Abstract
Background:
This study assessed whether antiangiogenic treatment may potentiate immune checkpoint blockade in patients with advanced renal cell carcinoma (RCC).
Materials and Methods:
This was an open-label, two-part, multicenter study involving treatment-naïve patients with advanced RCC. Part 1 consisted of a Phase I dose-escalation and expansion of pazopanib plus pembrolizumab (combination therapy). Cohorts A and B received pazopanib in combination with pembrolizumab, whereas Cohort C received pazopanib monotherapy for 9 weeks before receiving the combination therapy. Part 2 was planned as a randomized three-arm study but was not conducted.
Results:
Overall, 42 patients were enrolled (10 each in Cohorts A and B, 22 in Cohort C). The MTD was not reached and the RP2D was not declared as Cohort C was closed early because of safety concerns. The ORRs were 60% and 20% in Cohorts A and B, respectively. In Cohort C, the ORRs were 33%, 25%, and 0% in the combination therapy, pembrolizumab monotherapy, and pazopanib monotherapy groups, respectively. The median PFS was 21.95 months and 41.40 months in Cohorts A and B, respectively. Grade 3/4 AEs were observed in 90% of patients in Cohorts A and B. In Cohort C, the frequencies of Grade 3/4 AEs, SAEs, and AEs leading to dose reduction were typically high in the combination therapy group.
Conclusion:
Despite preliminary signs of efficacy, significant hepatotoxicity was observed in Cohorts A and B. The sequential schedule of pazopanib followed by pazopanib plus pembrolizumab showed reduced hepatotoxicity; however, other safety issues emerged with this approach.
Keywords: VEGF-TKI, TKI/IO, immune checkpoint inhibitor, antiangiogenic, immuno-oncology
Micro-abstract
This open-label, two-part, multicenter study involving 42 treatment-naïve patients with advanced RCC assessed the possibility of an immune checkpoint blockade in combination with antiangiogenic treatment. The overall clinical benefit rate of 60% showed encouraging efficacy across cohorts. However, significant safety issues associated with this treatment indicated that this combination is unsafe.
INTRODUCTION
Renal cell carcinoma (RCC) is the most common type of kidney cancer, and advanced RCC or metastatic RCC (mRCC) is almost always a fatal disease.1 The prognosis for patients with advanced RCC or mRCC is poor, with a 5-year survival rate of less than 10%.2
Overexpression of vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor α (PDGFR-α) has been identified in a majority of patients with RCC.3–6 Over the past decade, treatment with tyrosine kinase inhibitors (TKIs) targeting antiangiogenic pathways has been the standard of care for patients with mRCC. However, the disease invariably becomes resistant to TKI therapy,7, 8 warranting more durable responses. Beyond its proangiogenic role in RCC tumor biology, preclinical studies have implicated the VEGF pathway in tumor immune evasion.9 Accordingly, a rationale exists for combining VEGF-directed therapy with immune checkpoint inhibitors based on this cross-talk between the VEGF pathway, tumor microenvironment, and tumor-infiltrating lymphocytes.9 VEGF inhibition may modulate the host tumor microenvironment and reduce immune tumor suppression via several mechanisms, whereas the checkpoint inhibitors activate the host’s antitumor immune response by blocking negative regulatory immune signals.10, 11
Durable responses have been reported with several different checkpoint inhibitors across a broad range of human cancers, substantiating the hypothesis that cancer immunotherapy through checkpoint inhibitors is active in a range of indications.12–15 Proof-of-concept studies of immune checkpoint inhibitors have shown activity in mRCC. Nivolumab, a fully human immunoglobulin G4 (IgG4) anti– programmed death 1 (PD-1) antibody, demonstrated objective responses (~20%) and a manageable safety profile in patients with mRCC.14, 16 In a large Phase II cohort study of patients with mRCC, pembrolizumab, an anti–PD-1 antibody, demonstrated promising efficacy and acceptable tolerability.17, 18 Atezolizumab, an anti–programmed death ligand 1 (PD-L1) antibody, demonstrated durable antitumor effects in patients with mRCC.19 The preliminary results on immune checkpoint inhibitors led to a series of clinical trials that resulted in regulatory approval of nivolumab and the combination of ipilimumab plus nivolumab for the treatment of advanced RCC.
Treatment of mRCC with checkpoint inhibitors in combination with targeted agents is expected to achieve improved clinical outcomes compared with monotherapy.20 Several trials have evaluated checkpoint inhibitors in combination with targeted agents in patients with mRCC, increasing the options in frontline mRCC therapy. At the time this study began in September 2013, no data had been generated for targeted agents in combination with checkpoint inhibitors in RCC.
This open-label, two-part, multicenter study of pazopanib in combination with pembrolizumab was the first planned exploration designed to assess whether antiangiogenic treatment with pazopanib may potentiate immunotherapy with pembrolizumab in patients with treatment-naïve advanced RCC. Pazopanib, an oral angiogenesis inhibitor targeting VEGFR-1, −2, and −3; PDGFR-α and -β; and the stem cell factor receptor c-KIT, is indicated for the treatment of advanced RCC and advanced soft tissue sarcoma.21, 22 Pembrolizumab, a humanized IgG4 monoclonal antibody, binds with a high affinity to PD-1, inhibiting its interaction with PD-L1 and PD-L2. Although pembrolizumab is indicated for the treatment of various solid tumors and Hodgkin’s lymphoma, it was not yet indicated for RCC treatment at the onset of this study. The results of this study were previously disclosed as an oral presentation at the 2017 American Society of Clinical Oncology (ASCO) Annual Meeting.23
MATERIALS and METHODS
Patients
Eligible patients were aged 18 years or above; had locally advanced RCC or mRCC with predominantly clear-cell histology and no prior systemic therapy; had at least one measurable lesion as evaluated according to the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.124; had an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0 or 1 (on a scale from 0 to 5, with lower scores indicating lower disability)25; had adequate organ function; and had a left ventricular ejection fraction ≥ the lower limit of normal (determined using echocardiography or multiple-gated acquisition). The study was approved by the independent ethics committee or institutional review board for each center, and informed consent was obtained from each patient before performing study-specific procedures or assessments.
Objectives
The primary objective of the study was to determine the safety, tolerability, maximum tolerated dose (MTD), and recommended Phase II dose (RP2D) of pazopanib in combination with pembrolizumab in treatment-naïve patients with advanced RCC. Secondary objectives included determining the pharmacokinetics (PK) and clinical activity of this combination therapy.
Study design and treatments
This was an open-label, two-part, multicenter study of pazopanib and/or pembrolizumab in treatment-naïve patients with advanced RCC and mRCC.
Part 1 consisted of a Phase I dose-escalation study of pazopanib plus pembrolizumab (combination therapy), followed by an expansion study to determine the MTD and RP2D. Part 2 was a randomized three-arm study to evaluate the clinical efficacy and safety of pazopanib plus pembrolizumab compared with single-agent pazopanib and single-agent pembrolizumab. Part 2 of the study was not conducted.
Part 1: dose escalation
A modified 3+3 design was followed for dose escalation. The patients included in a cohort were assigned to one dose level for the duration of the study. Dose modifications were allowed based on the observed toxicities.
In Part 1, at least three patients were planned to receive the combination therapy (pazopanib + pembrolizumab). Starting doses were 800 mg orally once daily (QD; continuous dosing) for pazopanib and 2 mg/kg intravenously (IV) every 2 weeks (Q2W) for pembrolizumab. The maximum combination dose level was 800 mg QD (continuous dosing) pazopanib and 10 mg/kg IV (Q2W) pembrolizumab.
Patients were evaluated for a minimum of 8 weeks before the next dose level cohort was enrolled. MTD was defined as the highest dose of pazopanib in combination with the highest dose of pembrolizumab at which no more than one of six patients experienced dose-limiting toxicities (DLTs) after a minimum of 8 weeks of treatment. If two or more patients in a six-patient cohort experienced a DLT, the MTD was considered as exceeded.
DLT was defined as a drug-related adverse event (AE) starting in the first 8 weeks of treatment and meeting one of the following criteria: febrile neutropenia; Grade 4 thrombocytopenia; Grade 4 nonhematological toxicity; Grade 3 clinically significant nonhematological drug-related toxicities that could not be managed with adequate supportive therapy within 14 days of the onset of the event; aspartate aminotransferase (AST)/alanine aminotransferase (ALT) >3.0 × the upper limit of normal (ULN) with concomitant elevation in bilirubin; and any ≥Grade 2 eye pain or reduction in visual acuity that did not respond to topical therapy and did not improve to Grade 1 severity within 2 weeks of the initiation of topical therapy or required systemic treatment or a ≥Grade 3 toxicity occurring beyond the first 8 weeks of treatment.
Patients who withdrew from the study before the completion of 8 weeks of treatment for reasons other than DLT could be replaced. Of the six patients enrolled, five were evaluable and one had a DLT.
Overall, 20 patients were enrolled in the dose-expansion Cohorts A and B of Part 1 before Cohort C was added.
Cohort A (patients treated with the combination therapy [n=10]; six from the dose-escalation period and four newly enrolled): pazopanib (800 mg QD) plus pembrolizumab (2 mg/kg every 3 weeks [Q3W])
Cohort B (patients treated with the combination therapy [n=10]): pazopanib (600 mg QD) plus pembrolizumab (2 mg/kg Q3W)
The frequency of the infusion was implemented Q3W based on emerging data from the use of pembrolizumab as a single agent.
To mitigate the toxicity observed in Cohorts A and B, hence a dose expansion Cohort C was started to assess if the introduction of a 9-week safety run-in period with pazopanib would improve tolerability. Cohort C included a run-in period of 9 weeks with single-agent pazopanib to select patients who would tolerate pazopanib as a single agent before starting the combination. Following the 9-week safety run-in, patients were assessed based on laboratory and safety parameters to confirm eligibility to receive the combination therapy. Part 2 of the study was closed before enrolling patients, and the Part 1 treatment regimen for Cohort C was modified to allow continuation on single-agent pembrolizumab or pazopanib following the run-in period.
Biomarkers
Blood and tumor tissue samples were collected during the study. The date and time of biopsy for these tissue samples (fresh or archived) were recorded. Furthermore, to characterize the biomarkers related to the activity of pazopanib and pembrolizumab in this patient population, tissue biomarkers were assessed in baseline tissues (original diagnosis or a recent biopsy), tissues obtained at the end of the run-in period (Part 1, Cohort C), and tissues obtained at disease progression.
Analysis of blood biomarkers included whole blood and plasma cytokines and angiogenic factors. Whole blood peripheral blood mononuclear cells and cDNA were collected, but not analyzed. These samples may be analyzed at a later date.
Data analysis and statistical considerations
No formal statistical hypotheses were tested. Only descriptive methods were used in the analysis of the data obtained. Efficacy and safety analyses were based on all patients who received at least one dose of pazopanib or pembrolizumab (all-treated population). Analysis of the PK for pazopanib and pembrolizumab was based on all patients in the all-treated population who provided at least one evaluable PK concentration for pazopanib and pembrolizumab, respectively.
Role of the funding source
This study was sponsored by Novartis, in collaboration with Merck & Co. The responsibility for site monitoring resided with the GSK (until March 2015) and Novartis (after March 2015) field monitors. Final analyses and writing of the report were performed by Novartis and confirmed by Merck & Co. All authors had full access to the data and contributed to the development and approval of the manuscript. The corresponding author had full access to the data throughout the study and had final responsibility for the decision to submit for publication.
RESULTS
A total of 42 patients were enrolled from three centers in the United States (US) and two centers in the United Kingdom (UK). One patient did not receive any treatment because of ascites on Day 1 and was not clinically suitable for treatment.
Demographics
In all three cohorts, demographic characteristics were similar except for age (median age: 61, 57, and 61 years in Cohorts A, B, and C, respectively) and sex (50%, 70%, and 71% of patients were men in Cohorts A, B, and C, respectively; Table 1). 5 patients had liver metastasis at baseline.
Table 1.
Summary of demographic and baseline disease characteristics – All-treated population
| Cohort Aa (N=10) |
Cohort Bb (N=10) |
Cohort Cc (N=21) |
|
|---|---|---|---|
| Age, median, years | 61.0 | 57.0 | 61.0 |
| Min-max | 45–72 | 46–74 | 39–77 |
|
| |||
| Sex, n (%) | |||
| Male | 5 (50) | 7 (70) | 15 (71) |
|
| |||
| Stage at screening, n (%) | |||
| IV | 10 (100) | 10 (100) | 21(100) |
|
| |||
| Measurable disease at screening, n (%) | 10 (100) | 10 (100) | 21 (100) |
| Non-target lesion at screening, n (%) | 9 (90) | 8 (80) | 18 (86) |
|
| |||
| Metastatic disease at screening, n (%) | 10 (100) | 10 (100) | 21 (100) |
| Prior anticancer radiotherapy, n (%) | 0 | 2 (20) | 1 (5) |
|
| |||
| Any prior nephrectomy, n (%) | 9 (90) | 9 (90) | 18 (86) |
| Any cancer-related surgeries or procedures, n (%) | 2 (20) | 6 (60) | 3 (14) |
Cohort A: Patients treated with the pazopanib 800 mg QD + pembrolizumab 2 mg/kg Q3W combination.
Cohort B: Patients treated with the pazopanib 600 mg QD + pembrolizumab 2 mg/kg Q3W combination.
Cohort C run-in period: Each patient received pazopanib monotherapy at a starting dose of 800 mg orally daily for 9 weeks (pazopanib run-in period); Cohort C post–run-in period: each patient received pembrolizumab monotherapy or combination therapy (before the USM); pembrolizumab monotherapy 2 mg/kg Q3W; or remained on pazopanib monotherapy or continued with the combination therapy (after USM).
max, maximum; min, minimum; Q3W, every 3 weeks; QD, once daily; USM, urgent safety measure.
In the Cohort C post–run-in period, the demographic characteristics were similar in all three treatment groups.
Dose escalation
In this period, six patients were enrolled and treated with pazopanib 800 mg QD plus pembrolizumab 2 mg/kg IV Q2W. All patients were then included in Cohort A in the dose-expansion period.
Dose-expansion period
In Cohort A, 10 patients were enrolled (six patients from the dose-escalation period and four newly enrolled) and were treated with pazopanib 800 mg QD plus pembrolizumab 2 mg/kg IV Q3W. In Cohort B, 10 new patients were enrolled, and all patients received treatment with pazopanib 600 mg QD plus pembrolizumab 2 mg/kg IV Q3W. In Cohort C, 22 new patients were enrolled, and 21 patients were treated (run-in period only or run-in and post–run-in periods). One patient did not receive any treatment because of ascites on Day 1 and was not clinically suitable for treatment. Patient dispositions in the different periods are presented in Figure 1.
Figure 1.
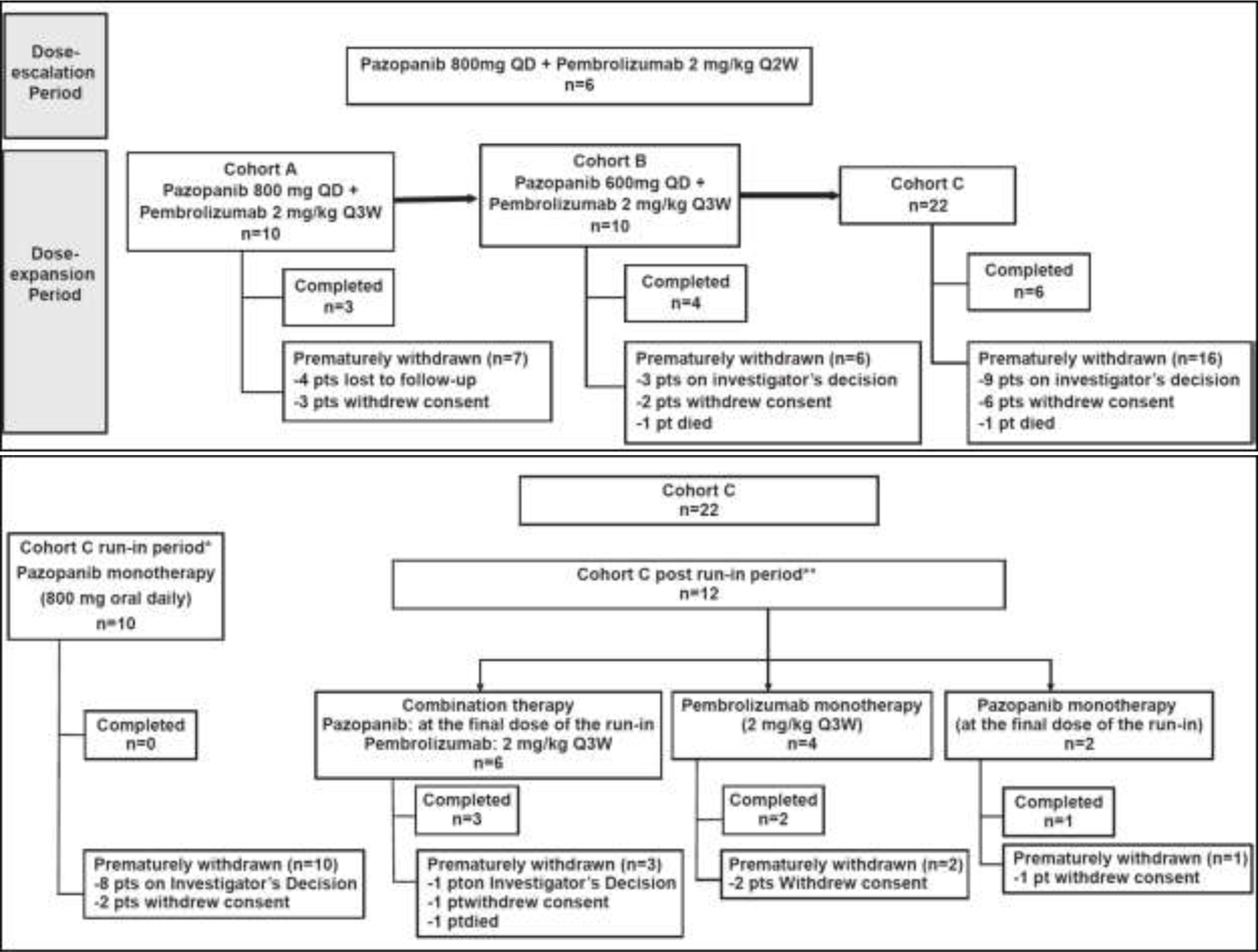
Patient Dispositions in Different Periods.
Abbreviations: pts = patients; Q2W = once every 2 weeks; Q3W = once every 3 weeks; q.d. = once daily.
*Cohort C run-in period n=10: Patients who entered in the run-in period and did not continue after run-in period. One patient who did not receive treatment, was counted in the Cohort C run-in period. This patient is one of the 8 who prematurely withdrawn from the study following Investigator’s decision.
**Cohort C post run-in period n=12: Patients who entered in the run-in period and entered in the post run-in period.
Of the 22 patients enrolled in the dose-expansion Cohort C, only 12 patients continued receiving treatment after the run-in period. At the end of the run-in period, based on the strict safety criteria, only six patients were eligible to pursue the combination therapy (pazopanib at the final dose of the run-in and pembrolizumab 2 mg/kg Q3W), four received pembrolizumab monotherapy at 2 mg/kg Q3W, and two received pazopanib monotherapy at the final dose of run-in. Although the hepatotoxicity observed in Cohorts A and B was limited, with the safety run-in introduced in Cohort C, additional AEs emerged, leading to dose reductions (40%) and interruptions (80%). In addition, three out of six patients treated with the combination therapy in Cohort C experienced DLTs (Grade 3 pneumonitis, Grade 3 bowel perforation, and Grade 4 lipase increased). Therefore, despite encouraging signs of clinical activity, the potential harm from the treatment was found to outweigh the potential benefits. Consequently, the sponsor, in agreement with the steering committee, decided to stop enrollment for Part 1, and the initiation of the combination therapy in patients already in the run-in period (Cohort C) was also stopped. Part 2 of the study was not initiated, and the Part 1 treatment regimen for Cohort C was modified to allow continuation on single-agent pembrolizumab or pazopanib following the run-in period.
Maximum tolerated dose and recommended Phase II dose
The MTD was not reached and the RP2D was not declared as the last cohort (Cohort C) was closed to further recruitment because of safety concerns.
Safety
Overview of adverse events
All patients experienced at least one AE related to the study treatment in all the cohorts. In Cohorts A and B, 90% of patients each had at least one Grade 3/4 AE. In Cohort C, the frequencies of Grade 3/4 AEs, serious adverse events (SAEs), and AEs leading to dose reduction were typically higher in the combination therapy treatment group versus the two monotherapy treatment groups (Table 2). During the run-in period, the most commonly reported AEs (>50% of patients) were diarrhea (57%) and nausea (52%).
Table 2.
Overview of AEs for Cohorts A, B, and C – All-treated population
| Cohort A | Cohort B | Cohort C | |||
|---|---|---|---|---|---|
| Pazopanib (800 mg QD) + pembrolizumab (2 mg/kg Q3W) (N=10) n (%) |
Pazopanib (600 mg QD) + pembrolizumab (2 mg/kg Q3W) (N=10) n (%) |
Pazopanib (at the final dose of the run-in) + pembrolizumab (2 mg/kg Q3W) (N=6) n (%) |
Pembrolizumab (2 mg/kg Q3W) (N=4) n (%) |
Pazopanib (at the final dose of the run-in) (N=11) n (%) |
|
| Any AE | 10 (100) | 10 (100) | 6 (100) | 4 (100) | 11 (100) |
| AEs related to study treatment | 10 (100) | 10 (100) | 6 (100) | 4 (100) | 11 (100) |
| Grade 3/4 AEs | 9 (90) | 9 (90) | 6 (100) | 2 (50) | 6 (55) |
| AEs leading to permanent discontinuation of study treatment | 8 (80) | 8 (80) | 2 (33) | 3 (75) | 1 (9) |
| AEs leading to dose reduction | 7 (70) | 5 (50) | 2 (33) | 1 (25) | 5 (45) |
| AEs leading to dose interruption | 10 (100) | 9 (90) | 5 (83) | 1 (25) | 6 (55) |
| Any SAE | 6 (60) | 6 (60) | 3 (50) | 1 (25) | 3 (27) |
| SAEs related to study treatment | 5 (50) | 4 (40) | 3 (50) | 1 (25) | 2 (18) |
| Fatal SAEs | 0 | 1 (10) | 0 | 0 | 0 |
| Fatal SAEs related to study treatment | 0 | 0 | 0 | 0 | 0 |
AE, adverse event; Q3W, every 3 weeks; QD, once daily; SAE, serious adverse event.
Dose-limiting toxicities
DLTs were reported in two patients in Cohort A, five patients in Cohort B, and four patients in Cohort C. Cohort A: Grade 3 ALT increased (two patients; 20%), Grade 3 AST increased (two patients; 20%), and Grade 3 blood bilirubin increased (one patient; 10%); Cohort B: Grade 4 ALT increased (one patient; 10%), Grade 3 AST increased (three patients; 30%), Grade 3 ALT increased (one patient; 10%), and Grade 1 and Grade 3 lipase increased (one patient each; 10%); Cohort C: DLTs were reported in one patient during the run-in period (treatment with pazopanib monotherapy) and in three patients during the post–run-in period (treatment with the combination therapy).
Run-in period (patients treated with pazopanib monotherapy): Grade 3 ALT increased (one patient); post– run-in period (patients treated with the combination therapy): Grade 3 large intestine perforation (one patient), Grade 4 lipase increased (one patient), and Grade 2 and Grade 3 pneumonitis (one patient each).
A summary of AEs for Cohorts A, B, and C is presented in Table 3. All patients had at least one AE. The most commonly reported AEs for Cohorts A and B were ALT increased (70% in each cohort) and AST increased (70% in Cohort A and 80% in Cohort B). During the post–run-in period, the most frequent AEs were diarrhea and hypertension (five patients each) in the combination therapy treatment group; diarrhea, ALT increased, and AST increased (four patients each) in the pembrolizumab monotherapy treatment group; and nausea (eight patients), diarrhea, fatigue, and vomiting (seven patients each) in the pazopanib monotherapy treatment group. During the run-in period, the most commonly reported AEs (>50% of patients) were diarrhea (12 patients; 57%) and nausea (11 patients; 52%). Diarrhea was also the most commonly reported AE during the post–run-in period (7/12 patients; 58.3%; Table 3). A summary of AEs by maximum Grade 3–5 for Cohorts A, B, and C is presented in Table 4.
Table 3.
Summary of AEs for Cohorts A, B, and C – All-treated population
| Cohort A | Cohort B | Cohort C | |||
|---|---|---|---|---|---|
| AE | Pazopanib (800 mg QD) + pembrolizumab (2 mg/kg Q3W) (N=10) n (%) |
Pazopanib (600 mg QD) + pembrolizumab (2 mg/kg Q3W) (N=10) n (%) |
Pazopanib (at the final dose of the run-in) + pembrolizumab (2 mg/kg Q3W) (N=6) n (%) |
Pembrolizumab (2 mg/kg Q3W) (N=4) n (%) |
Pazopanib (at the final dose of the run-in) (N=11) n (%) |
| Any event | 10 (100) | 10 (100) | 6 (100) | 4 (100) | 11 (100) |
| Nausea | 7 (70) | 3 (30) | 4 (67) | 0 | 8 (73) |
| Diarrhea | 6 (60) | 8 (80) | 5 (83) | 4 (100) | 7 (64) |
| Vomiting | 6 (60) | 1 (10) | 2 (33) | 0 | 7 (64) |
| Abdominal pain | 2 (20) | 3 (30) | 0 | 0 | 0 |
| Constipation | 2 (20) | 3 (30) | 0 | 2 (50) | 2 (18) |
| Dry mouth | 0 | 4 (40) | 0 | 0 | 0 |
| Arthralgia | 6 (60) | 4 (40) | 0 | 2 (50) | 0 |
| Pain in extremity | 4 (40) | 2 (20) | 0 | 0 | 0 |
| Musculoskeletal chest pain | - | - | 2 (33) | 0 | 0 |
| Fatigue | 4 (40) | 6 (60) | 3 (50) | 2 (50) | 7 (64) |
| Mucosal inflammation | 4 (40) | 1 (10) | 0 | 0 | 0 |
| Chills | 0 | 3 (30) | 0 | 0 | 0 |
| Pyrexia | 0 | 3 (30) | 0 | 0 | 0 |
| Upper respiratory tract infection | 4 (40) | 2 (20) | 0 | 0 | 0 |
| Sinusitis | 3 (30) | 0 | 0 | 0 | 0 |
| Alanine aminotransferase increased | 7 (70) | 7 (70) | 2 (33) | 4 (100) | 5 (45) |
| Aspartate aminotransferase increased | 7 (70) | 8 (80) | 1 (17) | 4 (100) | 4 (36) |
| Lipase increased | 6 (60) | 4 (40) | 4 (67) | 1 (25) | 0 |
| Amylase increased | 3 (30) | 3 (30) | 2 (33) | 1 (25) | 0 |
| Blood alkaline phosphatase increased | 3 (30) | 1 (10) | 3 (50) | 3 (75) | 3 (27) |
| Headache | 6 (60) | 4 (40) | 0 | 0 | 0 |
| Dysgeusia | 2 (20) | 3 (30) | 3 (50) | 1 (25) | 5 (45) |
| Dyspnea exertional | 4 (40) | 1 (10) | 3 (50) | 0 | 2 (18) |
| Oropharyngeal pain | 3 (30) | 1 (10) | 0 | 0 | 0 |
| Cough | 2 (20) | 3 (30) | 2 (33) | 1 (25) | 2 (18) |
| Pneumonitis | 2 (33) | 0 | 0 | ||
| Pruritus | 4 (40) | 4 (40) | 0 | 0 | 0 |
| Rash | 2 (20) | 5 (50) | 0 | 0 | 0 |
| Dry skin | 1 (10) | 3 (30) | 0 | 0 | 0 |
| Hair color changes | 1 (10) | 4 (40) | 2 (33) | 1 (25) | 1 (9) |
| Rash maculopapular | 0 | 3 (30) | 0 | 2 (50) | 1 (9) |
| Hypertension | 3 (30) | 8 (80) | 5 (83) | 2 (50) | 3 (27) |
| Hyperglycemia | 3 (30) | 2 (20) | |||
| Decreased appetite | 0 | 3 (30) | 2 (33) | 1 (25) | 5 (45) |
AE, adverse event; Q3W, every 3 weeks; QD, once daily.
Table 4.
Summary of AEs by maximum Grade 3–5 (≥10% of patients in any of the three cohorts) for Cohorts A, B, and C – All-treated population
| Cohort Aa (N=10) n (%) |
Cohort Bb (N=10) n (%) |
Cohort Cc (N=21) n (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| AE | Grade 3 | Grade 4 | Grade 5 | Grade 3 | Grade 4 | Grade 5 | Grade 3 | Grade 4 | Grade 5 |
| Any event | 7 (70) | 2 (20) | 0 | 7 (70) | 2 (20) | 1 (10) | 12 (57) | 2 (10) | 0 |
| Diarrhea | 1 (10) | 0 | 0 | 0 | 0 | 0 | 1 (5) | 0 | 0 |
| Nausea | 0 | 0 | 0 | 1 (10) | 0 | 0 | 1 (5) | 0 | 0 |
| Abdominal pain | 0 | 0 | 0 | 1 (10) | 0 | 0 | 0 | 0 | 0 |
| Pathological fracture | 1 (10) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Back pain | 0 | 0 | 0 | 1 (10) | 0 | 0 | 0 | 0 | 0 |
| Fatigue | 0 | 0 | 0 | 1 (10) | 0 | 0 | 1 (5) | 0 | 0 |
| Urinary tract infection | 0 | 0 | 0 | 1 (10) | 0 | 0 | 0 | 0 | 0 |
| ALT increased | 6 (60) | 1 (10) | 0 | 4 (40) | 1 (10) | 0 | 3 (14) | 0 | 0 |
| AST increased | 6 (60) | 0 | 0 | 5 (50) | 0 | 0 | 2 (10) | 0 | 0 |
| Lipase increased | 5 (50) | 1 (10) | 0 | 3 (30) | 1 (10) | 0 | 2 (10) | 2 (10) | 0 |
| Amylase increased | 2 (20) | 0 | 0 | 3 (30) | 0 | 0 | 1 (5) | 0 | 0 |
| Blood ALP increased | 1 (10) | 0 | 0 | 1 (10) | 0 | 0 | 1 (5) | 0 | 0 |
| Blood bilirubin increased | 1 (10) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gamma-glutamyl transferase increased | 0 | 0 | 0 | 0 | 0 | 0 | 2 (10) | 0 | 0 |
| Dyspnea | 0 | 0 | 0 | 1 (10) | 0 | 0 | 0 | 0 | 0 |
| Pulmonary embolism | 0 | 0 | 0 | 1 (10) | 0 | 0 | 1 (5) | 0 | 0 |
| Rash macular | 0 | 0 | 0 | 1 (10) | 0 | 0 | 0 | 0 | 0 |
| Hypertension | 2 (20) | 0 | 0 | 2 (20) | 0 | 0 | 5 (24) | 0 | 0 |
| Venous thrombosis | 0 | 0 | 0 | 1 (10) | 0 | 0 | 0 | 0 | 0 |
| Hyperglycemia | 2 (20) | 0 | 0 | 1 (10) | 0 | 0 | 0 | 0 | 0 |
| Decreased appetite | 0 | 0 | 0 | 1 (10) | 0 | 0 | 1 (5) | 0 | 0 |
| Hypoglycemia | 0 | 0 | 0 | 1 (10) | 0 | 0 | 0 | 0 | 0 |
| Atrioventricular block | 1 (10) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cardiac failure congestive | 1 (10) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tubulointerstitial nephritis | 1 (10) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anemia | 0 | 0 | 0 | 1 (10) | 0 | 0 | 0 | 0 | 0 |
| Portal vein thrombosis | 0 | 0 | 0 | 0 | 0 | 1 (10) | 0 | 0 | 0 |
Cohort A: Patients treated with the combination therapy: pazopanib (800 mg QD) + pembrolizumab (2 mg/kg Q3W).
Cohort B: Patients treated with the combination therapy: pazopanib (600 mg QD) + pembrolizumab (2 mg/kg Q3W).
Cohort C run-in period: Each patient received pazopanib monotherapy at a starting dose of 800 mg oral daily for 9 weeks (pazopanib run-in period); Cohort C post–run-in period: each patient received pembrolizumab monotherapy or combination therapy (before the USM); pembrolizumab monotherapy 2 mg/kg Q3W; or remained on pazopanib monotherapy or continued with the combination therapy (after USM).
AE, adverse event; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Q3W, every 3 weeks; QD, once daily; USM, urgent safety measure.
Adverse events suspected to be study drug related
Pazopanib
The incidences of AEs related to pazopanib were similar in Cohorts A and B. ALT increased, AST increased, diarrhea (in Cohorts A and B), nausea (in Cohort A only), and hypertension (in Cohort B only) were the only AEs observed in more than 50% of patients. In the Cohort C post–run-in period, the most frequent AEs related to pazopanib were diarrhea, nausea, and hypertension in the combination therapy treatment group; ALT increased and AST increased in the pembrolizumab monotherapy treatment group; and nausea, diarrhea, and vomiting in the pazopanib monotherapy treatment group (Table 5).
Table 5.
Summary of AEs related to pazopanib (≥30% of patients in any of the three treatment groups) for Cohorts A, B, and C – All-treated population
| Cohort A | Cohort B | Cohort C | |||
|---|---|---|---|---|---|
| AEs | Pazopanib (800 mg QD) + pembrolizumab (2 mg/kg Q3W) (N=10) n (%) |
Pazopanib (600 mg QD) + pembrolizumab (2 mg/kg Q3W) (N=10) n (%) |
Pazopanib + pembrolizumab (N=6) n (%) |
Pembrolizumab (N=4) n (%) |
Pazopanib (N=11) n (%) |
| Any event Diarrhea |
10 (100) 6 (60) |
10 (100) 6 (60) |
6 (100) 5 (83) |
4 (100) 2 (50) |
11 (100) 6 (55) |
| Nausea Vomiting |
6 (60) 5 (50) |
3 (30) 1 (10) |
3 (50) 1 (17) |
0 0 |
6 (55) 6 (55) |
| Fatigue Mucosal inflammation |
4 (40) 4 (40) |
5 (50) 1 (10) |
2 (33) 0 |
1 (25) 0 |
4 (36) 0 |
| Alanine aminotransferase increased Aspartate aminotransferase increased |
7 (70) 7 (70) |
6 (60) 7 (70) |
1 (17) 1 (17) |
3 (75) 3 (75) |
5 (45) 3 (27) |
| Blood alkaline phosphatase increased Pruritus |
3 (30) 3 (30) |
0 1 (10) |
2 (33) 0 |
2 (50) 0 |
2 (18) 0 |
| Rash Hair color changes |
2 (20) 1 (10) |
3 (30) 4 (40) |
0 2 (33) |
0 1 (25) |
0 1 (9) |
| Rash maculopapular Hypertension |
0 3 (30) |
3 (30) 7 (70) |
0 3 (50) |
0 0 |
0 1 (9) |
| Dysgeusia | - | - | 2 (33) | 1 (25) | 4 (36) |
AE, adverse event; Q3W, every 3 weeks; QD, once daily.
Pembrolizumab
In Cohorts A and B, the most commonly reported (in ≥50% of patients in both cohorts) AEs suspected to be related to pembrolizumab were AST increased and ALT increased. In the Cohort C post–run-in period, diarrhea was the most commonly reported AE related to pembrolizumab for both treatment groups (33% of patients in the combination therapy treatment group and 50% of patients in the pembrolizumab monotherapy treatment group; Table 6).
Table 6.
Summary of AEs related to pembrolizumab for Cohorts A, B, and C – All-treated population
| Cohort A | Cohort B | Cohort C | ||
|---|---|---|---|---|
| AEs | Pazopanib (800 mg QD) + pembrolizumab (2 mg/kg Q3W) (N=10) n (%) |
Pazopanib (600 mg QD) + pembrolizumab (2 mg/kg Q3W) (N=10) n (%) |
Pazopanib + pembrolizumab (N=6) n (%) |
Pembrolizumab (N=4) n (%) |
| Any event | 10 (100) | 10 (100) | 0 | 0 |
| Aspartate aminotransferase increased Alanine aminotransferase increased |
7 (70) 6 (60) |
6 (60) 5 (50) |
0 0 |
0 0 |
| Lipase increased Diarrhea |
0 5 (50) |
0 3 (30) |
2 (33) 2 (33) |
1 (25) 2 (50) |
| Arthralgia Pneumonitis |
0 0 |
0 0 |
0 2 (33) |
2 (50) 0 |
AE, adverse event; Q3W, every 3 weeks; QD, once daily.
Deaths and other serious or clinically significant adverse events
Deaths
A total of two patients died; one patient from Cohort B died while on treatment (death was not suspected to be related to the study treatment, neither with pembrolizumab nor with pazopanib) and the other from Cohort C died of an unknown cause during follow-up, 7 months after the last dose of pazopanib in the combination therapy.
Serious adverse events
Serious adverse events suspected to be related to pazopanib
In Cohorts A and B, the most commonly reported SAE related to pazopanib was ALT increased (30% of patients in Cohort A and 40% of patients in Cohort B). In Cohort B, AST increased (three patients; 30%) was also frequently reported as an SAE, suspected to be related to pazopanib.
In Cohort C, liver function test increased, diarrhea, large intestine perforation, dehydration, hepatic function abnormal, and pneumonitis (one patient each) were reported as SAEs related to pazopanib.
Serious adverse events suspected to be related to pembrolizumab
In Cohorts A and B, ALT increased and AST increased were the most common SAEs suspected to be related to pembrolizumab. The proportion of patients having these drug-related SAEs was lower in Cohort A (ALT increased: 20% of patients; AST increased: 10% of patients) than in Cohort B (ALT increased: 40% of patients; AST increased: 20% of patients).
In Cohort C, diarrhea, large intestine perforation, and pneumonitis (one patient each) were the SAEs suspected to be related to pembrolizumab.
Extent of exposure
Pazopanib
Pazopanib exposure was similar in Cohort A and Cohort B, with numerical differences that could be attributed to the small number of patients (n=10) in each cohort. Overall, 90% and 70% of patients in Cohorts A and B, respectively, were treated for less than 18 months (Table 7).
Table 7.
Summary of exposure to pazopanib for Cohorts A, B, and C – All-treated population
| Cohort A | Cohort B | Cohort C | ||||
|---|---|---|---|---|---|---|
| Pazopanib (800 mg QD) + pembrolizumab (2 mg/kg Q3W) (N=10) |
Pazopanib (600 mg QD) + pembrolizumab (2 mg/kg Q3W) (N=10) |
Pazopanib (at the final dose of the run-in) + pembrolizumab (2 mg/kg Q3W) (N=6) n (%) |
Pembrolizumab (2 mg/kg Q3W) (N=4) n (%) |
Pazopanib (at the final dose of the run-in) (N=11) n (%) |
||
| Patient daily dose (mg) a | Mean (SD) | 568.4 (224.52) | 503.9 (120.26) | 610.7 (189.43) | 706.3 (187.50) | 691.6 (110.36) |
| Median Min-max |
586.9 238–800 |
556.6 265–600 |
610.9 335–800 |
800.0 425–800 |
688.4 538–800 |
|
| Time on study treatment (months) b | Mean (SD) Median |
8.0 (7.43) 4.4 |
10.5 (13.29) 3.3 |
12.3 (8.32) 11.4 |
1.8 (0.19) 1.9 |
2.0 (1.15) 1.9 |
| Min-max <3 months |
1–23 3 (30) |
1–41 5 (50) |
4–25 0 |
2–2 4 (100) |
0–4 9 (82) |
|
| Time on study treatment categories, n (%) | 3 to <6 months 6 to <12 month s |
3 (30) 1 (10) |
1 (10) 1 (10) |
2 (33) 1 (17) |
0 0 |
2 (18) 0 |
| 12 to <18 months ≥18 months |
2 (20) 1 (10) |
0 3 (30) |
2 (33) 1 (17) |
0 0 |
0 0 |
|
The patient daily dose (the cumulative dose divided by the duration of exposure) was calculated for each patient first, and the summary statistics were calculated based on the patient’s average daily dose.
The time on study drug does not exclude dose interruptions.
max, maximum; min, minimum; Q3W, every 3 weeks; QD, once daily; SD, standard deviation.
In the Cohort C run-in and post–run-in periods, patients in the combination therapy treatment group received pazopanib for a longer duration (median exposure: 11.4 months) compared with those in the monotherapy treatment groups (pembrolizumab, median exposure: 1.9 months; pazopanib, median exposure: 1.9 months). None of the patients in either of the monotherapy treatment groups received pazopanib for 6 months or more.
Dose reduction/dose interruptions of pazopanib
Overall, seven (70%), six (60%), and nine (43%) patients had pazopanib dose reductions and seven (70%), seven (70%), and 11 (52%) patients had pazopanib dose interruptions in Cohorts A, B, and C, respectively. The primary reason for pazopanib dose reductions was AEs in 78%, 71%, and 92% of patients and the primary reason for pazopanib dose interruptions was AEs in 57%, 70%, and 88% of patients in Cohorts A, B, and C, respectively. Most pazopanib dose interruptions lasted for less than 7 days (~60%, and in most cases, patients were able to restart treatment).
Adverse events leading to dose adjustment and/or dose interruption
The most commonly reported AEs requiring dose adjustment or interruption were ALT increased and AST increased (five patients in each of the three cohorts). In general, AEs were resolved after the dose was decreased or temporarily interrupted or after the event was treated with appropriate interventionThe dose used of systemic steroids to treat AEs ranged from 4mg to 100mg. The AEs requiring treatment with glucocorticoids include pneumonitis (n=3), transaminitis (n=1), diarrhea (n=2), rash (n=3) LFTs and increased lipase (n=1 each).
Pembrolizumab
Compared with Cohort B, the mean and median times on pembrolizumab monotherapy were higher in Cohort A; however, in both cohorts, 50% of patients received treatment for 18 months or more.
In the Cohort C post–run-in period, exposure to pembrolizumab was similar in the combination therapy group versus the pembrolizumab monotherapy group. Most patients in each treatment group were treated for less than 18 months.
Dose interruptions of pembrolizumab
Most patients had pembrolizumab dose delays in Cohort A (90% of patients) and Cohort B (80% of patients). Dose delays of pembrolizumab were less frequent in patients in Cohort C (24% of patients). No dose interruption of pembrolizumab occurred in Cohorts A and B. One patient had a pembrolizumab dose interruption in Cohort C.
Efficacy results
Best response:
The overall response rates (ORRs; complete response [CR] or partial response [PR]) were 60% (6/10 patients, two CRs and four PRs) and 20% (2/10 patients, one CR and one PR) in Cohorts A and B, respectively. Two patients (20%) in Cohort A and one patient (10%) in Cohort B had CR. Progressive disease (PD) was reported in one patient in Cohort B. Stable disease (SD) was observed in four patients (40%) in Cohort A and in seven patients (70%) in Cohort B. The clinical benefit rate (CBR) was the same for both cohorts (60%).
In Cohort C, the ORRs (CR or PR) were 33%, 25%, and 0% in patients from the combination therapy, pembrolizumab monotherapy, and pazopanib monotherapy treatment groups, respectively. CR was reported in one patient (25%) treated with pembrolizumab monotherapy. PD was reported in one patient each from the combination therapy and pembrolizumab monotherapy groups. SD was observed in three patients (50%) treated with the combination therapy, two patients (50%) treated with pembrolizumab, and six patients (55%) treated with pazopanib monotherapy (Table 8).
Table 8.
Summary of investigator-assessed best confirmed response for Cohorts A, B, and C (RECIST 1.1 criteria) – All-treated population
| Cohort A | Cohort B | Cohort C | |||
|---|---|---|---|---|---|
|
| |||||
| Pazopanib (800 mg QD) + pembrolizumab (2 mg/kg Q3W) (N=10) n (%) |
Pazopanib (600 mg QD) + pembrolizumab (2 mg/kg Q3W) (N=10) n (%) |
Pazopanib (at the final dose of the run-in) + pembrolizumab (2 mg/kg Q3W) (N=6) n (%) |
Pembrolizumab (2 mg/kg Q3W) (N=4) n (%) |
Pazopanib (at the final dose of the run-in) (N=11) n (%) |
|
|
Best response
| |||||
| CR | 2 (20) | 1 (10) | 0 | 1 (25) | 0 |
| PR | 4 (40) | 1 (10) | 2 (33) | 0 | 0 |
|
| |||||
| SD | 4 (40) | 7 (70) | 3 (50) | 2 (50) | 6 (55) |
| PD | 0 | 1 (10) | 1 (17) | 1 (25) | 0 |
|
| |||||
| Overall response rate | |||||
| CR + PR | 6 (60) | 2 (20) | 2 (33) | 1 (25) | 0 |
|
| |||||
| 95% CIa | (26.2, 87.8) | (2.5, 55.6) | (4.3, 77.7) | (0.6, 80.6) | (0.0, 28.5) |
|
Clinical benefit rate
| |||||
| CR or PR or ≥6-month SD | 6 (60) | 6 (60) | 3 (50) | 2 (50) | 0 |
| 95% CIa | (26.2, 87.8) | (26.2, 87.8) | (11.8, 88.2) | (6.8, 93.2) | (0.0, 28.5) |
|
| |||||
| 18-month PFS rate (95% CI) b | 0.56 (0.22, 0.96) | 0.88 (0.28, 0.99) | 0.20 (0.01, 0.72) | 0.50 (0.16, 1.00) | 1.00 (0.72, 1.00) |
The 95% exact binomial CI (Clopper and Pearson, 1934) was calculated using the FREQ procedure (with the EXACT statement).
The 95% exact binomial CI (Clopper and Pearson, 1934) was calculated using the FREQ procedure (with the EXACT statement).
CI, confidence interval; CR, complete response; PD, progressive disease; PFS, progression-free survival; PR, partial response; Q3W, every 3 weeks; QD, once daily; RECIST, Response Evaluation Criteria in Solid Tumours; SD, stable disease.
In the pazopanib monotherapy treatment group, 45% of the patients were not evaluable (best overall response could not be derived in these patients because only one evaluation was available, and no confirmation response could be derived) and the other 55% had SD.
Progression-free survival
The 18-month progression-free survival (PFS) rates were 56% and 88% in Cohorts A and B, respectively. The median PFS (95% confidence interval [CI]) was 21.95 (7.62, not applicable) months in Cohort A and 41.40 (2.76, 41.69) months in Cohort B. Similar findings were observed in the efficacy endpoints when the modified RECIST criteria were used.
In the Cohort C post–run-in period, the 18-month PFS rate was 20% in patients treated with the combination therapy and 50% in patients treated with pembrolizumab monotherapy. No events occurred at or before 18 months in the group treated with pazopanib monotherapy.
The median PFS (95% CI) was 15.90 (4.90, 26.05) months in patients treated with the combination therapy and 13.16 (1.58, not applicable) months in patients treated with pembrolizumab monotherapy. Similar findings were observed for the efficacy endpoints when the modified RECIST criteria were used.
Pharmacokinetics
At steady state, pazopanib Ctrough concentrations were similar between the pazopanib monotherapy and combination therapy groups in the Cohort C run-in and post–run-in periods. Pazopanib concentrations as shown by Cmax increased with an increase in dose from 600 to 800 mg. In Cohort B, the geometric mean Ctrough plasma pazopanib concentrations at Weeks 9 and 10 were 17.4 μg/mL and 32.7 μg/mL, respectively. The geometric mean trough plasma pazopanib concentration at Week 4 was 28.1 μg/mL in the Cohort C run-in period. The geometric mean trough plasma pazopanib concentration was 32.1 μg/mL at Week 10 in the Cohort C post–run-in period.
In Cohort A, pembrolizumab steady-state concentrations were generally attained after Cycle 7, and the geometric mean Ctrough concentrations were between 42.5 µg/mL and 53.5 µg/mL at steady state. The coefficient of variation of the geometric mean trough concentrations of pembrolizumab in Cohort A was between 13.4% and 46.7% beyond Cycle 7 at steady state.
In Cohort B, pembrolizumab steady-state concentrations were generally attained after Cycle 13, and the geometric mean Ctrough concentrations were between 37.2 µg/mL and 42.4 µg/mL at steady state. The coefficient of variation of the geometric mean trough concentrations of pembrolizumab in Cohort B was between 6.1% and 11.9% beyond Cycle 13 at steady state.
In the Cohort C post–run-in period, pembrolizumab steady-state concentrations were attained after Cycle 7, and the geometric mean Ctrough concentrations were between 23.6 µg/mL and 35.1 µg/mL at steady state. The coefficient of variation of the geometric mean trough concentrations of pembrolizumab in Cohort C was between 26.9% and 44.7% at steady state.
Exploratory biomarkers
The expression of immune cell proteins in tissue (monitored using immunohistochemistry) and in circulating whole blood (monitored using flow cytometry) was not constant across patients in the three cohorts. The mean tumor-relative mRNA levels at baseline were similar among the three cohorts for the immune cell–related genes APOE, CD163, CD209, CD68, CD8A, CD8B, CHIT1, CSF1R, CXCL5, FLT3LG, FOXP3, GNLY, GZMA, GZMH, MARCO, MSR1, PDCD1, and PRF1. There were no apparent differences across cohorts and no large patient-to-patient variability for all immune cell biomarkers tested.
DISCUSSION
Single-agent therapy with immune checkpoint inhibitors has demonstrated clinical benefit in patients with mRCC.26 Studies have shown a role for VEGF in immune evasion as well as tumor angiogenesis in several tumor types.9 Moreover, in mRCC, VEGF has been reported to increase the levels of regulatory T cells, thereby maintaining immunosuppression.27 Combination of immune checkpoint inhibitors and VEGF inhibitors is a proposed hypothesis for the interplay between the immune system and angiogenesis in RCC. Preclinical studies have tested the combination of VEGF signaling blockade and immune checkpoint inhibition, and have demonstrated synergistic effects of this combination.9 Pazopanib is a standard of care treatment for mRCC.22 Immunomodulatory effects of pazopanib have been demonstrated through in vitro and in vivo studies, providing a strong rationale for the use of pazopanib in combination with immune checkpoint inhibitors.9 The efficacy of single-agent pembrolizumab has been demonstrated recently in KEYNOTE-427.28 The current Phase II study was designed to assess the safety and efficacy of pazopanib plus pembrolizumab in mRCC.
The combination showed encouraging efficacy with an overall CBR of 60%. Nevertheless, significant hepatotoxicity, with the most frequent (≥30%) SAEs being ALT and AST elevations, made this combination unsafe to take forward. Notably, hepatotoxicity reduction through sequential administration of pazopanib followed by the combination therapy (pazopanib + pembrolizumab) was successful. However, other safety issues emerged in patients treated with this approach.
TKI-immune checkpoint inhibitor combinations have demonstrated superior efficacy with manageable toxicity in selected pivotal studies, leading to the approval of such combinations. Combined treatment with pembrolizumab plus axitinib in treatment-naïve patients with previously advanced RCC resulted in a significantly longer overall survival (OS) and PFS and a significantly higher objective response rate than with sunitinib, with a similar overall toxicity in the two groups.29 A longer PFS was observed with avelumab plus axitinib versus sunitinib alone in patients with previously untreated advanced RCC.30 This study led to the approval of the avelumab plus axitinib combination for treating patients with treatment-naïve advanced RCC. A randomized Phase III trial of atezolizumab combined with bevacizumab showed a longer PFS compared with sunitinib in previously untreated PD-L1 positive patients with mRCC; however, no OS benefit was observed with this combination.31 Nivolumab and cabozantinib showed superior efficacy in terms of PFS, OS, and response rate, and the benefit was consistent compared with sunitinib in numerous subgroups, which led to the approval of this combination as a first-line therapy for RCC.32
The clinical activity of VEGF-targeted therapies in combination with PD-L1/PD-1 blockade has been demonstrated in different studies,26, 33, 34 but these combinations showed substantial toxicities in a majority of the studies, thereby limiting their use34 even though some of these combinations, such as axitinib plus avelumab30 and axitinib plus pembrolizumab, were successful in demonstrating superior efficacy.29, 35 Selection of suitable TKI appears to play an important role in tolerability of TKI and immune checkpoint inhibitor combinations.36
Unlike other TKIs used in RCC, pazopanib has a black box warning for causing severe and fatal hepatotoxicity.21 Fatal hepatic failure was reported in 0.2% of patients in the pivotal trial; many of the Grade 3/4 toxicities noted with pazopanib in patients with RCC were hepatic in nature, with elevations of AST, ALT, and bilirubin levels.22 A meta-analysis of pazopanib trials showed the occurrence of transaminase elevations in most patients treated with pazopanib at 3–9 weeks after therapy initiation, which resolved with a median time of 30 days following onset. In addition, it was noted that the rechallenge after resolution of the event was successful without liver failure. However, close monitoring of liver chemistry was recommended by the authors.37 Furthermore, in a real-world study, which retrospectively assessed the incidence of pazopanib-induced liver toxicity in patients with mRCC in the UK, the authors noticed lower hepatotoxicity rates (30%) compared with that in clinical trials, with most cases being mild and requiring no treatment modifications; age, PS, or presence of liver metastases did not show any association with hepatotoxicity.38 Pazopanib-induced active hepatitis39 and lethal hepatotoxicity40 have been recently described in case reports. In a patient who was treated with nivolumab in the third-line setting followed by fourth-line pazopanib, the authors hypothesized that lethal hepatotoxicity induced by pazopanib was because of the enhanced immunological reactions that were initially induced by nivolumab; the use of immunosuppressive treatment, such as glucocorticoids, is recommended in cases of severe hepatotoxicity due to treatment with a TKI followed by an immune checkpoint inhibitor.40 Thus far, the mechanism of pazopanib-induced hepatotoxicity remains unclear, although several preclinical and clinical studies have attempted to understand the possible mechanism.41–43
The CheckMate-016 study, which was in part designed to assess the combination of nivolumab with sunitinib or pazopanib in mRCC, also reported substantial toxicity with greater frequencies of high-grade treatment-related AEs and AEs leading to discontinuation than those previously observed with nivolumab, sunitinib, or pazopanib monotherapy.34 Although sustained antitumor activity was observed with the nivolumab and pazopanib combination (ORR 45%), DLTs including abnormal liver function led to the discontinuation of the study.44 It appears that the tolerability of such combinations is highly dependent on the choice of TKI, with a possible synergistic or additive toxicity effect.36
Several factors might have been associated with the outcomes in patients treated with these combinations, and there is a significant need to identify the prognostic factors associated with these outcomes. Biomarker analysis in the IMmotion 150 study demonstrated that relative expression of angiogenesis T-effector/interferon-γ response and myeloid inflammatory gene expression signatures were strongly and differentially associated with PFS within and across treatments. Therefore, further understanding of these molecular profiles might offer mechanistic insights into how angiogenesis blockage could overcome the resistance to the immune checkpoint blockade.45
CONCLUSION
Despite preliminary signs of efficacy, the harm from the combination of pazopanib and pembrolizumab appeared to outweigh the potential benefits in patients with treatment-naive advanced or metastatic RCC. Hence, further clinical development of the combination will not be pursued in this population. This pazopanib plus pembrolizumab trial highlights the importance of well-conducted clinical research and is a cautionary note about the application of PD-1 blockade–based combination therapy. It shows that differences in the choice of TKI influence the tolerability when combined with immune checkpoint inhibitor therapy. TKI/checkpoint inhibitor combinations remain a potential option for many patients with mRCC; however, there is a need to balance both safety and efficacy in future algorithms.
Supplementary Material
Highlights.
This study was the first to assess combination of pazopanib with pembrolizumab in patients with aRCC.
The combination showed an encouraging efficacy, with an overall CBR of 60%.
However, significant hepatotoxicity made this combination unsafe to pursue further.
This trial is a cautionary note about the application of PD-1 blockade–based combination therapy.
Differences in TKI might influence the tolerability when combined with CPI immune therapy.
CLINICAL PRACTICE POINTS.
The TKI/immune checkpoint inhibitor combinations that have been approved for the treatment of advanced RCC or mRCC are axitinib plus pembrolizumab and axitinib plus avelumab. With no previous data available on the TKI/immune oncology combination at the inception of this study, this was the first study designed to evaluate the safety and efficacy of this combination.
The current study of pazopanib in combination with pembrolizumab was the first planned exploration designed to assess whether antiangiogenic treatment with pazopanib may potentiate immunotherapy with pembrolizumab in patients with advanced RCC. The combination showed an encouraging efficacy, with an overall CBR of 60%. However, significant hepatotoxicity made this combination unsafe to pursue further.
This pazopanib plus pembrolizumab trial highlights the importance of well-conducted clinical research and is a cautionary note about the application of PD-1 blockade–based combination therapy. It shows that differences in TKI choice influence tolerability when combined with checkpoint inhibitor therapy. TKI/IO remains a potential option for many patients with mRCC; however, both safety and efficacy need to be balanced in future algorithms.
Acknowledgments
We thank Anuradha Bandaru of Novartis Healthcare Pvt Ltd for medical writing and editorial support.
This study was sponsored by Novartis, in collaboration with Merck & Co. The responsibility for site monitoring resided with the GSK (until March 2015) and Novartis (after March 2015) field monitors. Final analyses and writing of the report were performed by Novartis and confirmed by Merck & Co. All authors had full access to the data and contributed to the development and approval of the manuscript. The corresponding author had full access to the data throughout the study and had final responsibility for the decision to submit for publication.
Funding
This study was sponsored by Novartis, in collaboration with Merck & Co. (Clinicaltrials.gov identifier NCT02014636)
Abbreviations
- AE
adverse event
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CBR
clinical benefit rate
- CI
confidence interval
- CR
complete response
- DLT
dose-limiting toxicity
- ECOG PS
Eastern Cooperative Oncology Group performance status
- IgG
immunoglobulin G
- IV
intravenous
- mRCC
metastatic renal cell carcinoma
- MTD
maximum tolerated dose
- ORR
overall response rate
- OS
overall survival
- PD
progressive disease
- PD-1
programmed death 1
- PDGFR
platelet-derived growth factor receptor
- PD-L1
programmed death ligand 1
- PFS
progression-free survival
- PK
pharmacokinetics
- PR
partial response
- Q2W
every 2 weeks
- Q3W
every 3 weeks
- QD
once daily
- RCC
renal cell carcinoma
- RP2D
recommended Phase II dose
- SAE
serious adverse event
- SD
stable disease
- TKI
tyrosine kinase inhibitor
- UK
United Kingdom
- US
United States
- VEGFR
vascular endothelial growth factor receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
MHV reports grants from BMS; personal fees from Corvus, Exelixis, and Eisai; and grants and personal fees from Pfizer, outside the submitted work. DFMcD reports grants from BMS, Pfizer, Merck, Alkemes, Genetech, Exelixis, and X4 Pharma and personal fees from BMS, EMP Sereno, Eli Lilly Company, Merck, Pfizer, and Alkemes, outside the submitted work. TA reports personal fee from Bayer, Biontech, Beigene, Servier, Roche, outside the submitted work. MV reports personal fees from MSD, AstraZeneca, and BMS, outside the submitted work. PA and IN are employees of Novartis Pharma AG, Basel, Switzerland. AR is an employee of Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Data sharing statement
Novartis is committed to sharing the access to patient-level data and supporting clinical documents from eligible studies with qualified external researchers. These requests are reviewed and approved by an independent review panel based on scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations.
This trial data availability is according to the criteria and process described on www.clinicalstudydatarequest.com.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BM, Colao A. Complications of Cushing’s syndrome: state of the art. The lancet Diabetes & endocrinology 2016;4:611–629. [DOI] [PubMed] [Google Scholar]
- 3.Jacobsen J, Rasmuson T, Grankvist K, Ljungberg B. Vascular endothelial growth factor as prognostic factor in renal cell carcinoma. J Urol 2000;163:343–347. [PubMed] [Google Scholar]
- 4.Sulzbacher I, Birner P, Traxler M, Marberger M, Haitel A. Expression of platelet-derived growth factor-alpha alpha receptor is associated with tumor progression in clear cell renal cell carcinoma. Am J Clin Pathol 2003;120:107–112. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi A, Sasaki H, Kim SJ, et al. Markedly increased amounts of messenger RNAs for vascular endothelial growth factor and placenta growth factor in renal cell carcinoma associated with angiogenesis. Cancer Res 1994;54:4233–4237. [PubMed] [Google Scholar]
- 6.Tsuchiya N, Sato K, Akao T, et al. Quantitative analysis of gene expressions of vascular endothelial growth factor-related factors and their receptors in renal cell carcinoma. Tohoku J Exp Med 2001;195:101–113. [DOI] [PubMed] [Google Scholar]
- 7.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011;331:1565–1570. [DOI] [PubMed] [Google Scholar]
- 8.O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol 2019;16:151–167. [DOI] [PubMed] [Google Scholar]
- 9.Nair S, Boczkowski D, Moeller B, Dewhirst M, Vieweg J, Gilboa E. Synergy between tumor immunotherapy and antiangiogenic therapy. Blood 2003;102:964–971. [DOI] [PubMed] [Google Scholar]
- 10.Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer 2019;18:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amin A, Hammers H. The Evolving Landscape of Immunotherapy-Based Combinations for Frontline Treatment of Advanced Renal Cell Carcinoma. Front Immunol 2018;9:3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbst RS, Gordon MS, Gregg DF, et al. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. J. Clin. Oncol, 31 (suppl) (2013), p. 3000. [Google Scholar]
- 16.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol 2015;33:1430–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDermott DF, Lee JL, Szczylik C, et al. Pembrolizumab monotherapy as first-line therapy in advanced clear cell renal cell carcinoma (accRCC): Results from cohort A of KEYNOTE-427. Journal of Clinical Oncology 2018; 36: suppl 4500–4500.. [Google Scholar]
- 18.Donskov F, McDermott DF, Lee JL, et al. KEYNOTE-427 Cohort A: Pembrolizumab Monotherapy as First-Line Therapy in Advanced Clear Cell Renal Cell Carcinoma (ccRCC). Annals of Oncology (2018) 29 (suppl_8): viii303–viii331. [Google Scholar]
- 19.McDermott DF, Sosman JA, Sznol M, et al. Atezolizumab, an Anti-Programmed Death-Ligand 1 Antibody, in Metastatic Renal Cell Carcinoma: Long-Term Safety, Clinical Activity, and Immune Correlates From a Phase Ia Study. J Clin Oncol 2016;34:833–842. [DOI] [PubMed] [Google Scholar]
- 20.Albiges L, Fay AP, Xie W, et al. Efficacy of targeted therapies after PD-1/PD-L1 blockade in metastatic renal cell carcinoma. Eur J Cancer 2015;51:2580–2586. [DOI] [PubMed] [Google Scholar]
- 21.US Food & Drug Administration. Votrient (pazopanib) prescribing information https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/022465s029lbl.pdf.; 2020. Accessed 01 March 2021.
- 22.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28:1061–1068. [DOI] [PubMed] [Google Scholar]
- 23.Chowdhury S, Mcdermott DF, Voss MH, et al. A phase I/II study to assess the safety and efficacy of pazopanib (PAZ) and pembrolizumab (PEM) in patients (pts) with advanced renal cell carcinoma (aRCC). Journal of Clinical Oncology 35, no. 15_suppl (May 20, 2017) 4506–4506. [Google Scholar]
- 24.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–655. [PubMed] [Google Scholar]
- 26.Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med 2018;50:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adotevi O, Pere H, Ravel P, et al. A decrease of regulatory T cells correlates with overall survival after sunitinib-based antiangiogenic therapy in metastatic renal cancer patients. J Immunother 2010;33:991–998. [DOI] [PubMed] [Google Scholar]
- 28.Nosaki K, Saka H, Hosomi Y, et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1–positive advanced non–small-cell lung cancer: Pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer 2019;135:188–195. [DOI] [PubMed] [Google Scholar]
- 29.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019;380:1116–1127. [DOI] [PubMed] [Google Scholar]
- 30.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019;380:1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rini BI, Powles T, Atkins MB, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 2019;393:2404–2415. [DOI] [PubMed] [Google Scholar]
- 32.Choueiri TK, Powles T, Burotto MT, et al. Nivolumab + cabozantinib vs sunitinib in first-line treatment for advanced renal cell carcinoma: First results from the randomized phase III CheckMate 9ER trial. Annals of Oncology (2020) 31 (suppl_4): S1142–S1215. [Google Scholar]
- 33.Choueiri TK, Larkin J, Oya M, et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): an open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol 2018;19:451–460. [DOI] [PubMed] [Google Scholar]
- 34.Amin A, Plimack ER, Ernstoff MS, et al. Safety and efficacy of nivolumab in combination with sunitinib or pazopanib in advanced or metastatic renal cell carcinoma: the CheckMate 016 study. J Immunother Cancer 2018;6:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powles T, Plimack ER, Soulieres D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol 2020;21:1563–1573. [DOI] [PubMed] [Google Scholar]
- 36.Drake G Clinical Advances in Hematology & Oncology Volume 14, Issue 2. https://www.hematologyandoncology.net/files/2016/02/Drake-1.pdf. (Accessed February 2020). [Google Scholar]
- 37.Powles T, Bracarda S, Chen M, et al. Characterisation of liver chemistry abnormalities associated with pazopanib monotherapy: a systematic review and meta-analysis of clinical trials in advanced cancer patients. Eur J Cancer 2015;51:1293–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jain A, Handorf E, Mantia-Smaldone G. The use of adjuvant treatment in stage I endometrioid endometrial cancer in the National Cancer Database (NCDB). Journal of Clinical Oncology 33, no. 15_suppl (May 20, 2015) 5599–5599. [Google Scholar]
- 39.Ezponda A, De La Huebra IG, Calvo M, Idoate MA, Vivas I. Chronic active hepatitis induced by Pazopanib mimicking hypervascular liver metastases in a patient with recurrent soft tissue sarcoma: A case report. Oncol Lett 2018;16:4043–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Todo M, Kondo H, Hayashi T, et al. Delayed nivolumab-induced hepatotoxicity during pazopanib treatment for metastatic renal cell carcinoma: An autopsy case. IJU Case Rep 2019;2:272–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cetin B, Yilmaz GE, Armagan B, et al. Pazopanib-Induced Hepatotoxicity in an Experimental Rat Model. Chemotherapy 2018;63:39–45. [DOI] [PubMed] [Google Scholar]
- 42.Henriksen JN, Bottger P, Hermansen CK, et al. Pazopanib-Induced Liver Toxicity in Patients With Metastatic Renal Cell Carcinoma: Effect of UGT1A1 Polymorphism on Pazopanib Dose Reduction, Safety, and Patient Outcomes. Clin Genitourin Cancer 2020;18:62–68 e62. [DOI] [PubMed] [Google Scholar]
- 43.Choudhury Y, Toh YC, Xing J, et al. Patient-specific hepatocyte-like cells derived from induced pluripotent stem cells model pazopanib-mediated hepatotoxicity. Sci Rep 2017;7:41238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amin A, Plimack ER, Infante JR, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with sunitinib or pazopanib in patients (pts) with metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology 32, no. 15_suppl (May 20, 2014) 5010–5010. [Google Scholar]
- 45.McDermott DF, Huseni MA, Atkins MB, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med 2018;24:749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


