Abstract
Objective
Alterations in thyroid function tests (TFTs) have been recorded during SARS-CoV-2 infection as associated to either a destructive thyroiditis or a non-thyroidal illness.
Methods
We studied 144 consecutive COVID-19 patients admitted to a single center in intensive or subintensive care units. Those with previous thyroid dysfunctions or taking interfering drugs were excluded. Differently from previous reports, TSH, FT3, FT4, thyroglobulin (Tg), anti-Tg autoantibodies (TgAb) were measured at baseline and every 3–7 days. C-reacting protein (CRP), cortisol and IL-6 were also assayed.
Results
The majority of patients had a normal TSH at admission, usually with normal FT4 and FT3. Low TSH levels were found either at admission or during hospitalization in 39% of patients, associated with low FT3 in half of the cases. FT4 and Tg levels were normal, and TgAb-negative. TSH and FT3 were invariably restored at the time of discharge in survivors, whereas were permanently low in most deceased cases, but only FT3 levels were predictors of mortality. Cortisol, CRP and IL-6 levels were higher in patients with low TSH and FT3 levels.
Conclusions
Almost half of our COVID-19 patients without interfering drugs had normal TFTs both at admission and during follow-up. In this series, the transient finding of low TSH with normal FT4 and low FT3 levels, inversely correlated with CRP, cortisol and IL-6 and associated with normal Tg levels, is likely due to the cytokine storm induced by SARS-Cov-2 with a direct or mediated impact on TSH secretion and deiodinase activity, and likely not to a destructive thyroiditis.
Introduction
Due to the ACE2 expression, thyroid may become a target of coronavirus infection (1, 2, 3, 4) and indeed a thyroid involvement has been demonstrated by histology during the SARS-Cov-1 (5) and SARS-Cov-2 (6) outbreaks, as thyroid follicular cells damage or lymphocytic infiltration, respectively. An alteration in thyroid function tests (TFT) mainly characterized by the reduction of TSH levels has been recorded during SARS-CoV-2 infection as associated to either a destructive thyroiditis or a non-thyroidal illness (NTI). Indeed, cases of destructive thyrotoxicosis (7, 8, 9) or subacute thyroiditis (10, 11, 12, 13, 14, 15, 16) have been reported in patients with COVID-19 infection. On the other hand, SARS-CoV-2 coronavirus can cause immune response hyperactivity leading to the release of pro-inflammatory cytokines which may cause a cytokine storm (15). In this context, changes in serum thyroid hormone (TH) levels in the context of a NTI are commonly observed in sepsis and in patient with acute respiratory distress syndrome as a consequence of an acute inflammation, which affects deiodinase activity and suppresses the hypothalamic–pituitary–thyroid axis (HPT). During NTI, there is a decrease in free T3 (FT3), correlating with an increase of inflammatory cytokines, and a normal or moderately decreased free T4 (FT4) concentrations. Serum TSH is usually normal, and tends to reduce only in the most severe chronic conditions. Nevertheless, since the alterations of olfaction and taste typical of COVID-19 are in keeping with a SARS-CoV-2 neuro-tropism, the possibility of a hypothalamic–pituitary involvement should not be discarded, as it was previously suggested during SARS-CoV-1 outbreak (3, 16, 17).
Here, we report prospective data collected in a large cohort of 144 consecutive COVID-19 patients during hospitalization, with the aim to get insights into the debated pathogenesis of altered thyroid function tests (TFTs) related to this infection. Since one of the possible explanations of the discrepant results reported in the literature is the interference exerted on serum free TH and TSH determinations by drugs frequently used for the treatment of these patients (3), all the possibly interfered cases were excluded from the study or analyzed separately. Moreover, as data on atypical COVID-19 associated painless thyroiditis were recently published, still lacking thyroglobulin (Tg) determination and not consistent with our findings (7, 8, 9), Tg levels were retrospectively measured on the stored samples corresponding to a reduced/suppressed TSH.
Patients and methods
Patients
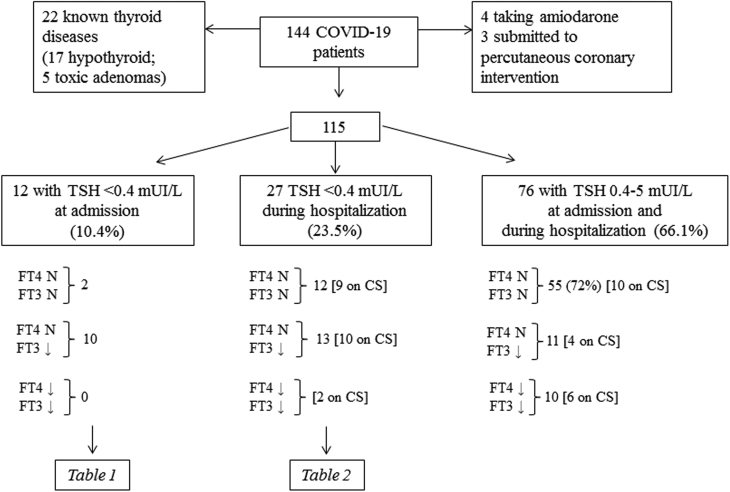
We enrolled 144 consecutive patients (97 males and 47 women, mean age 68.1 ± 14.67 range 26–96 years) affected with COVID-19 pneumonia admitted to the intensive care unit (ICU) or sub-intensive care unit (SICU) at the San Luca Hospital, Istituto Auxologico Italiano in Milan, Italy, between March and May 2020 due to respiratory insufficiency of variable severity (Fig. 1). The overall mortality rate was 25% (36/144). The mean length of the hospital stay was 21 ± 19 days (range 2–108 days).
Figure 1.
Selection criteria at admission and follow-up data of patients included in the study. Patients were then divided according to the TSH levels: suppressed at admission, suppressed during hospitalization, normal. Free thyroid hormone levels are also reported and patients treated with corticosteroids were (CS) highlighted.
In 130/144 patients, high flow oxygen (n = 54), or continuous positive airway pressure (CPAP) treatment (n = 40), non-invasive ventilation (n = 19), endo-tracheal intubation (n =11), tracheostomy (n =6) were required due to severe blood oxygenation impairment.
Twenty-two patients were excluded because of a previous diagnosis of thyroid dysfunction (17 and 5 patients with primary hypothyroidism or multinodular toxic goiter, respectively). We also excluded seven patients submitted to coronarography (n = 3) in the 3 weeks prior to admission or taking amiodarone for atrial fibrillation (n = 4) (Fig. 1). At baseline, none of the patients was taking steroids, while 41 out of the 115 patients included in the study received corticosteroids (CS) during hospitalization as a treatment for pneumonia.
During follow-up, TSH was measured at baseline and at every 3 days while FT4 and FT3 were assessed weekly (mean 8 ± 2 days). Thyroglobulin (Tg) and anti-Tg autoantibodies (TgAb) were retrospectively measured on the serum sample corresponding to a reduced/suppressed TSH, in order to diagnose or exclude destructive thyroiditis. Cortisol levels were also retrospectively measured at baseline, at the time of TSH suppression and discharge in patients not consuming interfering drugs. C-reacting protein (CRP) and IL-6 were measured according to clinical practice guidelines; samples obtained from patients treated with tocilizumab were not included.
At the time of discharge, a blood measurement for reflex TSH measurement was obtained in all patients.
The study was approved by the ethics committee of Istituto Auxologico Italiano (COV-endo study, n 05C021). The participants or their parents gave informed consent to include their clinical data in the present study.
Methods
SARS-Cov-2 infection was confirmed in all patients by RT-PCR from naso–pharyngeal swab. The indication for invasive or non-invasive mechanical ventilation was based on the Brescia-COVID Respiratory Severity Scale (BCRSS)/Algorithm (18). TSH, FT4 and FT3 were assessed by an electrochemiluminescence assay (Roche). In order to limit in vitro displacement leading to spurious hyperthyroxinaemia, we took serum samples more than 10 h after the last injection of high-molecular weight heparin. For assessing serum Tg levels, we used Elecsys® Tg II-Roche Diagnostics (Basilea–Switzerland, analytical sensitivity 0.04 mcg/L) and for TgAb Elecsys® anti-Tg Roche Diagnostics (upper limit normal 115 kU/L). The possible interference by positive IgM, reported to be produced 1 month after the onset of destructive thyroiditis (19), was excluded because in our series Tg was measured at the time of TSH suppression. Serum total cortisol and IL-6 were assayed with the electrochemiluminescent Elecsys® cortisol immunoassays (Roche Diagnostics). CRP was measured by Tina-quant® C-Reactive Protein assay IV immunoturbidimetric assay (Roche Diagnostics)
Serum samples obtained after dopamine administration at the time of ICU admission were excluded. In the present series, none of the patients used neuroepileptics. Quetiapine, sertraline or haloperidol were taken by six, three and one patients, respectively, all of them not showing significant TSH changes. Data related to the possible presence of common comorbidities (i.e. hypertension, type 2 diabetes, obesity) were derived from medical reports, collected and managed using REDCap electronic data capture tools hosted at Istituto Auxologico Italiano (20). Thyroid volume was estimated in some patients with a portable US scanner or by evaluation of the chest CT scan.
The temporal length of TSH reduction was calculated as the number of days between the first low TSH and the first normal TSH values.
Statistical analysis
Clinical data were expressed as median (range) and mean ± standard deviation for continuous variables and as frequencies and percentage for categorical or nominal data. A normally distributed paired variable was compared with paired t-test. The linear association between two continuous variables was assessed by the Pearson correlation coefficient.
Two-tailed Fisher's exact test was used to compare categorical data as appropriate. A multivariate logistic regression model was then employed to determine variables associated independently with mortality. The predictive model was determined by stepwise selection using P = 0.05 as the entry value for the model.
Results
Twelve out of the 115 patients (10.4%) without an history of thyroid dysfunctions had low TSH levels (median: 0.209 mU/L; range: 0.07–0.39, normal values: 0.4–5 mU/L) at admission. The majority of them had low levels of FT3, too (median: 2.2 pmol/L; range: 1.9–4.4; normal value:s 2.9–7.1), none of them was treated with CS during hospitalization (Fig. 1, Table 1, Supplementary Table 1, see section on supplementary materials data given at the end of this article).
Table 1.
Thyroid function tests in patients with reduced TSH (median: 0.2, range: 0.07–0.39 mU/L) at admission. The median time of TSH suppression was 5 days (range: 2–9). Blood sampling was done weekly (mean: 8 ± 2 days). The most representative samples between admission and discharge are reported (the whole of the results at each time point are reported in Supplementary Table 1). The day after admission at which the blood sampling (BS) was done is reported in parentheses. The last measurement available corresponds to either discharge or death. Thyroglobulin (TG) was measured at the time of TSH suppression. None of these patients were taking steroids.
| # |
Sex |
Age |
*TSH reduction |
Levels at admission | Levels during hospitalization | Levels post-discharge | Oxygen support |
US or CT result |
TG |
Outcome |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TSH | FT4 | FT3 | TSH | FT4 | FT3 | BS day | TSH | FT4 | FT3 | BS day | TSH | FT4 | FT3 | BS day | ||||||||
| 1 | M | 83 | 4 | 0.12 | 21.5 | 2.8 | 1.56 | 13 | 2.3 | 12 | 1.88 | 17.5 | 3.6 | 45 | 1.47 | na | na | 211 | NIMV | Normal | 13.2 | S |
| 2 | M | 73 | 4 | 0.19 | 17.3 | 1.9 | 0.58 | 15.8 | 1.5 | 22 | 1.70 | 20.9 | 2.9 | 47 | – | – | – | – | Goggles | Normal | 3.7 | S |
| 3 | F | 67 | 5 | 0.23 | 14.3 | 2.7 | 0.30 | 15.2 | 2.9 | 4 | 1.07 | 17.3 | 3.1 | 25 | 1.10 | na | na | 126 | CPAP | Normal | 5.2 | S |
| 4 | M | 57 | 5 | 0.07 | 15.5 | 2.1 | 2.08 | 20.1 | 5.2 | 14 | 0.93 | 16.3 | 5.2 | 37 | 1.29 | na | na | 127 | IOT | Normal | 3.1 | S |
| 5 | M | 72 | 5 | 0.08 | 15.3 | 2.2 | 0.05 | 11.6 | 1.5 | 5 | 2.42 | 21.2 | 3.5 | 65 | – | – | – | – | IOT | Normal | 3.6 | S |
| 6 | F | 67 | 4 | 0.31 | 16.6 | 2.0 | 0.38 | na | na | 4 | 1.45 | 17.3 | 2.3 | 28 | – | – | – | – | Goggles | Normal | 3.4 | S |
| 7 | M | 72 | 2 | 0.39 | 16.3 | 3.3 | 0.34 | na | na | 2 | 1.17 | 15.4 | 3.1 | 14 | 0.77 | na | na | 175 | Goggles | na | 7.2 | S |
| 8 | F | 65 | 8 | 0.37 | na | na | 0.05 | 18.2 | 2.9 | 6 | 1.50 | 19.3 | 4.4 | 11 | – | – | – | – | CPAP | NG | 61.4 | S |
| 9 | M | 68.4 | 2 | 0.30 | 14.2 | 1.9 | – | – | – | – | 0.41 | 13.2 | 2.0 | 2 | – | - | - | - | IOT | na | 9.3 | D |
| 10 | M | 89 | 6 | 0.14 | 19.7 | 2.6 | 0.09 | 18.7 | 2.9 | 4 | 0.05 | na | na | 6 | – | – | – | – | NIMV | Normal | 7.6 | D |
| 11 | M | 83 | 2 | 0.30 | 15.2 | 2.0 | 0.48 | 15.6 | 2.5 | 3 | 1.51 | 13.6 | 2.0 | 26 | – | – | – | – | VM | Normal | 3.2 | D |
| 12 | M | 70 | 9 | 0.09 | 16.9 | 4.4 | 0.13 | 15.7 | 2.8 | 2 | 2.11 | 14.9 | 1.9 | 14 | – | – | – | – | IOT | NG | 41.3 | D |
Reference range for: TSH = 0.4–5.0 mU/L; FT4 = 11.5–24.5 pmol/L; FT3 = 2.9–7.1 pmol/L.
Duration in days of TSH reduction.
CPAP, continuous positive airway pressure; CT, computed tomography; D, deceased; IOT, endo-tracheal intubation; Na, not assessed; NG, nodular goiter; NIMV, noninvasive mechanical ventilation, S, survivor; TG, thyroglobulin (normal values <85 mcg/L); US, ultrasound; VM,Venturi mask.
On the other hand, 27/115 cases (23.5%) developed TSH levels < 0.4 mU/L during hospitalization (median 0.289 mU/L; range 0.11–0.393). In 13 cases (10 of whom on CS), FT3 levels below the normal range were also found (median 2.1 mU/L; range 1.6–2.8), and 2 patients treated with CS had low FT3 and FT4 levels. FT4 levels were normal in the remaining 25 cases (Fig. 1, Table 2, Supplementary Table 2). It is worth to note that TSH levels were < 0.2 mU/L, at admission or during the follow-up, in 17/39 patients, whereas a slighter reduction (≥ 0.2 < 0.4 mU/L) was found in the remaining. Longitudinal evaluations done during the hospital stay showed that the TSH suppression was transient in all the survivors with a median time to normalization of 5 days (range 2–9 days; mean ± s.d. 4.26 ± 1.98). At the time of discharge, all the survivors, except two cases (2.3 and 2.7 pmol/L), had normal FT3 levels (range 3–5.2 pmol/L) (Tables 1 and 2, Supplementary Tables 1 and 2), whereas TSH and FT3 were permanently low in 6/11 and in 7/11 deceased cases, respectively. Tg levels were invariably found to be normal in the absence of a possible TgAb interference in all the 39 patients with low TSH, either at admission or during follow-up (median 7.6 mcg/L, range: 3–70.3 mcg/L). Only three patients had higher Tg levels (70.3, 61.4 and 41.3 mcg/L) but were found to have a nodular goiter. TgAb levels (normal limit <115 kU/L) were <10 in nine patients, 11–17 in 27, and 20–50 in the remaining three. None of them complained any manifestation suggestive of subacute thyroiditis (SAT) or thyrotoxicosis.
Table 2.
Thyroid function tests in the 27 patients with normal TSH at baseline but with reduced TSH during hospitalization. The median time of TSH suppression was 5 days (range: 2–9). Blood sampling was done weekly (mean 8 ± 2 days). The most representative samples between admission and discharge are reported (the whole of the results at each time point are reported in Supplementary Table 2). The day after admission at which the blood sampling (BS) was done is reported. The last measurement available corresponds to either discharge or death. Thyroglobulin (TG) was measured in 25/27 patients at the time of TSH suppression.
| # |
Sex |
Age |
*TSH reduction |
Levels at admission | Levels during hospitalization | Levels post-discharge | Oxygen support |
US/ CT result |
TG |
Outcome |
Steroids start day |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TSH | FT4 | FT3 | TSH | FT4 | FT3 | BS day | TSH | FT4 | FT3 | BS day | TSH | FT4 | FT3 | BS day | |||||||||
| 1 | M | 46 | 4 | 0.94 | 14.2 | 3.0 | 0.16 | 11.2 | 2.2 | 5 | 2.27 | 15.9 | 4.5 | 33 | 2.23 | 16.8 | 4.8 | 153 | IOT | Normal | 1.67 | S | 3 |
| 2 | F | 67 | 3 | 0.54 | 18.5 | 2.5 | 0.29 | 17.2 | 2.5 | 11 | 1.01 | 17.5 | 4 | 48 | – | – | – | – | IOT | Normal | 10.9 | S | 7 |
| 3 | M | 84 | 8 | 0.41 | 17.3 | 2.9 | 0.21 | 16.2 | 2.9 | 9 | 0.80 | 15.3 | 3.1 | 21 | – | – | – | – | CPAP | Normal | NA | S | 1 |
| 4 | M | 61 | 7 | 0.58 | 16.3 | 2.9 | 0.19 | 16.7 | 2.3 | 5 | 1.61 | 17.4 | 3.2 | 13 | – | – | – | –- | CPAP | Normal | 6.3 | S | 4 |
| 5 | M | 53 | 3 | 1.48 | 19.4 | 2.6 | 0.37 | 16.5 | 2.3 | 6 | 1.08 | 14.3 | 2.1 | 12 | – | – | – | – | IOT | Normal | 6.56 | D | 4 |
| 6 | M | 71 | 2 | 2.37 | 15.9 | 3.1 | 0.23 | 16.7 | 2.9 | 11 | 4.05 | 17.1 | 3.3 | 15 | 2.48 | NA | NA | 249 | Goggles | Normal | 11.1 | S | 1 |
| 7 | M | 67 | 2 | 1.16 | 15.6 | 3.1 | 0.35 | 12.2 | 2.0 | 2 | 1.44 | 14.4 | 2.9 | 19 | – | – | – | – | IOT | Normal | 1.64 | D | 8 |
| 8 | M | 57 | 4 | 2.05 | 19.8 | 3.9 | 0.39 | 13.2 | 2.9 | 17 | 1.42 | 15.1 | 3.2 | 27 | – | – | – | – | IOT | Normal | 22.1 | S | 1 |
| 9 | M | 47 | 5 | 0.67 | 15.2 | 3.2 | 0.21 | 14.4 | 3.0 | 4 | 3.01 | 17.6 | 4.1 | 9 | – | – | – | – | CPAP | Normal | 6.5 | S | 1 |
| 10 | M | 78 | 6 | 0.86 | 14.1 | 3.1 | 0.12 | NA | NA | 6 | 0.19 | 12.3 | 2.9 | 8 | – | – | – | – | NIMV | Normal | 4.4 | D | 2 |
| 11 | F | 56 | 2 | 1.08 | 16.8 | 3.1 | 0.36 | 15.9 | 1.9 | 9 | 1.87 | 14.3 | 3.1 | 26 | 1.9 | NA | NA | 110 | NIMV | Normal | 14.1 | S | 3 |
| 12 | M | 74 | 4 | 0.91 | 17.8 | 2.7 | 0.34 | 16.4 | 3.3 | 7 | 1.80 | 17.2 | 3.5 | 14 | – | – | – | – | CPAP | NG | 70.3 | S | 4 |
| 13 | M | 58 | 6 | 1.47 | 19.2 | 4.2 | 0.29 | 17.5 | 3.2 | 8 | 0.41 | 15.5 | 2.7 | 14 | – | – | – | – | CPAP | Normal | 9.77 | S | 5 |
| 14 | M | 26 | 4 | 1.21 | 18.2 | 4.6 | 0.36 | 18.9 | 4.4 | 14 | 1.3 | 17.4 | 3.9 | 20 | – | – | – | – | CPAP | Normal | 5.6 | S | 9 |
| 15 | M | 69 | 2 | 1.04 | 19.2 | 3.9 | 0.28 | 20.1 | 3.3 | 4 | 1.15 | 21.4 | 2.9 | 14 | 1.64 | 17.2 | 3.5 | 163 | CPAP | Normal | 10.5 | S | 4 |
| 16 | M | 84 | 5 | 0.74 | 13.2 | 2.8 | 0.33 | 7.5 | 1.7 | 16 | 0.17 | 8.2 | 1.9 | 21 | – | – | – | – | IOT | Normal | 2.74 | D | 5 |
| 17 | M | 77 | 3 | 0.74 | 14.3 | 3.3 | 0.29 | 16.4 | 2.8 | 4 | 2.44 | 15.7 | 3.2 | 13 | 2.27 | NA | NA | 182 | CPAP | Normal | 8.47 | S | 1 |
| 18 | M | 56 | 4 | 0.62 | 18.2 | 4.1 | 0.28 | 16.9 | 3.8 | 4 | 1.10 | 15.8 | 3.1 | 9 | – | – | – | – | CPAP | Normal | NA | S | 5 |
| 19 | M | 61 | 9 | 0.81 | 17.3 | 3.8 | 0.25 | 22.2 | 2.5 | 11 | 0.97 | 19.5 | 2.9 | 40 | – | – | – | – | VM | NG | 27.1 | S | 7 |
| 20 | M | 74 | 2 | 0.92 | 18.2 | 2.6 | 0.26 | 18.7 | 1.9 | 3 | 1.80 | 20.3 | 3.1 | 28 | – | – | – | – | CPAP | Normal | 23.5 | S | 12 |
| 21 | M | 66 | 6 | 1.45 | 16.9 | 5.1 | 0.15 | 14.6 | 4.5 | 6 | 2.24 | 16.7 | 3.2 | 40 | 3.9 | 14.2 | NA | 170 | CPAP | Normal | 6.99 | S | 4 |
| 22 | F | 86 | 4 | 0.68 | 16.2 | 2.1 | 0.38 | 14.2 | 2.8 | 5 | 0.55 | 16.1 | 3.3 | 12 | – | – | – | – | VM | Normal | 8.2 | S | NO |
| 23 | M | 68 | 9 | 1.27 | 17.6 | 4.2 | 0.11 | 16.9 | 3.6 | 5 | 1.87 | 18.1 | 4 | 20 | – | – | – | – | NONE | Normal | 15 | S | NO |
| 24 | M | 71 | 4 | 0.41 | 18.1 | 3.7 | 0.21 | 15.7 | 3 | 6 | 0.63 | 12.7 | 4 | 16 | 0.8 | 13.4 | 4.3 | 228 | VM | Normal | 0.2 | S | NO |
| 25 | M | 77 | 5 | 0.66 | 16 | 2.8 | 0.39 | NA | NA | 2 | 0.11 | 13.2 | 2.1 | 5 | – | – | – | – | NIMV | Normal | 4.1 | D | NO |
| 26 | M | 64 | 5 | 0.73 | 17.2 | 2.5 | 0.39 | 16.8 | 2.4 | 1 | 0.12 | 14.3 | 1.6 | 5 | – | – | – | – | IOT | Normal | 32.3 | D | NO |
| 27 | M | 65 | 3 | 0.63 | 19.3 | 4.2 | 0.34 | NA | NA | 4 | 0.19 | 18.2 | 3.4 | 6 | – | – | – | – | NIMV | Normal | 8.28 | D | NO |
Reference ranges: TSH = 0.4–5.0 mU/L; FT4 = 11.5–24.5 pmol/L; FT3 = 2.9–7.1 pmol/L; TG normal values < 85 mcg/L.
Duration of TSH reduction in days.
CPAP, continuous positive airway pressure; D, deceased; IOT, endo-tracheal intubation; NA, not assessed; NG, nodular goiter; NIMV, noninvasive mechanical ventilation; S, survivor; VM, Venturi mask.
The remaining 76 patients (66.1%) had a normal TSH at admission (0.41–5.0 mU/L), and the vast majority of them (55/76, 72%) had normal FT3 and FT4, whereas FT3 alone or both FT3/FT4 were low in 21 cases (Fig. 1).
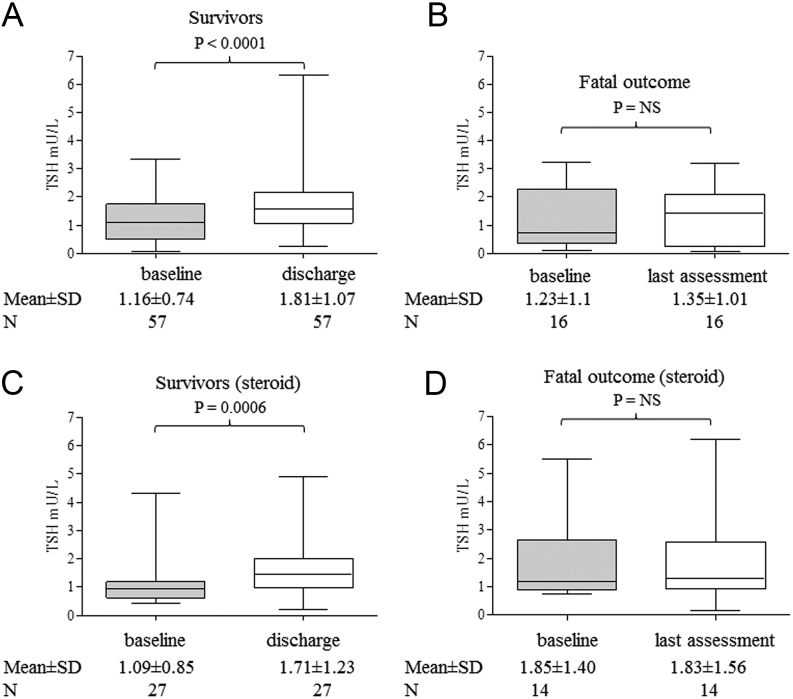
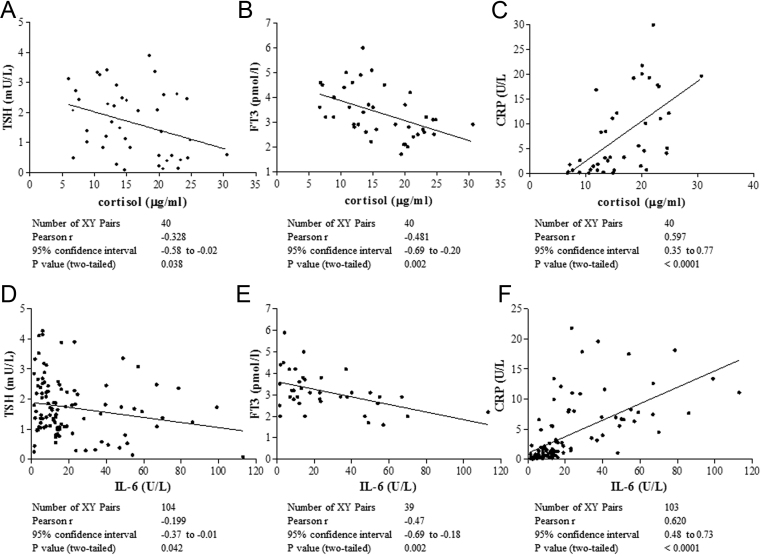
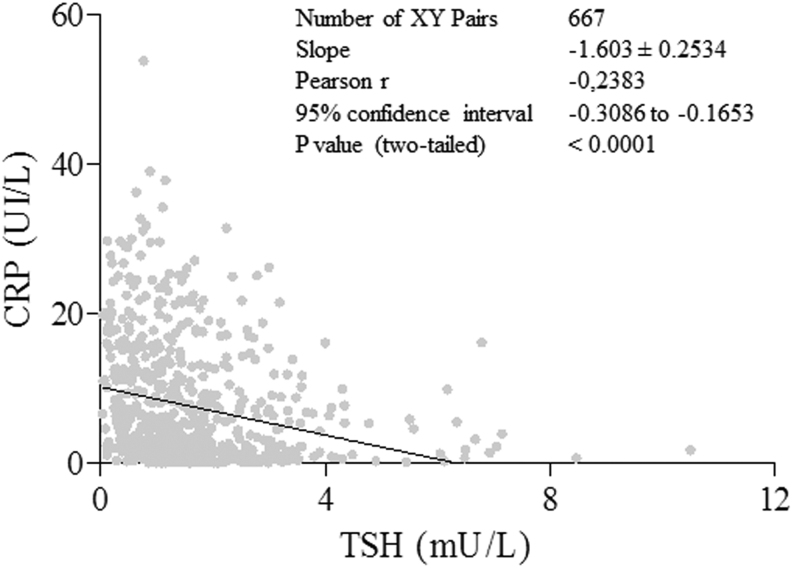
Interestingly, considering the total of 115 cases, TSH levels at discharge were significantly higher than those at admission in survivors, in contrast with deceased cases. This finding appears not due to an effect of exogenous steroids on TSH secretion, being observed both in patients without (Fig. 2, panel A and B) or with CS treatment (Fig. 2, panels C and D). To evaluate the effects on TFTs of endogenous cortisol, whose secretion is associated to stressful events, we retrospectively measured cortisol levels on stored sample of 40 patients not treated with steroids or other interfering drugs. We found a significant inverse correlation between the cortisol levels and TSH and FT3 (r = −0.3, P = 0.04 and r = −0.5, P = 0.002, respectively) and a direct correlation with CRP levels, as expected (r = 0.6; P < 0.0001) (Fig. 3, upper panels). The pro-inflammatory milieu was also found to correlate with TFTs, because IL-6 levels were significantly and inversely correlated with TSH and FT3 levels (r−0.2; P = 0.04 and r = −0.5; P = 0.02) and directly correlated with CRP (Fig. 3 lower panels). Accordingly, in the whole series including 667 samples, we found a significant and inverse correlation between CRP and TSH, after exclusion of all TSH values obtained during CS treatment (Fig. 4). To test the possible impact of TFTs on mortality in our series, we evaluated by uni-and multi-variate analyses for several predictors (Supplementary Table 3). At univariate analysis, predictors of mortality were: age > 65 (P = 0.0004), coronary artery disease (P = 0.03), diabetes (P = 0.05), chronic obstructive pulmonary disease (P = 0.04), atrial fibrillation (P = 0.04), severity of respiratory dysfunction at admission (P = 0.01), coinfections during hospitalization (P = 0.0002), disseminated intravascular coagulation (P = 0.04), liver (P = 0.005) and kidney acute dysfunctions (P < 0.0001), low FT3 (P < 0.0001), low FT4 (P = 0.01) but not low TSH levels. At multivariate analysis, only age > 65, coronary artery disease, co-infections, acute kidney injury and low FT3 were retained in the model.
Figure 2.
Mean ± s.d. TSH levels at the time of hospital admission and of discharge or death in patients who survived (panels A and C) and in those with a fatal outcome (panels B and D). Patients requiring steroids during the hospital stay were analyzed separately. The number of patients analyzed is reported.
Figure 3.
Significant inverse correlation between TSH, FT3 and C-reactive protein (CRP) levels with total cortisol (top panels) and IL-6 (bottom panels). Top panels: patients taking steroids or aldosterone antagonists were excluded from the analysis. Mean cortisol levels were not significantly different between patients treated with antiretroviral therapy (n = 25) or not (n = 15), data not shown. Bottom panels: values obtained after administration of tocilizumab were excluded from the analysis.
Figure 4.
Significant inverse correlation between TSH and C-reactive protein (CRP) levels in 667 samples. Patients treated with steroid were excluded from the analysis.
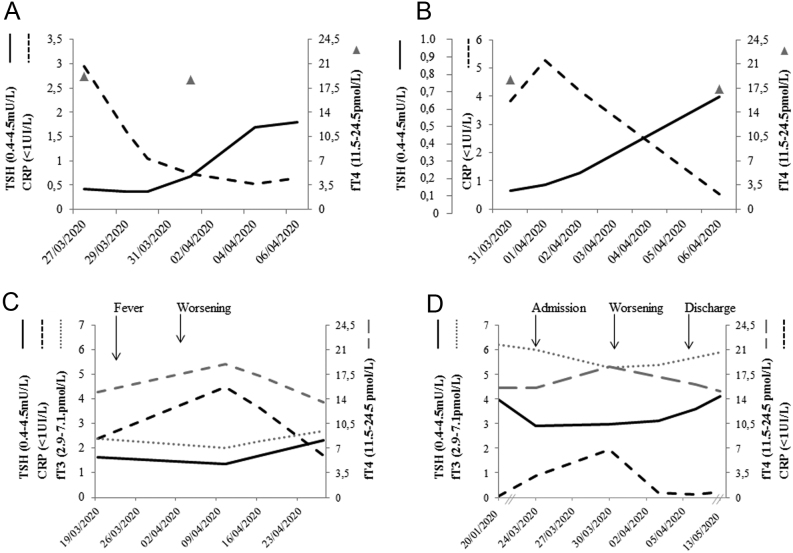
In Fig. 5, we report that TFTs change in some representative cases. Two female patients (aged 74 and 89 years) admitted to a low intensity ward are reported in panels A and B. They had undergone total thyroidectomy and radioiodine ablation for differentiated thyroid cancer several years before and were on clinical/biochemical remission, thus giving us the unique opportunity to evaluate TSH fluctuations in the absence of thyroid tissue. They were on L-T4 replacement treatment and TSH levels were repeatedly normal before COVID-19 diagnosis. They were treated with antivirals, chloroquine, antibiotics, heparin and oxygen support (CPAP in one case and goggles for oxygen/nasal feeding in the other) for a bilateral interstitial pneumonia, while they did not receive CS. At admission, they both had a reduced TSH, which normalized along with improvement in CRP. L-T4 dose was not modified during the hospital stay and the patients' compliance with treatment was not affected as they remained fully conscious. Baseline FT4 levels were normal (19.2 and 18.7 pmol/L, respectively), and no significant variations were noted in following samplings. In panel C, we report an 88-year-old euthyroid female patient with a normal thyroid gland and negative Tg-Ab and TPO-Ab followed in a low intensity ward for bilateral COVID-19 pneumonia and treated only by antivirals and antibiotics. Thyroid function test assessed during the hospital stay showed a minor reduction in TSH level (from 1.7 to 1.3 mU/L), rising levels of FT4 (from 15 to 19.2 pmol/L, range: 11.5–24.5) and decreasing levels of FT3 (from 2.4 to 2.0 pmol/L, range: 2.9–7.1) during the acute phase, while this pattern reverted upon disease improvement (TSH: 2.31 mU/L, FT4: 13.6 pmol/L and FT3: 2.7 pmol/L).
Figure 5.
Changes in thyroid function tests in four representative patients with COVID-19 pneumonia. Panels A and B: two female patients previously submitted to total thyroidectomy and radioiodine ablation for a differentiated thyroid cancer and in complete remission. They were on L-T4 replacement treatment and TSH levels were repeatedly normal before COVID-19 diagnosis. At admission, they both had a reduced TSH, which normalized along with the improvement of CRP. The patient in panel A is an ICU doctor with a bilateral pneumonia who asked to be discharged to home after 9 days of hospitalization with domiciliary oxygen supply. The patient in panel 2 had a milder disease, and oxygen supply was prescribed during the hospital stay. Panel C: thyroid function test assessed during the hospital stay in a female patient with mild COVID-19 pneumonia. During the acute phase, a slight reduction of TSH levels was observed, with rising levels of FT4 and decreasing levels of FT3, but still in the normal range. This pattern reverted upon disease improvement. Panel D: in a male patient, for whom pre-COVID 19 infection parameters were available, TSH decrease, paralleling CRP increase, FT4 increase and Ft3 decrease were observed at admission and during hospitalization in a low intensity care unit. Interestingly, 1 month after discharge, thyroid function tests fully recovered their original setup point. No neck pain was reported in the patients depicted in panels C and D and thyroglobulin levels were normal at the time of TSH reduction (6.6 and 19.6 mcg/L, respectively). CRP, C-reactive protein.
A similar pattern was observed in a 40-year-old male patient for whom we had the availability of biochemical data not only during hospitalization but also some months before admission and at 1 month after discharge (panel D). In this patient, we observed TSH reduction along with disease progression associated with an increase in FT4 and a parallel decrease in FT3. Interestingly, 1 month after discharge, thyroid function tests fully recovered to their original setup point. Of note, no symptoms of SAT were documented in these latter patients, and Tg levels were normal at the time of TSH reduction (6.6 and 19.6 mcg/L, respectively).
Discussion
A number of serious effects on various organs and systems have been reported in humans during the COVID-19 pandemic, including a potential association between the infection and thyroid dysfunctions.
The 48% (55/115) of our COVID-19 patients hospitalized in ICU and SICU did not experience any alteration in TFTs either at admission or during the whole follow-up. A lower number of cases (21/115, 18%) had free thyroid hormone variations in the presence of normal TSH levels, as the consequence of either a low T3 syndrome or due to the corticosteroid impact on peripheral T4 deiodination. On the other hand, TSH levels below the normal range were found in 10.4% of cases (12/115) at admission and in 23.5% (27/115) during the follow-up, and TSH levels were <0.2 mU/L in 17/39 patients. The majority of patients with low TSH levels had normal FT4 and low FT3 levels, and we hypothesized that this situation could be due to the combination of a classic NTI associated with a suppressive effect on TSH exerted by exogenous or endogenous CS, as observed for acute and chronic inflammatory conditions (21, 22) and here due to the typical proinflammatory milieu induced by SARS- Cov 2 infection. The combination of central and peripheral dysfunctions in the pathogenesis of NTI due to severe infections has been demonstrated in an animal model of lipopolysaccharide-induced acute illness. In particular, pro-inflammatory cytokines such as IL-18 appear responsible for the central component of the NTI (23). Interestingly, serum IL-18 concentrations were found to be remarkably increased in patients with COVID-19 and correlated with markers of renal, hepatic and cardiac injury, suggesting that IL-18 contributes to multiorgan injury (24). Similarly, the injection of IL-6 to mimic acute inflammatory stress in healthy humans was found to induce a significant reduction in TSH levels, and an effect of this inflammatory cytokine either direct or mediated via an increase in cortisol was suggested (25). Several analyses were done to test this hypothesis in our series. First, a possible inhibitory effect of CS on TSH secretion is unlikely because TSH concentrations of patients with and without steroids treatment were similarly low at baseline, and both increased at discharge, although a possible effect on either TSH or peripheral T4 to T3 conversion cannot be excluded in some of our patients (Supplementary Table 2). On the other hand, we found an inverse significant correlation between endogenous cortisol and IL-6 levels and TSH and FT3 levels, further supporting the impact at the central (TSH inhibition) and peripheral (deiodinase inhibition) levels of the proinflammatory milieu induced by SARS-Cov-2. The changes in TSH and FT3/FT4 ratio found in the patients not treated with CS and described in detail (Fig. 5, panels C and D) are in support of a direct combined effect at the central and peripheral levels. In both patients, we found an initial lowering of TSH levels, which is likely due to a direct inflammatory effect at the hypothalamic−-pituitary level and not to destructive thyroiditis in light of the normal FT4 and Tg levels. Upon disease progression, the FT3/FT4 ratio decreased due to a combined reduction of FT3 and an increase of FT4, which is suggestive of an impaired peripheral conversion of FT4 to FT3 (22, 26). Moreover, the availability of serial TSH measurements allowed us to confirm previous data indicating that the decline of the TSH is transient (27) and to demonstrate that the patient-specific set point of the HPT axis was perfectly re-established at remission.
To further highlight that destructive thyroiditis is an unlikely cause of the TSH decrease observed during hospitalization, at least in our series, we had the unique opportunity to study two thyroidectomized women on complete remission from thyroid cancer. In these patients, the TSH decrease could not be due to a thyrotoxic event but rather represents a central effect of the inflammatory cytokines, as supported by the inverse correlation between TSH and CRP. Consistently, TSH levels remained low in patients who died due to complications of SARS-Cov2 pneumonia.
Based on these data, we believe that in our series the alterations of the HPT axis are the result of the cytokine storm which may complicate COVID-19 infection, as observed in serious illness, and induces an NTI with a combined effect on pituitary, which leads to a decrease in TSH levels, and at the peripheral level, with a reduced FT4 to FT3 conversion. This is in line with recent studies suggesting that NTI is the most common thyroid dysfunction seen in COVID patients (27, 28). In particular, consistent with our findings, Khoo et al. found in 13% of 383 patients hospitalized for COVID-19 a mild reduction of TSH levels and hypothesized a possible role of IL-6 based on the negative correlation with TSH (27). Moreover, Gao et al. reported a decrease in TSH, FT3 levels and FT3/FT4 ratio paralleling the severity of the COVID-19 infection, and reaching the lowest levels in deceased patients (28). Finally, in a series of 50 patients hospitalized for COVID-19 and retrospectively analyzed, the decrease in TSH was found to be highly prevalent (56%) and to positively correlate, together with total T3 levels, to the severity of the infection (29).
On the other hand, a thyrotoxicosis due to a typical subacute De Quervain's thyroiditis (SAT) and likely linked to the COVID-19 infection has also been reported in a total of ten cases at the time of the writing of this manuscript (10, 11, 12, 13, 14, 30, 31). In all cases, a typical picture of SAT is described, with neck pain radiating to the jaw, palpitations, fever and asthenia, suppressed TSH, high FT4 and Tg, ultrasound signs and absent uptake at scintigraphy. Interestingly, the onset is reported in most cases to be between 2 and 5 weeks after the resolution of the viral disease with a strong response to steroids administration.
Still, the occurrence of an atypical painless thyroiditis in affected hospitalized patients has been reported, with variable prevalence (6–20%) likely related to the different time points of TSH sampling among series (7, 8, 9). In particular, in the first series, patients hospitalized in a high-intensity of care unit (HICU) and testing positive for COVID-19 were compared with a group of HICU patients collected prior to the COVID-19 pandemic. The authors found that in the COVID-19 cases, the TSH levels were lower and FT4 levels were higher than in the non COVID-19 patients, with FT3 levels equally low in the two groups. They attributed these findings to a subacute thyroiditis without neck pain, despite alterations of TSH/FT4 levels much less evident than expected during a destructive thyroiditis. After a mean of 55 days after discharge, they found three cases with focal hypoechoic areas corresponding to reduced Technetium-99m uptake. Tg determinations were not available to strengthen these findings, and the authors interpreted these data as being due to an atypical thyroiditis with a combination of thyrotoxicosis and NTI syndrome (7). In the second study, free thyroid hormones were measured in only 25% of patients, but low TSH levels were found, accompanied in half of the cases by increased FT4 levels, and normal FT3. The authors concluded in favor of a destructive thyroiditis, rather than of an NTI (8), but Tg data or radioiodine uptake, crucial to confirm a destructive thyroiditis in the absence of neck pain, were not available. In the third study, TSH reduction was found in 11/191 (6%) patients, without FT3 and or FT4 alterations in ten of them. The authors concluded the occurrence of a destructive thyroiditis, although three out of the 11 cases had Graves disease with positive TRAbs and neither Tg determinations nor any thyroid imaging were performed (9).
The occurrence of a SAT or of a silent thyroiditis as the underlying cause for TSH decrease is unlikely in our series. Thyroid uptake was not performed, and ultrasound was limited to the bed-side estimation of the thyroid volume, possibly loosing minor alterations of the echogenicity. Nevertheless, neck pain was always absent, and none of our patients at the time of TSH suppression had increased levels of Tg, which is a reliable marker of destructive thyroiditis, independent of its pathogenesis (immuno-mediated, drug-induced or viral) (32). Another argument against the occurrence of destructive thyroiditis comes from the longitudinal observation of the TSH levels which, in patients cured of the Sars-Cov-2 infection, normalize in few days, not consistent with the typical triphasic course (thyrotoxicosis, hypothyroidism, and euthyroidism) of destructive thyroiditis, which usually lasts several weeks.
The main strength of our study are: (a) the availability of Tg levels which excluded that the high prevalence of low TSH levels in COVID-19 patients could be due to thyrotoxicosis; (b) the possibility to test two thyroidectomized patients, thus excluding the thyroid contribution from the analysis of SARS-Cov-2 effects on TSH levels; (c) the separate analysis of patients treated without or with steroids, to evaluate the confounding effect on thyroid function tests of this drug frequently used in the care of COVID-19 patients; (d) an inverse significant correlation between endogenous cortisol and IL-6 levels and TSH and FT3 levels, supporting the central and peripheral effects of the inflammatory milieu induced by SARS-Cov-2 virus.
The main weakness of our study is the absence of a control group. In previous studies, control groups including heterogeneous patients admitted to ICU for any reasons, including cardiovascular or neurological diseases were used, though that choice is questionable, especially when patients treated with angioplasty or exposed to iodinated contrast media or interfering drugs (e.g. dopamine, steroids, e.v furosemide, antiepileptic etc.) are not excluded. The ideal control group, but extremely difficult to get, would be represented by patients admitted to ICU for an infective disease leading to respiratory insufficiency. Nevertheless, we believe that the longitudinal determinations at admission but also during the follow-up, and even after discharge gives a strong support to the interpretation of our data.
In conclusion, taken together, our data suggest that, in our series, the cytokine release due to the COVID-19 infection causes a combined effect at the hypothalamic–pituitary level and in peripheral tissues. Indeed, the slight reduction in TSH with normal FT4 levels along with low-normal FT3 levels should be interpreted as a change in thyroid hormone metabolism and/or pituitary responsiveness rather than to thyrotoxicosis. Moreover, TFT alterations have been shown to be transient. Thus, unless more insight into the effects of SARS-Cov-2 on the HPT axis is needed, we do not recommend the assessment of thyroid function during hospitalization for acute COVID-19 infection, as the results might be a source of misdiagnosis and a waste of resources. Conversely, clinical and biochemical monitoring after discharge/clinical improvement are appropriate, as SAT may complicate the recovery phase of these patients.
Future independent studies are certainly needed to provide a more detailed picture of how Covid-19 affects the hypothalamus/pituitary/thyroid axis.
Supplementary materials
This is linked to the online version of the paper at https://doi.org/10.1530/EJE-20-1391.
Declaration of interest
Laura Fugazzola is on the editorial board of EJE. Laura Fugazzola was not involved in the review or editorial process for this paper, on which he/she is listed as an author.
Funding
The work was partially supported by the Ricerca Corrente Funds of Istituto Auxologico Italiano (Acronym: COV-endo study, n 05C021_2020)
Author contribution statement
IC and LF designed the study, IC analyzed the data and wrote the draft of the manuscript. IC and LR collected the data. IB, AD, ET performed the serological assays. GBP, ET, CT actively followed the patients during hospitalization. LP and LF revised the manuscript.
Supplementary Material
Supplemental Table 1: Thyroid function tests in patients with reduced TSH (median 0.2, range 0.07–0.39 mU/L) at admission. The median time of TSH suppression was 5 days (range 2–9). Blood sampling were done weekly (mean 8±2 days). The last measurement available corresponds to either discharge or death. None of these patients was taking steroids
Supplemental Table 2: Thyroid function tests in the 27 patients with normal TSH at baseline and showing a reduced TSH during hospitalization. The median time of TSH suppression was 5 days (range 2–9). Blood sampling were done weekly (mean 8±2 days). The last measurement available corresponds to either discharge or death.
Supplemental Table 3: univariate (top panel) and multivariate (bottom panel) analysis of the variables associated with mortality. Significant variables are highlighted in bold.
Acknowledgements
The authors thank Marta Di Stefano, Silvia Federici, Vittoria Favero, Noemi Giancola, Luca Giovannelli, Giovanni Goggi, Federica Sileo, Matteo Trevisan for their assistance in the clinical management of the patients included in the present study.
References
- 1. Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infectious Diseases of Poverty 2020945. ( 10.1186/s40249-020-00662-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rotondi M, Coperchini F, Ricci G, Denegri M, Croce L, Ngnitejeu ST, Villani L, Magri F, Latrofa F, Chiovato L. Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: a clue for COVID-19-related subacute thyroiditis. Journal of Endocrinological Investigation 202061–6. ( 10.1007/s40618-020-01436-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen W, Tian Y, Li Z, Zhu J, Wei T, Lei J. Potential interaction between SARS-CoV-2 and thyroid: a review. Endocrinology 2021162 bqab004. ( 10.1210/endocr/bqab004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Croce L, Gangemi D, Ancona G, Liboà F, Bendotti G, Minelli L, Chiovato L. The cytokine storm and thyroid hormone changes in COVID-19. Journal of Endocrinological Investigation 2021. ( 10.1007/s40618-021-01506-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, Mou HM, Wang LH, Zhang HR, Fu WJet al. A pathological report of three COVID-19 cases by minimal invasive autopsies. Chinese Journal of Pathology 202049411–417. ( 10.3760/cma.j.cn112151-20200312-00193) [DOI] [PubMed] [Google Scholar]
- 6. Wei L, Sun S, Xu CH, Zhang J, Xu Y, Zhu H, Peh SC, Korteweg C, McNutt MA, Gu J. Pathology of the thyroid in severe acute respiratory syndrome. Human Pathology 20073895–102. ( 10.1016/j.humpath.2006.06.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Muller I, Cannavaro D, Dazzi D, Covelli D, Mantovani G, Muscatello A, Ferrante E, Orsi E, Resi V, Longari Vet al. SARS-CoV-2-related atypical thyroiditis. Lancet. Diabetes & Endocrinology 20208739–741. ( 10.1016/S2213-8587(2030266-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lania A, Sandri MT, Cellini M, Mirani M, Lavezzi E, Mazziotti G. Thyrotoxicosis in patients with COVID-19: the THYRCOV study. European Journal of Endocrinology 2020183381–387. ( 10.1530/EJE-20-0335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lui DTW, Lee CH, Chow WS, Lee ACH, Tam AR, Fong CHY, Law CY, Leung EKH, To KKW, Tan KCBet al. Thyroid dysfunction in relation to immune profile, disease status and outcome in 191 patients with COVID-19. Journal of Clinical Endocrinology & Metabolism 2021106e926–e935. ( 10.1210/clinem/dgaa813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mattar SAM, Koh SJQ, Rama Chandran S, Cherng BPZ. Subacute thyroiditis associated with COVID-19. BMJ Case Reports 202013 e237336. ( 10.1136/bcr-2020-237336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asfuroglu Kalkan E, Ates I. A case of subacute thyroiditis associated with Covid-19 infection. Journal of Endocrinological Investigation 2020431173–1174. ( 10.1007/s40618-020-01316-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis after Sars-COV-2 infection. Journal of Clinical Endocrinology & Metabolism 2020105 dgaa276. ( 10.1210/clinem/dgaa276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ruggeri RM, Campennì A, Siracusa M, Frazzetto G, Gullo D. Subacute thyroiditis in a patient infected with SARS-COV-2: an endocrine complication linked to the COVID-19 pandemic. Hormones 20201–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Campos-Barrera E, Alvarez-Cisneros T, Davalos-Fuentes M. Subacute thyroiditis associated with COVID-19. Case Reports in Endocrinology 202020208891539. ( 10.1155/2020/8891539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine & Growth Factor Reviews 20205325–32. ( 10.1016/j.cytogfr.2020.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leow MK, Kwek DS, Ng AW, Ong KC, Kaw GJ, Lee LS. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS). Clinical Endocrinology 200563197–202. ( 10.1111/j.1365-2265.2005.02325.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wei L, Sun S, Zhang J, Zhu H, Xu Y, Ma Q, McNutt MA, Korteweg C, Gu J. Endocrine cells of the adenohypophysis in severe acute respiratory syndrome (SARS). Biochemistry and Cell Biology 201088723–730. ( 10.1139/O10-022) [DOI] [PubMed] [Google Scholar]
- 18. Duca A, Piva S, Focà E, Latronico N, Rizzi M. Calculated decisions: Brescia-COVID respiratory severity scale (BCRSS)/algorithm. Emergency Medicine Practice 202022(Supplement) CD1–CD2. [PubMed] [Google Scholar]
- 19. Ricci D, Brancatella A, Marinò M, Rotondi M, Chiovato L, Vitti P, Latrofa F. The detection of serum IgMs to thyroglobulin in subacute thyroiditis suggests a protective role of IgMs in thyroid autoimmunity. Journal of Clinical Endocrinology & Metabolism 2020105 e2261–e2270. ( 10.1210/clinem/dgaa038) [DOI] [PubMed] [Google Scholar]
- 20. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby Jet al. The REDCap consortium: building an international community of software partners. Journal of Biomedical Informatics 201995103208. ( 10.1016/j.jbi.2019.103208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeGroot LJ. The non-thyroidal illness syndrome. In Endotext. South Dartmouth, MA, 2000. (available at: MDText.com, Inc. Last Update: February 1, 2015. [Google Scholar]
- 22. Warner MH, Beckett GJ. Mechanisms behind the non-thyroidal illness syndrome: an update. Journal of Endocrinology 20102051–13. ( 10.1677/JOE-09-0412) [DOI] [PubMed] [Google Scholar]
- 23. Boelen A, Kwakkel J, Platvoet-ter Schiphorst M, Mentrup B, Baur A, Koehrle J, Wiersinga WM. Interleukin-18, a proinflammatory cytokine, contributes to the pathogenesis of non-thyroidal illness mainly via the central part of the hypothalamus-pituitary-thyroid axis. European Journal of Endocrinology 2004151497–502. ( 10.1530/eje.0.1510497) [DOI] [PubMed] [Google Scholar]
- 24. Satış H, Özger HS, Aysert Yıldız P, Hızel K, Gulbahar Ö, Erbaş G, Aygencel G, Guzel Tunccan O, Öztürk MA, Dizbay Met al. Prognostic value of interleukin-18 and its association with other inflammatory markers and disease severity in COVID-19. Cytokine 2021137155302. ( 10.1016/j.cyto.2020.155302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Torpy DJ, Tsigos C, Lotsikas AJ, Defensor R, Chrousos GP, Papanicolaou DA. Acute and delayed effects of a single-dose injection of interleukin-6 on thyroid function in healthy humans. Metabolism: Clinical & Experimental 1998471289–1293. ( 10.1016/s0026-0495(9890338-9) [DOI] [PubMed] [Google Scholar]
- 26. Boelen A, Kwakkel J, Thijssen-Timmer DC, Alkemade A, Fliers E, Wiersinga WM. Simultaneous changes in central and peripheral components of the hypothalamus-pituitary-thyroid axis in lipopolysaccharide-induced acute illness in mice. Journal of Endocrinology 2004182315–323. ( 10.1677/joe.0.1820315) [DOI] [PubMed] [Google Scholar]
- 27. Khoo B, Tan T, Clarke SA, Mills EG, Patel B, Modi M, Phylactou M, Eng PC, Thurston L, Alexander ECet al. Thyroid function before, during and after COVID-19. Journal of Clinical Endocrinology & Metabolism 2020 2021106e803–e811. ( 10.1210/clinem/dgaa830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gao W, Guo W, Guo Y, Shi M, Dong G, Wang G, Ge Q, Zhu J, Zhou X. Thyroid hormone concentrations in severely or critically ill patients with COVID-19. Journal of Endocrinological Investigation 202021–10. ( 10.1007/s40618-020-01460-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen M, Zhou W, Xu W. Thyroid function analysis in 50 patients with COVID-19: A retrospective study. Thyroid 2021318–11. ( 10.1089/thy.2020.0363) [DOI] [PubMed] [Google Scholar]
- 30. Brancatella A, Ricci D, Cappellani D, Viola N, Sgrò D, Santini F, Latrofa F. Is subacute thyroiditis an underestimated manifestation of SARS-CoV-2 infection? Insights From a case series. Journal of Clinical Endocrinology & Metabolism 2020105 dgaa537. ( 10.1210/clinem/dgaa537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ippolito S, Dentali F, Tanda ML. SARS-CoV-2: a potential trigger for subacute thyroiditis? Insights from a case report. Journal of Endocrinological Investigation 2020431171–1172. ( 10.1007/s40618-020-01312-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MNet al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 2016261343–1421. ( 10.1089/thy.2016.0229) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Thyroid function tests in patients with reduced TSH (median 0.2, range 0.07–0.39 mU/L) at admission. The median time of TSH suppression was 5 days (range 2–9). Blood sampling were done weekly (mean 8±2 days). The last measurement available corresponds to either discharge or death. None of these patients was taking steroids
Supplemental Table 2: Thyroid function tests in the 27 patients with normal TSH at baseline and showing a reduced TSH during hospitalization. The median time of TSH suppression was 5 days (range 2–9). Blood sampling were done weekly (mean 8±2 days). The last measurement available corresponds to either discharge or death.
Supplemental Table 3: univariate (top panel) and multivariate (bottom panel) analysis of the variables associated with mortality. Significant variables are highlighted in bold.