Abstract
There is limited evidence on vaccine effectiveness against asymptomatic or mild Omicron infections. We estimated that recent third doses of messenger RNA or inactivated vaccines reduced the risk of self-reported infection by 52% (95% confidence interval, 17%–73%) among randomly sampled adults during the Omicron BA.2–dominated surge in Hong Kong.
Keywords: vaccine effectiveness, SARS-CoV-2, asymptomatic, booster dose
Using self-reported vaccination and testing results for coronavirus disease 2019 among a random adult population in Hong Kong, we estimated that third doses of messenger RNA or inactivated vaccines could provide moderate level of protection during the Omicron BA.2 surge.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant has spread rapidly around the world. One reason for the increased transmissibility of Omicron is its ability to infect people who have preexisting immunity to previous strains [1]. While there is good evidence that coronavirus disease 2019 (COVID-19) vaccines maintain a high level of protection against severe disease associated with Omicron infections [2], there is less evidence on the extent to which vaccines can protect against mild or asymptomatic infections and thus potentially can reduce transmission.
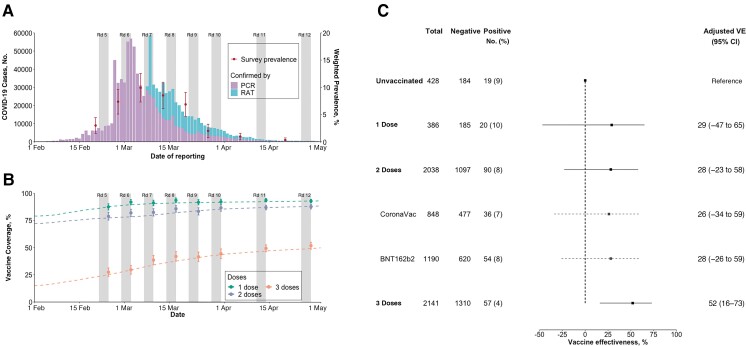
Hong Kong was able to suppress community transmission of COVID-19 during the first 2 years of the pandemic, with <1.5 laboratory-confirmed cases for every 1000 persons by 31 December 2021, while serologic studies indicated that there had not been much silent transmission [3]. From January through April 2022, a large community pandemic of Omicron BA.2 lineage occurred in Hong Kong's 7.4 million population, resulting in >1 million confirmed cases (Figure 1), among which there were >9000 deaths [4]. Two vaccines are widely available in Hong Kong, the inactivated CoronaVac vaccine (Sinovac) and the mRNA vaccine BNT162b2 (BioNTech/Fosun Pharma/Pfizer). Doses administrated are tracked daily [5]. Vaccination uptake of ≥2 doses among adults increased from 79% on 1 February 2022 to 93% by 30 April 2022, and the uptake of third doses increased from 15% to 50% in the same period (Figure 1 and Supplementary Figure 1) [2].
Figure 1.
A, Weighted prevalence of self-reported severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections among participants (estimates shown as dots with confidence intervals) and coronavirus disease 2019 (COVID-19) cases confirmed by polymerase chain reaction (PCR) and rapid antigen testing (RAT) from local surveillance data. Gray bars (in both A and B) represent the times when the surveys were conducted. Abbreviation: Rd, round. B, Population-level (dashed lines) and self-reported (dots and vertical lines) coverage by vaccine dose. C. Adjusted vaccine effectiveness (VE) compared with unvaccinated participants. VE was derived as 1 minus the odds ratio, which was estimated from a logistic regression of those testing positive, after adjustment for age, sex, education, occupation, chronic disease condition, and survey round. Abbreviation: CI, confidence interval.
METHODS
We estimated the vaccine effectiveness (VE) of self-reported vaccinations by dose and type against positive testing for SARS-CoV-2 with polymerase chain reaction (PCR) or rapid antigen testing (RAT) during the Omicron BA.2 epidemic in Hong Kong. We conducted regular telephone surveys every 1–2 weeks during the pandemic [6]. In the current study, we focused on 8 surveys conducted during Hong Kong's fifth wave (Figure 1). We collected information on vaccination history, COVID-19 testing, and demographics from randomly selected adults in the general community (details in Supplementary Tables 1 and 2).
We used random iterative method weighting to calculate the COVID-19 prevalence, defined as the proportion of positive test results with either PCR or RAT among all participants responding to each survey. We compared the weighted COVID-19 prevalence and vaccine coverage among survey participants with those calculated from the external data sources (ie, census data for people with confirmed COVID-19 cases and vaccine doses administered), to assess the sample representativeness of the general population in Hong Kong.
We included participants who self-reported conducting COVID-19 tests during the previous week. We performed multiple imputations of the values of independent variables (except for sex and chronic disease condition) when missing, using data collected from participants who self-reported COVID-19 tests. VE was derived as 1 minus the adjusted odds ratio, which was estimated from a logistic regression of those testing positive among participants with self-reported COVID-19 tests, after adjustment for age, sex, occupation, education, chronic disease condition, and calendar time (to account for depletion of susceptibles).
RESULTS
A total of 5105 Hong Kong adults responded to our 8 surveys between 21 February and 28 April 2022, 112 (2%) of who were excluded with other or unspecified vaccines. Among the included 4993 individuals, 8% (n = 386), 41% (n = 2038), and 43% (n = 2141) reported receiving 1, 2, or 3 doses of SARS-CoV-2 vaccines at the time of being interviewed (Figure 1, Supplementary Figure 1, and Supplementary Table 2). A total of 3006 participants (60%) reported conducting COVID-19 tests with specified testing methods in the week before our survey, with 186 testing positive (21%, 40%, and 38% tested with PCR, RAT, or both); the majority (70%) were tested voluntarily (Supplementary Table 2). The temporal distribution of weighted COVID-19 prevalence and vaccination coverage among all survey participants from each round closely reflected the epidemic progression and vaccine coverage in the population (Figure 1 and Supplementary Figure 1).
Compared with unvaccinated participants, we found statistically significant protection against test positivity with PCR or RAT among individuals who received 3 doses of a COVID-19 vaccine (VE, 52% [95% confidence interval, 17%–73%) (Figure 1). We also found that 1 or 2 doses of either CoronaVac or BNT162b2 provided no significant protection against self-reported Omicron infections (Figure 1). Our findings were consistent across all laboratory testing methods (Supplementary Table 3).
DISCUSSION
Our findings indicate that 3 doses of either CoronaVac or BNT162b2 may provide protection against test positivity for the Omicron variant. We were not able to break down by vaccine type for people who received booster doses, owing to the limited sample size of those with positive test results. Enhanced immunogenicity and protections against severe cases have been reported after a BNT162b2 booster dose following both homologues and heterologous primary vaccination. In particular, neutralizing antibodies against Omicron were substantially increased after a booster dose of BNT162b2, among individuals who were primed with either BNT162b2 or CoronaVac [7–9].
We were unable to account for the time since vaccination in our survey participants, which may bias our estimated VE (Supplementary Figure 2). For instance, the Hong Kong vaccine census suggested that, on 8 March 2022 (ie, case peak), 23.9% and 12.5% of people who had received the first dose of CoronaVac or BNT162b2 were within 15–90 days of vaccination (ie, benefiting from the highest level of protection), respectively, which may partially explain the positive point estimates for VE for a single dose of CoronaVac or BNT162b2.
We included only survey participants who self-reported conducting COVID-19 tests in the VE estimation analysis. Compared with the general adult population in Hong Kong, excluded survey participants were more likely to be older adults, aged >60 years old (Supplementary Table 1 and Supplementary Figure 3), who had a lower vaccination uptake rate, and we may therefore have underestimated VE [2].
In summary, our results suggested that 3 doses of COVID-19 vaccine may provide a moderate level of protection, at least for a short time after the third dose, against self-reported infection during the Omicron BA.2–dominated surge.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Bingyi Yang, WHO Collaborating Centre for Infectious Disease Epidemiology and Control, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China.
Irene O L Wong, WHO Collaborating Centre for Infectious Disease Epidemiology and Control, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China.
Jingyi Xiao, WHO Collaborating Centre for Infectious Disease Epidemiology and Control, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China.
Tim K Tsang, WHO Collaborating Centre for Infectious Disease Epidemiology and Control, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China; Laboratory of Data Discovery for Health Limited, Hong Kong Science and Technology Park, New Territories, Hong Kong, China.
Qiuyan Liao, WHO Collaborating Centre for Infectious Disease Epidemiology and Control, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China.
Benjamin J Cowling, WHO Collaborating Centre for Infectious Disease Epidemiology and Control, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China; Laboratory of Data Discovery for Health Limited, Hong Kong Science and Technology Park, New Territories, Hong Kong, China.
Notes
Author contributions . All authors meet the ICMJE criteria for authorship. B. Y. and B. J. C. conceived the study. I. O. L. W., J. X., and Q. L. investigated and prepared the data. B. Y. performed the data analyses. B. Y., T. K. T., and B. J. C. interpreted the results. B. Y. wrote the first draft of the manuscript, and all authors provided critical review and revision of the text and approved the final version.
Financial support . This project was supported by the Food and Health Bureau and Government of the Hong Kong Special Administrative Region (grant COVID19F04) and the Chinese Center for Disease Control and Prevention-sponsored COVID-19 Vaccines Evaluation Program (COVEP).
References
- 1. Yu J, Collier AY, Rowe M, et al. Neutralization of the SARS-CoV-2 Omicron BA.1 and BA.2 variants. N Engl J Med 2022; 386:1579–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McMenamin ME, Nealon J, Lin Y, et al. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect Dis 2022. doi: 10.1016/S1473-3099(22)00345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boon SS, Wong MCS, Ng RWY, et al. Seroprevalence of unidentified SARS-CoV-2 infection in Hong Kong during 3 pandemic waves. JAMA Netw Open 2021; 4:e2132923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mefsin YM, Chen D, Bond HS, et al. Epidemiology of infections with SARS-CoV-2 Omicron BA.2 variant, Hong Kong, January-March 2022. Emerg Infect Dis 2022; 28:1856–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. The Government of the Hong Kong Special Administrative Region . COVID-19 vaccination programme. https://www.covidvaccine.gov.hk/en/. Accessed 16 June 2022.
- 6. Yang B, Wu P, Lau EHY, et al. Changing disparities in coronavirus disease 2019 (COVID-19) burden in the ethnically homogeneous population of Hong Kong through pandemic waves: an observational study. Clin Infect Dis 2021; 73:2298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pérez-Then E, Lucas C, Monteiro VS, et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat Med 2022; 28:481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Costa Clemens SA, Weckx L, Clemens R, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet 2022; 399:521–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zuo F, Abolhassani H, Du L, et al. Heterologous immunization with inactivated vaccine followed by mRNA-booster elicits strong immunity against SARS-CoV-2 Omicron variant. Nat Commun 2022; 13:2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.