21], [22]. Once formed, HSPGs are transported from the Golgi to the endothelial cell membrane by secretory vesicle exocytosis [18]. After HSPGs are expressed in the eGCX, endothelial- or leukocyte-derived endosulfatases (Sulf) 1 and 2 may further modify HS side chains on HSPGs through the selective cleavage of 6-O-sulfo groups. The vast permutations in number of disaccharide repeats, hexuronic acid expression, and sulfation patterns within attached HS side chains give rise to extraordinary diversity in HSPG structure at the vascular endothelial surface.
Fig. 1.
Heparan sulfate (HS) disaccharide structural modification/sulfation process. Polymerized HS chains attached to core proteoglycans destined for the endothelial surface may undergo several sulfation modifications within the endothelial cell Golgi apparatus prior to apical surface expression. First, N-deacetylase/N-sulfotransferase replaces the acetyl group for a sulfate at the amino linkage to the second carbon of a glucosamine residue. Next, HS 2-O-sulfotransferase may attach a sulfate group to the hexuronic acid residue (either d-glucuronic acid or l-iduronic acid). Glucosamine residues within the various disaccharides may undergo further sulfation by HS 3-O-sulfotransferase and/or 6-O-sulfotransferase.
The physicochemical properties (e.g., size, composition, and charge) of each individual HSPG shape its function within the eGCX. The molecular size, complexity, and negative fixed charge density of HSPGs contribute to the eGCX’s function as a sentry for circulating cell interactions with luminal surface adhesion molecules and selective permeability to circulating proteins through steric hindrance and electrostatic repulsion [23], [24], [25]. Distinct sulfation patterns within the HS moieties of HSPGs dictate their affinity to bind specific proteins, enabling the eGCX to have a tremendous capacity for interacting with and storing a broad range of circulating and locally derived proteins. Moreover, particular ligands such as fibroblast growth factor (FGF) [26], [27], [28] and vascular endothelial growth factor (VEGF) [29], [30], [31] engage the HS side chains of eGCX HSPGs as coreceptors to synergize ligand-cognate receptor interactions at the endothelial cell surface to affect putative endothelial signaling (see more specific detail below).
Endothelial surface HSPGs regulate transmural fluid movement in the microcirculation through what is considered the revised Starling principle [32]. In this update to the traditional understanding of the physiologic principles driving microvascular transmural fluid movement, Hu and Weinbaum [1] uncovered that the eGCX adds an additional layer of complexity to transvascular fluid flux by creating an oncotic pressure gradient between the vascular lumen and the sub-eGCX space through its reflection of plasma macromolecules. Therefore, transvascular fluid flux is not simply dictated by hydrostatic and oncotic pressure gradients between the intra- and extravascular spaces by instead involves a more complex relationship between eGCX integrity, endothelial cell–cell junctional integrity, and changes in intraluminal hydrostatic and oncotic pressure [33], [34], [35].
HSPGs are also integral in the transduction of circulatory shear stress to the endothelial intracellular environment, activating signaling mechanisms that support competent endothelial focal adhesions and intercellular junction integrity [36], [37] while providing a feed-forward mechanism to support robust eGCX expression [38]. Shear stress transduction through HSPGs also activates eNOS, generating nitric oxide critical to the regulation of surrounding vascular smooth muscle tone [39], [40]. Additionally, HSPG binding and activation of anti-coagulant mediators such as antithrombin helps prevent baseline levels of coagulation and clot formation [41]. In summary, an intact eGCX promotes a local anti-inflammatory, anti-coagulant/anti-thrombotic environment that maintains vascular patency and barrier integrity.
Modifications to eGCX HSPGs
A number of important mechanisms promoting structural and biochemical changes to HSPGs within the vascular eGCX have been uncovered that contribute to aberrant vascular biology. Cleavage of HSPGs and GAGs from the eGCX is a hallmark of vascular damage associated with many pathologic conditions, and plasma levels of shed HS and HSPG ectodomains have been investigated as prognostic biomarkers and measures of injury/illness severity [42], [43], [44]. As several recent review manuscripts extensively cover the mechanisms driving eGCX degradation resulting in vasculopathy [45], [46], [47], herein we focus on structural eGCX remodeling that arises from alterations in endothelial HSPG biosynthesis and post-translational sulfation modifications to HS.
Mechanisms of impaired eGCX HSPG synthesis
Endothelial GCX integrity is impacted by inflammatory insults, hemodynamic perturbations, and comorbidities that promote variable HSPG synthesis and expression. The local and systemic inflammatory responses to acute insults such as trauma, hemorrhagic shock, burns, or sepsis, for example, generate a cytokine response that augments HSPG synthesis within vascular endothelial cells. In isolated cell culture, tumor necrosis factor alpha (TNFα) has been shown to increase SDC2 mRNA expression in human primary umbilical vein endothelial cells (HUVEC) but suppress SDC1 in Ea.hy926 HUVEC-A549 epithelial hybridoma cells [7], [48]. Interleukin-1β, on the other hand, appears to suppress SDC1 and SDC2 expression while upregulating SDC4 in HUVECs [49]. FGF2 and transforming growth factor β1 have been demonstrated to upregulate SDC4 mRNA and SDC-4 proteoglycan expression in bovine aortic endothelial cells [50], [51]. Similarly, the proline-rich peptide PR-39 released during soft tissue injury has been demonstrated to upregulate both SDC-1 and −4 proteoglycan expression on the surface of bovine adrenal microvascular endothelial cells [52]. In light of the fact that acute inflammation promotes increased HSPG degradation by eGCX-targeting sheddases [53], [54], [55], [56], results from these in vitro studies may point to potential compensatory mechanisms upregulating HSPG synthesis in the face of increased HSPG turn-over. Furthermore, there may also be an interdependence between the expression of specific HSPGs. Work by Hara et al. [57], complemented by studies performed by Vuong et al. [49], suggests that endothelial SDC-4 expression may be inversely related to SDC-1 expression. Thus, loss of homeostatic SDC-1 expression within the eGCX may lead to the compensatory upregulation of SDC-4 at the endothelial surface, though the precise mechanism and purpose of this compensatory response remains unclear.
MicroRNA (miRNA) may also contribute to the regulation of endothelial HSPG synthesis. Using a mouse model of acute ischemia/reperfusion, Wu et al. [58] discovered that upregulation of miR-19b contributes to lung injury observed following hemorrhage and resuscitation, corresponding with decreased SDC1 gene expression within lung tissue. The authors corroborated in vivo findings by demonstrating a miR-19b-mediated decrease in SDC-1 protein expression in human primary lung microvascular endothelial cells (HLMVEC). However, the mechanisms responsible for miR-19b upregulation following hemorrhagic shock were unresolved. Other work suggests that miR-126, an endothelial-derived microRNA shown to preserve endothelial monolayer integrity [59], [60], may increase eGCX SDC-4 expression in an autocrine/paracrine manner [61]. On the other hand, activity by the more ubiquitous miR-149 was shown to diminish eGCX GPC1 gene and GPC-1 protein expression [62]. However, at present it is unclear how miR-126 or miR-149 are regulated during inflammation and to what degree these microRNAs contribute to HSPG expression during inflammation in vivo.
Systemic inflammation may also attenuate HS expression in the eGCX. Yang et al. [63] observed that recovery of eGCX thickness within mouse pulmonary microcirculation required 24 h following sterile enzymatic erosion of HS from the eGCX; however, mouse pulmonary microvascular eGCX thinning in a cecal ligation and puncture (CLP) model of sepsis required at least 72 h for recovery. The authors found that delayed eGCX recovery in mice with sepsis was unrelated to ongoing HS shedding but instead occurred in response to impaired FGF receptor-1 (FGFR1) signaling, resulting in decreased EXT1 expression and decreased HS synthesis. Though further research is needed to clarify how FGFR1 signaling is depressed during sepsis, this work is an important step forward in understanding the mechanisms driving eGCX HS expression and suggests a cell signaling pathway regulating HS synthesis as a potential therapeutic target during systemic inflammation.
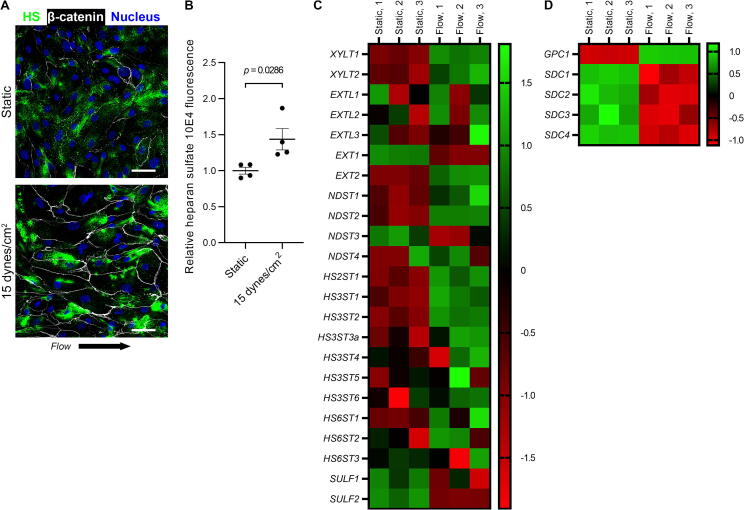
Disturbances in shear stress at the endothelial surface likely contribute to variations in eGCX HSPG synthesis and expression. Zeng et al. [64] observed that rat fat pad endothelial cells exposed to laminar shear stress express higher eGCX levels of SDC-1, GPC-1, and HS relative to static culture. When exposed to turbulent flow, Harding et al. [65] found that rat fat pad endothelial cells express significantly lower levels of HS within the eGCX relative to laminar flow. Conversely, Russo et al. [66] reported that SDC-4 expression and whole cell expression of sulfated GAGs decreased in immortalized rabbit aortic endothelial cells in response to laminar shear stress compared to static culture. Koo et al. [67] observed increased HS and SDC-1 expression on the surface of HUVECs conditioned in higher shear stress (∼12 dynes/cm2 [68]) relative to HUVECs maintained in either static culture or near-static flow conditions (∼1 dyne/cm2 [68]). However, these authors found that GPC1 mRNA and GPC-1 protein expression decreased with higher shear. We similarly observed increased HS expression over HLMVECs conditioned with laminar shear stress (Fig. 2, A and B), concurrent with increased XYLT1, XYLT2, and EXT2 gene expression and variably increased expression for EXTL1-3 (Fig. 2C). Intriguingly, we also found that flow conditioning HLMVECs increased GPC1 gene expression while relatively uniformly suppressing SDC1-4 (Fig. 2, C and D). Taken together, these studies suggest that surface shear stress may impact HSPG expression in a manner dependent upon both the magnitude of change in shear stress and the endothelial cell type. Moreover, these studies suggest that changes in the expression of individual HSPG subtypes may not be uniform in response to shear stress alterations. Further investigation is required to clarify how variable flow profiles and shear stress magnitude impact the expression of HSPG subtypes within the eGCX of different circulation beds in humans both during physiologic and pathophysiologic conditions.
Fig. 2.
Shear stress increases heparan sulfate (HS) expression within the glycocalyx on human primary lung microvascular endothelial cells (HLMVEC), concurrent with increased gene expression of HS-synthesizing enzymes. A) Stacked confocal micrographs of HLMVECs incubated for 48 h in either static conditions (top panel) or 15 dynes/cm2 of laminar shear stress (bottom panel) demonstrating staining for HS 10E4 epitope (cells demarcated by β-catenin with DAPI overlay). Scale bar represents 20 µm. B) Relative staining intensity for HS 10E4 epitope on HLMVECs following 48 h of static culture or laminar flow (from 4 representative 20x images). Data are presented as mean ± SEM and analyzed by Mann-Whitney U test. C) Heat map displaying the relative fold change in gene expression for select HS-synthesizing enzymes measured in HLMVECs incubated in static or flow conditions (n = 3 per group) by an nCounter (NanoString, Seattle, WA) digital multiplexed gene expression array. Overall, shear stress promoted increased mRNA expression for xylotransferases, exostosin-like glycosyltransferases, and exostosin 2 while also increasing mRNA expression for most sulfotransferases. Flow conditioning decreased mRNA expression for sulfatases. D) Interestingly, flow conditioning promoted higher expression of GPC1 mRNA while uniformly reducing SDC1-4 mRNA in HLMVECs.
Advancing age and development of cardiovascular comorbidities also appear to impact baseline HSPG expression. As blood vessels age, they accrue fibrotic changes and calcifications that gradually diminish vascular compliance [69]. Groundbreaking work by Mahmoud et al. [70] revealed that these biomechanical changes in the subendothelial architecture may be an important driver of reduced eGCX thickness by means of reduced GPC-1 expression. However, it remains unknown how vessel stiffness impacts the expression of other HSPGs within the eGCX. Hyperlipidemia may also contribute to changes in endothelial surface HSPG expression as suggested by Kang et al. [71] in a study showing that a 12-week high-cholesterol diet caused eGCX thinning and reduction in sulfated GAG content of the eGCX of carotid arteries. Moreover, GPC1 and EXT1 gene expression was reduced within the carotid arteries (not endothelial-specific) of high-cholesterol-fed rats while SDC4 gene expression was unchanged (SDC1 was not reported). These studies provide important insights into the co-morbidities and pathobiology that may be driving age-related changes to HSPG synthesis and expression within the eGCX.
Mechanisms driving sulfation modifications to HS
Localized and systemic inflammation alters the expression level and activity of sulfotransferases and endosulfatases regulating HS sulfation, which leads to distinct changes in sulfation patterns and bioactivity of HSPGs within the eGCX. While these changes would be predicted to significantly alter putative cell and molecular interactions with eGCX HSPGs, at present relatively little is known about the impact of inflammation on the sulfation of eGCX HSPGs and downstream effects. Here, we present what evidence has more recently come to light about these changes and speculate on potential mechanisms that may be at play.
Rops et al. [72] observed that TNFα and interleukin-1β treatment of mouse glomerular endothelial cells increased expression of NDST1, NDST2, HS6ST1 and HS6ST2 and decreased expression of SULF2 through the activation of canonical nuclear factor kappa-light-chain-enhancer of activated B cells signaling. These findings corresponded with a higher presence of N- and 6-O-sulfo groups within eGCX HS molecules and were confirmed within the cortices of kidneys from two separate murine models of local nephritic inflammation [72]. Similar sulfation changes to HS were observed in the eGCX of HUVECs in response to TNFα treatment. Carava et al. [73] found that the presence of disulfated [UA-GlcNS(6S)] disaccharides increased within the eGCX of HUVECs following a 24-hour treatment with TNFα corresponding with reductions in the amount of monosulfated [UA-GlcNAc(6S)] and [UA-GlcNS] disaccharides and unsulfated disaccharides, suggesting significant increases in NDST and HS6ST activities. Smits et al. [74] took these findings a step further, observing that TNFα promoted the expression of the unique [IdoA(2S)-GlcNS(6S)]3 hexasaccharide motif within eGCX HS molecules on HUVECs (rarely observed in healthy human tissue) that is instrumental for leukocyte adhesion to the endothelial cell surface. In a murine CLP model of sepsis, Oshima et al. [75] observed that the pulmonary vascular eGCX reconstituted within 72 h of the initial systemic inflammatory insult and eGCX injury and was largely enriched in [UA-GlcNAc(6S)] disaccharides with only a slight increase in [UA-GlcNS(6S)] expression, a finding that correlated with abrogation of SULF1 expression within lung tissue of septic mice. Taken together, these findings point to an important interplay between cytokine–induced modifications to the eGCX that may feed-forward to support leukocyte-endothelial interactions. However, more work is needed to determine the impact of these and other sulfation changes to the eGCX on the function of various vascular beds in vital organs.
Hypoxia and tissue ischemia may similarly initiate sulfation modifications to eGCX HSPGs by augmenting the activity of various sulfotransferases and/or both endosulfatases. Li et al. [76] found that incubating HUVECs and mouse primary cardiac microvascular endothelial cells in a hypoxic environment significantly increased expression of NDST1, NDST2, and HS2ST in both cell types without substantial changes in HS6ST1 or HS6ST2. Similar findings were observed following adenoviral transduction-mediated overexpression of hypoxia inducible factor-1α, suggesting a putative transcriptional regulator of these sulfotransferases. However, the authors did not investigate the impact of hypoxia on specific changes in the sulfation of eGCX HS side chains. Using a mouse model of coronary ischemia, Korf-Klingebiel et al. [77] found that tissue hypoxia increased expression for SULF1 and SULF2 in tissue samples from infarcted myocardia. More granular analysis of gene expression within individual cell types from infarcted mouse myocardia revealed that endothelial cells and fibroblasts largely accounted for increased SULF1 while monocytes were the primary source of increased SULF2.
Perturbations in exostosin expression may also impact HS sulfation via regulation of NDST activity. Current evidence suggests that NDST1 competes with EXT1 for binding to EXT2 and subsequent transport to the Golgi where it is available to deacetylate and sulfate GlcNAc residues as the initiating step for HS sulfation [78]. Thus, changes in the relative amounts of EXT1 and EXT2 may influence shuttling of NDST enzymes and their subsequent availability to modify HS residues as evidenced by increased NDST1 activity in cardiac tissue of transgenic mice overexpressing EXT2 protein [78]. However, the translational applicability of exostosin-mediated HS sulfation modifications and the relevance to eGCX-specific sulfation patterns has not been explored. Modulations in exostosin expression are typically associated with overall abrogation of HS expression as observed in hereditary multiple exostoses, and the relationship between EXT and sulfation modifications has been less studied. However, as discussed above, Yang et al. discovered that EXT1 gene expression is attenuated in lung tissue from septic mice, corresponding with elevated circulating levels of N-sulfated HS fragments [63]. Although the authors did not specifically interrogate the role of EXT1 in regulating HS sulfation modifications, their findings support the possibility that decreased EXT1 expression could allow for increased interactions between EXT2 and NDST to promote NDST shuttling and HS sulfation in sepsis. Further studies are warranted to validate these speculations and to more clearly establish the role for exostosins in regulating HS remodeling in sepsis and other disease processes in which vascular dysfunction is relevant.
In summary, existing literature, though sparse, supports an important role for inflammatory processes in structural remodeling of eGCX HSPGs. However, sulfation patterning of HS disaccharides involves complex and multifaceted interactions of many enzymes, and our understanding of how inflammation and other disease factors impact these orchestrated interactions is only in its infancy.
Impact of eGCX HSPG expression and sulfation modifications on vascular function
Alterations in eGCX HSPG expression and modifications to HS side chains have important ramifications on endothelial homeostasis that may can contribute to vascular pathobiology. Techniques such as high-performance liquid chromatography-tandem mass spectrometry and surface plasmon resonance have aided in characterizing how the finely tuned sulfation properties of HS may impact various endothelial cell signaling axis in vitro. Moreover, development of endothelial-specific murine knockout models permit researchers in the fields of glycobiology and vascular biology to challenge/validate in vitro findings and elucidate their physiologic relevance to vascular health and disease. Here we describe the latest available research into the implications of impaired eGCX HSPG expression and/or sulfation modifications to eGCX HSPGs in endothelial pathobiology (summarized in Table 1).
Table 1.
Impact of altered heparan sulfate proteoglycan expression and heparan sulfate side chain sulfation modifications within the endothelial apical glycocalyx on endothelial cell biology.
| HSPG Modification | Mechanism | Effect on Endothelium | Reference |
|---|---|---|---|
| ↓ SDC-1 expression | ↓ SDC1 expression | ↓ PI3K/protein kinase B pathway activation ↑ Endothelial monolayer permeability ↑ ICAM1 gene expression; ↑ monocyte adhesion |
[79] |
| ↓ GPC-1 expression | ↓ GPC1 expression | ↓ eNOS activity | [6], [80], [81] |
| ↑ GlcNAc N-sulfation | ↑ NDST expression | ↑ Leukocyte adhesion and trafficking Furin attachment for SARS-CoV-2 spike protein preactivation |
[68], [72], [82], [83], [84], [85], [86], [87], [88] |
| ↑ GlcNAc 2-O-sulfation | ↑ HS2ST expression | FGF2-induced angiogenesis Inhibit neutrophil-derived cathelin-related antimicrobial peptide |
[76], [89] |
| ↑ UA 3-O-sulfation | ↑ HS3ST expression | ↑ Antithrombin binding ↑ Antithrombin-mediated anti-inflammation ↑ Herpes simplex viral entry/infectivity Furin attachment for SARS-CoV-2 spike protein preactivation |
[90], [91], [92], [93], [94], [88] |
| ↑ GlcNAc 6-O-sulfation | ↑ HS6ST1-3 expression | FGFR1-mediated angiogenesis VEGF-induced angiogenesis ↑ Leukocyte adhesion |
[95], [96], [95], [72] |
| ↑ GlcNAc 6-O-sulfation | ↓ SULF1 expression | ↓ Post-sepsis leukocyte adhesion | [75] |
| ↓ GlcNAc 6-O-sulfation | ↑ SULF1,2 expression | ↑ Post-myocardial infarction angiogenesis | [77] |
Abbreviations: eNOS, endothelial nitric oxide synthase; FGF, fibroblast growth factor; FGFR, fibroblast growth factor receptor; GlcNAc, glucosamine residue; GPC, glypican; HS2ST, heparan sulfate 2-O-sulfotransferase; HS3ST, heparan sulfate 3-O-sulfotransferase; HS6ST, heparan sulfate 6-O-sulfotransferase; HSPG, heparan sulfate proteoglycan; ICAM, intercellular adhesion molecule; NDST, N-deacetylase/N-sulfotransferase; PI3K, Phosphatidylinositol 3-kinase; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SDC, syndecan; SULF, endosulfatase; UA, hexuronic acid.
Due to the strong anionic charges imparted to the HSPG by HS side chains, the level of HS and/or HSPG expression at the endothelial apical surface is a critical determinant of eGCX, and thereby endothelial, macromolecular permeability (see revised Starling principle above). Thus, factors that interfere with HS and/or HSPG expression within the eGCX may contribute to increased vascular permeability [97]. This principle has been generally demonstrated in models of enzymatic eGCX injury whereby cleavage of endothelial surface HS or HSPG increases paracellular passage of albumin or reduces transendothelial electrical resistance [98], [99], [100]. Although, to our knowledge, the impact of a selective reduction in the expression levels of specific eGCX HSPGs on endothelial permeability remains to be clarified. Additionally, intracellular domains of SDC molecules, particularly SDC-1 [79], are integral in transducing apical shear stress to the actin cytoskeleton and downstream intracellular signaling that promotes competent cell–cell adhesions during homeostatic flow conditions [101]. Xu et al. [102] found that increased albumin movement across monolayers of TNFα-treated human glomerular or mouse renal endothelial cells depended upon activation of RhoA/Rho-associated kinase and myosin light chain kinase signaling that resulted in downstream actin stress fiber formation, increased number of intercellular fenestrae, and greater transendothelial albumin leakage. In light of the fact that TNFα may downregulate SDC-1 expression over the endothelial apical surface [48], it is plausible that TNFα-related endothelial permeability may involve, at least in part, diminished SDC-1 expression that impairs homeostatic endothelial signaling governing the formation of actin stress fibers. This hypothesis is supported by in vivo data in which Voyvodic et al. [79] observed greater vascular permeability in SDC1-/- knockout mice to the inflammatory stimulus by mustard oil relative to wild type mice. However, further investigation is needed to more firmly link alterations in eGCX HSPG expression and the mechanisms regulating vascular permeability.
Reduced HS/HSPG expression and altered HS sulfation within the eGCX may propagate vascular inflammation by increasing endothelial cell-leukocyte interactions and strengthening the adhesion of leukocytes to the endothelial surface. In vitro and in vivo work suggests that reduction in eGCX HS/HSPG expression may expose surface adhesion molecules (e.g., intercellular adhesion molecule-1, vascular cell adhesion molecule-1, platelet endothelial cell adhesion molecule-1, P- and E-selectins) to circulating leukocytes and platelets, thereby immobilizing and activating these cells. Enzymatic heparan sulfate cleavage reduces leukocyte rolling while enhancing leukocyte adhesion at the surface of both cultured human primary abdominal aortic endothelial cells and murine cremasteric venular endothelial cells [2], [103]. Similarly, broad-spectrum inhibition of matrix metalloproteinases reduced leukocyte adhesion to the surface of mesenteric venules in male Wistar rats [104]. Interestingly, Voyvodic et al. [79] demonstrated that loss of SDC1 expression from mouse lung endothelial cells increased ICAM1 mRNA expression that coincided with increased monocyte adhesion to a monolayer of lung endothelial cells from SDC1-/- knockout mice. Together these data suggest that impairment in HS and/or HSPG expression in the eGCX may promote a phenotypic change in the endothelial cell toward proinflammatory signaling. Furthermore, N- and 6-O-sulfation of glucosamine residues in HS have been shown to play a key role in inflammation propagation by mediating leukocyte adhesion to the vascular endothelium (Fig. 3A). Upregulation of NDST1 and HS6ST is observed in a variety of inflammatory conditions [82], [83], and increased expression of N- and 6-O-sulfated HS domains on endothelial cells in vitro results in firmer adhesion of leukocytes to the endothelial monolayer [72], [83]. Similarly, myriad experiments using mice with endothelial-specific NDST1-/- knockout (Ndst1flox/floxTie2Cre+) consistently demonstrate substantial reduction in leukocyte adhesion to the vascular endothelium [82], [84], [85], [86], [87], [105], supporting a proinflammatory role by N-sulfated HS in the eGCX. The role of 6-O-sulfated HS, however, may be more contextually nuanced. Oshima et al. observed that wild type mice recovering from CLP-induced sepsis developed less leukocyte infiltration following intratracheal instillation of lipopolysaccharide (LPS), concurrent with both a reduction in SULF1 expression within isolated pulmonary endothelial cells and an increase in pulmonary levels of HS with 6-O-sulfation [75]. Further, these authors found that pretreating mice recovering from CLP-induced sepsis with recombinant Sulf1 intravenously before intratracheal LPS administration significantly increased pulmonary inflammation, confirming the role of decreased Sulf1 expression in regulating the immunosuppressive phenotype observed in mice following CLP-induced sepsis. Interestingly, the authors did not observe any difference in pulmonary leukocyte recruitment following intratracheal LPS administration to mice with endothelial-specific, conditional SULF1/2-/- knockout (Sulf1flox/floxSulf2flox/floxVEcadCreERT2+), suggesting that the 6-O-sulfation enrichment appears to promote an anti-inflammatory vascular phenotype specifically following sepsis. Moreover, they found that ICAM1 gene expression is attenuated in HLMVECs following SULF1 knockdown with small interfering RNA. Together, these data point to a complex interaction between the inflammatory milieu and the vascular endothelium by which inflammatory mediators influence the expression of specific enzymes regulating HSPG sulfation that, in turn, direct the endothelial cell’s interaction with circulating leukocytes.
Fig. 3.
Cartoon thematically depicting the impact of sulfation on heparan sulfate (HS)-mediated leukocyte trafficking, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infectivity, antithrombin activity, and growth factor signaling at the luminal surface of the vascular endothelium. A) Inflammatory cytokines promote upregulation of N-deacetylase/N-sulfotransferase (NDST) and HS 6-O-sulfotransferase (HS6ST) activity within the Golgi of endothelial cells. This results in increased endothelial glycocalyx (eGCX) expression of proteoglycan HS side chains with N- and 6-O-sulfation of N-acetyl glucosamine residues (represented by a green ring in inset; blue ring represents the hexuronic acid), respectively, that support leukocyte adhesion and transmigration. B) Furin preactivation of SARS-CoV-2 virion spike proteins (represented by the change in spike protein color to yellow) is enhanced by interaction with eGCX HS molecules that are enriched in N- and 3-O-sulfation by NDST and HS 3-O-sulfatotransferases (HS3ST). Following furin preactivation, HS molecules further support virion spike protein adherence to surface angiotensin-converting enzyme 2 (ACE2) receptors that is essential for cell infectivity. C) Antithrombin binding to 3-O-sulfated HS moieties within the eGCX may promote a conformational change that increases its efficiency to inactive thrombin, thereby contributing to coagulation regulation at the endothelial surface. Further, antithrombin binding to 3-O-sulfated HS side chains in the endothelial glycocalyx promotes homeostatic, anti-inflammatory endothelial cell signaling. D) The eGCX harbors fibroblast growth factor 2 (FGF2) near HS regions enriched in 2-O-sulfation by HS 2-O-sulfotransferase (HS2ST) [155]. Moreover, eGCX HS side chains enriched in 6-O-sulfation by HS 6-O-sulfotransferase (HS6ST) synergize cognate receptor binding with FGF2 (and other FGF variants) and vascular endothelial growth factor (VEGF) at the endothelial surface with putative effects on angiogenesis and vascular permeability. For simplicity, the proteoglycan depicted in all panels is syndecan; however, it is currently unknown how sulfation varies within the HS side chains of specific proteoglycans within the eGCX. Figure generated using Biorender. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Endothelial surface HSPGs may contribute to the pathogenesis and systemic manifestations of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for the coronavirus disease 2019 (COVID-19) pandemic (Fig. 3B). It is now well-established that SARS-CoV-2 infectivity occurs through virion spike protein binding to angiotensin-converting enzyme 2 (ACE2) receptors over the surface of host cells with spike protein activation occurring through the protease activity of the host enzyme furin [106]. HS appears to be integrally involved in each of these steps of host cell infection. Targosz-Korecka et al. [107] demonstrated that SARS-CoV-2 spike proteins bind tightly to the surface of human primary pulmonary artery endothelial cells through interactions with eGCX HS. Clausen et al. [108] corroborated this finding, demonstrating that binding of SARS-CoV-2 virion spike protein to eGCX HS promotes a conformational change in the spike protein receptor binding domain that enhances spike protein interactions with and anchoring to the endothelial surface ACE2 receptor. Moreover, furin availability for SARS-CoV-2 spike protein preactivation may depend on the presence of eGCX HS, specifically HS molecules rich in N- and 3-O-sulfation [88]. Notably, advanced age and underlying co-morbidities (e.g., obesity, hypertension) are considerable risk factors for severe SARS-CoV-2 infection [109]. However, these risk factors for worse SARS-CoV-2 are also associated with thinning of the eGCX [110], [111], which would be predicted to result in reduced furin and HS availability to support spike protein interactions with ACE2 receptors and, therefore, less viral infectivity. Though it is possible that a less robust eGCX may permit greater interactions between the SARS-CoV-2 virion and host endothelium and epithelium, making those with advanced age and cardiovascular co-morbidities more vulnerable to SARS-CoV-2 infection with worse disease severity, further research is needed to resolve the more granular mechanisms (including the role of eGCX HSPGs) that make these particular populations more susceptible to COVID-19.
HSPGs within the eGCX have traditionally been considered a key contributor to the antithrombotic phenotype of the vascular endothelial luminal surface by harboring and accelerating the activity of hemostatic regulators such as antithrombin, heparin cofactor II, thrombomodulin, and tissue factor pathway inhibitor [112], [113], [114]. Antithrombin activation, in particular, is historically thought to rely on 3-O-sulfation of HS glucosamine residues for eGCX binding and activation [41], [90] (Fig. 3C). In support of this notion, HajMohammadi et al. [115] observed that HS3ST1 overexpression in CHO4B cells, which lack constitutive HS3ST1 expression and basal thrombin inhibition, significantly elevated the level of thrombin inhibition to that of mouse cardiac microvascular endothelial cells. Moreover, Weiss et al. [116] demonstrated that knockdown of zinc finger transcriptional protein ZNF263, a repressor of HS3ST1 expression, from HeLa cells resulted in a significant increase in cell surface antithrombin binding and accelerated factor Xa inhibition. However, further investigations and endothelial-specific studies have challenged the anti-coagulation paradigm of antithrombin-HS interactions. Richard et al. [117] demonstrated that, though removal of 3-O-sulfo groups from heparin (a highly sulfated heparan sulfate analog) substantially reduced the affinity of antithrombin for heparin, antithrombin bound to 3-O-desulfated heparin retained the same degree of reactivity toward factor Xa. HajMohammadi et al. similarly observed that although HS3ST1-/- knockout mice exhibit reduced antithrombin binding to the vascular endothelial surface, they do not have increased vascular fibrin deposition at baseline or following global hypoxia (to incite tissue factor release) nor do they exhibit a propensity for thrombosis after local endothelial surface injury by ferric chloride [115]. Further, the authors found that HS3ST1 overexpression in mouse primary cardiac microvascular endothelial cells did not result in higher levels of thrombin inhibition. Interestingly, they also observed that HS molecules isolated from tissues in HS3ST1-/- mice retained 2–25 % residual anti-Xa activity in the presence of antithrombin, which may suggest that other 3-O-sulfotransferase isoforms contribute to HS 3-O-sulfation that supports antithrombin activity at the endothelial surface. Taken together, these data do not conclusively demonstrate a role for GAG 3-O-sulfation in antithrombin activation but instead support a need for further investigation into this elusive area of endothelial biology.
Though the impact of eGCX HSPG on the anticoagulant property of antithrombin remains an area of controversy, studies consistently demonstrate that antithrombin interactions with 3-O-sulfated HS of eGCX HSPGs play a central role in suppressing vascular inflammation [118] (Fig. 3C). Shworak and colleagues [93] observed that antithrombin administration significantly reduced mortality in LPS-treated wild type mice but had no impact on mortality in LPS-treated HS3ST1-/- mice. Moreover, antithrombin treatment reduced leukocyte adhesion to the endothelium of cremasteric venules in LPS-treated wild type mice but surprisingly increased leukocyte adhesion in LPS-treated HS3ST1-/- mice [119]. Finally, in a large cohort of 2,144 adult cardiology patients, Shworak et al. discovered that patients with worse coronary artery disease demonstrated a greater preponderance of homozygosity for the rs16881446G recessive allele, a single nucleotide polymorphism found within the human HS3ST1 intron [119]. Work by Ma et al. [120] corroborates the essential role that 3-O-sulfated HS plays in promoting antithrombin’s endothelial anti-inflammatory activity. Using Ea.hy926 endothelial cells, these authors found that HS3ST1 knockdown significantly diminished antithrombin’s ability to preserve cell–cell junctional integrity in the face of LPS treatment by reducing antithrombin-HS-mediated 5′ adenosine monophosphate-activated protein kinase activation that, in turn, promoted c-Jun N-terminal kinase proinflammatory signaling. Interestingly, Smits et al. demonstrated that 3-O-sulfated HS is most highly expressed in vivo at arterial branch points and vascular regions exposed to high shear stress [93], areas of the vascular tree with known susceptibility to atherosclerotic plaque formation. As endothelial expression of 3-O-sulfated HS motifs may decline with advancing age [121], these data implicate a potential contribution of waning antithrombin-HS-mediated protective endothelial cell signaling in age-associated cardiovascular disease.
eGCX HSPGs are instrumental in transducing luminal shear stress to endothelial cell signaling axes that govern vascular smooth muscle tonicity, in particular through eNOS activation and downstream nitric oxide production [39], [40]. Therefore, reduced expression of HSPGs at the endothelial apical surface may impair homeostatic vasoregulation, culminating in tissue hypoperfusion. Loss of SDC-1 expression in statically cultured murine lung endothelial cells was shown to reduce phosphatidylinositol-3 kinase/protein kinase B signaling activity [79], a commonly accepted upstream promoter of eNOS activation [122]. Conversely, Ebong et al. [6] observed no reduction in eNOS phosphorylation in bovine aortic endothelial cells conditioned with shear stress (15 dynes/cm2, 24 h) following SDC-1 knockdown. Arguably, this in vitro work under flow conditions more convincingly suggests that SDC-1 does not have a role in shear stress activation of eNOS; however, differences in endothelial cell type must be considered and systematically evaluated. Ebong et al. also found that GPC-1 knockdown in bovine aortic endothelial cells returned eNOS phosphorylation to levels of statically cultured wild type cells [6]. Similar findings were demonstrated in murine aortic endothelial cells and HUVECs [70], pointing to a more central role for GPC-1 in the mechanism driving flow-induced eNOS phosphorylation. In light of prior work implicating that platelet endothelial cell adhesion molecule-1 (PECAM-1) is involved in the process of shear stress-induced eNOS activation [123], [124], Bartosch et al. [81] advanced our understanding of the role GPC-1 has in shear stress-induced eNOS signaling by investigating the interaction between GPC-1 and PECAM-1 as a cooperative linchpin in this complex cascade. Through a series of elegant experiments combining in vitro work with atomic force microscopy and in vivo studies with GPC1-/- knockout mice, the authors demonstrated that PECAM-1 serves as an essential linkage between GPC-1-mediated shear sensing and intracellular eNOS activation [81]. While the specific molecular interactions between GPC-1 and PECAM-1 remain to be clarified, this important study sheds insight into the complexity by which HSPGs contribute to endothelial mechanosignaling. Further, it is logical to conjecture that any disease or biological process that impairs endothelial cell expression of GPC-1 may attenuate eNOS activity and thus compromise homeostatic vasoregulation. In fact, the same laboratory that linked GPC-1 to PECAM-1-mediated eNOS activation demonstrated that HUVECs cultured on stiffer substrates (thereby mimicking arteriosclerosis) attenuated GPC1 mRNA and GPC-1 protein expression while simultaneously suppressing eNOS phosphorylation to levels found in HUVECs cultured on softer substrate after GPC1 knockdown [70]. Thus, processes such as arteriosclerosis or advancing age may suppress GPC-1 expression within the eGCX that, in turn, may culminate in impaired vasoregulation by reducing nitric oxide signaling.
Finally, sulfation modifications to eGCX HSPG are instrumental in regulating growth factor interactions with endothelial apical surface cognate receptors that direct vascular angiogenesis (Fig. 3D). Li et al. observed that incubation of HUVECs or mouse primary cardiac microvascular endothelial cells in a hypoxic environment increased HS2ST expression and total HS GAG expression in both cell types [76]. They also observed that co-treatment with FGF2, a potent instigator of angiogenesis [125], in the setting of hypoxia resulted in substantially greater cell replication of both cell types as compared to FGF2 treatment alone. Together, these findings suggest that hypoxia increases expression of 2-O sulfation within eGCX HSPGs, an HS motif to which FGF2 has known affinity [126], [127], thus priming the endothelial surface for proangiogenic FGF signaling. Furthermore, the presence of 6-O sulfation within eGCX HS side chains appears to be essential for FGFR1-mediated angiogenesis by strengthening the formation of the endothelial surface FGF2-HS-FGFR1 ternary complex [95], [96]. Prior work using surface plasmon resonance suggests that HS 6-O-sulfation significantly enhances recombinant human VEGF165 binding to its endothelial surface cognate VEGF receptor 2 [128]. Ferreras et al. [95] observed that loss of 6-O sulfation in HS in HUVECs through HS6ST1 and HS6ST2 silencing resulted in substantial reduction of endothelial cell sprouting and tube formation in response to VEGF165. However, surprisingly Korf-Klingebiel et al. found that reduced SULF1 expression in HUVECs, which would presumably increase 6-O-sulfo group expression in eGCX HS, abrogated endothelial recovery from scratch injury in response to VEGF165 [77]. Taken together, these data suggest that HS interactions with VEGF signaling are perhaps more complicated than initially thought, prompting a call for further investigation into other potential signaling contributors (e.g., transglutaminase 2 [129]).
Therapeutic strategies and future directions
In light of recent discoveries that have identified the eGCX as a valuable therapeutic target in ameliorating aberrant vascular biology, investigators have begun to explore strategies to promote HSPG synthesis or remodeling within the eGCX. Many of these strategies utilize mimetics of HS or other eGCX components to reconstitute the eGCX following erosion, while others involve targeting pathways of HSPG biosynthesis. Moreover, approaches to fine-tune the sulfation properties of HSPGs have also emerged as potential strategies for controlling eGCX interactions with circulating cells and proteins that have important influence over mechanisms regulating endothelial cell function. In this section, we provide an overview of the most recent and promising strategies that bolster the expression HSPGs to reform a robust, healthy eGCX.
Promotion of HSPG expression
Many preclinical studies have demonstrated therapeutic benefits for abrogating endothelial dysfunction following eGCX erosion using treatments that promote HSPG expression at the endothelial cell surface. Recently, the HS mimetic fucoidan was shown to restore eGCX expression in HLMVECs following treatment with plasma from critically ill COVID19 patients. Fucoidan treatment and eGCX recovery corresponded with a reduction in endothelial activation, improvement in barrier function and restoration of an anticoagulant cell surface [130]. Sulodexide is a commercially-available, purified mixture of natural GAGs consisting of 80 % HS and 20 % dermatan sulfate that has shown promise for aiding eGCX reconstitution in preclinical models of sepsis [131] and vascular injury [132] and in patients with type 2 diabetes mellitus [133] and peripheral vascular disease [134], [135]. Notably, sulodexide inhibits the activity of heparanase-1, the only known mammalian endoglucuronidase with activity on eGCX HS [136], and is itself resistant to enzymatic degradation, indicating that sulodexide may both reconstitute the eGCX and prevent ongoing enzymatic HS degradation. Sulodexide has also been shown to prevent LPS-induced eGCX damage in brain microvascular endothelial cells and to improve eGCX integrity, vascular permeability and mortality following induction of sepsis in mice [131]. Recently, sulodexide treatment in patients with early stage COVID-19 was associated with attenuated levels of C-reactive protein (i.e., less systemic inflammation) and d-dimer (i.e., less thrombus burden), decreased need for supplemental oxygen, and reduced the need for hospitalization [137]. EC-SEAL is another GAG mimetic that consists of selectin-binding peptides attached to a dermatan sulfate backbone that is designed to bind selectins on injured endothelium to prevent leukocyte and platelet binding. EC-SEAL has been shown to prevent platelet [138] and leukocyte [139] binding to activated endothelial cells in vitro and to reduce thrombus formation in vivo following ligation of the inferior vena cava in mice [138].
Administration of fresh frozen plasma (FFP) is gaining attention as a first-line therapeutic strategy against eGCX damage caused by trauma-hemorrhagic shock [140], [141] and burn injuries [142]. In rodent models of severe hemorrhage, administration of FFP results in significantly less eGCX damage compared to administration of crystalloid solutions like lactated Ringer’s solution [143], [144]. Partial restoration of SDC-1 expression was demonstrated following hemodynamic stabilization with FFP via mechanisms related to both the antithrombin [145] and fibrinogen [146] components of plasma. Furthermore, albumin, a key component of FFP, has exceptional binding to the eGCX and has been shown to reinforce eGCX barrier function by contributing to steric hindrance and electrostatic repulsion of circulating proteins and cells [147]. Moreover, albumin’s restorative effects on the eGCX may also be related to its role in delivering sphingosine-1-phosphate to the endothelium, which stabilizes the eGCX by both inhibiting SDC-1 shedding and promoting SDC-1 synthesis [148], [149], [150].
Targeting cellular pathways that regulate HSPG biosynthesis also holds promise for enhancing eGCX reconstitution following insult. For example, EXT1 expression and eGCX repair after heparinase-mediated cleavage is mediated by FGFR1 signaling in the pulmonary endothelium of mice, and impairments in FGFR1 signaling are associated with delayed eGCX recovery [63]. Thus, FGFR1-promoting therapies (e.g., FGF2, sphingosine-1-phosphate) are likely candidates to facilitate EXT1 expression and HS biosynthesis to promote eGCX reconstitution following pathologic damage.
Nutraceuticals have also been employed by researchers as an alternative therapeutic approach for promoting eGCX expression. Arterosil® and EndocalyxTM are two commercially available nutraceutical products marketed to enhance the integrity of the vascular eGCX. The major component of Arterosil®, rhamnan sulfate extracted from algae, has been shown to enhance HS expression on endothelial cell monolayers and prevent the permeability of low density lipoprotein [151]. EndocalyxTM contains a unique blend of HS-mimicking polysaccharides and GlcNAc (for HS synthesis) along with hyaluronan and antioxidants. EndocalyxTM was shown to improve age-related deficits in eGCX integrity, restore vascular barrier function and increase nitric oxide bioavailability in aged mice [152]. A clinical trial is currently underway to establish the efficacy of EndocalyxTM in patients with type 2 diabetes mellitus complicated by albuminuria [153]. Though additional therapeutic applications for nutraceuticals remain to be tested, their use in the treatment approach for acute manifestations of eGCX injury, as seen in traumatic injury or sepsis [37], [154], is rational.
Tailoring HSPG sulfation
Given the importance of HSPG structural modifications in regulating pathologic endothelial functions, therapies that control HS sulfation could be used to fine-tune the functional properties of a damaged or freshly reconstituted eGCX layer. As a proof of concept to support this approach, Korf-Klingebiel et al. used adenoviral transfections to overexpress SULF1 and SULF2 in cardiac tissue prior to myocardial infarction [77]. Endosulfatase overexpression corresponded with decreased HS sulfation within the infarcted tissue leading to less VEGF binding to HS. Functionally, the attenuated interactions between VEGF and HS resulted in enhanced capillarization of the infarct border zone, reduced scarring and improved left ventricular systolic function. In a second approach, the small molecule and HS-antagonist surfen was used to block HS interactions with protein ligands following myocardial infarction. Similar to overexpressing sulfatases, surfen-treated mice showed decreased VEGF binding to HS resulting in reduced scar formation and enhanced cardiac function [77]. Although these strategies did not specifically target the vascular endothelium, this work does establish a premise for modulating HSPG sulfation patterns as a therapeutic strategy to improve pathologic outcomes.
Conclusions
Our understanding of the vital role of the eGCX in vascular health and disease has grown immensely in recent years. The eGCX not only acts as a physical barrier to protect the underlying endothelial surface, but it also actively participates in the homeostatic mechanisms that regulate vascular patency, inflammation, and coagulation. Disruption of the eGCX by activated sheddases, reduced HSPG expression and modulation of HS sulfation can have local and systemic consequences on endothelial mechanisms that drive pathologic processes for a wide variety of conditions. Although there is compelling evidence from in vitro studies and animal work to suggest that strategies to promote eGCX synthesis or remodeling will improve or tailor vascular functions, for the most part, the efficacy of these therapies for treating vasculopathy in humans remains elusive. Considering the progress in the field, we anticipate significant translational developments of therapies aimed at eGCX restoration and remodeling to rapidly evolve.
CRediT authorship contribution statement
Danielle Pretorius: Writing – original draft. Robert P. Richter: Conceptualization, Writing – review & editing. Tanya Anand: Writing – review & editing. Jessica C. Cardenas: Writing – review & editing. Jillian R. Richter: Conceptualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank the University of Alabama at Birmingham Nanostring Laboratory for the nCounter technical and data processing support. This work was supported in part by the National Institutes of Health grants (NIGMS) K08 GM144788-01 to RPR, R35 GM146859-01 to JCC, and R35 GM137958-02 to JRR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Data availability
Data will be made available on request.
References
- 1.Hu X., Weinbaum S. A new view of Starling's hypothesis at the microstructural level. Microvasc. Res. 1999;58(3):281–304. doi: 10.1006/mvre.1999.2177. [DOI] [PubMed] [Google Scholar]
- 2.Constantinescu A.A., Vink H., Spaan J.A. Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler. Thromb. Vasc. Biol. 2003;23(9):1541–1547. doi: 10.1161/01.ATV.0000085630.24353.3D. [DOI] [PubMed] [Google Scholar]
- 3.Bernfield M., Kokenyesi R., Kato M., Hinkes M.T., Spring J., Gallo R.L., Lose E.J. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu. Rev. Cell. Biol. 1992;8(1):365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg R.D., Shworak N.W., Liu J., Schwartz J.J., Zhang L. Heparan sulfate proteoglycans of the cardiovascular system. Specific structures emerge but how is synthesis regulated? J. Clin. Invest. 1997;99(9):2062–2070. doi: 10.1172/JCI119377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinbaum S., Tarbell J.M., Damiano E.R. The structure and function of the endothelial glycocalyx layer. Annu. Rev. Biomed. Eng. 2007;9(1):121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 6.Ebong E.E., Lopez-Quintero S.V., Rizzo V., Spray D.C., Tarbell J.M. Shear-induced endothelial NOS activation and remodeling via heparan sulfate, glypican-1, and syndecan-1. Integr. Biol. (Camb.) 2014;6(3):338–347. doi: 10.1039/c3ib40199e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halden Y., Rek A., Atzenhofer W., Szilak L., Wabnig A., Kungl A.J. Interleukin-8 binds to syndecan-2 on human endothelial cells. Biochem. J. 2004;377(Pt 2):533–538. doi: 10.1042/BJ20030729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jannaway M., Yang X., Meegan J.E., Coleman D.C., Yuan S.Y., Komarova Y. Thrombin-cleaved syndecan-3/-4 ectodomain fragments mediate endothelial barrier dysfunction. PLoS One. 2019;14(5):e0214737. doi: 10.1371/journal.pone.0214737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tkachenko E., Rhodes J.M., Simons M. Syndecans: new kids on the signaling block. Circ. Res. 2005;96(5):488–500. doi: 10.1161/01.RES.0000159708.71142.c8. [DOI] [PubMed] [Google Scholar]
- 10.van Deurs B., Roepstorff K., Hommelgaard A.M., Sandvig K. Caveolae: anchored, multifunctional platforms in the lipid ocean. Trends Cell Biol. 2003;13(2):92–100. doi: 10.1016/s0962-8924(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 11.Zeng, Y., Waters, M., Andrews, A., Honarmandi, P., Ebong, E.E., Rizzo, V., et al., (2013). Fluid shear stress induces the clustering of heparan sulfate via mobility of glypican-1 in lipid rafts. Am. J. Physiol. Heart Circ. Physiol.,305 (6), H811-820. [DOI] [PMC free article] [PubMed]
- 12.Sezgin E., Levental I., Mayor S., Eggeling C. The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017;18(6):361–374. doi: 10.1038/nrm.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harvey R.D., Calaghan S.C. Caveolae create local signalling domains through their distinct protein content, lipid profile and morphology. J. Mol. Cell. Cardiol. 2012;52(2):366–375. doi: 10.1016/j.yjmcc.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernfield M., Götte M., Park P.W., Reizes O., Fitzgerald M.L., Lincecum J., Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 1999;68(1):729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 15.Filmus J., Capurro M., Rast J. Glypicans. Genome Biol. 2008;9(5):224. doi: 10.1186/gb-2008-9-5-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prydz K., Dalen K.T. Synthesis and sorting of proteoglycans. J. Cell Sci. 2000;113(Pt 2):193–205. doi: 10.1242/jcs.113.2.193. [DOI] [PubMed] [Google Scholar]
- 17.Multhaupt H.A., Couchman J.R. Heparan sulfate biosynthesis: methods for investigation of the heparanosome. J. Histochem. Cytochem. 2012;60(12):908–915. doi: 10.1369/0022155412460056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreuger J., Kjellen L. Heparan sulfate biosynthesis: regulation and variability. J. Histochem. Cytochem. 2012;60(12):898–907. doi: 10.1369/0022155412464972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Busse-Wicher M., Wicher K.B., Kusche-Gullberg M. The exostosin family: proteins with many functions. Matrix Biol. 2014;35:25–33. doi: 10.1016/j.matbio.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Mulloy B., Forster M.J., Jones C., Davies D.B. N.m.r. and molecular-modelling studies of the solution conformation of heparin. Biochem. J. 1993;293(Pt 3):849–858. doi: 10.1042/bj2930849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L. Glycosaminoglycan (GAG) biosynthesis and GAG-binding proteins. Prog. Mol. Biol. Transl. Sci. 2010;93:1–17. doi: 10.1016/S1877-1173(10)93001-9. [DOI] [PubMed] [Google Scholar]
- 22.Denys A., Allain F. The emerging roles of heparan sulfate 3-o-sulfotransferases in cancer. Front. Oncol. 2019;9:507. doi: 10.3389/fonc.2019.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stace T.M., Damiano E.R. An electrochemical model of the transport of charged molecules through the capillary glycocalyx. Biophys. J. 2001;80(4):1670–1690. doi: 10.1016/S0006-3495(01)76139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haaren, P.M.A.v., VanBavel, E., Vink, H., Spaan, J.A.E., (2005). Charge modification of the endothelial surface layer modulates the permeability barrier of isolated rat mesenteric small arteries. Am. J. Physiol. Heart Circ. Physiol.,289 (6), H2503-H2507. [DOI] [PubMed]
- 25.Mulivor, A.W., Lipowsky, H.H., (2002). Role of glycocalyx in leukocyte-endothelial cell adhesion. Am. J. Physiol. Heart Circ. Physiol.,283 (4), H1282-1291. [DOI] [PubMed]
- 26.Yayon A., Klagsbrun M., Esko J.D., Leder P., Ornitz D.M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- 27.Kan M., Wu X., Wang F., McKeehan W.L. Specificity for fibroblast growth factors determined by heparan sulfate in a binary complex with the receptor kinase. J. Biol. Chem. 1999;274(22):15947–15952. doi: 10.1074/jbc.274.22.15947. [DOI] [PubMed] [Google Scholar]
- 28.Chang Z., Meyer K., Rapraeger A.C., Friedl A. Differential ability of heparan sulfate proteoglycans to assemble the fibroblast growth factor receptor complex in situ. FASEB J. 2000;14(1):137–144. doi: 10.1096/fasebj.14.1.137. [DOI] [PubMed] [Google Scholar]
- 29.Robinson C.J., Stringer S.E. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J. Cell Sci. 2001;114(Pt 5):853–865. doi: 10.1242/jcs.114.5.853. [DOI] [PubMed] [Google Scholar]
- 30.Grunewald F.S., Prota A.E., Giese A., Ballmer-Hofer K. Structure-function analysis of VEGF receptor activation and the role of coreceptors in angiogenic signaling. Biochim. Biophys. Acta. 2010;1804(3):567–580. doi: 10.1016/j.bbapap.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Xu D., Fuster M.M., Lawrence R., Esko J.D. Heparan sulfate regulates VEGF165- and VEGF121-mediated vascular hyperpermeability. J. Biol. Chem. 2011;286(1):737–745. doi: 10.1074/jbc.M110.177006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinbaum S. 1997 Whitaker Distinguished Lecture: Models to solve mysteries in biomechanics at the cellular level; a new view of fiber matrix layers. Ann. Biomed. Eng. 1998;26(4):627–643. doi: 10.1114/1.134. [DOI] [PubMed] [Google Scholar]
- 33.Rehm M., Zahler S., Lötsch M., Welsch U., Conzen P., Jacob M., et al. Endothelial glycocalyx as an additional barrier determining extravasation of 6% hydroxyethyl starch or 5% albumin solutions in the coronary vascular bed. Anesthesiology. 2004;100(5):1211–1223. doi: 10.1097/00000542-200405000-00025. [DOI] [PubMed] [Google Scholar]
- 34.Jacob M., Bruegger D., Rehm M., Welsch U., Conzen P., Becker B.F. Contrasting effects of colloid and crystalloid resuscitation fluids on cardiac vascular permeability. Anesthesiology. 2006;104(6):1223–1231. doi: 10.1097/00000542-200606000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Hahn R.G., Dull R.O., Zdolsek J. The extended starling principle needs clinical validation. Acta Anaesthesiol. Scand. 2020;64(7):884–887. doi: 10.1111/aas.13593. [DOI] [PubMed] [Google Scholar]
- 36.Thi M.M., Tarbell J.M., Weinbaum S., Spray D.C. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a “bumper-car” model. Proc. Natl. Acad. Sci. U.S.A. 2004;101(47):16483–16488. doi: 10.1073/pnas.0407474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richter R.P., Ashtekar A.R., Zheng L., Pretorius D., Kaushlendra T., Sanderson R.D., Gaggar A., Richter J.R. Glycocalyx heparan sulfate cleavage promotes endothelial cell angiopoietin-2 expression by impairing shear stress-related AMPK/FoxO1 signaling. JCI. Insight. 2022;7(15) doi: 10.1172/jci.insight.155010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang G., Kostidis S., Tiemeier G.L., Sol W.M.P.J., de Vries M.R., Giera M., Carmeliet P., van den Berg B.M., Rabelink T.J. Shear stress regulation of endothelial glycocalyx structure is determined by glucobiosynthesis. Arterioscler. Thromb. Vasc. Biol. 2020;40(2):350–364. doi: 10.1161/ATVBAHA.119.313399. [DOI] [PubMed] [Google Scholar]
- 39.Florian, J.A., Kosky, J.R., Ainslie, K., Pang, Z., Dull, R.O., Tarbell, J.M., (2003). Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ. Res.,93 (10), e136-142. [DOI] [PubMed]
- 40.Pahakis M.Y., Kosky J.R., Dull R.O., Tarbell J.M. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem. Biophys. Res. Commun. 2007;355(1):228–233. doi: 10.1016/j.bbrc.2007.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Agostini A.I., Watkins S.C., Slayter H.S., Youssoufian H., Rosenberg R.D. Localization of anticoagulantly active heparan sulfate proteoglycans in vascular endothelium: antithrombin binding on cultured endothelial cells and perfused rat aorta. J. Cell Biol. 1990;111(3):1293–1304. doi: 10.1083/jcb.111.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez E.G., Ostrowski S.R., Cardenas J.C., Baer L.A., Tomasek J.S., Henriksen H.H., Stensballe J., Cotton B.A., Holcomb J.B., Johansson P.I., Wade C.E. Syndecan-1: A quantitative marker for the endotheliopathy of trauma. J. Am. Coll. Surg. 2017;225(3):419–427. doi: 10.1016/j.jamcollsurg.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Hippensteel J.A., Uchimido R., Tyler P.D., Burke R.C., Han X., Zhang F., McMurtry S.A., Colbert J.F., Lindsell C.J., Angus D.C., Kellum J.A., Yealy D.M., Linhardt R.J., Shapiro N.I., Schmidt E.P. Intravenous fluid resuscitation is associated with septic endothelial glycocalyx degradation. Crit. Care. 2019;23(1) doi: 10.1186/s13054-019-2534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang D., Li L., Chen Y.u., Ma J., Yang Y., Aodeng S., Cui Q., Wen K., Xiao M., Xie J., Xu Y., Li Y. Syndecan-1, an indicator of endothelial glycocalyx degradation, predicts outcome of patients admitted to an ICU with COVID-19. Mol. Med. 2021;27(1) doi: 10.1186/s10020-021-00412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Becker B.F., Jacob M., Leipert S., Salmon A.H., Chappell D. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. Br. J. Clin. Pharmacol. 2015;80(3):389–402. doi: 10.1111/bcp.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sullivan R.C., Rockstrom M.D., Schmidt E.P., Hippensteel J.A. Endothelial glycocalyx degradation during sepsis: Causes and consequences. Matrix. Biol. Plus. 2021;12 doi: 10.1016/j.mbplus.2021.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patterson E.K., Cepinskas G., Fraser D.D. Endothelial glycocalyx degradation in critical illness and injury. Front. Med. 2022;9 doi: 10.3389/fmed.2022.898592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kainulainen V., Nelimarkka L., Jarvelainen H., Laato M., Jalkanen M., Elenius K. Suppression of syndecan-1 expression in endothelial cells by tumor necrosis factor-alpha. J. Biol. Chem. 1996;271(31):18759–18766. doi: 10.1074/jbc.271.31.18759. [DOI] [PubMed] [Google Scholar]
- 49.Vuong T.T., Reine T.M., Sudworth A., Jenssen T.G., Kolset S.O. Syndecan-4 is a major syndecan in primary human endothelial cells in vitro, modulated by inflammatory stimuli and involved in wound healing. J. Histochem. Cytochem. 2015;63(4):280–292. doi: 10.1369/0022155415568995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hara T., Yabushita S., Yamamoto C., Kaji T. Cell density-dependent fibroblast growth factor-2 signaling regulates syndecan-4 expression in cultured vascular endothelial cells. Int. J. Mol. Sci. 2020;21(10):3698. doi: 10.3390/ijms21103698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hara T., Yoshida E., Fujiwara Y., Yamamoto C., Kaji T. Transforming growth factor-beta1 modulates the expression of syndecan-4 in cultured vascular endothelial cells in a biphasic manner. J. Cell. Biochem. 2017;118(8):2009–2017. doi: 10.1002/jcb.25861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gallo R.L., Ono M., Povsic T., Page C., Eriksson E., Klagsbrun M., Bernfield M. Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc. Natl. Acad. Sci. U.S.A. 1994;91(23):11035–11039. doi: 10.1073/pnas.91.23.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rajavashisth T.B., Xu X.-P., Jovinge S., Meisel S., Xu X.-O., Chai N.-N., Fishbein M.C., Kaul S., Cercek B., Sharifi B., Shah P.K. Membrane type 1 matrix metalloproteinase expression in human atherosclerotic plaques: evidence for activation by proinflammatory mediators. Circulation. 1999;99(24):3103–3109. doi: 10.1161/01.cir.99.24.3103. [DOI] [PubMed] [Google Scholar]
- 54.Liu P.-L., Tsai J.-R., Hwang J.-J., Chou S.-H., Cheng Y.-J., Lin F.-Y., Chen Y.-L., Hung C.-Y., Chen W.-C., Chen Y.-H., Chong I.-W. High-mobility group box 1-mediated matrix metalloproteinase-9 expression in non-small cell lung cancer contributes to tumor cell invasiveness. Am. J. Respir. Cell Mol. Biol. 2010;43(5):530–538. doi: 10.1165/rcmb.2009-0269OC. [DOI] [PubMed] [Google Scholar]
- 55.Kothari P., Pestana R., Mesraoua R., Elchaki R., Khan K.M.F., Dannenberg A.J., Falcone D.J. IL-6-mediated induction of matrix metalloproteinase-9 is modulated by JAK-dependent IL-10 expression in macrophages. J. Immunol. 2014;192(1):349–357. doi: 10.4049/jimmunol.1301906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zou Z., Li L.i., Li Q., Zhao P., Zhang K., Liu C., Cai D., Maegele M., Gu Z., Huang Q. The role of S100B/RAGE-enhanced ADAM17 activation in endothelial glycocalyx shedding after traumatic brain injury. J. Neuroinflammation. 2022;19(1) doi: 10.1186/s12974-022-02412-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hara T., Sato A., Yamamoto C., Kaji T. Syndecan-1 downregulates syndecan-4 expression by suppressing the ERK1/2 and p38 MAPK signaling pathways in cultured vascular endothelial cells. Biochem. Biophys. Rep. 2021;26 doi: 10.1016/j.bbrep.2021.101001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu F., Wang J.Y., Chao W., Sims C., Kozar R.A. miR-19b targets pulmonary endothelial syndecan-1 following hemorrhagic shock. Sci. Rep. 2020;10(1):15811. doi: 10.1038/s41598-020-73021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chistiakov D.A., Orekhov A.N., Bobryshev Y.V. The role of miR-126 in embryonic angiogenesis, adult vascular homeostasis, and vascular repair and its alterations in atherosclerotic disease. J. Mol. Cell. Cardiol. 2016;97:47–55. doi: 10.1016/j.yjmcc.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 60.Wang H.F., Wang Y.Q., Dou L., Gao H.M., Wang B., Luo N., et al. Influences of up-regulation of miR-126 on septic inflammation and prognosis through AKT/Rac1 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019;23(5):2132–2138. doi: 10.26355/eurrev_201903_17257. [DOI] [PubMed] [Google Scholar]
- 61.Mondadori dos Santos A., Metzinger L., Haddad O., M'Baya-Moutoula E., Taibi F., Charnaux N., et al. miR-126 is involved in vascular remodeling under laminar shear stress. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/497280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chamorro-Jorganes A., Araldi E., Rotllan N., Cirera-Salinas D., Suarez Y. Autoregulation of glypican-1 by intronic microRNA-149 fine tunes the angiogenic response to FGF2 in human endothelial cells. J. Cell Sci. 2014;127(Pt 6):1169–1178. doi: 10.1242/jcs.130518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Y., Haeger S.M., Suflita M.A., Zhang F., Dailey K.L., Colbert J.F., Ford J.A., Picon M.A., Stearman R.S., Lin L., Liu X., Han X., Linhardt R.J., Schmidt E.P. Fibroblast growth factor signaling mediates pulmonary endothelial glycocalyx reconstitution. Am. J. Respir. Cell Mol. Biol. 2017;56(6):727–737. doi: 10.1165/rcmb.2016-0338OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeng Y.e., Tarbell J.M., Vinci M.C. The adaptive remodeling of endothelial glycocalyx in response to fluid shear stress. PLoS One. 2014;9(1):e86249. doi: 10.1371/journal.pone.0086249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harding I.C., Mitra R., Mensah S.A., Herman I.M., Ebong E.E. Pro-atherosclerotic disturbed flow disrupts caveolin-1 expression, localization, and function via glycocalyx degradation. J. Transl. Med. 2018;16(1):364. doi: 10.1186/s12967-018-1721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Russo T.A., Banuth A.M.M., Nader H.B., Dreyfuss J.L., Pintus G. Altered shear stress on endothelial cells leads to remodeling of extracellular matrix and induction of angiogenesis. PLoS One. 2020;15(11):e0241040. doi: 10.1371/journal.pone.0241040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koo, A., Dewey, C.F., Jr., Garcia-Cardena, G., (2013). Hemodynamic shear stress characteristic of atherosclerosis-resistant regions promotes glycocalyx formation in cultured endothelial cells. Am. J. Physiol. Cell Physiol.,304 (2), C137-146. [DOI] [PMC free article] [PubMed]
- 68.Dai G., Kaazempur-Mofrad M.R., Natarajan S., Zhang Y., Vaughn S., Blackman B.R., Kamm R.D., García-Cardeña G., Gimbrone M.A. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc. Natl. Acad. Sci. U.S.A. 2004;101(41):14871–14876. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Machin D.R., Phuong T.T., Donato A.J. The role of the endothelial glycocalyx in advanced age and cardiovascular disease. Curr. Opin. Pharmacol. 2019;45:66–71. doi: 10.1016/j.coph.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mahmoud M., Mayer M., Cancel L.M., Bartosch A.M., Mathews R., Tarbell J.M. The glycocalyx core protein Glypican 1 protects vessel wall endothelial cells from stiffness-mediated dysfunction and disease. Cardiovasc. Res. 2021;117(6):1592–1605. doi: 10.1093/cvr/cvaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kang, H., Sun, A., Wu, Q., Yang, J., Zhang, W., Qu, Y., et al., (2020). Atherogenic diet-diminished endothelial glycocalyx contributes to impaired vasomotor properties in rat. Am. J. Physiol. Heart Circ. Physiol.,319 (4), H814-H823. [DOI] [PubMed]
- 72.Rops A.L., van den Hoven M.J., Baselmans M.M., Lensen J.F., Wijnhoven T.J., van den Heuvel L.P., van Kuppevelt T.H., Berden J.H., van der Vlag J. Heparan sulfate domains on cultured activated glomerular endothelial cells mediate leukocyte trafficking. Kidney Int. 2008;73(1):52–62. doi: 10.1038/sj.ki.5002573. [DOI] [PubMed] [Google Scholar]
- 73.Caravà E., Moretto P., Caon I., Parnigoni A., Passi A., Karousou E., Vigetti D., Canino J., Canobbio I., Viola M. HA and HS changes in endothelial inflammatory activation. Biomolecules. 2021;11(6):809. doi: 10.3390/biom11060809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smits N.C., Kurup S., Rops A.L., ten Dam G.B., Massuger L.F., Hafmans T., Turnbull J.E., Spillmann D., Li J.-P., Kennel S.J., Wall J.S., Shworak N.W., Dekhuijzen P.N.R., van der Vlag J., van Kuppevelt T.H. The heparan sulfate motif (GlcNS6S-IdoA2S)3, common in heparin, has a strict topography and is involved in cell behavior and disease. J. Biol. Chem. 2010;285(52):41143–41151. doi: 10.1074/jbc.M110.153791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oshima, K., Han, X., Ouyang, Y., El Masri, R., Yang, Y., Haeger, S.M., et al., (2019). Loss of endothelial sulfatase-1 after experimental sepsis attenuates subsequent pulmonary inflammatory responses. Am. J. Physiol. Lung Cell Mol. Physiol.,317 (5), L667-L677. [DOI] [PMC free article] [PubMed]
- 76.Li J., Shworak N.W., Simons M. Increased responsiveness of hypoxic endothelial cells to FGF2 is mediated by HIF-1alpha-dependent regulation of enzymes involved in synthesis of heparan sulfate FGF2-binding sites. J. Cell Sci. 2002;115(Pt 9):1951–1959. doi: 10.1242/jcs.115.9.1951. [DOI] [PubMed] [Google Scholar]
- 77.Korf-Klingebiel M., Reboll M.R., Grote K., Schleiner H., Wang Y., Wu X., Klede S., Mikhed Y., Nobre A., Bauersachs J., Klintschar M., Rudat C., Kispert A., Niessen H.W., Lübke T., Dierks T., Wollert K.C. Heparan sulfate-editing extracellular sulfatases enhance VEGF bioavailability for ischemic heart repair. Circ. Res. 2019;125(9):787–801. doi: 10.1161/CIRCRESAHA.119.315023. [DOI] [PubMed] [Google Scholar]
- 78.Presto J., Thuveson M., Carlsson P., Busse M., Wilén M., Eriksson I., Kusche-Gullberg M., Kjellén L. Heparan sulfate biosynthesis enzymes EXT1 and EXT2 affect NDST1 expression and heparan sulfate sulfation. Proc. Natl. Acad. Sci. U.S.A. 2008;105(12):4751–4756. doi: 10.1073/pnas.0705807105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Voyvodic P.L., Min D., Liu R., Williams E., Chitalia V., Dunn A.K., Baker A.B. Loss of syndecan-1 induces a pro-inflammatory phenotype in endothelial cells with a dysregulated response to atheroprotective flow. J. Biol. Chem. 2014;289(14):9547–9559. doi: 10.1074/jbc.M113.541573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bartosch A.M.W., Mathews R., Tarbell J.M. Endothelial glycocalyx-mediated nitric oxide production in response to selective AFM pulling. Biophys. J. 2017;113(1):101–108. doi: 10.1016/j.bpj.2017.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bartosch A.M.W., Mathews R., Mahmoud M.M., Cancel L.M., Haq Z.S., Tarbell J.M. Heparan sulfate proteoglycan glypican-1 and PECAM-1 cooperate in shear-induced endothelial nitric oxide production. Sci. Rep. 2021;11(1):11386. doi: 10.1038/s41598-021-90941-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang L., Fuster M., Sriramarao P., Esko J.D. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat. Immunol. 2005;6(9):902–910. doi: 10.1038/ni1233. [DOI] [PubMed] [Google Scholar]
- 83.LWMM Rops A., Loeven M.A., van Gemst J.J., Eversen I., Van Wijk X.M., Dijkman H.B., van Kuppevelt T.H., Berden J.H.M., Rabelink T.J., Esko J.D., van der Vlag J. Modulation of heparan sulfate in the glomerular endothelial glycocalyx decreases leukocyte influx during experimental glomerulonephritis. Kidney Int. 2014;86(5):932–942. doi: 10.1038/ki.2014.115. [DOI] [PubMed] [Google Scholar]
- 84.Chen H., Ambadapadi S., Wakefield D., Bartee M., Yaron J.R., Zhang L., et al. Selective deletion of heparan sulfotransferase enzyme, Ndst1, in donor endothelial and myeloid precursor cells significantly decreases acute allograft rejection. Sci. Rep. 2018;8(1):13433. doi: 10.1038/s41598-018-31779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ge X.N., Bastan I., Ha S.G., Greenberg Y.G., Esko J.D., Rao S.P., Sriramarao P. Regulation of eosinophil recruitment and allergic airway inflammation by heparan sulfate proteoglycan (HSPG) modifying enzymes. Exp. Lung Res. 2018;44(2):98–112. doi: 10.1080/01902148.2018.1451574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Talsma D.T., Katta K., Ettema M.A.B., Kel B., Kusche-Gullberg M., Daha M.R., Stegeman C.A., van den Born J., Wang L. Endothelial heparan sulfate deficiency reduces inflammation and fibrosis in murine diabetic nephropathy. Lab. Invest. 2018;98(4):427–438. doi: 10.1038/s41374-017-0015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Golden G.J., Toledo A.G., Marki A., Sorrentino J.T., Morris C., Riley R.J., Spliid C., Chen Q., Cornax I., Lewis N.E., Varki N., Le D., Malmström J., Karlsson C., Ley K., Nizet V., Esko J.D., Conlon B., Whiteley M. Endothelial heparan sulfate mediates hepatic neutrophil trafficking and injury during staphylococcus aureus sepsis. mBio. 2021;12(5) doi: 10.1128/mBio.01181-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zeng J., Meng Y., Chen S.-Y., Zhao G., Wang L., Zhang E.-X., Qiu H. Structural characteristics of Heparan sulfate required for the binding with the virus processing Enzyme Furin. Glycoconj. J. 2022;39(3):315–325. doi: 10.1007/s10719-021-10018-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hayashida A., Amano S., Gallo R.L., Linhardt R.J., Liu J., Park P.W. 2-O-Sulfated domains in syndecan-1 heparan sulfate inhibit neutrophil cathelicidin and promote staphylococcus aureus corneal infection. J. Biol. Chem. 2015;290(26):16157–16167. doi: 10.1074/jbc.M115.660852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lindahl U., Backstrom G., Thunberg L., Leder I.G. Evidence for a 3-O-sulfated D-glucosamine residue in the antithrombin-binding sequence of heparin. Proc. Natl. Acad. Sci. U.S.A. 1980;77(11):6551–6555. doi: 10.1073/pnas.77.11.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Olson S.T., Richard B., Izaguirre G., Schedin-Weiss S., Gettins P.G. Molecular mechanisms of antithrombin-heparin regulation of blood clotting proteinases. A paradigm for understanding proteinase regulation by serpin family protein proteinase inhibitors. Biochimie. 2010;92(11):1587–1596. doi: 10.1016/j.biochi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dinarvand P., Yang L., Villoutreix B.O., Rezaie A.R. Expression and functional characterization of two natural heparin-binding site variants of antithrombin. J. Thromb. Haemost. 2018;16(2):330–341. doi: 10.1111/jth.13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smits N.C., Kobayashi T., Srivastava P.K., Skopelja S., Ivy J.A., Elwood D.J., Stan R.V., Tsongalis G.J., Sellke F.W., Gross P.L., Cole M.D., DeVries J.T., Kaplan A.V., Robb J.F., Williams S.M., Shworak N.W. HS3ST1 genotype regulates antithrombin's inflammomodulatory tone and associates with atherosclerosis. Matrix Biol. 2017;63:69–90. doi: 10.1016/j.matbio.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xia G., Chen J., Tiwari V., Ju W., Li J.-P., Malmström A., Shukla D., Liu J. Heparan sulfate 3-o-sulfotransferase isoform 5 generates both an antithrombin-binding site and an entry receptor for herpes simplex virus, Type 1. J. Biol. Chem. 2002;277(40):37912–37919. doi: 10.1074/jbc.M204209200. [DOI] [PubMed] [Google Scholar]
- 95.Ferreras C., Rushton G., Cole C.L., Babur M., Telfer B.A., van Kuppevelt T.H., Gardiner J.M., Williams K.J., Jayson G.C., Avizienyte E. Endothelial heparan sulfate 6-O-sulfation levels regulate angiogenic responses of endothelial cells to fibroblast growth factor 2 and vascular endothelial growth factor. J. Biol. Chem. 2012;287(43):36132–36146. doi: 10.1074/jbc.M112.384875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang S., Ai X., Freeman S.D., Pownall M.E., Lu Q., Kessler D.S., Emerson C.P. QSulf1, a heparan sulfate 6-O-endosulfatase, inhibits fibroblast growth factor signaling in mesoderm induction and angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 2004;101(14):4833–4838. doi: 10.1073/pnas.0401028101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schmidt E.P., Yang Y., Janssen W.J., Gandjeva A., Perez M.J., Barthel L., Zemans R.L., Bowman J.C., Koyanagi D.E., Yunt Z.X., Smith L.P., Cheng S.S., Overdier K.H., Thompson K.R., Geraci M.W., Douglas I.S., Pearse D.B., Tuder R.M. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 2012;18(8):1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singh A., Satchell S.C., Neal C.R., McKenzie E.A., Tooke J.E., Mathieson P.W. Glomerular endothelial glycocalyx constitutes a barrier to protein permeability. J. Am. Soc. Nephrol. 2007;18(11):2885–2893. doi: 10.1681/ASN.2007010119. [DOI] [PubMed] [Google Scholar]
- 99.Puerta-Guardo H., Glasner D.R., Harris E., Kuhn R.J. Dengue virus NS1 disrupts the endothelial glycocalyx, leading to hyperpermeability. PLoS Pathog. 2016;12(7):e1005738. doi: 10.1371/journal.ppat.1005738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramnath R.D., Butler M.J., Newman G., Desideri S., Russell A., Lay A.C., Neal C.R., Qiu Y., Fawaz S., Onions K.L., Gamez M., Crompton M., Michie C., Finch N., Coward R.J., Welsh G.I., Foster R.R., Satchell S.C. Blocking matrix metalloproteinase-mediated syndecan-4 shedding restores the endothelial glycocalyx and glomerular filtration barrier function in early diabetic kidney disease. Kidney Int. 2020;97(5):951–965. doi: 10.1016/j.kint.2019.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baeyens N., Mulligan-Kehoe M.J., Corti F., Simon D.D., Ross T.D., Rhodes J.M., Wang T.Z., Mejean C.O., Simons M., Humphrey J., Schwartz M.A. Syndecan 4 is required for endothelial alignment in flow and atheroprotective signaling. Proc. Natl. Acad. Sci. U.S.A. 2014;111(48):17308–17313. doi: 10.1073/pnas.1413725111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu C., Wu X., Hack B.K., Bao L., Cunningham P.N. TNF causes changes in glomerular endothelial permeability and morphology through a Rho and myosin light chain kinase-dependent mechanism. Physiol. Rep. 2015;3(12):e12636. doi: 10.14814/phy2.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McDonald K.K., Cooper S., Danielzak L., Leask R.L., Sperandio M. Glycocalyx degradation induces a proinflammatory phenotype and increased leukocyte adhesion in cultured endothelial cells under flow. PLoS One. 2016;11(12):e0167576. doi: 10.1371/journal.pone.0167576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lipowsky, H.H., Gao, L., Lescanic, A., (2011). Shedding of the endothelial glycocalyx in arterioles, capillaries, and venules and its effect on capillary hemodynamics during inflammation. Am. J. Physiol. Heart Circ. Physiol.,301 (6), H2235-2245. [DOI] [PMC free article] [PubMed]
- 105.Dai E., Liu L.-Y., Wang H., McIvor D., Sun Y.M., Macaulay C., King E., Munuswamy-Ramanujam G., Bartee M.Y., Williams J., Davids J., Charo I., McFadden G., Esko J.D., Lucas A.R., Bereswill S. Inhibition of chemokine-glycosaminoglycan interactions in donor tissue reduces mouse allograft vasculopathy and transplant rejection. PLoS One. 2010;5(5):e10510. doi: 10.1371/journal.pone.0010510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. U.S.A. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Targosz-Korecka M., Kubisiak A., Kloska D., Kopacz A., Grochot-Przeczek A., Szymonski M. Endothelial glycocalyx shields the interaction of SARS-CoV-2 spike protein with ACE2 receptors. Sci. Rep. 2021;11(1):12157. doi: 10.1038/s41598-021-91231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Clausen T.M., Sandoval D.R., Spliid C.B., Pihl J., Perrett H.R., Painter C.D., et al. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020;183(4):1043–1057 e1015. doi: 10.1016/j.cell.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Elezkurtaj S., Greuel S., Ihlow J., Michaelis E.G., Bischoff P., Kunze C.A., et al. Causes of death and comorbidities in hospitalized patients with COVID-19. Sci. Rep. 2021;11(1):4263. doi: 10.1038/s41598-021-82862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee, D.H., Dane, M.J., van den Berg, B.M., Boels, M.G., van Teeffelen, J.W., de Mutsert, R., et al., (2014). Deeper penetration of erythrocytes into the endothelial glycocalyx is associated with impaired microvascular perfusion. PLoS One,9 (5), e96477. [DOI] [PMC free article] [PubMed]
- 111.Ikonomidis I., Voumvourakis A., Makavos G., Triantafyllidi H., Pavlidis G., Katogiannis K., Benas D., Vlastos D., Trivilou P., Varoudi M., Parissis J., Iliodromitis E., Lekakis J. Association of impaired endothelial glycocalyx with arterial stiffness, coronary microcirculatory dysfunction, and abnormal myocardial deformation in untreated hypertensives. J. Clin. Hypertens. (Greenwich) 2018;20(4):672–679. doi: 10.1111/jch.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Damus P.S., Hicks M., Rosenberg R.D. Anticoagulant action of heparin. Nature. 1973;246(5432):355–357. doi: 10.1038/246355a0. [DOI] [PubMed] [Google Scholar]
- 113.Marcum, J.A., Fritze, L., Galli, S.J., Karp, G., Rosenberg, R.D., (1983). Microvascular heparin-like species with anticoagulant activity. Am. J. Physiol. Heart Circ. Physiol.,245 (5 Pt 1), H725-733. [DOI] [PubMed]
- 114.Ho G., Broze G.J., Jr., Schwartz A.L. Role of heparan sulfate proteoglycans in the uptake and degradation of tissue factor pathway inhibitor-coagulation factor Xa complexes. J. Biol. Chem. 1997;272(27):16838–16844. doi: 10.1074/jbc.272.27.16838. [DOI] [PubMed] [Google Scholar]
- 115.HajMohammadi S., Enjyoji K., Princivalle M., Christi P., Lech M., Beeler D., Rayburn H., Schwartz J.J., Barzegar S., de Agostini A.I., Post M.J., Rosenberg R.D., Shworak N.W. Normal levels of anticoagulant heparan sulfate are not essential for normal hemostasis. J. Clin. Invest. 2003;111(7):989–999. doi: 10.1172/JCI15809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Weiss R.J., Spahn P.N., Toledo A.G., Chiang A.W.T., Kellman B.P., Li J., Benner C., Glass C.K., Gordts P.L.S.M., Lewis N.E., Esko J.D. ZNF263 is a transcriptional regulator of heparin and heparan sulfate biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 2020;117(17):9311–9317. doi: 10.1073/pnas.1920880117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Richard B., Swanson R., Olson S.T. The signature 3-O-sulfo group of the anticoagulant heparin sequence is critical for heparin binding to antithrombin but is not required for allosteric activation. J. Biol. Chem. 2009;284(40):27054–27064. doi: 10.1074/jbc.M109.029892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rezaie A.R., Giri H. Anticoagulant and signaling functions of antithrombin. J. Thromb. Haemost. 2020;18(12):3142–3153. doi: 10.1111/jth.15052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shworak N.W., Kobayashi T., de Agostini A., Smits N.C. Anticoagulant heparan sulfate to not clot–or not? Prog. Mol. Biol. Transl. Sci. 2010;93:153–178. doi: 10.1016/S1877-1173(10)93008-1. [DOI] [PubMed] [Google Scholar]
- 120.Ma Y., Wang J., Gao J., Yang H., Wang Y., Manithody C., Li J.i., Rezaie A.R. Antithrombin up-regulates AMP-activated protein kinase signalling during myocardial ischaemia/reperfusion injury. Thromb. Haemost. 2015;113(02):338–349. doi: 10.1160/TH14-04-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kalita M., Chua J.S., Boothello R.S., Joice A., Antelope O., Roy A., Anandh Babu P.V., Saijoh Y., Desai U.R., Kuberan B. Visualizing antithrombin-binding 3-O-sulfated heparan sulfate motifs on cell surfaces. Chem. Commun. (Camb.) 2020;56(92):14423–14426. doi: 10.1039/d0cc05893a. [DOI] [PubMed] [Google Scholar]
- 122.Iwakiri, Y., Tsai, M.H., McCabe, T.J., Gratton, J.P., Fulton, D., Groszmann, R.J., et al., (2002). Phosphorylation of eNOS initiates excessive NO production in early phases of portal hypertension. Am. J. Physiol. Heart Circ. Physiol.,282 (6), H2084-2090. [DOI] [PubMed]
- 123.Dusserre N., L’Heureux N., Bell K.S., Stevens H.Y., Yeh J., Otte L.A., Loufrani L., Frangos J.A. PECAM-1 interacts with nitric oxide synthase in human endothelial cells: implication for flow-induced nitric oxide synthase activation. Arterioscler. Thromb. Vasc. Biol. 2004;24(10):1796–1802. doi: 10.1161/01.ATV.0000141133.32496.41. [DOI] [PubMed] [Google Scholar]
- 124.Fleming I., Fisslthaler B., Dixit M., Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J. Cell. Sci. 2005;118(Pt 18):4103–4111. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- 125.Cao R., Eriksson A., Kubo H., Alitalo K., Cao Y., Thyberg J. Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ. Res. 2004;94(5):664–670. doi: 10.1161/01.RES.0000118600.91698.BB. [DOI] [PubMed] [Google Scholar]
- 126.Guimond S., Maccarana M., Olwin B.B., Lindahl U., Rapraeger A.C. Activating and inhibitory heparin sequences for FGF-2 (basic FGF). Distinct requirements for FGF-1, FGF-2, and FGF-4. J. Biol. Chem. 1993;268(32):23906–23914. [PubMed] [Google Scholar]
- 127.Ashikari-Hada S., Habuchi H., Sugaya N., Kobayashi T., Kimata K. Specific inhibition of FGF-2 signaling with 2-O-sulfated octasaccharides of heparan sulfate. Glycobiology. 2009;19(6):644–654. doi: 10.1093/glycob/cwp031. [DOI] [PubMed] [Google Scholar]
- 128.Teran M., Nugent M.A. Synergistic binding of vascular endothelial growth factor-a and its receptors to heparin selectively modulates complex affinity. J. Biol. Chem. 2015;290(26):16451–16462. doi: 10.1074/jbc.M114.627372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Beckouche N., Bignon M., Lelarge V., Mathivet T., Pichol-Thievend C., Berndt S., et al. The interaction of heparan sulfate proteoglycans with endothelial transglutaminase-2 limits VEGF165-induced angiogenesis. Sci. Signal. 2015;8(385):ra70. doi: 10.1126/scisignal.aaa0963. [DOI] [PubMed] [Google Scholar]
- 130.Yuan L., Cheng S., Sol W.M.P.J., van der Velden A.I.M., Vink H., Rabelink T.J., van den Berg B.M. Heparan sulfate mimetic fucoidan restores the endothelial glycocalyx and protects against dysfunction induced by serum of COVID-19 patients in the intensive care unit. ERJ Open Res. 2022;8(2):00652-2021. doi: 10.1183/23120541.00652-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Song J.W., Zullo J.A., Liveris D., Dragovich M., Zhang X.F., Goligorsky M.S. Therapeutic restoration of endothelial glycocalyx in sepsis. J. Pharmacol. Exp. Ther. 2017;361(1):115–121. doi: 10.1124/jpet.116.239509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li T., Liu X., Zhao Z., Ni L., Liu C. Sulodexide recovers endothelial function through reconstructing glycocalyx in the balloon-injury rat carotid artery model. Oncotarget. 2017;8(53):91350–91361. doi: 10.18632/oncotarget.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Broekhuizen L.N., Lemkes B.A., Mooij H.L., Meuwese M.C., Verberne H., Holleman F., Schlingemann R.O., Nieuwdorp M., Stroes E.S.G., Vink H. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia. 2010;53(12):2646–2655. doi: 10.1007/s00125-010-1910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Harenberg J. Review of pharmacodynamics, pharmacokinetics, and therapeutic properties of sulodexide. Med. Res. Rev. 1998;18(1):1–20. doi: 10.1002/(sici)1098-1128(199801)18:1<1::aid-med1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 135.Lasierra-Cirujeda J., Coronel P., Aza M., Gimeno M. Use of sulodexide in patients with peripheral vascular disease. J. Blood Med. 2010;1:105–115. doi: 10.2147/JBM.S10558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vlodavsky I., Ilan N., Sanderson R.D. Forty years of basic and translational heparanase research. Adv. Exp. Med. Biol. 2020;1221:3–59. doi: 10.1007/978-3-030-34521-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gonzalez-Ochoa A.J., Raffetto J.D., Hernández A.G., Zavala N., Gutiérrez O., Vargas A., Loustaunau J. Sulodexide in the treatment of patients with early stages of COVID-19: A randomized controlled trial. Thromb. Haemost. 2021;121(07):944–954. doi: 10.1055/a-1414-5216. [DOI] [PubMed] [Google Scholar]
- 138.Wodicka J., Chambers A., Sangha G., Goergen C., Panitch A. Development of a glycosaminoglycan derived, selectin targeting anti-adhesive coating to treat endothelial cell dysfunction. Pharmaceuticals (Basel) 2017;10(4):36. doi: 10.3390/ph10020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wodicka J.R., Morikis V.A., Dehghani T., Simon S.I., Panitch A. Selectin-targeting peptide-glycosaminoglycan conjugates modulate neutrophil-endothelial interactions. Cell. Mol. Bioeng. 2019;12(1):121–130. doi: 10.1007/s12195-018-0555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Haywood-Watson R.J., Holcomb J.B., Gonzalez E.A., Peng Z., Pati S., Park P.W., Wang WeiWei, Zaske A.M., Menge T., Kozar R.A., McNeil P. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS ONE. 2011;6(8):e23530. doi: 10.1371/journal.pone.0023530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Peng Z., Pati S., Potter D., Brown R., Holcomb J.B., Grill R., Wataha K., Park P.W., Xue H., Kozar R.A. Fresh frozen plasma lessens pulmonary endothelial inflammation and hyperpermeability after hemorrhagic shock and is associated with loss of syndecan 1. Shock. 2013;40(3):195–202. doi: 10.1097/SHK.0b013e31829f91fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vigiola Cruz M., Carney B.C., Luker J.N., Monger K.W., Vazquez J.S., Moffatt L.T., et al. Plasma ameliorates endothelial dysfunction in burn injury. J. Surg. Res. 2019;233:459–466. doi: 10.1016/j.jss.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 143.Kozar R.A., Peng Z., Zhang R., Holcomb J.B., Pati S., Park P., Ko T.C., Paredes A. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth. Analg. 2011;112(6):1289–1295. doi: 10.1213/ANE.0b013e318210385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Torres L.N., Sondeen J.L., Ji L., Dubick M.A., Torres Filho I. Evaluation of resuscitation fluids on endothelial glycocalyx, venular blood flow, and coagulation function after hemorrhagic shock in rats. J. Trauma Acute Care Surg. 2013;75(5):759–766. doi: 10.1097/TA.0b013e3182a92514. [DOI] [PubMed] [Google Scholar]
- 145.Lopez E., Peng Z., Kozar R.A., Cao Y., Ko T.C., Wade C.E., et al. Antithrombin III contributes to the protective effects of fresh frozen plasma following hemorrhagic shock by preventing syndecan-1 shedding and endothelial barrier disruption. Shock. 2020;53(2):156–163. doi: 10.1097/SHK.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 146.Wu F., Kozar R.A. Fibrinogen protects against barrier dysfunction through maintaining cell surface syndecan-1 in vitro. Shock. 2019;51(6):740–744. doi: 10.1097/SHK.0000000000001207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aldecoa C., Llau J.V., Nuvials X., Artigas A. Role of albumin in the preservation of endothelial glycocalyx integrity and the microcirculation: a review. Ann. Intensive Care. 2020;10(1):85. doi: 10.1186/s13613-020-00697-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zeng, Y., Adamson, R.H., Curry, F.R., Tarbell, J.M., (2014). Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am. J. Physiol. Heart Circ. Physiol.,306 (3), H363-372. [DOI] [PMC free article] [PubMed]
- 149.Zeng Y., Liu X.H., Tarbell J., Fu B. Sphingosine 1-phosphate induced synthesis of glycocalyx on endothelial cells. Exp. Cell Res. 2015;339(1):90–95. doi: 10.1016/j.yexcr.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 150.Mensah, S.A., Cheng, M.J., Homayoni, H., Plouffe, B.D., Coury, A.J., Ebong, E.E., (2017). Regeneration of glycocalyx by heparan sulfate and sphingosine 1-phosphate restores inter-endothelial communication. PLoS One,12 (10), e0186116. [DOI] [PMC free article] [PubMed]
- 151.Cancel, L.M., Tarbell, J.M., (2013). Rhamnan sulfate enhances the endothelial glycocalyx and decreases the LDL permeability of human coronary artery endothelial cells in vitro. FASEB J.,27 (S1), 896.893-896.893.
- 152.Machin D.R., Nguyen D., Bramwell R.C., Lesniewski L.A., Donator A.J. Dietary glycocalyx precursor supplementation ameliorates age-related vascular dysfunction. FASEB J. 2019;33(1) [Google Scholar]
- 153.Dietary Interventions on Glycocalyx Dimensions in South Asian Patients With Diabetic Nephropathy. (Glycotreat). [cited 30/09 2021] Available from: https://clinicaltrials.gov/ct2/show/NCT03889236.
- 154.Richter R.P., Joiner D.M., Griffin R.L., Jansen J.O., Kerby J.D., Wade C.E., Holcomb J.B., Cardenas J.C., Richter J.R. Endotheliopathy is associated with a 24-hour Fibrinolysis phenotype described by low TEG lysis and High D-dimer after trauma: a secondary analysis of the PROPPR study. Ann. Surg. Open. 2022;3(1):e116. doi: 10.1097/AS9.0000000000000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Saksela O., Moscatelli D., Sommer A., Rifkin D.B. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J. Cell Biol. 1988;107(2):743–751. doi: 10.1083/jcb.107.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.