This cohort study examines data from Swedish population registries to determine the relationship between genetic risk factors for major depression and bipolar disorder.
Key Points
Question
What is the association between genetic risk factors for major depression (MD) and bipolar disorder (BD) in the Swedish population and risks for a range of psychiatric disorders?
Findings
In this cohort study including 2.7 million Swedish adults, BD and MD shared a genetic vulnerability to mood disorders. However, individuals at high genetic liability to MD and BD had substantial and relatively specific risks for anxiety disorders and psychosis, respectively.
Meaning
This study suggests that from a genetic perspective, BD and MD are neither very closely nor only distally related but have both shared and relatively distinct genetic risks.
Abstract
Importance
The nature of the genetic relationship between major depression and bipolar disorder remains unclear and might be clarified by considering disorders outside of the mood spectrum.
Objective
To better understand the relationship between genetic liabilities for major depression (MD) and bipolar disorder (BD).
Design, Setting, and Participants
A cohort study was conducted with data for individuals born in Sweden to Swedish parents from 1960 to 1990, with follow-up through December 31, 2018. The data included family genetic risk scores for MD and BD and International Classification of Diseases codes for a range of disorders as reported in primary care, specialist, and hospital registries. Data analysis was conducted from April 2022 to July 2022.
Exposures
High and low genetic liability were defined as being in the upper and lower 2 risk deciles. Risk was compared in individuals at high genetic liability to (1) MD only, (2) BD only, and (3) both MD and BD and those at (4) high genetic liability to BD and low genetic liability to MD and (5) high genetic liability to MD and low genetic liability to BD.
Main Outcomes and Measures
Risk for nonpsychotic MD and BD, psychotic MD and BD, anxiety disorders, obsessive-compulsive disorder, schizoaffective disorder (SAD), schizophrenia, and other nonaffective psychosis.
Results
Data were included for 2 736 950 individuals with a mean (SD) age at follow-up of 43.9 (9.1) years. High genetic liability to only BD increased risk for nonpsychotic BD, psychotic BD, and SAD. High genetic liability to only MD augmented risk for nonpsychotic MD, anxiety disorders, and nonpsychotic BD. High genetic liability to both BD and MD had the strongest association with risk for nonpsychotic BD, anxiety disorders, and nonpsychotic MD. High genetic liability to BD and low genetic liability to MD increased risk for psychotic BD, nonpsychotic BD, and SAD with no increased risk for nonpsychotic MD or anxiety disorders. High genetic liability to MD and low genetic liability to BD increased risk for nonpsychotic MD, nonpsychotic BD, and anxiety disorders with no increased risk for psychotic BD.
Conclusions and Relevance
In this study, hypotheses that BD and MD are either genetically distinct or genetically closely interrelated were not supported. Both BD and MD were associated with a genetic vulnerability to mood disorders, but even that liability was partially selective. However, compared with individuals at high liability to MD, those at elevated genetic liability for BD had a substantially increased risk for psychosis. Compared with individuals at elevated genetic liability to BD, those at high genetic risk for MD had a considerably augmented risk for anxiety disorders. Clarifying genetic relationships between psychiatric syndromes can be substantially aided by the consideration of profiles of risk for a range of disorders.
Introduction
Within psychiatric genetics, few relationships have remained as enigmatic as that between major depression (MD) and bipolar disorder (BD).1 The 2 syndromes were subsumed within Emil Kraepelin’s diagnosis of manic depressive insanity,2,3 although objections were soon lodged as to its breadth.4,5 Its division into MD and BD was first proposed by Karl Leonhard in 1959.6 Although the DSM-III,7 DSM-III-R,8 and DSM-IV9 placed both disorders in a single category of affective or mood disorders, in the DSM-5, they received their own chapters.10
The validity of the MD-BD distinction proposed by Leonhard was supported by most1,11,12,13,14,15 but not all family studies,16,17 showing that while relatives of both MD and BD probands had substantially elevated risks for MD, large increases in rates of BD were seen only in the relatives of BD probands. Other analyses suggest that MD and BD are disorders resulting from the same genetic liability, with BD reflecting a more severe and MD a milder form of illness.18,19
The genetic relationship between the 2 disorders has also been assessed by genetic correlation, examples including estimates of +0.65 (95% CI, 0.58-0.75) from a high-quality twin study,20 +0.47 (0.06) from polygenic risk scores,21 and +0.32 (95% CI, 0.01-0.52) from an expanded adoption design.22 These moderate correlations are inconsistent with Kraepelin’s conception of these disorders arising from the same disease process.
We seek to further understand the association between the genetic liabilities to BD and MD through a top-down genetic approach in which, using family genetic risk scores (FGRSs),23,24,25,26 we classify the Swedish population into deciles of low to high genetic liability for BD and MD. To gain novel perspectives on this question, we examine large samples of individuals with (1) high genetic liability to BD, (2) high genetic liability to MD, (3) high genetic liability to both BD and MD, (4) high genetic liability to BD and low genetic liability to MD, and (5) high genetic liability to MD and low genetic liability to BD. In each of these population samples, we examine the risk for 9 mood, anxiety, and psychotic disorders.
We evaluate 3 plausible theories of relationships between genetic risk factors for MD and BD,1,18 that they (1) are largely the same differing only in severity,18,19 (2) are largely independent, or (3) share some dimensions of genetic liability and differ in others.
Methods
We collected information on individuals from Swedish population-based registers with national coverage linking each person’s unique personal identification number (which was replaced with a serial number by Statistics Sweden to preserve confidentiality). Ethical approval for this study was granted by the regional ethical review board in Lund (No. 2008/409 and later amendments). Participant consent was not required.
The database consisted of all individuals born in Sweden from 1960 to 1990 to Swedish parents followed up through December 31, 2018. The database included registrations for nonpsychotic MD (NP-MD), psychotic MD (P-MD), nonpsychotic BD (NP-BD), psychotic BD (P-BD), anxiety disorder (AD), obsessive-compulsive disorder (OCD), schizophrenia (SZ), schizoaffective disorder (SAD), other nonaffective psychosis (ONAP). Codes for these disorders from the eighth, ninth, and tenth revisions of the International Classification of Diseases were reported in primary care, specialist, and hospital registries (eAppendix 1 and eTables 1 and 2 in the Supplement contain details, including the diagnostic hierarchies). We also included 2 FGRSs, 1 for MD and 1 for BD. The FGRSs are calculated from morbidity risks for disorders in first- through fifth-degree relatives, controlling for cohabitation effects, and thus arise from phenotypes in extended pedigrees, not from molecular genetic data (eAppendix 2 in the Supplement).
For analysis, we divided the sample according to level of FGRSs for BD and MD into groups of individuals at high (9th-10th), median (5th-6th), and low (1st-2nd) deciles of genetic liability. We used this approach because BD is rare enough that substantial concentrations of cases are seen only in pedigrees of probands in the highest deciles of genetic risk.
Using these definitions, we selected 5 sample groups from the general population, depicted in Figure 1 with cases shown in orange and controls in blue. In groups 1 and 2, we compared individuals at high vs median genetic risk for BD and MD, respectively. Group 3 compared individuals at high genetic risk for BD and MD to those at median risk for both disorders. Groups 4 and 5 compared individuals at median genetic risk for both disorders with those at high genetic risk for BD and low genetic risk for MD and those at high genetic risk for MD and low genetic risk for BD, respectively.
Figure 1. Ascertainment of Participants for 5 Population Samples Representing the Swedish Population Born 1960 to 1990.
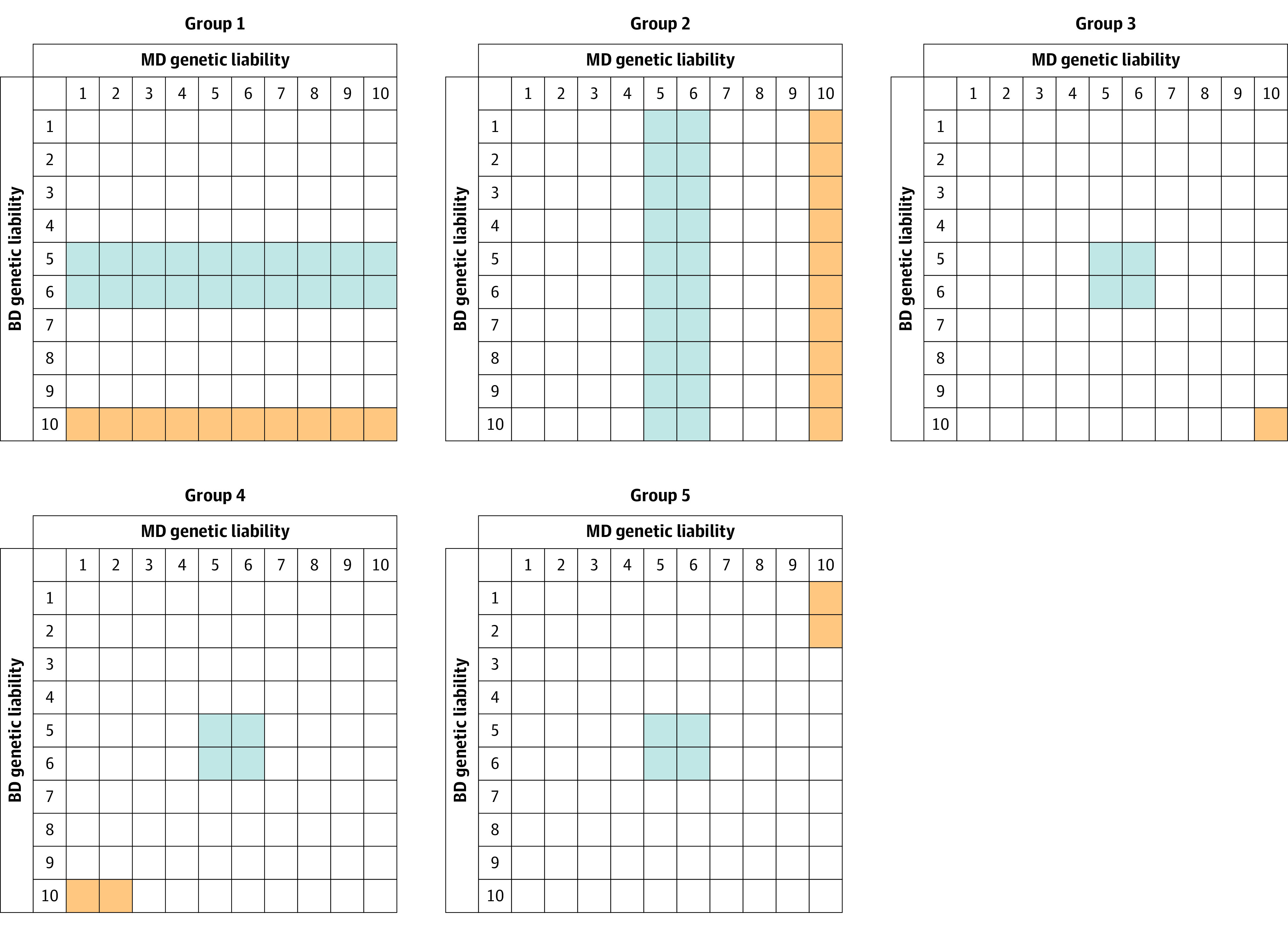
Columns are deciles of major depression (MD) genetic liability as assessed by the familial genetic risk score; rows are deciles of bipolar disorder (BD) familial genetic liability. Orange indicates cases; blue, controls.
Within each sample, we calculated the tetrachoric correlation between the 2 selected groups for the occurrence of each of the 9 disorders. For example, a 2 × 2 table from which the correlation would be calculated is the number of individuals in the high-risk group without and with a particular disorder and the number of individuals in the median-risk group without and with that same disorder. Higher correlations reflect greater differences in risk for that disorder between the high- and median-risk groups.
We used tetrachoric correlations, which represent the correlation for a latent underlying normally distributed liability to illness,27 as opposed to more standard odds ratios because of the ease of interpretability28 and their insensitivity to changes in base rates,29 given the differing rates of disorders (eg, NP-MD vs P-BD) in our sample.
To account for the difference in registration for the 9 disorders, we included 3 regression terms for year of birth, sex, and county of residence on the thresholds. Further, we created 2 subsamples of individuals registered with MD (registration of NP-MD or P-MD) and with BD (registration of NP-BD or P-BD). Within each sample, we calculated the least-squares means of BD-FGRS and MD-FGRS for individuals with nonpsychotic MD/BD and psychotic MD/BD controlling for year of birth, sex, and county of residence. Analyses were conducted from April 2022 to July 2022 using Mplus version 7.3130 and SAS version 9.4.31
Results
This study included data for 2 736 950 individuals with a mean (SD) age at follow-up of 43.9 (9.1) years. The logic of the selection for case and control samples for the 5 key analyses is outlined in Figure 1, which represents the entire population of Sweden born from 1960 to 1990 divided by the decile of their genetic liabilities to BD and MD calculated by their FGRS. Table 1 provides the sample sizes for the 5 analyses we conducted, outlined above, as well as the prevalence in each of them for 9 disorders in the cases and controls in each analysis. For the 5 analysis groups shown in Figure 2, Table 2 provides pairwise P values for the correlations for each of the 9 disorders across all 5 analyses.
Table 1. Sample Sizes and Prevalence of 9 Diagnostic Categories in the Cases and Controls From 5 Selected Population Samplesa.
| Disorder | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases, % | Controls, % | Cases, % | Controls, % | Cases, % | Controls, % | Cases, % | Controls, % | Cases, % | Controls, % | |
| No. of individuals | 273 686 | 547 372 | 273 694 | 547 389 | 35 694 | 109 448 | 44 009 | 109 448 | 72 188 | 109 448 |
| Nonpsychotic MD | 19.9 | 15.3 | 25.1 | 15.3 | 28.4 | 14.6 | 13.8 | 14.6 | 23.5 | 14.6 |
| Nonpsychotic BD | 3.6 | 1.1 | 2.5 | 1.4 | 5.1 | 1.0 | 2.4 | 1.0 | 2.1 | 1.0 |
| AD | 22.3 | 15.6 | 26.1 | 16.6 | 31.1 | 15.1 | 15.6 | 15.1 | 26.0 | 15.1 |
| OCD | 1.3 | 0.7 | 1.5 | 0.9 | 1.8 | 0.7 | 1.0 | 0.7 | 1.9 | 0.7 |
| Psychotic MD | 0.3 | 0.2 | 0.3 | 0.2 | 0.3 | 0.1 | 0.2 | 0.1 | 0.2 | 0.1 |
| Psychotic BD | 0.06 | 0.02 | 0.03 | 0.03 | 0.06 | 0.02 | 0.07 | 0.02 | 0.02 | 0.02 |
| SAD | 0.4 | 0.1 | 0.2 | 0.1 | 0.4 | 0.1 | 0.3 | 0.1 | 0.2 | 0.1 |
| SZ | 0.6 | 0.3 | 0.4 | 0.3 | 0.6 | 0.3 | 0.5 | 0.3 | 0.3 | 0.3 |
| ONAP | 1.5 | 0.8 | 1.2 | 0.8 | 1.8 | 0.7 | 1.3 | 0.7 | 1.0 | 0.7 |
Abbreviations: AD, anxiety disorders; BD, bipolar disorder; MD, major depression; OCD, obsessive-compulsive disorder; ONAP, other nonaffective psychosis; SZ, schizophrenia; SAD, schizoaffective disorder.
For definitions of the sampling of groups 1-5, see Figure 1.
Figure 2. Tetrachoric Correlations for 9 Disorders With Varying Combinations of Genetic Liabilities to Bipolar Disorder (BD) and Major Depression (MD).
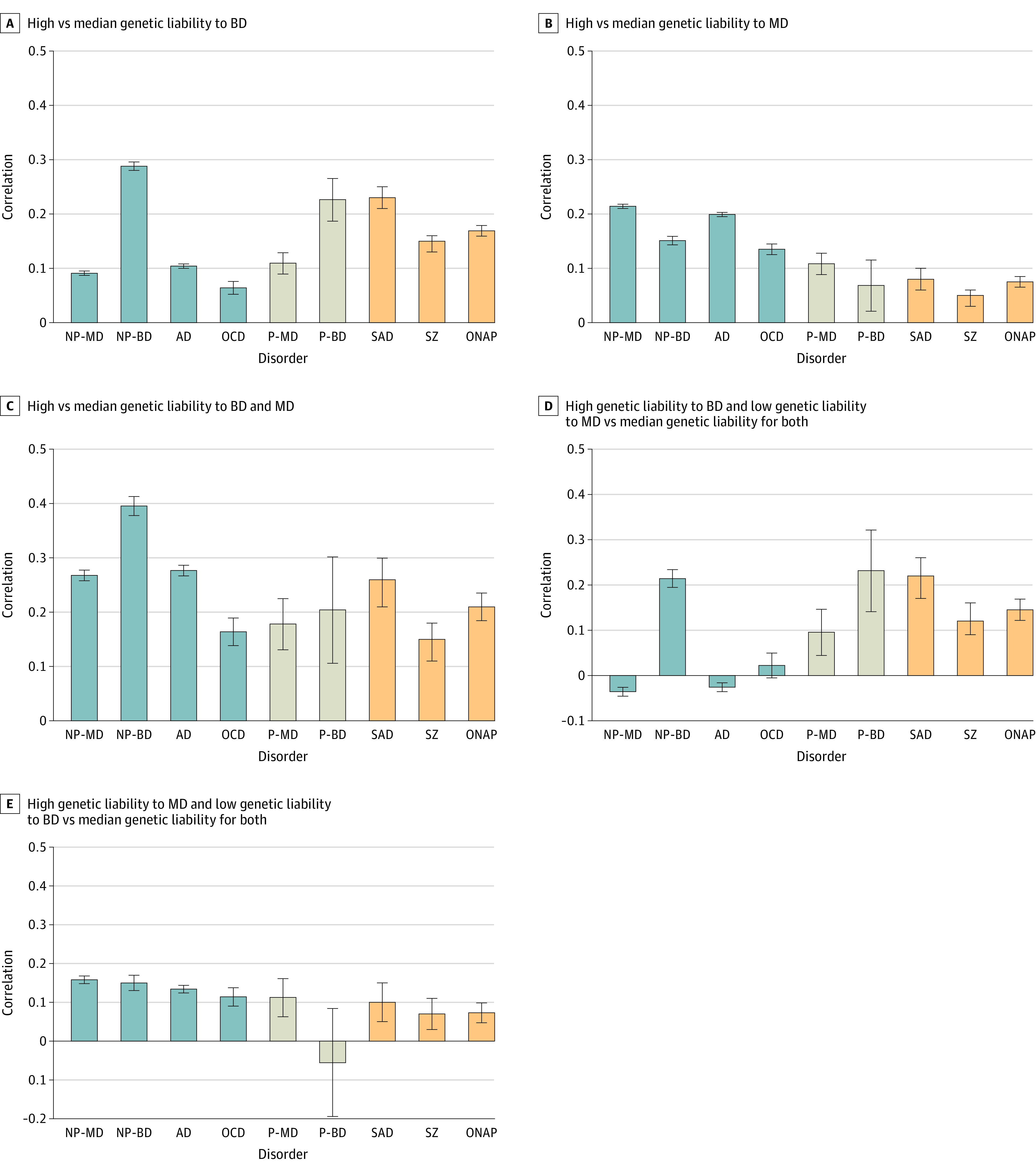
These correlations derive from 2 × 2 tables based on the number of individuals in the high-risk and median-risk groups without and with a particular disorder. The correlation therefore reflects the degree of difference in the rates of the disorder in the 2 groups such that higher the correlation, the greater the difference in risk. Error bars indicate 95% CIs. AD indicates anxiety disorder; NP-BD, nonpsychotic bipolar disorder; NP-MD, nonpsychotic major depression; OCD, obsessive-compulsive disorder; ONAP, other nonaffective psychosis; P-BD, psychotic bipolar disorder; P-MD, psychotic major depression; SZ, schizophrenia; SAD, schizoaffective disorder.
Table 2. Test of Equality of Tetrachoric Correlations for the Relevant Comparisons in Figure 2 Between Each of 9 Disorders Across 5 Analysis Groupsa.
| Disorder | Groups compared | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 vs 2 | 1 vs 3 | 1 vs 4 | 1 vs 5 | 2 vs 3 | 2 vs 4 | 2 vs 5 | 3 vs 4 | 3 vs 5 | 4 vs 5 | |
| Nonpsychotic MD | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
| Nonpsychotic BD | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | .99b | <.001 | <.001 | <.001 |
| AD | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
| OCD | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | .87b | <.001 | <.001 | <.001 |
| Psychotic MD | .82b | <.001 | .94b | .75b | <.001 | .93b | .71b | .03b | .06b | .69b |
| Psychotic BD | <.001 | .11b | .45b | .001b | .08b | .004b | .26b | .35b | .005b | .001b |
| SAD | <.001 | .23b | .68b | <.001 | <.001 | <.001 | .82b | .10b | <.001 | <.001 |
| SZ | <.001 | .95b | .12b | <.001 | <.001 | <.001 | .08b | .36b | <.001 | .13b |
| ONAP | <.001 | <.001 | .42b | <.001 | <.001 | <.001 | .49b | <.001 | <.001 | <.001 |
Abbreviations: AD, anxiety disorders; BD, bipolar disorder; MD, major depression; OCD, obsessive-compulsive disorder; ONAP, other nonaffective psychosis; SZ, schizophrenia; SAD, schizoaffective disorder.
The analysis groups represented by these P values are presented in Figure 2.
No significant difference.
Figure 2A shows that our sample with high vs median genetic liability to BD had the greatest elevation in risk for NP-BD, SAD, and P-BD, followed by ONAP and SZ. Modest elevations, in order of effect size, were also seen for P-MD, AD, NP-MD, and OCD.
The pattern in Figure 2B, comparing those with high vs median genetic liability for MD, was qualitatively different. Disorders with the greatest increased risk were NP-MD, AD, NP-BD, OCD, and P-MD. More modest risk elevations were seen for SAD, ONAP, SZ, and P-BD. As seen in Table 2, with the exception of P-MD, the risk elevations for the other disorders all differed significantly between those at high genetic risk for BD vs MD.
The risk for disorders in those at high vs median genetic liability for both BD and MD, as seen in Figure 2C, represented our smallest sample size, so CIs were wide, especially for rarer disorders. The magnitudes of increased risk were greater for all disorders compared with those seen in Figure 2A and B. Of greater interest is that the pattern of results differed. Highest risk was now seen for NP-BD, followed by AD, NP-MD, and SAD. Lower but significant elevations in risk were seen for ONAP, P-BD, P-MD, OCD, and finally SZ. Compared with individuals at high genetic liability to only BD, those with high liability to both BD and MD were at significantly higher risk for all disorders except P-BD, SAD, and SZ. Compared with individuals at high genetic liability to only MD, those with high liability to both disorders were at significantly higher risk for all disorders except P-MD.
Defining cases as individuals with high genetic liability to BD and low genetic liability to MD produced the results seen in Figure 2D. Such individuals were at highest risk for P-BD and NP-BD, followed by SAD, ONAP, SZ, and P-MD. Interestingly, they were not at significantly increased risk for NP-MD, AD, or OCD. Compared with those at high genetic liability to BD, these individuals had significantly reduced risks for NP-MD, NP-BD, AD, and OCD.
The sample in group 5 had a high genetic liability to MD and a low genetic liability to BD (Figure 2E). These individuals were at highest risk for NP-MD, followed closely by NP-BD, AD, OCD, and P-MD. Modest increases in risk were seen for SAD, SZ, and ONAP, with no increased risk for P-BD. Compared with those at high genetic liability to MD (Figure 2B), these individuals had significantly reduced risks for only NP-MD and AD.
In the final analysis, we followed up evidence that genetic risk for BD more strongly predisposes to psychotic forms of affective illness compared with genetic risk for MD (Table 3). Controlling for year of birth, sex, and primary care registration, the mean level of MD-FGRS did not distinguish P-MD from NP-MD and was significantly lower in P-BD than NP-BD. By contrast, levels of BD-FGRS were significantly and substantially higher in P-MD vs NP-MD and P-BD vs NP-BD.
Table 3. Mean Genetic Liability as Assessed by Family Genetic Risk Scores for Major Depression and Bipolar Disorder in Cases With and Without Psychosisa.
| MD genetic liability, mean z score (95% CI) | BD genetic liability, mean z score (95% CI) | |
|---|---|---|
| MD (nonpsychotic) | 0.28 (0.28-0.29) | 0.09 (0.08-0.09) |
| MD (psychotic) | 0.27 (0.23-0.30) | 0.20 (0.17-0.24) |
| P value (equality of means) | .36 | <.001 |
| BD (nonpsychotic) | 0.34 (0.33-0.35) | 0.59 (0.57-0.60) |
| BD (psychotic) | 0.24 (0.15-0.33) | 0.82 (0.67-0.97) |
| P value (equality of means) | .04 | .003 |
Abbreviations: BD, bipolar disorder; MD, major depression.
Means adjusted for year of birth, sex, and primary care registration.
Discussion
We sought, in these analyses, to gain further insight into the association between the genetic liabilities to MD and BD by examining their effect on risk for 9 mood, anxiety, and psychotic disorders. Unlike the prior family, twin, and polygenic risk score perspectives on this problem, we took a top-down approach, selecting from the Swedish population subgroups with high, median, and low levels of genetic liability for MD and BD. We compared the pattern of risk for selected disorders between and across these groups, observing the association of these 2 liabilities with diagnostic outcomes from 3 perspectives: alone, when added together, and when subtracted from one another. We suggest 4 noteworthy patterns of results, which we review in turn.
First, consistent with most family studies,1,7,8,9,10,11 the patterns of elevated risk for affective disorders in our populations with high vs median liability to BD and MD differ substantially. The disorders with the strongest associations with a high BD genetic liability were NP-BD, P-BD, and SAD. Two of the 3 disorders with the strongest associations with a high MD genetic liability were different: NP-MD and AD. Only cases of NP-BD were substantially elevated in both high-risk groups. Risks for 2 other psychotic disorders, ONAP and SZ, were also significantly higher in the high-liability BD than in the high-liability MD group, while the opposite pattern was seen for AD and OCD. These results suggest that BD and MD have both shared and relatively distinct genetic liabilities, with BD risk associated largely with mood and psychotic disorders while MD risk affects mood and anxiety disorders. Even in their liability to mood disorders, however, there is some specificity. While BD and MD liability increased risk for depressive and bipolar syndromes, each has a stronger association within their own domain.
Second, what do we learn by examining a population sample of individuals at high risk for both BD and MD? We see synergistic effects on 6 disorders, where the concatenation of high genetic liability for both mood syndromes is significantly associated with increased risk beyond that found with only high BD or only high MD liability. Thus, these disorders are those whose risk is appreciably associated with both BD and MD liability. These 6 disorders include 3 core mood disorders (NP-MD, NP-BD, and P-MD) and, perhaps surprisingly, 2 nonmood internalizing disorders (AD and OCD) and the most nonspecific psychotic disorder (ONAP). The 3 disorders where risks did not differ in the high BD vs high BD+MD groups were SAD, SZ, and, most importantly, P-BD. These results suggest that for these disorders, high genetic liability to MD has little to no effect on risk.
Third, what further insights were gained from the 2 “subtractive” analyses? When we selected individuals from the general population at high BD but low MD risk, we saw significant reductions in risk for 4 disorders compared with those with only high BD risk. All 4 were either mood or anxiety disorders, none of which include psychotic symptoms: NP-MD, NP-BD, AD, and OCD. In fact, for 2 of these disorders (NP-MD and AD), no association with increased risk over controls is seen in this selected sample. That is, individuals with high BD liability and low MD liability show no elevation in risks for NP-MD and AD. This subtractive technique provides a distinct approach to detecting the disorders influenced by our genetic liability, reaffirming the weakness of the associations between MD genetic liability and psychotic syndromes and BD liability and AD.
Next, we compared individuals from the general population at high MD but low BD risk with those with only high MD risk. There were significant reductions in risk for only 2 disorders: NP-MD and AD. These results suggest, by subtractive analysis, that BD genetic liability is associated with a modest excess risk to AD, a finding not fully consistent with the other subtractive analysis. It is also noteworthy that in this group, the increased risk for P-BD was zero. Individuals with high MD liability but low BD liability had no increased risk for P-BD, again suggesting the very weak connection between MD genetic risk and vulnerability to psychosis.
The final analysis was the relationship of BD and MD genetic liability with the probability of those with MD and BD developing P-MD and P-BD, respectively. Congruent with other findings, the genetic liabilities to BD and MD differ strikingly in their association with risk for psychotic symptoms in the setting of a mood disorder. Liability to BD substantially increases that risk while liability to MD does not.
Our results can be usefully viewed in the context of the rich prior literature on the familial/genetic relationship between MD and BD. Reviewing a large literature of uncontrolled and controlled family studies of BD and MD, Tsuang and Faraone1 conclude that both BD and MD are familial disorders with partial but incomplete overlap in the pattern of mood disorders found in their families. Broadly consistent with our findings of a partial but far from complete overlap of genetic risk for the 2 disorders are that (1) a classic study of manic-depressive illness found a much higher than expected resemblance for unipolar and bipolar disorder in concordant monozygotic twins,32 (2) prior estimates for the genetic correlation ranged from +0.30 to +0.65 across methods,20,21,22 and (3) our previous study showed differences in the relationships of MD and BD FGRS in cases of major mood and psychotic disorders.23
Our results are not consistent with prior analyses of genetically informative designs, which support a multiple-threshold model in which cases of MD and BD differ quantitatively on the same dimension of liability BD18,19,33 as we show a number of qualitative differences in the pattern of disorders in individuals at high genetic risk to MD and BD. We replicated the results of Weissman et al14 in finding a much greater risk for generalized anxiety disorder in relatives of MD vs BD probands.
Limitations
Our results should be viewed in the context of 2 major methodological concerns. First, our FGRS is an estimate of genetic risk reflecting aggregation of disease in close and distant relatives and is quite different from the molecular polygenic risk score. It has the advantage of being based on the phenotypic liability directly rather than an index of that liability through single-nucleotide polymorphisms. However, our adjustments for cohabitation are approximate but only have small effects on the overall score.23,24,25 Our final genetic risk scores are not highly sensitive to the various corrections involved in their calculation, as their deletion produces results that highly correlate with those from the full model with similar predictive power. We validated the FGRS by comparing it with a recently proposed quantitative family-history score,34 showing in Swedish samples that, when matched for the relatives examined, the 2 scores correlated +0.94 (0.02). Furthermore, we tested the FGRS by simulation, showing that it performs as expected as do our corrections for cohabitation (eAppendix 3 and eFigures 1-4 in the Supplement).
Second, the validity of our analyses is dependent on the quality of diagnoses in the Swedish registries, which have been well supported for diagnoses of BD, SZ, and OCD.35,36,37,38 The validity of MD and AD diagnoses is supported by their prevalence, sex ratio, sibling and twin correlations, and associations with known psychosocial risk factors.39,40 We further evaluated the MD and BD diagnoses by using the national pharmacy registry. Among the NP-MD and P-MD cases, 83% and 93% of individuals received antidepressant prescriptions, respectively. For the NP-BD and P-BD cases, 78% and 87% of individuals received prescriptions for lithium or mood stabilizers.
Conclusions
The results of this study are not consistent with hypotheses that BD and MD are genetically distinct or genetically closely interrelated. Rather, they support a more nuanced view, involving genetic liabilities to 3 psychopathological dimensions. BD and MD share a genetic vulnerability to mood disorders (ie, both BD and MD), but that liability is partially selective. High genetic risk for BD is associated with increased risk for BD more than MD risk and vice versa. However, compared with individuals with high genetic liability to BD, those with high genetic liability to MD had a much greater risk for anxiety disorders and a much lower risk for psychosis. Furthermore, the BD-associated risk for psychosis acts more robustly on risk for psychotic symptoms in those with affective syndromes, including SAD, than in the more purely psychotic disorders of schizophrenia and ONAP. Clarifying the genetic relationship between pairs of psychiatric disorders can be aided by moving beyond a consideration of risks solely for those 2 disorders to examine profiles of risk to a broader set of conditions.
eAppendix 1. Description of registers
eTable 1. Definition of phenotypes
eTable 2. Decision table for registrations of SAD and SZ
eAppendix 2. Calculation of the Familial Genetic Risk Score (FGRS)
eAppendix 3. Simulation methods
eFigure 1. Results of Simulations of Pedigrees Containing 1st-5th Degree Relatives Analyzed by FGRS as a Function of Heritability and Prevalence (c2 = 0%)
eFigure 2. Results of Simulations of Pedigrees Containing 1st-5th Degree Relatives Analyzed by FGRS as a Function of Heritability and Prevalence (c2 = 2.5%)
eFigure 3. Results of Simulations of Pedigrees Containing 1st-5th Degree Relatives Analyzed by FGRS as a Function of Heritability and Prevalence (c2 = 5%)
eFigure 4. Results of Simulations of Pedigrees Containing 1st-5th Degree Relatives Analyzed by FGRS as a Function of Heritability and Prevalence (c2 = 10%)
References
- 1.Tsuang MT, Faraone SV. The Genetics of Mood Disorders. The Johns Hopkins University Press; 1990. [Google Scholar]
- 2.Trede K, Salvatore P, Baethge C, Gerhard A, Maggini C, Baldessarini RJ. Manic-depressive illness: evolution in Kraepelin’s textbook, 1883-1926. Harv Rev Psychiatry. 2005;13(3):155-178. doi: 10.1080/10673220500174833 [DOI] [PubMed] [Google Scholar]
- 3.Kraepelin E. Psychiatrie: Ein Lehrbuch fur Studirende und Aerzte Vol 2 vols. 6th ed. Barth; 1899. [Google Scholar]
- 4.Angst J, Sellaro R. Historical perspectives and natural history of bipolar disorder. Biol Psychiatry. 2000;48(6):445-457. doi: 10.1016/S0006-3223(00)00909-4 [DOI] [PubMed] [Google Scholar]
- 5.Kendler KS, Engstrom EJ. Criticisms of Kraepelin’s Psychiatric Nosology: 1896-1927. Am J Psychiatry. 2018;175(4):316-326. doi: 10.1176/appi.ajp.2017.17070730 [DOI] [PubMed] [Google Scholar]
- 6.Leonhard K. Aufteilung der Endogenen Psychosen. 2nd ed. Akademie Verlag; 1959. [Google Scholar]
- 7.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. American Psychiatric Association; 1980. [Google Scholar]
- 8.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 3rd ed, revised. American Psychiatric Association; 1987. [Google Scholar]
- 9.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; 1994. [Google Scholar]
- 10.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013. [Google Scholar]
- 11.Perris C. A study of bipolar (manic-depressive) and unipolar recurrent depressive psychoses. Acta Psychiatr Scand Suppl. 1966;194:1-188. [PubMed] [Google Scholar]
- 12.Angst J, Frey R, Lohmeyer B, Zerbin-Rüdin E. Bipolar manic-depressive psychoses: results of a genetic investigation. Hum Genet. 1980;55(2):237-254. doi: 10.1007/BF00291773 [DOI] [PubMed] [Google Scholar]
- 13.Smeraldi E, Negri F, Melica AM. A genetic study of affective disorders. Acta Psychiatr Scand. 1977;56(5):382-398. doi: 10.1111/j.1600-0447.1977.tb06679.x [DOI] [PubMed] [Google Scholar]
- 14.Weissman MM, Gershon ES, Kidd KK, et al. Psychiatric disorders in the relatives of probands with affective disorders: the Yale University–National Institute of Mental Health Collaborative Study. Arch Gen Psychiatry. 1984;41(1):13-21. doi: 10.1001/archpsyc.1984.01790120015003 [DOI] [PubMed] [Google Scholar]
- 15.Rice J, Reich T, Andreasen NC, et al. The familial transmission of bipolar illness. Arch Gen Psychiatry. 1987;44(5):441-447. doi: 10.1001/archpsyc.1987.01800170063009 [DOI] [PubMed] [Google Scholar]
- 16.Fieve RR, Go R, Dunner DL, Elston R. Search for biological/genetic markers in a long-term epidemiological and morbid risk study of affective disorders. J Psychiatr Res. 1984;18(4):425-445. doi: 10.1016/0022-3956(84)90031-1 [DOI] [PubMed] [Google Scholar]
- 17.Tsuang MT, Winokur G, Crowe RR. Morbidity risks of schizophrenia and affective disorders among first degree relatives of patients with schizophrenia, mania, depression and surgical conditions. Br J Psychiatry. 1980;137:497-504. doi: 10.1192/bjp.137.6.497 [DOI] [PubMed] [Google Scholar]
- 18.Kendler KS, Pedersen NL, Neale MC, Mathé AA. A pilot Swedish twin study of affective illness including hospital- and population-ascertained subsamples: results of model fitting. Behav Genet. 1995;25(3):217-232. doi: 10.1007/BF02197180 [DOI] [PubMed] [Google Scholar]
- 19.Baron M. Genetic models of affective disorder: application to twin data. Acta Genet Med Gemellol (Roma). 1980;29(4):289-294. doi: 10.1017/S0001566000007807 [DOI] [PubMed] [Google Scholar]
- 20.McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry. 2003;60(5):497-502. doi: 10.1001/archpsyc.60.5.497 [DOI] [PubMed] [Google Scholar]
- 21.Lee SH, Ripke S, Neale BM, et al. ; Cross-Disorder Group of the Psychiatric Genomics Consortium; International Inflammatory Bowel Disease Genetics Consortium (IIBDGC) . Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45(9):984-994. doi: 10.1038/ng.2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kendler KS, Ohlsson H, Sundquist J, Sundquist K. An extended Swedish national adoption study of bipolar disorder illness and cross-generational familial association with schizophrenia and major depression. JAMA Psychiatry. 2020;77(8):814-822. doi: 10.1001/jamapsychiatry.2020.0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kendler KS, Ohlsson H, Sundquist J, Sundquist K. family genetic risk scores and the genetic architecture of major affective and psychotic disorders in a Swedish national sample. JAMA Psychiatry. 2021;78(7):735-743. doi: 10.1001/jamapsychiatry.2021.0336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kendler KS, Ohlsson H, Sundquist J, Sundquist K. The patterns of family genetic risk scores for eleven major psychiatric and substance use disorders in a Swedish national sample. Transl Psychiatry. 2021;11(1):326. doi: 10.1038/s41398-021-01454-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kendler KS, Ohlsson H, Sundquist J, Sundquist K. The impact of sex, age at onset, recurrence, mode of ascertainment and medical complications on the family genetic risk score profiles for alcohol use disorder. Psychol Med. 2021;1-9. doi: 10.1017/S0033291721003317 [DOI] [PubMed] [Google Scholar]
- 26.Kendler KS, Ohlsson H, Bacanu S, Sundquist J, Sundquist K. Differences in genetic risk score profiles for drug use disorder, major depression, and ADHD as a function of sex, age at onset, recurrence, mode of ascertainment, and treatment. Psychol Med. 2022;1-13. doi: 10.1017/S0033291721005535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekström J. The phi-coefficient, the tetrachoric correlation coefficient, and the Pearson-Yule Debate. 2011. https://escholarship.org/uc/item/7qp4604r
- 28.Falconer DS. Introduction to Quantitative Genetics. 3rd ed. Wiley; 1989. [Google Scholar]
- 29.Babchishin KM, Helmus LM. The influence of base rates on correlations: an evaluation of proposed alternative effect sizes with real-world data. Behav Res Methods. 2016;48(3):1021-1031. doi: 10.3758/s13428-015-0627-7 [DOI] [PubMed] [Google Scholar]
- 30.Muthén LK, Muthén BO. Mplus User’s guide: 1998-2015. 7th ed. Muthén & Muthén; 2015. [Google Scholar]
- 31.SAS Institute . SAS/STAT online documentation. Accessed August 18, 2022. https://support.sas.com/en/software/sas-stat-support.html
- 32.Bertelsen A, Harvald B, Hauge M. A Danish twin study of manic-depressive disorders. Br J Psychiatry. 1977;130:330-351. doi: 10.1192/bjp.130.4.330 [DOI] [PubMed] [Google Scholar]
- 33.Baron M, Klotz J, Mendlewicz J, Rainer J. Multiple-threshold transmission of affective disorders. Arch Gen Psychiatry. 1981;38(1):79-84. doi: 10.1001/archpsyc.1981.01780260081009 [DOI] [PubMed] [Google Scholar]
- 34.Hujoel MLA, Gazal S, Loh P-R, Patterson N, Price AL. Liability threshold modeling of case-control status and family history of disease increases association power. Nat Genet. 2020;52(5):541-547. doi: 10.1038/s41588-020-0613-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lichtenstein P, Björk C, Hultman CM, Scolnick E, Sklar P, Sullivan PF. Recurrence risks for schizophrenia in a Swedish national cohort. Psychol Med. 2006;36(10):1417-1425. doi: 10.1017/S0033291706008385 [DOI] [PubMed] [Google Scholar]
- 36.Sellgren C, Landén M, Lichtenstein P, Hultman CM, Långström N. Validity of bipolar disorder hospital discharge diagnoses: file review and multiple register linkage in Sweden. Acta Psychiatr Scand. 2011;124(6):447-453. doi: 10.1111/j.1600-0447.2011.01747.x [DOI] [PubMed] [Google Scholar]
- 37.Ekholm B, Ekholm A, Adolfsson R, et al. Evaluation of diagnostic procedures in Swedish patients with schizophrenia and related psychoses. Nord J Psychiatry. 2005;59(6):457-464. doi: 10.1080/08039480500360906 [DOI] [PubMed] [Google Scholar]
- 38.Rück C, Larsson KJ, Lind K, et al. Validity and reliability of chronic tic disorder and obsessive-compulsive disorder diagnoses in the Swedish National Patient Register. BMJ Open. 2015;5(6):e007520. doi: 10.1136/bmjopen-2014-007520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kendler KS, Ohlsson H, Lichtenstein P, Sundquist J, Sundquist K. The genetic epidemiology of treated major depression in Sweden. Am J Psychiatry. 2018;175(11):1137-1144. doi: 10.1176/appi.ajp.2018.17111251 [DOI] [PubMed] [Google Scholar]
- 40.Sundquist J, Ohlsson H, Sundquist K, Kendler KS. Common adult psychiatric disorders in Swedish primary care where most mental health patients are treated. BMC Psychiatry. 2017;17(1):235. doi: 10.1186/s12888-017-1381-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Description of registers
eTable 1. Definition of phenotypes
eTable 2. Decision table for registrations of SAD and SZ
eAppendix 2. Calculation of the Familial Genetic Risk Score (FGRS)
eAppendix 3. Simulation methods
eFigure 1. Results of Simulations of Pedigrees Containing 1st-5th Degree Relatives Analyzed by FGRS as a Function of Heritability and Prevalence (c2 = 0%)
eFigure 2. Results of Simulations of Pedigrees Containing 1st-5th Degree Relatives Analyzed by FGRS as a Function of Heritability and Prevalence (c2 = 2.5%)
eFigure 3. Results of Simulations of Pedigrees Containing 1st-5th Degree Relatives Analyzed by FGRS as a Function of Heritability and Prevalence (c2 = 5%)
eFigure 4. Results of Simulations of Pedigrees Containing 1st-5th Degree Relatives Analyzed by FGRS as a Function of Heritability and Prevalence (c2 = 10%)


