Abstract
It is unclear whether hepatic artery infusion chemotherapy (HAIC) or transcatheter arterial chemoembolization (TACE) is more efficient in the combination therapy of hepatocellular carcinoma (HCC). Head-to-head comparisons among HAIC-related therapies are lacking. For this network meta-analysis, PubMed, EMBASE and Cochrane Library databases were searched up to April 1, 2022. Randomized controlled trials (RCTs) were eligible if they evaluated the use or prolongation of TACE or HAIC in patients with advanced HCC and reported or collected survival data. A network meta-analysis was performed to synthesize data and make direct and indirect comparisons between treatments. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated to explore the efficacy of various treatment options on overall survival (OS), odds ratios (ORs) with 95% CI were used for overall response rate (ORR), whereas risk ratios (RRs) with 95% CI were used for serious adverse events (SAEs). The analysis of 7 trials including a total of 1,073 patients found that sorafenib with HAIC-oxaliplatin improved survival (HR=0.33, 95% CI: 0.25-0.44); the ORR was also improved in patients treated with sorafenib plus HAIC-oxaliplatin and sorafenib plus PF-HAIC (OR=22.18, 95% CI: 10.69-52.56; and OR=2.72, 95% CI: 1.43-5.36, respectively). The incidence of liver injury was elevated in patients treated with sorafenib plus TACE (OR=5.93, 95% CI: 2.70-15.41). However, no differences in the incidences of other SAEs were identified among the treatment groups. The present meta-analysis provides preliminary evidence for the comparative safety and efficacy of HAIC and TACE combined with sorafenib, and indicates the dominance of HAIC-oxaliplatin in HCC interventional therapy. However, high-quality RCTs are required to further confirm the efficacy of HAIC-oxaliplatin. The present study has been registered with PROSPERO (registration no. CRD42021288497).
Keywords: hepatocellular carcinoma, hepatic artery infusion chemotherapy, transcatheter arterial chemoembolization, efficacy, combination therapy
Introduction
Hepatocellular carcinoma (HCC) is currently the sixth most frequently occurring malignant tumor and ranks fourth in terms of cancer-related mortality (1). Sorafenib, an oral tyrosine kinase inhibitor, has been associated with increased survival in patients with HCC (2). However, because 12–32% of patients with HCC have vascular invasion as well as distant metastases at diagnosis (3), the efficacy of sorafenib in these patients is limited (4,5). As a result, combination therapy may be more beneficial. Current therapy options include vascular interventions, local ablation therapy, external radiation therapy, targeted drug therapy, immunotherapy and systemic chemotherapy (6). Hepatic arterial intervention therapy is widely used for its benefits, which include improved tumor-targeting ability, reduced effect on surrounding normal tissues and a lower incidence of serious adverse events (SAEs). However, using transcatheter arterial chemoembolization (TACE) to achieve complete embolization in the treatment of giant HCC is challenging and may increase the incidence of SAEs; additionally, multiple TACE interventions may cause liver injury and negatively affect patient prognosis (7). Hepatic artery infusion chemotherapy (HAIC) with portal vein tumor thrombosis (PVTT) is recommended as the first-line treatment for HCC in Japan due to its stability, safety and high conversion rate (8). Moreover, its efficacy in large HCC has been shown to be superior to that of TACE in other studies (9,10).
Although the overall response rate (ORR) of cisplatin and 5-fluorouracil (5-FU)-based HAIC (PF-HAIC), the most commonly used HAIC regimen in Japan, can reach 27.6 to 40.5% (11), it is questionable whether HAIC is superior to TACE in combination therapy. A previous meta-analysis showed that HAIC combined with sorafenib was not effective in prolonging survival compared with sorafenib alone (12), while a meta-analysis by Li et al (13) demonstrated that TACE combined with sorafenib significantly prolonged survival compared with sorafenib alone, despite a greater number of reported adverse events (AEs) during treatment. Given that these studies included patients with advanced HCC, whether high-dose chemotherapy is more effective than vascular embolization is debatable. Studies conducted in recent years have shown that the clinical efficacy of HAIC-oxaliplatin is much improved when used as a combination therapy. A head-to-head study of 5-FU, leucovorin and oxaliplatin (FOLFOX)-HAIC vs. TACE also demonstrated a clear advantage of the former in terms of efficacy and safety (14); however, numerous questions remain regarding this study (15,16). Therefore, additional randomized controlled trials (RCTs) are warranted to determine whether the efficacy of HAIC is influenced by the treatment modality or therapeutic regimen.
There is a lack of evidence from trials in which different interventional modalities and various regimens for HAIC have been directly compared; thus, a network meta-analysis can assist in the comparison of two or more therapies that have not been directly compared. In the present study, to aid physicians in the selection of appropriate interventions and regimens, HCC was stratified into primary and secondary cancers due to their different pathogenesis, and only clinical prospective RCTs related to primary HCC were included to eliminate bias in the analysis. A network meta-analysis of the included RCTs was performed to assess the survival and incidence of AEs in patients with unresectable liver cancer receiving different HAIC regimens or TACE.
Materials and methods
Protocol and registration
The present study followed the PRISMA guidelines (17) and was preliminarily registered on PROSPERO (registration no. CRD42021288497). The systematic review was conducted in accordance with the previously established protocol outlined in Appendix S1.
Literature retrieval process
Potentially relevant and eligible studies published before April 2022 were systemically retrieved from electronic databases including PubMed, Cochrane Library and Embase. The PubMed database was searched with keywords including ‘unresectable hepatocellular carcinoma’, ‘unresectable HCC’, ‘HAIC’, ‘hepatic arterial infusion chemotherapy’, ‘TACE’ and ‘transcatheter arterial chemoembolization’. The search strategy is detailed in Appendix S2.
Study inclusion and exclusion criteria
The study inclusion criteria were as follows: RCTs; studies that included patients diagnosed with advanced HCC; studies with complete data on methodology, patients’ characteristics, AEs and overall survival (OS); and studies comparing ≥2 arms that consisted of the modalities of interest, including sorafenib alone, TACE with sorafenib, and HAIC with sorafenib. The study exclusion criteria were as follows: Letters to the editor, study protocols, conference abstracts, case reports, non-RCTs, animal studies, editorials and posters. In addition, only reports in English were included in the study.
Literature screening
Two reviewers (XLC and HCY) independently evaluated all titles and abstracts. Additionally, all references cited by enrolled studies were manually reviewed to identify additional potentially eligible studies. Articles were further screened in a stepwise fashion according to their abstract and then full-text using the aforementioned inclusion and exclusion criteria. Any disagreements were resolved through consensus and the opinion of a third reviewer (QGF).
Data extraction and quality assessment
The first author's name, publication year, population characteristics, number of patients in each treatment arm and outcomes including OS, ORR, SAEs, hazard ratios (HRs) and 95% confidence intervals (CIs) were extracted. Moreover, information about the trial design, interventions and comparators, statistical analysis, primary and secondary outcomes and quality of life outcomes were also retrieved.
Risk of bias assessment and data analyses
De-duplication of retrieved documents was performed via EndNote X9 (Clarivate). The quality of the included studies was assessed using the Cochrane risk of bias tool (version 5.1.0; The Cochrane Institute). Two reviewers (XLC and HCY) evaluated each study for bias and rated it as follows: Low risk of bias, high risk of bias or unclear. Unclear was defined as either the lack of information to determine bias or uncertainty for bias.
The network meta-analysis was conducted in R package (4.1.2) using the gemtc package (1.0-1). This package uses a Bayesian Markov chain Monte Carlo sampler to run the model with 50,000 adaptation or ‘burn-in’ samples followed by 100,000 samples without thinning. Model convergence was established by the visual inspection of Brook-Gelman Rubin plots. As the analyses used a contrast-based approach for OS data, the analysis assumed that the data conformed to the proportional hazards assumption. Certain survival data were extracted from the survival curves according to the methods described by Parmar et al (18) and Wang and Zeng (19). Methods were as described in the Cochrane Handbook for Systematic Reviews of Interventions (20). For Kaplan-Meier data, the proportional hazards assumption was tested by fitting a Cox proportional hazards regression model to the time-to-event data and assessing whether the Schoenfeld residuals from the Cox model were independent of time. HRs with 95% CIs were calculated to explore the efficacy of various treatment options on OS, odds ratios (ORs) with 95% CI were used for ORR, whereas risk ratios (RRs) with 95% CI were used for SAEs.
Results
Screening
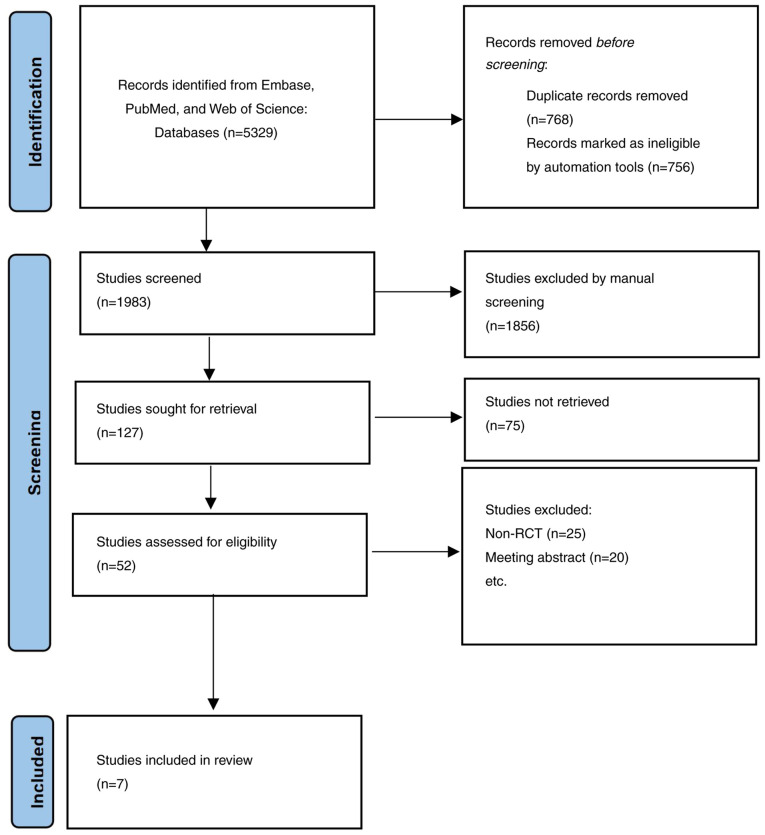
A total of 5,329 articles were retrieved for this study. EndNote X9 was used to eliminate 3,256 duplicates. A further 1,931 articles were excluded by reading the titles and abstracts. After reading 52 accessible articles in full, 20 articles related to trial registration and 25 retrospective studies were excluded. Eventually, a total of 7 studies (21–27) involving 1,073 patients were included, as presented in Fig. 1.
Figure 1.
Flow chart of the search strategy. RCT, randomized control trial.
Risk of bias assessment
Based on the Cochrane risk of bias tool, the methodological quality of the included studies was fair, as demonstrated in Table SI and Fig. S1. All 7 studies had adequate outcome measurement, enrolled a representative sample of patients and had a reasonable length of follow-up.
Characteristics of included studies
Table I presents the characteristics of the included studies. The 7 studies included a total of 1,073 patients (sorafenib alone, n=517; sorafenib plus HAIC, n=359; sorafenib plus TACE, n=197), of whom 925 were male and 148 were female. The most common etiology of HCC was hepatitis B virus infection [sorafenib plus HAIC, 49.86% (179/359); sorafenib alone, 57.25% (296/517); sorafenib plus TACE, 77.66% (153/197)], followed by hepatitis C virus infection [sorafenib plus HAIC, 26.18% (94/359); sorafenib alone, 22.24% (115/517), sorafenib plus TACE, 5.0% (10/197)]. Furthermore, 26.74% (96/359) patients treated with sorafenib plus HAIC, 18.96% (98/517) patients treated with sorafenib alone and 39.59% (78/197) treated with sorafenib plus TACE group had extrahepatic metastases. Only the studies by He et al (22), Zheng et al (21) and Lee et al (27) observed a statistically significant difference in OS between the two groups being compared in the respective study (HR=0.35, 95% CI: 0.26-0.48; HR=0.28, 95% CI: 0.15-0.53; and HR=0.59, 95% CI: 0.84-0.48, respectively). Since Lee et al (27) did not report the HR values with a 95% CI, the aforementioned data were extracted from the Kaplan-Meier curve provided in the article. All 7 studies enrolled HCC patients with PVTT, and all included patients were unsuitable for curative resection or local ablation.
Table I.
Characteristics of included studies.
| Author, year | Country | ECOG | Group | Interventional therapy details | mOS, months | N | Sex (M/F) | Age, years | Tumor size, cm | No. of tumors (single/multiple) | PVTT | Child-Pugh | BCLC | Data time frame | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ikeda, 2016 | Japan | 0-1 | SorCDDP | Cisplatin (65 mg/m2)/4–6 weeks | 10.6 | 65 | 56/9 | 49 (41–55) | 10.1 (7.7-13.2) | 30/95 | 40/25 | 5-6 | NA | June 2011-December 2013 | (25) |
| Sorafenib alone | 400 mg twice daily | 8.7 | 41 | 32/9 | 49 (40–56) | 10.1 (8.3-12.1) | 33/89 | 17/24 | |||||||
| Kudo, 2018 | Japan | 0-1 | Sorafenib + PF-HAIC | Cisplatin (20 mg/m2) on days 1 and 8 + 5-FU (330 mg/m2) on days 1–5 and 8–12/28 days | 11.8 | 102 | 89/13 | 69 (62–75) | NA | NA | 58/44 | 5-7 | 0/32/70 | November 2010-June 2014 | (24) |
| Sorafenib alone | 400 mg twice daily | 11.5 | 103 | 88/15 | 68 (62–75) | 64/39 | 0/27/76 | ||||||||
| Kondo, 2019 | Japan | 0-1 | SorCDDP | Cisplatin (65 mg/m2)/4–6 weeks | 15.2 | 35 | 28/7 | 72 (65–79) | NA | NA | 21/14 | 5-7 | 2/14/19 | August 2011-November 2014 | (23) |
| Sorafenib alone | 400 mg sorafenib twice daily | 10.0 | 33 | 27/6 | 70.9 (61.8-80) | 22/11 | 2/13/18 | ||||||||
| He, 2019 | China | 0-2 | Sorafenib + FOLFOX-HAIC | Oxaliplatin (85 mg/m2) + leucovorin (400 mg/m2) + 5-FU (400 mg/m2); 5-FU infusion 2,400 mg/m2 for 46 h/3 weeks | 13.4 | 125 | 111/14 | 49 (41–55) | 10.1 (7.7-13.2) | 30/95 | 125/0 | 5-6 | NA | April 2016-October 2017 | (22) |
| Sorafenib alone | 400 mg twice daily | 7.1 | 122 | 112/10 | 49 (40–56) | 10.1 (8.3-12.1) | 33/89 | 122/0 | |||||||
| Zheng, 2022 | China | 0-2 | Sorafenib + HAIC | Oxaliplatin (35 mg/m2) + 5-FU (600 mg/m2) + leucovorin (200 mg/m2 injected intravenously)/ 28 days | 16.3 | 32 | 30/2 | 56 (45–67) | 10.6 (6.6-14.6) | 15/17 | 32/0 | 5-7 | NA | June 2017-November 2019 | (21) |
| Sorafenib | 400 mg twice daily | 6.5 | 32 | 31/1 | 55 (45–65) | 10.7 (6.8-14.6) | 12/20 | 32/0 | |||||||
| Lee, 2020 | China | 0-2 | Sorafenib + TACE | Lipiodol + 10–20 mg Adriamycin/elution beads + Adriamycin/2–3 months | 11.0 | 27 | 23/4 | 60 (53-65.5) | 6.2 (3.5-13.5) | NA | 11/16 | 5-7 | NA | NA | (27) |
| Sorafenib | 200–400 mg twice daily | 5.0 | 17 | 15/2 | 59 (51–62) | 6.5 (3.4-12.5) | 5/12 | ||||||||
| Park, 2018 | South Korea | 0-2 | Sorafenib + TACE | Doxorubicin or cisplatin + embolization materials/28 days | 12.8 | 170 | 136/34 | 60.2 (50.6-69.8) | NA | NA | 68/102 | 5-7 | 3/39/128 | January 2013-December 2015 | (26) |
| Sorafenib | 200–400 mg twice daily | 10.8 | 169 | 147/22 | 61.3 (51.7-70.9) | 63/106 | 0/4/125 |
Continuous variables are expressed as median (range). ECOG, Eastern Cooperative Oncology Group; mOS, mean overall survival; PVTT, portal vein tumor thrombosis; BCLC, Barcelona Clinic Liver Cancer; SorCDDP, sorafenib with cisplatin-HAIC; HAIC, hepatic artery infusion chemotherapy; PF-HAIC, 5-FU-based HAIC; 5-FU, 5-fluorouracil; FOLFOX, 5-FU, leucovorin and oxaliplatin; TACE, transcatheter arterial chemoembolization; NA, not available.
Network meta-analysis
The 7 RCTs identified through the literature reviews enabled the creation of a seven-node network with two edges, which included sorafenib alone, sorafenib with cisplatin-HAIC (CDDP) (SorCDDP), sorafenib with HAIC-oxaliplatin, sorafenib with TACE, and sorafenib with PF-HAIC. Fig. 2 shows the network meta-analysis (NMA) map of the interventional therapies in the studies that were directly compared for efficacy in prolonging survival. According to deviance information criterion (DIC) and total residual deviance statistics, the I2 value was much higher than 50%, and the random-effects model had a superior fit to the data than the fixed-effects model, with the former model having fewer parameters and a lower DIC (28). It was not possible to assess inconsistency within the network due to the absence of any closed loops. The proportional hazards assumption was not violated in the studies with time-to-event data. The Kaplan-Meier curves in all 7 RCTs crossed over; however, violation of the proportional hazards assumption was not supported by the statistical data.
Figure 2.
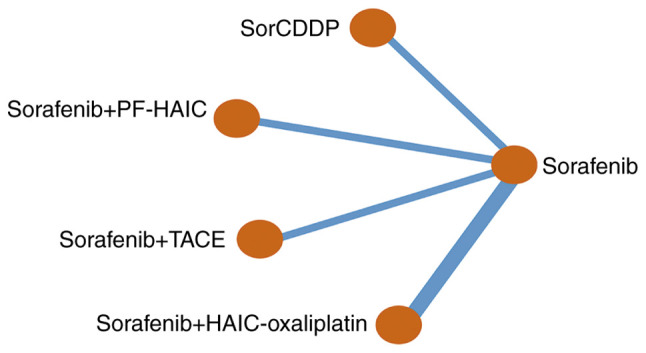
Network of the available direct comparisons in the included studies. SorCDDP, sorafenib with cisplatin-HAIC; PF-HAIC, 5-fluorouracil-based HAIC; TACE, transcatheter arterial chemoembolization; HAIC, hepatic artery infusion chemotherapy.
Efficacy
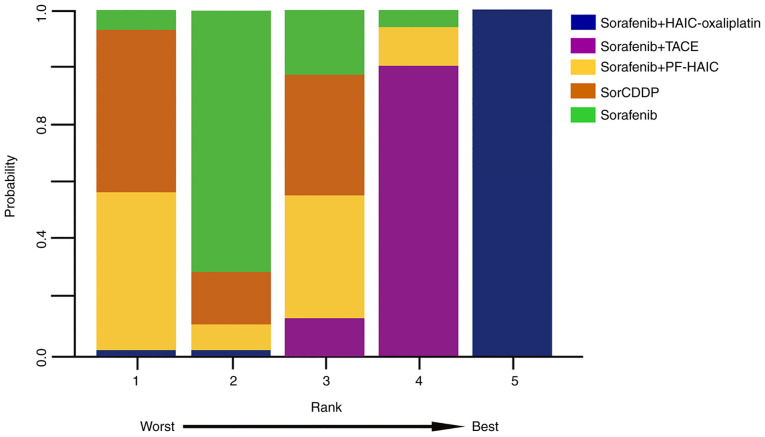
The NMA revealed a significant difference between the sorafenib plus HAIC-oxaliplatin regimen and other treatment strategies in terms of OS (HR=0.33, 95% CI: 0.25-0.44). By contrast, comparisons between sorafenib plus PF-HAIC, SorCDDP and sorafenib plus TACE identified little difference with mortality HRs of 1.01 (95% CI: 0.74-1.38), 1.22 (95% CI: 0.88-1.71) and 0.79 (95% CI: 0.62-1.01), respectively. Fig. 3 depicts the ranking of the treatments with regard to the likelihood of being the superior treatment. Rank probability plots revealed that there would be a 99.9% likelihood of sorafenib plus FOLFOX-HAIC being the most effective treatment, followed by sorafenib plus TACE at 86.6% and SorCDDP at 75.6%. Furthermore, a comparison based on mRECIST criteria found that patients treated with sorafenib plus FOLFOX-HAIC had significantly higher ORR than those receiving other treatment regimens (OR=22.0, 95% CI: 10.6-25.7).
Figure 3.
Rankogram of treatment modality. HAIC, hepatic artery infusion chemotherapy; TACE, transcatheter arterial chemoembolization; PF-HAIC, 5-fluorouracil-based HAIC; SorCDDP, sorafenib with cisplatin-HAIC.
SAEs
The analysis of SAEs revealed that the risk of liver injury was higher in the sorafenib plus TACE group compared with other groups (HR=5.93, 95% CI: 2.70-15.41); there was no significant difference in the risk of liver injury between patients receiving sorafenib plus HAIC compared with those receiving sorafenib alone. Furthermore, no significant differences in the constitutional symptoms and gastrointestinal reactions were detected between all the treatment groups. Table II summarizes the results of the network meta-analyses regarding OS, ORR and SAEs.
Table II.
Comparisons of efficacy in terms of OS and severe adverse events in advanced hepatocellular carcinoma.
| A, OS [HR (95% CI)] | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Author, year | Treatment group | Sorafenib + HAIC-oxaliplatin | Sorafenib + TACE | Sorafenib + PF-HAIC | SorCDDP | (Refs.) |
| Zheng, 2022; He, 2019 | Sorafenib + HAIC-oxaliplatin | - | - | - | - | (21,22) |
| Park, 2018; Lee, 2020 | Sorafenib + TACE | 0.42 (0.29-0.62) | - | - | - | (26,27) |
| Kudo, 2018 | Sorafenib + PF-HAIC | 0.33 (0.22-0.50) | 0.78 (0.52-1.17) | - | - | (24) |
| Kondo, 2018; Ikeda, 2016 | SorCDDP | 0.27 (0.18-0.42) | 0.64 (0.42-0.97) | 0.83 (0.52-1.30) | - | (23,25) |
| All of the above | Sorafenib (Control) | 0.33 (0.25-0.44) | 0.79 (0.62-1.01) | 1.01 (0.74-1.38) | 1.22 (0.88-1.71) | (21–27) |
|
| ||||||
| B, Overall response rate [OR (95% CI)] | ||||||
|
| ||||||
| Author, year | Treatment group | Sorafenib + HAIC-oxaliplatin | Sorafenib + TACE | Sorafenib + PF-HAIC | SorCDDP | (Refs.) |
|
| ||||||
| Zheng, 2022; He, 2019 | Sorafenib + HAIC-oxaliplatin | - | - | - | - | (21,22) |
| Park, 2018; Lee, 2020 | Sorafenib + TACE | 9.26 (3.03-29.26) | - | - | - | (26,27) |
| Kudo, 2018 | Sorafenib + PF-HAIC | 8.16 (3.01-23.79) | 0.88 (0.32-2.54) | - | - | (24) |
| Kondo, 2018; Ikeda, 2016 | SorCDDP | 7.73 (2.05-27.81) | 0.83 (0.22-2.96) | 0.95 (0.27-3.03) | - | (23,25) |
| All of the above | Sorafenib (Control) | 22.18 (10.69-52.56) | 2.4 (1.10-5.58) | 2.72 (1.43-5.36) | 2.88 (1.12-8.58) | (21–27) |
|
| ||||||
| C, Liver injury [OR (95% CI)] | ||||||
|
| ||||||
| Author, year | Treatment group | Sorafenib + HAIC-oxaliplatin | Sorafenib + TACE | Sorafenib + PF-HAIC | SorCDDP | (Refs.) |
|
| ||||||
| Zheng, 2022; He, 2019 | Sorafenib + HAIC-oxaliplatin | - | - | - | - | (21,22) |
| Park, 2018; Lee, 2020 | Sorafenib + TACE | 0.22 (0.07-0.68) | - | - | - | (26,27) |
| Kudo, 2018 | Sorafenib + PF-HAIC | 1.34 (0.49-3.81) | 5.98 (2.07-19.30) | - | - | (24) |
| Kondo, 2018; Ikeda, 2016 | SorCDDP | 1.35 (0.51-3.62) | 6.02 (2.17,18.53) | 1.01 (0.39-2.55) | - | (23,25) |
| All of the above | Sorafenib (Control) | 1.34 (0.64-2.90) | 5.93 (2.70-15.41) | 1.00 (0.49-2.00) | 0.99 (0.54-1.86) | (21–27) |
|
| ||||||
| D, Constitutional symptoms [OR (95% CI)] | ||||||
|
| ||||||
| Author, year | Treatment group | Sorafenib + HAIC-oxaliplatin | Sorafenib + TACE | Sorafenib + PF-HAIC | SorCDDP | (Refs.) |
|
| ||||||
| Zheng, 2022; He, 2019 | Sorafenib + HAIC-oxaliplatin | - | - | - | - | (21,22) |
| Park, 2018; Lee, 2020 | Sorafenib + TACE | 1.47 (0.35-6.59) | - | - | - | (26,27) |
| Kudo, 2018 | Sorafenib + PF-HAIC | 1.39 (0.33-6.23) | 0.94 (0.25-3.65) | - | - | (24) |
| Kondo, 2018; Ikeda, 2016 | SorCDDP | 0.53 (0.06-3.41) | 0.36 (0.04-2.07) | 0.38 (0.04-2.15) | - | (23,25) |
| All of the above | Sorafenib (Control) | 1.81 (0.63-5.92) | 1.24 (0.48-3.28) | 1.31 (0.52-3.40) | 3.36 (0.83-25.50) | (21–27) |
|
| ||||||
| E, Gastrointestinal reactions [OR (95% CI)] | ||||||
|
| ||||||
| Author, year | Treatment group | Sorafenib + HAIC-oxaliplatin | Sorafenib + TACE | Sorafenib + PF-HAIC | SorCDDP | (Refs.) |
|
| ||||||
| Zheng, 2022; He, 2019 | Sorafenib + HAIC-oxaliplatin | - | - | - | - | (21,22) |
| Park, 2018; Lee, 2020 | Sorafenib + TACE | 0.72 (0.22-2.35) | - | - | - | (26,27) |
| Kudo, 2018 | Sorafenib + PF-HAIC | 2.05 (0.59-8.51) | 2.86 (0.63,14.83) | - | - | (24) |
| Kondo, 2018; Ikeda, 2016 | SorCDDP | 0.57 (0.07-3.35) | 0.78 (0.08-5.63) | 0.27 (0.03-2.05) | - | (23,25) |
| All of the above | Sorafenib (Control) | 0.9 (0.49-1.63) | 1.24 (0.45-3.53) | 0.44 (0.12-1.30) | 1.57 (0.30-12.06) | (21–27) |
The HR values in the table are the treatment modality in the horizontal coordinate compared to the treatment modality in the vertical coordinate. HR, hazard ratio; CI, credible interval; OR, odds ratio; HAIC, hepatic artery infusion chemotherapy; TACE, transcatheter arterial chemoembolization; PF-HAIC, 5-fluorouracil-based HAIC; SorCDDP, sorafenib with cisplatin-HAIC; OS, overall survival.
Discussion
Due to the lack of clinical RCTs in which different treatments of HCC are directly compared, the present meta-analysis used 7 RCT studies to indirectly evaluate the efficacy and safety of CDDP, PF-HAIC, HAIC-oxaliplatin and TACE as interventional modalities when combined with sorafenib.
The study findings revealed that combining CDDP, PF-HAIC and TACE with sorafenib did not significantly differ from sorafenib alone in terms of OS, indicating that inappropriate treatment modalities and regimens are not beneficial to HCC patient survival. However, the prognosis of patients receiving HAIC-oxaliplatin plus sorafenib was found to be significantly improved when compared with other treatment modalities. The survival benefit may be explained in part by the superior efficacy of oxaliplatin in HCC treatment. Compared with cisplatin, oxaliplatin has significantly improved pharmacokinetic, biochemical, cytotoxic and immunological properties (29). Oxaliplatin is an inhibitor of ribosomal RNA synthesis, which induces ribosome biogenesis stress during the active translation of RNA interference. Thus, oxaliplatin exerts its cytotoxic effects by inhibiting protein synthesis rather than via a DNA damage response (30). In addition, oxaliplatin induces antitumor immune responses to anthracyclines via the stimulation of pro-apoptotic cell calreticulin exposure, leading to immunogenic tumor cell death (31). Notably, sorafenib normalizes HCC vasculature and increases drug transport by interacting with platinum transporter proteins to increase the local enrichment of the oxaliplatin concentration in tumors (32). As a result, a multidrug combination based on oxaliplatin may be superior to cisplatin alone. The present findings indirectly support a previous study suggesting that HAIC-oxaliplatin plus sorafenib has greater efficacy than TACE for large HCC (≥7 cm) (14). It is hypothesized that this may be because the prolonged and continuous infusion of chemotherapeutic agents through the target artery increases the local drug concentration and tumor uptake compared with systemic intravenous chemotherapy (33). In addition, there may be a synergistic antitumor effect between sorafenib, oxaliplatin and 5-FU. Sorafenib has several targets, including Raf-1, vascular endothelial growth factor (VEGF) receptor 1–3 and platelet-derived growth factor receptor-β; the inhibition of Raf-1 can induce apoptosis and counteract the resistance of tumor cells to FOLFOX (34,35). It has been shown that 5-FU, in particular, increases tumor sensitivity to oxaliplatin by increasing multidrug resistance-associated protein 2 mRNA expression, and its synergistic efficacy with oxaliplatin mediates the expression of drug transporter proteins (22). TACE plus sorafenib is more effective than traditional HAIC, which is likely due to the tumor microenvironment created by TACE treatment-induced ischemia and hypoxia, which causes the upregulation of local inflammatory factors, including insulin-like growth factor-2 and VEGF (36). Moreover, sorafenib inhibits tyrosine kinases of the VEGF signaling pathway, inhibiting tumor angiogenesis and weakening the proliferation ability of tumor cells (37). The combination of sorafenib with TACE can improve patient survival, since sorafenib promotes the deposition of the drug-eluting TACE component lipiodol in HCC vessels, where it exhibits a tumor necrotizing effect (38). TACE induces ischemia and hypoxia in tumor tissues by the targeted embolization of arteries during treatment; however, this effect requires hepatic vein patency to ensure normal tissue metabolism, and since ischemic and hypoxic conditions may favor the resistance of tumor cells to therapeutic agents (39), promoting angiogenesis and the migration of certain cytokines, for example IL-8, this may lead to tumor metastasis (40). HAIC, by contrast, is continuously administered throughout the treatment process, and tumor cells are killed via a cytotoxic response, without any dependence of normal tissue metabolism on the hepatic vein and the resistance and metastasis induced by inflammatory factors. Therefore, HAIC may be a more effective and safer intervention for HCC than TACE. Nevertheless, more studies are required to clarify and demonstrate whether this efficacy is due to interventional modalities or the regimen used. Based on the current findings, we hypothesize that HAIC-oxaliplatin currently is a superior interventional combination therapy for HCC. However, whether this efficacy advantage is due to the type of treatment or the drug combination remains unclear and requires validation in high-quality head-to-head studies.
A comparison of the various SAEs in the present meta-analysis revealed that patients treated with TACE plus sorafenib had an elevated risk of liver injury. This could be because TACE and its associated metabolites stimulate the expression of pro-apoptotic genes and the activation of multiple death signaling pathways, thereby promoting apoptosis and inducing an inflammatory response that accelerates the progression of drug-induced liver injury. Moreover, embolic agents are able to enter small arteries and capillaries in non-cancerous areas, causing ischemia and hypoxia in normal tissues and exacerbating oxidative stress and liver injury (41). Furthermore, patients with HCC who have intrahepatic metastases typically have a history of hepatitis and cirrhosis, and their liver function is already compromised (42). According to previous research, liver injury frequently occurs in colorectal cancer-related chemotherapy using the FOLFOX regimen, most likely due to the toxicity of oxaliplatin metabolites, which affects the permeability of the hepatic sinusoidal cells and raises the pressure in the hepatic venous sinusoids, resulting in liver injury and sinusoidal portal hypertension (43,44). However, the present study indicates that HAIC-oxaliplatin plus sorafenib is significantly safer than TACE when combined with sorafenib. Regarding constitutional symptoms and gastrointestinal events, we hypothesize that patients with HCC who are treated with HAIC-oxaliplatin are at a higher risk of SAEs because they have a heavier tumor burden. Notably, the incidence of overall SAEs suggests that HAIC-oxaliplatin may be more appropriate than TACE and other HAIC therapies in the combination treatment of patients with a heavier tumor burden.
The combination of interventional therapy and oral drugs has markedly improved the survival of patients with advanced HCC. The REFLECT trial established the first-line status of lenvatinib (45), and Ding et al (46) demonstrated its superior efficacy compared with sorafenib in HCC combination therapy. Regorafenib and cabozantinib, which are the second-line drugs for advanced HCC, were also approved following the RESORCE and CELESTIAL trials (47,48), With the development of drug-eluting beads and the advancement of super-selective TACE (2,49), further studies are recommended to investigate the efficacy of these new oral drugs in combination with interventional modalities for advanced HCC.
There are several limitations to the present study. Firstly, only 7 RCTs met the inclusion criteria of the meta-analysis, and the potential bias of the studies could impact the results. Furthermore, because the pathogenesis and prevalence of hepatitis vary greatly across different regions and populations, the fact that the population in this study comprises only Asian patients may limit the generalizability of the study findings.
In conclusion, the present study indicates that in patients with advanced primary HCC, HAIC-oxaliplatin significantly improves patient survival when combined with sorafenib compared with other regimens or interventional modalities. Although there may be significant bias in assessing outcome indicators due to patient enrollment differences, the results suggest that the HAIC-oxaliplatin plus sorafenib combination should be more widely used. However, the study of HAIC dosing regimens for advanced HCC and exploration of combination treatment options in the future studies is recommended.
Supplementary Material
Acknowledgements
Not applicable.
Glossary
Abbreviations
- ORR
overall response rate
- 5-FU
5-fluorouracil
- FOLFOX, 5-FU
leucovorin and oxaliplatin
- HAIC
hepatic artery infusion chemotherapy
- TACE
transcatheter arterial chemoembolization
- HCC
hepatocellular carcinoma
- PF
cisplatin and 5-FU
- RCT
randomized controlled trial
- CDDP
cisplatin-HAIC
- SorCDDP
sorafenib with CDDP
- PVTT
portal vein tumor thrombosis
- DIC
deviance information criterion
- AEs
adverse events
- SAEs
serious AEs
- VEGF
vascular endothelial growth factor
Funding Statement
This work is supported by the Gansu Traditional Chinese Medicine Research Project (grant no. GZKP-2020-28) and the Lanzhou City Chengguan District Science and Technology Plan Project (grant no. 2020-2-11-4).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
XLC and HCY conceived and designed the research. XLC, HCY and QGF performed data acquisition, data analysis and manuscript preparation. QY, WKJ, SZR and WCZ assisted with data acquisition, data analysis and statistical analysis. WCZ and SZR confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Xu S, Bie ZX, Li YM, Li B, Kong FL, Peng JZ, Li XG. Drug-eluting bead bronchial arterial chemoembolization with and without microwave ablation for the treatment of advanced and standard treatment-refractory/ineligible non-small cell lung cancer: A comparative study. Front Oncol. 2022;12:851830. doi: 10.3389/fonc.2022.851830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong KM, King GG, Harris WP. The treatment landscape of advanced hepatocellular carcinoma. Curr Oncol Rep. 2022;24:917–927. doi: 10.1007/s11912-022-01247-7. [DOI] [PubMed] [Google Scholar]
- 4.Ni JY, Liu SS, Sun HL, Wang WD, Zhong ZL, Hou SN, Chen YT, Xu LF. Transcatheter hepatic arterial infusion chemotherapy vs sorafenib in the treatment of patients with hepatocellular carcinoma of Barcelona Clinic Liver Cancer stage C: A meta-analysis of Asian population. Onco Targets Ther. 2018;11:7883–7894. doi: 10.2147/OTT.S156844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moriguchi M, Aramaki T, Nishiofuku H, Sato R, Asakura K, Yamaguchi K, Tanaka T, Endo M, Itoh Y. Sorafenib versus hepatic arterial infusion chemotherapy as initial treatment for hepatocellular carcinoma with advanced portal vein tumor thrombosis. Liver Cancer. 2017;6:275–286. doi: 10.1159/000473887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, Bachini M, Borad M, Brown D, Burgoyne A, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:541–565. doi: 10.6004/jnccn.2021.0012. [DOI] [PubMed] [Google Scholar]
- 7.Lyu N, Wang X, Li JB, Lai JF, Chen QF, Li SL, Deng HJ, He M, Mu LW, Zhao M. Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: A biomolecular exploratory, randomized, phase III trial (FOHAIC-1) J Clin Oncol. 2022;40:468–480. doi: 10.1200/JCO.21.01963. [DOI] [PubMed] [Google Scholar]
- 8.Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, Okusaka T, Miyayama S, Tsuchiya K, Ueshima K, et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study Group of Japan. Liver Cancer. 2014;3:458–468. doi: 10.1159/000343875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai Z, He C, Zhao C, Lin X. Survival comparisons of hepatic arterial infusion chemotherapy with mFOLFOX and transarterial chemoembolization in patients with unresectable intrahepatic cholangiocarcinoma. Front Oncol. 2021;11:611118. doi: 10.3389/fonc.2021.611118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai WL, Lai KH, Liang HL, Hsu PI, Chan HH, Chen WC, Yu HC, Tsay FW, Wang HM, Tsai HC, Cheng JS. Hepatic arterial infusion chemotherapy for patients with huge unresectable hepatocellular carcinoma. PLoS One. 2014;9:e92784. doi: 10.1371/journal.pone.0092784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song DS, Song MJ, Bae SH, Chung WJ, Jang JY, Kim YS, Lee SH, Park JY, Yim HJ, Cho SB, et al. A comparative study between sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. J Gastroenterol. 2015;50:445–454. doi: 10.1007/s00535-014-0978-3. [DOI] [PubMed] [Google Scholar]
- 12.Regmi P, Hu HJ, Lv TR, Paudyal A, Sah RB, Ma WJ, Jin YW, Li FY. Efficacy and safety of sorafenib plus hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma. Surg Oncol. 2021;39:101663. doi: 10.1016/j.suronc.2021.101663. [DOI] [PubMed] [Google Scholar]
- 13.Li D, Pang Y, Xu L, Xu X. Efficacy and safety of sorafenib combined with TACE in the treatment of advanced hepatocellular carcinoma: A meta-analysis. J BUON. 2021;26:1355–1364. [PubMed] [Google Scholar]
- 14.Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, Wei W, Zhang YJ, Guo Y, Guo RP, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: A randomized phase III trial. J Clin Oncol. 2022;40:150–160. doi: 10.1200/JCO.21.00608. [DOI] [PubMed] [Google Scholar]
- 15.Cai YS, Wu H. Is FOLFOX-HAIC superior to transarterial chemoembolization in treating large hepatocellular carcinoma? Hepatobiliary Surg Nutr. 2022;11:164–165. doi: 10.21037/hbsn-21-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mei J, Yu H, Qin L, Jia Z. FOLFOX-HAIC for unresectable large hepatocellular carcinoma: The effectiveness has yet to be determined. J Clin Oncol. 2022;40:1841. doi: 10.1200/JCO.21.02533. [DOI] [PubMed] [Google Scholar]
- 17.Panagopoulos A, Solou K, Tatani I, Triantafyllopoulos IK, Lakoumentas J, Kouzelis A, Athanasiou V, Kokkalis ZT. What is the optimal surgical treatment for Neer type IIB (IIC) distal clavicle fractures? A systematic review and meta-analysis. J Orthop Surg Res. 2022;17:215. doi: 10.1186/s13018-022-03108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Zeng T. Response to: Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2013;14:391. doi: 10.1186/1745-6215-14-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng K, Zhu X, Fu S, Cao G, Li QW, Xu L, Chen H, Wu D, Yang R, Wang K, et al. Sorafenib plus hepatic arterial infusion chemotherapy versus sorafenib for hepatocellular carcinoma with major portal vein tumor thrombosis: A randomized trial. Radiology. 2022;303:455–464. doi: 10.1148/radiol.211545. [DOI] [PubMed] [Google Scholar]
- 22.He M, Li Q, Zou R, Shen J, Fang W, Tan G, Zhou Y, Wu X, Xu L, Wei W, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: A randomized clinical trial. JAMA Oncol. 2019;5:953–960. doi: 10.1001/jamaoncol.2019.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kondo M, Morimoto M, Kobayashi S, Ohkawa S, Hidaka H, Nakazawa T, Aikata H, Hatanaka T, Takizawa D, Matsunaga K, et al. Randomized, phase II trial of sequential hepatic arterial infusion chemotherapy and sorafenib versus sorafenib alone as initial therapy for advanced hepatocellular carcinoma: SCOOP-2 trial. BMC Cancer. 2019;19:954. doi: 10.1186/s12885-019-6198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kudo M, Ueshima K, Yokosuka O, Ogasawara S, Obi S, Izumi N, Aikata H, Nagano H, Hatano E, Sasaki Y, et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): A randomised, open label, phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3:424–432. doi: 10.1016/S2468-1253(18)30078-5. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda M, Shimizu S, Sato T, Morimoto M, Kojima Y, Inaba Y, Hagihara A, Kudo M, Nakamori S, Kaneko S, et al. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: Randomized phase II trial. Ann Oncol. 2016;27:2090–2096. doi: 10.1093/annonc/mdw323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JW, Kim YJ, Kim DY, Bae SH, Paik SW, Lee YJ, Kim HY, Lee HC, Han SY, Cheong JY, et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: The phase III STAH trial. J Hepatol. 2019;70:684–691. doi: 10.1016/j.jhep.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 27.Lee WC, Hung HC, Lee JC, Wang YC, Cheng CH, Wu TH, Lee CF, Wu TJ, Chou HS, Chan KM. Treatment strategy of adding transcatheter arterial chemoembolization to sorafenib for advanced stage hepatocellular carcinoma. Cancer Rep (Hoboken) 2021;4:e1294. doi: 10.1002/cnr2.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barili F, Parolari A, Kappetein PA, Freemantle N. Statistical primer: Heterogeneity, random- or fixed-effects model analyses? Interact Cardiovasc Thorac Surg. 2018;27:317–321. doi: 10.1093/icvts/ivy163. [DOI] [PubMed] [Google Scholar]
- 29.Theile D, Grebhardt S, Haefeli WE, Weiss J. Involvement of drug transporters in the synergistic action of FOLFOX combination chemotherapy. Biochem Pharmacol. 2009;78:1366–1373. doi: 10.1016/j.bcp.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Bruno PM, Liu Y, Park GY, Murai J, Koch CE, Eisen TJ, Pritchard JR, Pommier Y, Lippard SJ, Hemann MT. A subset of platinum-containing chemotherapeutic agents kills cells by inducing ribosome biogenesis stress. Nat Med. 2017;23:461–471. doi: 10.1038/nm.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- 32.He MK, Le Y, Li QJ, Yu ZS, Li SH, Wei W, Guo RP, Shi M. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: A prospective non-randomized study. Chin J Cancer. 2017;36:83. doi: 10.1186/s40880-017-0251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewandowski RJ, Geschwind JF, Liapi E, Salem R. Transcatheter intraarterial therapies: Rationale and overview. Radiology. 2011;259:641–657. doi: 10.1148/radiol.11081489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma SQ, Cao BR, Zhang H, Luo LP, Ren Y, Hu T, Chen CM. The lack of Raf-1 kinase feedback regulation enhances antiapoptosis in cancer cells. Oncogene. 2017;36:2014–2022. doi: 10.1038/onc.2016.384. [DOI] [PubMed] [Google Scholar]
- 35.Mizukoshi E, Yamashita T, Arai K, Terashima T, Kitahara M, Nakagawa H, Iida N, Fushimi K, Kaneko S. Myeloid-derived suppressor cells correlate with patient outcomes in hepatic arterial infusion chemotherapy for hepatocellular carcinoma. Cancer Immunol Immunother. 2016;65:715–725. doi: 10.1007/s00262-016-1837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pratama MY, Pascut D, Massi MN, Tiribelli C. The role of microRNA in the resistance to treatment of hepatocellular carcinoma. Ann Transl Med. 2019;7:577. doi: 10.21037/atm.2019.09.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chao Y, Chung YH, Han G, Yoon JH, Yang J, Wang J, Shao GL, Kim BI, Lee TY. The combination of transcatheter arterial chemoembolization and sorafenib is well tolerated and effective in Asian patients with hepatocellular carcinoma: Final results of the START trial. Int J Cancer. 2015;136:1458–1467. doi: 10.1002/ijc.29126. [DOI] [PubMed] [Google Scholar]
- 38.Zheng L, Guo CY, Chen CS, Xiao JC, Hu HT, Cheng HT, Zong DW, Jiang L, Li HL. Sorafenib improves lipiodol deposition in transarterial chemoembolization of Chinese patients with hepatocellular carcinoma: A long-term, retrospective study. Oncotarget. 2017;8:97613–97622. doi: 10.18632/oncotarget.18811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He X, Guo X, Zhang H, Kong X, Yang F, Zheng C. Mechanism of action and efficacy of LY2109761, a TGF-β receptor inhibitor, targeting tumor microenvironment in liver cancer after TACE. Oncotarget. 2017;9:1130–1142. doi: 10.18632/oncotarget.23193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin W, Wang H, Zhong M, Yu S, Zhao S, Liang S, Du J, Cheng B, Gu W, Ling C. Effect and molecular mechanisms of jiedu recipe on hypoxia-induced angiogenesis after transcatheter arterial chemoembolization in hepatocellular carcinoma. Evid Based Complement Alternat Med. 2021;2021:6529376. doi: 10.1155/2021/6529376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang P, Zhou W, Li C, Zhang M, Jiang Y, Jiang R, Ba H, Li C, Wang J, Yin B, et al. Kupffer-cell-expressed transmembrane TNF-α is a major contributor to lipopolysaccharide and D-galactosamine-induced liver injury. Cell Tissue Res. 2016;363:371–383. doi: 10.1007/s00441-015-2252-2. [DOI] [PubMed] [Google Scholar]
- 42.Chen LX, Zou SJ, Li D, Zhou JY, Cheng ZT, Zhao J, Zhu YL, Kuang D, Zhu XH. Prostate-specific membrane antigen expression in hepatocellular carcinoma, cholangiocarcinoma, and liver cirrhosis. World J Gastroenterol. 2020;26:7664–7678. doi: 10.3748/wjg.v26.i48.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bloom S, Kemp W, Lubel J. Portal hypertension: Pathophysiology, diagnosis and management. Internal Med J. 2015;45:16–26. doi: 10.1111/imj.12590. [DOI] [PubMed] [Google Scholar]
- 44.Fuentes-Lacouture MC, Barrera-Garavito EC, Gomez A, Mantilla W. Non-cirrhotic portal hypertension in a patient with colonic carcinoma treated with oxaliplatin. J Med Cases. 2021;12:99–101. doi: 10.14740/jmc3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 46.Ding X, Sun W, Li W, Shen Y, Guo X, Teng Y, Liu X, Zheng L, Li W, Chen J. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: A prospective randomized study. Cancer. 2021;127:3782–3793. doi: 10.1002/cncr.33677. [DOI] [PubMed] [Google Scholar]
- 47.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 48.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyayama S, Yamashiro M, Ikeda R, Matsumoto J, Takeuchi K, Sakuragawa N, Ueda T, Sanada T, Notsumata K, Terada T. Efficacy of superselective conventional transarterial chemoembolization using guidance software for hepatocellular carcinoma within three lesions smaller than 3 cm. Cancers (Basel) 2021;13:6370. doi: 10.3390/cancers13246370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.