Abstract
Objective
The pandemic of coronavirus disease (COVID-19) has rapidly spread globally and infected millions of people. The prevalence and prognostic impact of dysnatremia in COVID-19 is inconclusive. Therefore, we investigated the prevalence and outcome of dysnatremia in COVID-19.
Design
The prospective, observational, cohort study included consecutive patients with clinical suspicion of COVID-19 triaged to a Swiss Emergency Department between March and July 2020.
Methods
Collected data included clinical, laboratory and disease severity scoring parameters on admission. COVID-19 cases were identified based on a positive nasopharyngeal swab test for SARS-CoV-2, patients with a negative swab test served as controls. The primary analysis was to assess the prognostic impact of dysnatremia on 30-day mortality using a cox proportional hazard model.
Results
172 (17%) cases with COVID-19 and 849 (83%) controls were included. Patients with COVID-19 showed a higher prevalence of hyponatremia compared to controls (28.1% vs 17.5%, P < 0.001); while comparable for hypernatremia (2.9% vs 2.1%, P = 0.34). In COVID-19 but not in controls, hyponatremia was associated with a higher 30-day mortality (HR: 1.4, 95% CI: 1.10–16.62, P = 0.05). In both groups, hypernatremia on admission was associated with higher 30-day mortality (COVID-19 - HR: 11.5, 95% CI: 5.00–26.43, P < 0.001; controls - HR: 5.3, 95% CI: 1.60–17.64, P = 0.006). In both groups, hyponatremia and hypernatremia were significantly associated with adverse outcome, for example, intensive care unit admission, longer hospitalization and mechanical ventilation.
Conclusion
Our results underline the importance of dysnatremia as predictive marker in COVID-19. Treating physicians should be aware of appropriate treatment measures to be taken for patients with COVID-19 and dysnatremia.
Introduction
The ongoing pandemic of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), has rapidly spread worldwide and infected millions of people with increasing numbers of infection rate and deaths (World Health Organization). SARS-CoV-2 is characterized by clinical symptoms such as fever (83.82%), cough (81.8%), fatigue (69.6%), and shortness of breath (31%) (1). Data from large cohort studies have shown that physician diagnosed pneumonia rate of up to 91% in hospitalized patients with COVID-19 (2, 3).
In patients with pneumonia, dysnatremia is common. In fact, hyponatremia has been described with an incidence rate of up to 30% in hospitalized patients (4, 5, 6, 7, 8), while hypernatremia is less frequent with an incidence rate of 3% in hospitalized patients (9, 10, 11, 12, 13). The mechanisms causing dysnatremia in patients with pneumonia are not well-understood and include decreased water clearance, increased saliuretic therapy and an increased rate of the syndrome of inappropriate antidiuresis (SIAD) (7, 8, 14, 15).
The plasma sodium level in patients with pneumonia plays an important role for severity assessment and is therefore used in scores like the pneumonia severity index (PSI) (15). Many studies have shown the association between dysnatremia, especially hyponatremia and worse outcome parameters like mortality and length of hospital stay (19, 20). Large cohort studies have reported an association between hyponatremia and severity parameters such as higher rates of intensive care unit (ICU) admission, longer ICU stay and mechanical ventilation as an important prognostic marker in patients with pneumonia (16). A meta-analysis with 13 816 patients showed a significant association of hyponatremia improvement with reduction of overall mortality up to 60% compared to patients without hyponatremia improvement (11, 17).
To date, the prevalence and outcome of dysnatremia in patients tested positive for COVID-19 remains inconclusive. While most studies on electrolyte disturbances in patients with COVID-19 have shown overall significantly lower sodium blood concentrations, other studies in COVID-19 reported hypernatremia to be more frequent (2, 18), but no study reported comparison to a control group.
The aim of our study was to investigate the prevalence of dysnatremia and the impact on patient-outcome, that is, mortality rate and rehospitalization, and severity of disease in COVID-19. We hypothesized an increased rate of dysnatremia in patients with COVID-19 compared to controls and an association with increased mortality and rehospitalization. Furthermore, we hypothesized that the previously observed association of dysnatremia with clinical severity parameters is also true for patients with COVID-19.
Methods
Study design, population and inclusion criteria
The prospective, observational, cohort study included consecutively enrolled patients aged 18 years and older presenting with clinical suspected or confirmed SARS-CoV-2 infection to the emergency department (ED) of the University Hospital in Basel, Switzerland, during the first wave of COVID-19 pandemic between March 2020 and July 2020. All patients underwent nasopharyngeal SARS-CoV-2 PCR swab tests. Patients were considered COVID-19 positive, in the following referred to as cases, if at least one SARS-CoV-2 PCR swab test performed at day of ED presentation or within 14 days prior to or post ED presentation was positive in combination with clinical signs and symptoms of COVID-19 infection. Patients with negative SARS-CoV-2 PCR swab test were considered controls. All participating patients or their legally authorized representatives consented by signing a local general consent form. The study was registered on ClinicalTrials.gov (ClinicalTrials.gov NCT04366765), conducted according to the principles of the Declaration of Helsinki, and approved by the Ethical Committee Northwest and Central Switzerland. The authors designed the studies, gathered, and analyzed the data according to the STROBE guidelines (19), vouched for the data and analysis, wrote the paper, and decided to submit it for publication.
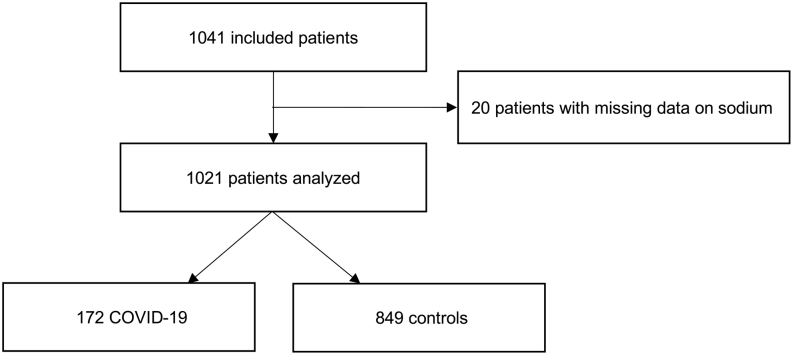
The total number of included patients (n = 1041) was grouped into patients with confirmed COVID-19 (n = 172; cases) and patients without COVID-19 but similar symptoms on admission (n = 849; controls). Twenty patients were excluded for absence of sodium levels measured on admission (Fig. 1). In case of multiple presentations to the ED during the study duration, we only considered the index presentation. According to the sodium levels on admission, patients were sub-grouped in normonatremic (135–145 mmol/L), hyponatremic (<135 mmol/L), and hypernatremic (>145 mmol/L).
Figure 1.
Study flowchart. Flowchart showing patient enrollment. In total, 1041 patients were included in the study. Altogether 1021 patients were analyzed; 20 patients were excluded for absence of sodium levels measured on admission.
Follow-up
Patients were contacted over telephone calls or in written form 30 days after discharge by research physicians or study nurses. Information about current health, rehospitalizations, and adverse events were obtained, guided by a predefined set of questions and item-checklists. Records of hospitals and primary care physicians, as well as national death registries, were queried for additional information, if applicable.
Clinical assessment
All patients underwent a thorough clinical assessment by the treating ED physician according to local standard operating procedure. Vital parameters including body temperature, respiratory rate, oxygen saturation, heart rate, and blood pressure were assessed in every patient. The patients' management was left to the discretion of the attending physicians and in accordance with local standard operating procedures.
Blood sampling
Blood samples were routinely drawn in every patient (both cases and controls) at time of ED presentation. Routine laboratory parameters, for example, sodium and c-reactive protein (CRP), were determined in every patient as part of the local standard operating procedure in COVID-19 suspects. Timing and type of subsequent laboratory measurements during hospital stay were left to the discretion of the treating physicians and were not part of this study protocol.
Study outcomes
The primary outcome was to explore the association of admission dysnatremia, that is, hyponatremia and hypernatremia, with 30-day mortality and 30-days rehospitalization in patients with confirmed COVID-19 as compared to controls.
Secondary outcomes were to assess the association of admission dysnatremia with adverse outcome such as length of hospital stay, need for ICU admission, and need for invasive mechanical ventilation in COVID-19 compared to controls. Furthermore, we investigated the association of dysnatremia on admission with clinical severity parameter, measured by vital parameters such as body temperature, respiratory rate, oxygen saturation, and National Early Warning Score (NEWS) (20) on admission in both groups, and with clinical symptoms, such as fever and shivering.
Statistical analysis
Baseline variables of continues data are shown as median with interquartile range (IQR) and categorical variables as number (n) with percentage (%), respectively.
The primary endpoint was the association of hyponatremia and hypernatremia with time to 30-day mortality using a Cox proportional hazard model for patients with COVID-19 and controls. In a multivariable Cox proportional hazard models, we adjusted for age, sex, and number of comorbidities. The same method was used to assess the association of sodium levels on admission with time to 30-day rehospitalization rate in both groups. Graphical time to event analyses was performed using the Kaplan-Meier method.
Secondary endpoints were analyzed using univariable and multivariable logistic regression models to assess the association of serum sodium levels, that is, subgroups hyponatremia, normonatremia and hypernatremia, on adverse outcome, for example, length of hospital stay, ICU admission, and need for mechanical ventilation. Furthermore, we used the same method to assess the association of sodium levels parameters presenting severity of disease, for example, body temperature, respiratory rate, oxygen saturation, and NEWS on admission, and on symptoms, for example, fever and shivering. In the multivariable regression model, we adjusted for further known variables influencing sodium levels such as sex, age and the presence of comorbidities. The sum in numbers of predefined comorbidities per patient was used for the adjustment, ranging from 0 to 12. Comorbidities included presence of coronary heart disease, heart failure, arterial hypertension, pneumopathy, renal failure, hepatopathy, obesity (BMI > 25 kg/m2), rheumatological disease, immunosuppression inclusive human immunodeficiency virus (HIV) infection, cerebrovascular disease, active neoplastic disease, and diabetes mellitus. The independent variables were included in a stepwise way.
Data were analyzed using R statistical software (21). A two-sided P-value of < 0.05 was considered to be statistically significant.
Results
Baseline characteristics
In the present analysis, 1021 patients were included, of whom 172 (17%) had a positive and 849 (83%) had a negative nasopharyngeal swab for SARS-CoV-2, with an overall median age of 59 years (IQR: 42–73), 45% females. Overall, the median length of hospitalization was 7 days (IQR: 4.5–13.5) in patients with COVID-19 and 6 days (IQR: 4–10) in controls. Baseline characteristics including comorbidities, medications and laboratory parameters, for example, CRP, of the subgroups are shown in Table 1.
Table 1.
Baseline characteristics of both groups. Data from 1021 patients; 849 controls and 172 with positive nasopharyngeal swab test for SARS-CoV-2 in each subgroup (normonatremia, hyponatremia, and hypernatremia on admission). Data are presented as n (%) and median (IQR: 25th –75th percentile).
| COVID-19 | Controls | |||||
|---|---|---|---|---|---|---|
| Normonatremia | Hyponatremia | Hypernatremia | Normonatremia | Hyponatremia | Hypernatremia | |
| Patients | ||||||
| n | 117 | 50 | 5 | 682 | 149 | 18 |
| Age (years) | 54 (41–65) | 63 (52–75) | 70 (65–71) | 57 (38–74) | 67 (54 –76) | 71 (57 –76) |
| Females | 55 (47) | 18 (36) | 3 (60) | 290 (43) | 62 (42) | 9 (50) |
| Comorbidities | ||||||
| Number of comorbidities | 1.7 (1.7) | 2.4 (2.0) | 3.8 (1.8) | 1.9 (1.9) | 2.4 (1.8) | 3.3 (2.8) |
| Diabetes mellitus | 18 (16) | 14 (28) | 2 (40) | 87 (13) | 34 (23) | 6 (33) |
| Hypertension | 45 (39) | 25 (50) | 4 (80) | 272 (40) | 66 (44) | 9 (50) |
| Obesity | 41 (35) | 21 (42) | 4 (80) | 216 (32) | 45 (30) | 5 (28) |
| Medication on admission | ||||||
| Diuretics | 20 (17) | 9 (18) | 3 (60) | 139 (21) | 28 (19) | 8 (44) |
| ACE-inhibitors | 11 (9) | 8 (16) | 2 (40) | 71 (10) | 21 (14) | 1 (6) |
| AT2-Blocker | 21 (18) | 11 (22) | 0 (0) | 106 (16) | 18 (12) | 4 (22) |
| Analgesics | 33 (28) | 11 (22) | 4 (80) | 143 (21) | 35 (23) | 5 (28) |
| B-blockers | 13 (11) | 8 (16) | 1 (20) | 131 (19) | 36 (24) | 5 (28) |
| Steroids | 1 (1) | 2 (4) | 1 (20) | 35 (5) | 11 (7) | 2 (11) |
| Immunosuppressants | 1 (1) | 1 (2) | 1 (20) | 27 (4) | 12 (8) | 2 (11) |
| Laboratory values on admission | ||||||
| Sodium (mmol/L) | 138 (136–141) | 132 (130–134) | 148 (148–155) | 139 (137–141) | 132 (129–133) | 147 (146–151) |
| Potassium (mmol/L) | 3.9 (3.7–4.1) | 4.0 (3.6– 4.4) | 4.7 (4.5–5.0) | 4.0 (3.8– 4.3) | 4.0 (3.7–4.3) | 4.0 (3.8–4.3) |
| CRP (mmol/L) | 11.6 (1.5–52.5) | 49.4 (27.7–107.0) | 127.0 (106.5–138.0) | 4.8 (0.9–31.4) | 56.1 (12.2– 132.3) | 5.3 (2.6–25.5) |
ACE, angiotensin converting enzyme; AT2, angiotensin 2; CRP, c-reactive protein; Obesity (BMI > 25 kg/m2); IQR, inter quartile range.
Prevalence of dysnatremia
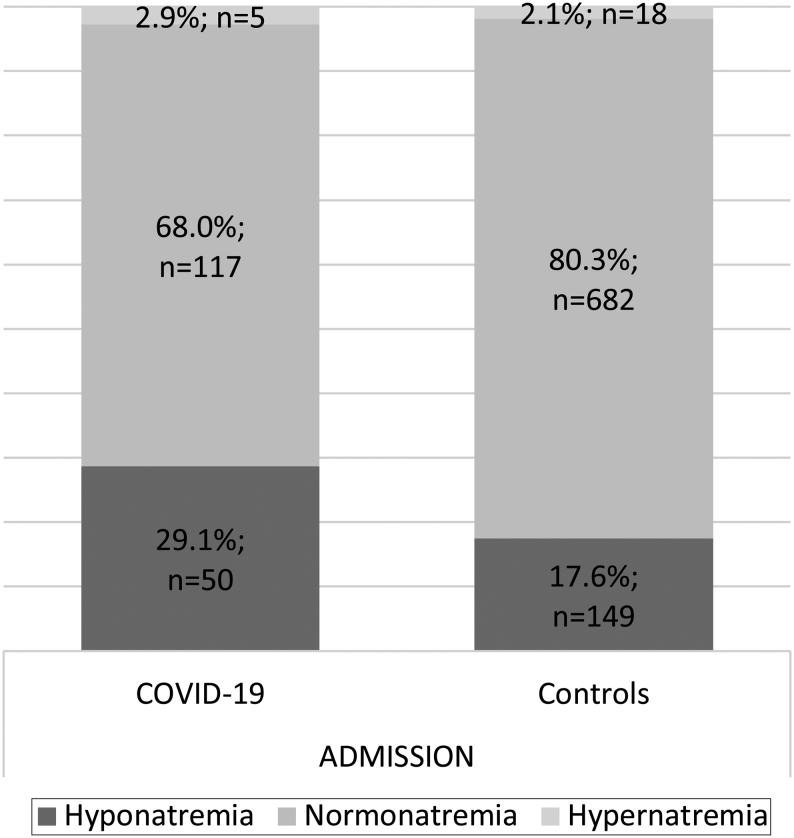
On admission, patients with COVID-19 as compared to controls showed a higher prevalence of hyponatremia (n = 50, 29.1% vs n = 149, 17.6%, OR 1.95, 95% CI 1.33–2.83, P < 0.001). In contrast, hypernatremia was comparably prevalent in both groups on admission (n = 5, 2.9% vs n = 18, 2.1%, P = 0.34) (Fig. 2, Table 1).
Figure 2.
Prevalence of dysnatremia on admission in COVID-19 and controls. Sodium levels on admission in patients with COVID-19 (n = 172) and controls (n = 849) in each subgroup (hyponatremia, normonatremia, and hypernatremia). Data are presented as numbers (n) and percentage of patients.
Association of admission dysnatremia with 30-day mortality and 30-day rehospitalization
Follow-up data for in-hospital events were available for all patients while follow-up data 30 days after discharge were available for 1015 of 1021 patients. Overall, 44 of 1015 patients died during the first 30 days after admission, with 7.0% (12 of 171 patients) significantly more often in COVID-19 as compared to 3.8% (32 of 844 patients) in controls (HR: 2.5, 95% CI: 1.24–4.89, P = 0.009). The leading cause of death was respiratory failure, followed by cardiovascular arrest, multi-organ failure, and septic shock.
In COVID-19, hyponatremia on admission in comparison to normonatremia showed a significant association with 30-day mortality (HR: 1.4, 95% CI: 1.10–16.62, P = 0.05), whereas no association in controls was observed (HR: 0.93, 95% CI: 0.36–2.46, P = 0.89).
In both groups, hypernatremia on admission showed a higher 30-day mortality in comparison to normonatremia (COVID-19 - HR: 11.5, 95% CI: 5.00–26.43, P < 0.001; controls - HR: 5.3, 95% CI: 1.60–17.64, P = 0.006). Figure 3A, B and C show Kaplan-Meier survival curves for admission sodium and all-cause mortality for all patients, patients with COVID-19, and controls within the first 30 days after admission.
Figure 3.
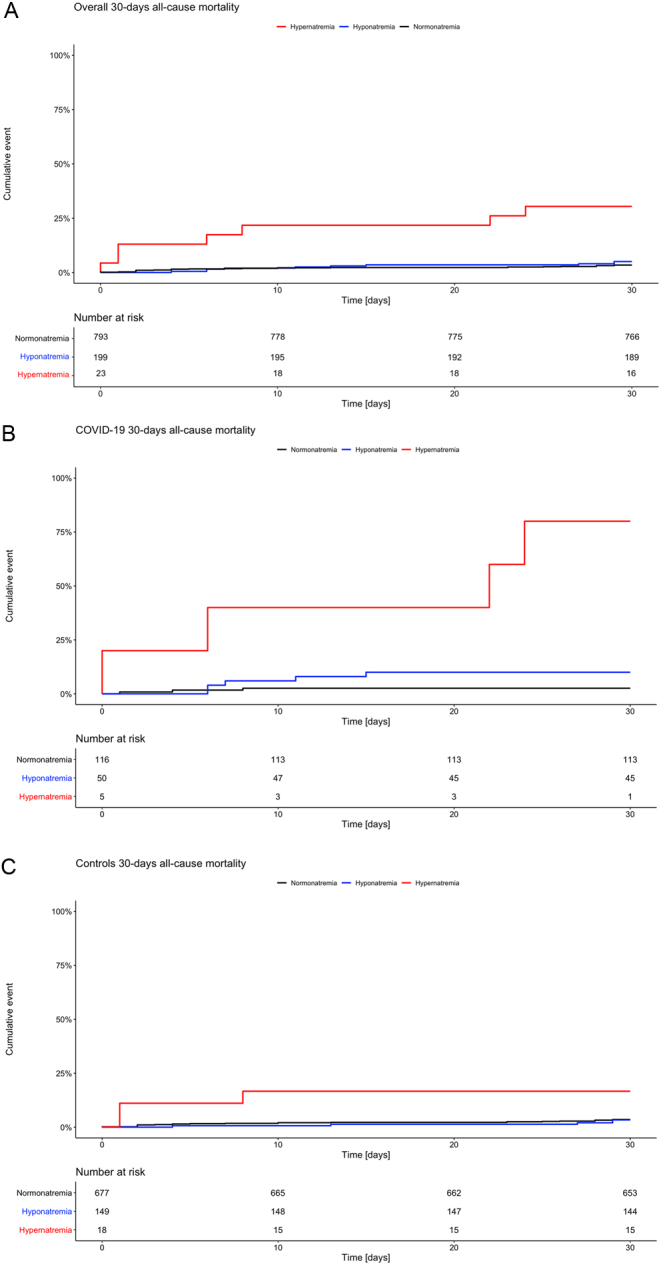
(A) Time to death graph of all patients. Kaplan-Meier curves displaying probability to death within the first 30 days after admission for all patients in each subgroup (hyponatremia, normonatremia, and hypernatremia) (n = 1015). Patients with hypernatremia (red) have had an increased risk of death compared to normonatremic patients with a hazard ratio of 2.1. (B) Time to death graph in COVID-19. Kaplan–Meier curves displaying probability to death within the first 30 days after admission in COVID-19 in each subgroup (hyponatremia, normonatremia, and hypernatremia) (n = 171). Patients with dysnatremia have had an increased risk of death compared to normonatremic patients, with a hazard ratio of 11.5 for hypernatremia (red) and with a hazard ratio of 1.4 for hyponatremia (blue). (C) Time to death graph in controls. Kaplan–Meier curves displaying probability to death within the first 30 days after admission in controls in each subgroup (hyponatremia, normonatremia, and hypernatremia) (n = 844). Patients with hypernatremia (red) have had an increased risk of death compared to normonatremic patients with a hazard ratio of 5.3.
Overall, the 30-day rehospitalization rate in survivors was 6.0% (n = 59 of 982 patients); 5.0% (n = 8 of 159) in patients with COVID-19 and 6.2% (n = 51 of 823) in controls (HR: 0.76, 95% CI: 0.36–1.62, P = 0.48). In an adjusted multivariable analysis, in both groups, hyponatremia as compared to normonatremia on admission showed no association to a higher 30-day rehospitalization rate (COVID-19- HR: 0.7, 95% CI: 0.15–3.88, P = 0.74; controls- HR: 1.7, 95% CI: 0.93–3.16, P = 0.08), which was similar for hypernatremia on admission as compared to normonatremia (COVID-19- HR: 0.7, 95% CI: 0.33–1.23, P = 0.89; controls- HR: 1.8, 95% CI: 0.40–7.86, P = 0.46).
Association of admission sodium levels with adverse outcome, severity parameters, and clinical symptoms
After adjusting for age, sex, and comorbidities, irrespective of the COVID-19 status, hyponatremia and hypernatremia were associated with adverse outcome. Specifically, patients with hyponatremia and hypernatremia as compared to normonatremia showed a significant longer hospital stay (hyponatremia- OR: 2.7, 95% CI: 2.59–2.93, P < 0.001; hypernatremia- OR: 2.5, 95% CI: 2.13–2.89, P < 0.001), were significantly associated with higher ICU admission rates (hyponatremia- OR: 2.8, 95% CI: 1.64–4.88, P < 0.001; hypernatremia- OR: 10.3, 95% CI: 3.54–27.42, P < 0.001), and were significantly linked to invasive mechanical ventilation (hyponatremia- OR: 3.3, 95% CI: 1.67–6.63, P < 0.001, hypernatremia- OR: 6.1, 95% CI: 1.28–22.00, P = 0.01) (Table 2).
Table 2.
Follow-up and outcome data. Data are presented as n (%) and median (IQR: 25th–75th percentile).
| COVID-19 | Controls | |||||
|---|---|---|---|---|---|---|
| Normonatremia | Hyponatremia | Hypernatremia | Normonatremia | Hyponatremia | Hypernatremia | |
| Patients, n | 116 | 50 | 5 | 677 | 149 | 18 |
| Follow-up data | ||||||
| Length of hospital stay (days) | 0 (0– 5) | 7 (5–12) | 10 (6–22) | 0 (0–5) | 5 (0–9) | 2 (1–10) |
| Deaths within 30 days | 3 (3) | 5 (10) | 4 (80) | 24 (4) | 5 (3) | 3 (17) |
| Time to death (days) | 4 (2.5–6) | 7 (6–9) | 14 (4.5–22.5) | 8 (2–25) | 27 (13–29) | 1 (1–4.5) |
| 30-day rehospitalization | 8 (7) | 5 (10) | 0 (0) | 57 (8) | 22 (15) | 4 (22) |
| ICU admission, | 18 (16) | 14 (28) | 4 (80) | 20 (3) | 14 (9) | 3 (17) |
| Intubation need, | 14 (12) | 9 (18) | 3 (60) | 10 (1) | 9 (6) | 1 (6) |
| i.v. Norepinephrine | 13 (11) | 9 (18) | 2 (40) | 8 (1) | 8 (5) | 2 (11) |
| ARDS | 10 (9) | 9 (18) | 3 (60) | 4 (1) | 2 (1) | 0 (0) |
ARDS, acute respiratory distress syndrome.
Irrespective of the COVID-19 status, hyponatremia and hypernatremia on admission were associated with severity parameters. In detail, hyponatremia and hypernatremia as compared to normonatremia were associated with a higher respiratory rate on admission (hyponatremia- OR: 1.4, 95% CI: 1.34–1.44, P < 0.001; hypernatremia- OR: 1.2 95% CI: 1.10–1.29, P = 0.001), and were associated with significantly lower oxygen levels (SpO2 ≤ 93%) compared to normonatremic patients (hyponatremia- OR: 1.8, 95% CI: 1.16–2.68, P = 0.006; hypernatremia- OR: 2.9, 95% CI: 1.19–5.98, P = 0.009), and showed a strong association with a higher NEWS on admission (hyponatremia- OR: 1.3, 95% CI: 1.23–1.47, P < 0.001; hypernatremia- OR: 2.5, 95% CI: 2.08–3.04, P < 0.001). Furthermore, hyponatremia on admission was associated with higher body temperature on admission (≥38°C) (OR: 2.7, 95% CI: 1.86–3.85, P < 0.001) compared to patients with normonatremia, whereas hypernatremia showed no difference compared to normonatremia (P = 0.98).
Clinical symptoms of subgroups on admission are shown in Table 3. Patients with COVID-19 reported significant more often fever on admission as compared to controls (OR: 1.6, 95% CI: 1.14–2.25, P = 0.005). Irrespective of the COVID-19 status, hyponatremia as compared to normonatremia on admission was associated with symptoms such as fever (OR: 2.1, 95% CI: 1.55–2.97, P < 0.001) and shivering (OR: 1.95, 95% CI: 1.33–2.86, P < 0.001). Hypernatremia on admission showed no difference as compared to normonatremia in both groups, patients with COVID-19 and controls, regarding the described symptoms.
Table 3.
Clinical characteristics of patients with normonatremia, hyponatremia and hypernatremia on admission. Data are presented as n (%) and median (IQR: 25th –75th percentile).
| COVID-19 | Controls | |||||
|---|---|---|---|---|---|---|
| Normonatremia | Hyponatremia | Hypernatremia | Normonatremia | Hyponatremia | Hypernatremia | |
| Patients, n | 117 | 50 | 5 | 682 | 149 | 18 |
| Symptoms on admission | ||||||
| Fever | 59 (51) | 29 (58) | 2 (40) | 243 (36) | 81 (54) | 6 (33) |
| Shivering | 22 (19) | 8 (16) | 0 (0) | 112 (17) | 43 (29) | 2 (11) |
| Cough | 79 (68) | 28 (56) | 3 (60) | 360 (53) | 72 (48) | 9 (50) |
| Dyspnea | 51 (44) | 20 (40) | 2 (40) | 357 (53) | 54 (36) | 8 (44) |
| Sore throat | 30 (26) | 5 (10) | 0 (0) | 195 (29) | 21 (14) | 3 (17) |
| Diarrhea | 22 (19) | 14 (28) | 0 (0) | 99 (15) | 25 (17) | 2 (11) |
| Nausea/vomiting | 21 (18) | 9 (18) | 0 (0) | 136 (20) | 35 (23) | 3 (17) |
| Clinical parameters | ||||||
| Temperature (°C) | 37.1 (36.6–37.8) | 37.4 (36.9–38.5) | 36.7 (36.5–36.8) | 36.9 (36.5–37.4) | 37.6 (36.8–38.3) | 36.8 (36.5–37.4) |
| Respiratory rate (/min) | 20 (16–23) | 23 (17–25) | 26 (24–26) | 18 (16–22) | 20 (16–24) | 21 (16–27) |
| O2 Saturation (%) | 97 (95–98) | 95 (93–97) | 95 (89–96) | 97 (96–98) | 96 (95–98) | 96 (93–98) |
| Heart rate (/min) | 88 (80–102) | 90 (82–104) | 99 (89–104) | 86 (74–100) | 94 (82–108) | 102 (80–115) |
| SBP (mmHg) | 136 (125–151) | 133 (120–152) | 102 (95–108) | 138 (123–157) | 131 (115–151) | 141 (122–167) |
| DBP (mmHg) | 84 (74, 90) | 80 (70, 88) | 56 (54–68) | 81 (72–90) | 80 (71– 86) | 85 (77–96) |
| O2-Therapy | 15 (13) | 14 (28) | 5 (100) | 79 (12) | 25 (17) | 9 (50) |
| NEWS Total, n (IQR) | 2 (0–4) | 4 (2–5) | 8 (7–8) | 2 (0–4) | 3 (1–5) | 5 (3–9) |
DBP, diastolic blood pressure; NEWS, national early warning score; SBP, systolic blood pressure.
Discussion
Our study has four main findings. First, the prevalence of hyponatremia on admission is significantly higher in patients with COVID-19 in comparison to controls. Secondly, hyponatremia in COVID-19 but not in controls showed a significant association to 30-day mortality in comparison to normonatremia. Thirdly, hypernatremia on admission was associated with 30-day mortality, in both, patients with and without COVID-19. Forthly, hyponatremia and hypernatremia on admission were associated with adverse outcome and severity parameters in both groups.
The prevalence of hyponatremia and hypernatremia in our study is similar to previously published data of a large cohort with COVID-19, where the incidence of hyponatremia was 30%, whilst hypernatremia was seen with a prevalence of 4% (22). Our study, however, in contrast to previous studies, includes a control group with patients without COVID-19 but similar symptoms, and we here show for the first time that patients with COVID-19 experience a significantly higher rate of hyponatremia compared to controls. In addition, we could confirm the association between hyponatremia and mortality in COVID-19 (22, 23), moreover, we show for the first time that this association is significantly higher in comparison to a control group. Different mechanisms leading to an increased prevalence of hyponatremia in pneumonia have been postulated for example, the syndrome of inappropriate antidiuresis (SIAD) (24, 25), drug-induced hyponatremia (mainly diuretics or psychotropics), and microbial-induced hyponatremia (Legionella pneumonia) (26). These mechanisms could also be linked to the unfavorable outcome. One hypothesis is that the immune system may lead to the development of hyponatremia in patients with pneumonia and more specifically in patients with COVID-19 (27). In a recent meta-analysis of COVID-19 patients, a high rate of increased IL-1 and IL-6 was observed (28). IL-6 is presumed to play a role in sodium homeostasis by non-osmotically releasing arginine vasopressin (AVP) (29, 30). In support of this hypothesis, Berni et al. showed in a small cohort of 29 patients with COVID-19 that hyponatremia improved after anti-IL-6 therapy (31). In our study, we see significantly higher levels of c-reactive protein (CRP) in hyponatremic patients which would be in line with these findings. However, further intervention studies are required to test this hypothesis. As an alternative hypothesis, Angiotensin-converting enzyme 2 receptor (ACE2) is expressed in epithelial cells of tissues including lung and kidneys and has been identified to interact with SARS-CoV2 for intracellular transport (32). Post et al. suggest a possible role of dysnatremia in the regulation of ACE2 (33). Low sodium levels are causing an upregulation of ACE2 in epithelial cells, and therefore possibly a higher rate of intracellular virus transport. The higher virus load and therefore increased disease severity could explain the higher mortality rate in patients with COVID-19 compared to controls. Overall, however, it is still unclear whether the association of hyponatremia and outcome mirrors causality or simply defines hyponatremia as a marker for severity of disease.
Hypernatremia was also associated with a higher mortality rate in our study, as shown previously for patients with pneumonia in general (34). Hypernatremia is often a reflection of water loss rather than sodium gain, mostly due to dehydration due to increased body temperature (35). Fever was one of the most common symptoms in our analysis and could therefore partly explain the high rate of hypernatremia. Another common cause is drug-induced dehydration, in particular overuse of diuretics (36). Interestingly, our data show the highest rate of diuretics in hypernatremic patients in both groups. Diuretics in addition to age-related physiologic changes such as decreased thirst drive can lead to reduced net body water (37). Furthermore, studies in critical ill patients showed high rates of dehydration caused by insensible water loss due to increased respiratory rate (38). Among all subgroups in our cohort, hypernatremic patients in COVID-19 and controls had the highest respiratory rates, which may have contributed to hypernatremia.
Previous studies showed increased rates of rehospitalization in hyponatremic and hypernatremic patients with pneumonia (4, 7). Interestingly, our study showed no link between hyponatremia or hypernatremia to 30-days rehospitalization in both, COVID-19 and controls, compared to normonatremia. However, our data on mortality indicated an unfavorable outcome in patients with COVID-19 and dysnatremia. Our sample might be too small to find such an association, which is supported as the 30-day rehospitalization rate in our cohort was lower as compared to other publications (39).
Both hyponatremia and hypernatremia as compared to normonatremia were linked to other adverse patients' outcome. Especially hyponatremia is an established and recognized criterion in severity scores for pneumonia, for example, the Pneumonia Severity Index (PSI) (15). Interestingly, a recent analysis showed a direct relation of hyponatremia and low PaO2/FiO2 ratio, an important index for respiratory performance (31). Respiratory failure was the leading cause of death in our analysis. Accordingly, symptoms and parameters representing respiratory failure such as low oxygen level on admission, fever, and mechanical ventilation were significantly linked to hyponatremia as compared to normonatremia in both groups.
There are some limitations to this study. First, we have only limited information about the etiology of dysnatremia, neither about psychotropic nor diuretic medication that could lead to drug induced SIAD. Other studies have investigated this question suggesting SIAD or hypovolemia to be main causes for dysnatremia. Due to the lack of information regarding the clinical volaemic status and further laboratory parameters, a reliable diagnosis of the etiology of hyponatremia was not possible.
Secondly, the study was insufficiently powered for some secondary analyses such as rehospitalization.
A major strength of our study is the comparison to a control group with suspected COVID-19 and thus similar symptoms, but a negative nasopharyngeal swab test for COVID-19. To the best of our knowledge, this is the first study with a control group and thus adds valuable information to the literature.
In conclusion, our data show that patients with COVID-19 presented significantly more often with hyponatremia on admission compared to controls. Hyponatremia and hypernatremia in COVID-19 is associated with increased mortality. This underlines the importance of considering dysnatremia as a predictive outcome marker in COVID-19.
Declaration of interest
R Twerenbold reports research support from the Swiss National Science Foundation (Grant No P300PB_167803), the Swiss Heart Foundation, the Swiss Society of Cardiology, the Cardiovascular Research Foundation Basel, the University of Basel and the University Hospital Basel and speaker honoraria/consulting honoraria from Abbott, Amgen, Astra Zeneca, Roche, Siemens, Singulex and Thermo Scientific BRAHMS.
Funding
The COVIVA study was supported by the Swiss Heart Foundation and the Cardiovascular Research Foundation Basel.
Author contribution statement
C Atila and C O Sailer have contributed equally and share first authorship. R Twerenbold and M Christ-Crain have contributed equally and share senior authorship.
Acknowledgements
The authors thank the clinical staff for their valuable help during the study conduction. We thank all participants for their participation in the study.
References
- 1. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Yet al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 20203231061–1069. ( 10.1001/jama.2020.1585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSCet al. Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine 20203821708–1720. ( 10.1056/NEJMoa2002032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Qian GQ, Yang NB, Ding F, Ma AHY, Wang ZY, Shen YF, Shi CW, Lian X, Chu JG, Chen Let al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM 2020113474–481. ( 10.1093/qjmed/hcaa089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krüger S, Ewig S, Giersdorf S, Hartmann O, Frechen D, Rohde G, Suttorp N, Welte TCAPNETZ study group. Dysnatremia, vasopressin, atrial natriuretic peptide and mortality in patients with community-acquired pneumonia: results from the german competence network CAPNETZ. Respiratory Medicine 20141081696–1705. ( 10.1016/j.rmed.2014.09.014) [DOI] [PubMed] [Google Scholar]
- 5. Corrado RE, Lee D, Lucero DE, Varma JK, Vora NM. Burden of adult community-acquired, health-care-associated, hospital-acquired, and ventilator-associated pneumonia: New York City, 2010 to 2014. Chest 2017152930–942. ( 10.1016/j.chest.2017.04.162) [DOI] [PubMed] [Google Scholar]
- 6. Cuesta M, Slattery D, Goulden EL, Gupta S, Tatro E, Sherlock M, Tormey W, O'Neill S, Thompson CJ. Hyponatraemia in patients with community-acquired pneumonia; prevalence and aetiology, and natural history of SIAD. Clinical Endocrinology 201990744–752. ( 10.1111/cen.13937) [DOI] [PubMed] [Google Scholar]
- 7. Nair V, Niederman MS, Masani N, Fishbane S. Hyponatremia in community-acquired pneumonia. American Journal of Nephrology 200727184–190. ( 10.1159/000100866) [DOI] [PubMed] [Google Scholar]
- 8. Schuetz P, Haubitz S, Christ-Crain M, Albrich WC, Zimmerli W, Mueller BProHOSP Study Group. Hyponatremia and anti-diuretic hormone in Legionnaires' disease. BMC Infectious Diseases 201313585. ( 10.1186/1471-2334-13-585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. American Journal of Medicine 2006119(Supplement 1) S30–S35. ( 10.1016/j.amjmed.2006.05.005) [DOI] [PubMed] [Google Scholar]
- 10. Hoorn EJ, Lindemans J, Zietse R. Development of severe hyponatraemia in hospitalized patients: treatment-related risk factors and inadequate management. Nephrology, Dialysis, Transplantation 20062170–76. ( 10.1093/ndt/gfi082) [DOI] [PubMed] [Google Scholar]
- 11. Corona G, Giuliani C, Verbalis JG, Forti G, Maggi M, Peri A. Hyponatremia improvement is associated with a reduced risk of mortality: evidence from a meta-analysis. PLOS ONE 201510 e0124105. ( 10.1371/journal.pone.0124105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borroni G, Maggi A, Sangiovanni A, Cazzaniga M, Salerno F. Clinical relevance of hyponatraemia for the hospital outcome of cirrhotic patients. Digestive and Liver Disease 200032605–610. ( 10.1016/s1590-8658(0080844-0) [DOI] [PubMed] [Google Scholar]
- 13. Palevsky PM, Bhagrath R, Greenberg A. Hypernatremia in hospitalized patients. Annals of Internal Medicine 1996124197–203. ( 10.7326/0003-4819-124-2-199601150-00002) [DOI] [PubMed] [Google Scholar]
- 14. Katan M, Muller B, Christ-Crain M. Copeptin: a new and promising diagnostic and prognostic marker. Critical Care 200812117. ( 10.1186/cc6799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, Coley CM, Marrie TJ, Kapoor WN. A prediction rule to identify low-risk patients with community-acquired pneumonia. New England Journal of Medicine 1997336243–250. ( 10.1056/NEJM199701233360402) [DOI] [PubMed] [Google Scholar]
- 16. Zilberberg MD, Exuzides A, Spalding J, Foreman A, Jones AG, Colby C, Shorr AF. Hyponatremia and hospital outcomes among patients with pneumonia: a retrospective cohort study. BMC Pulmonary Medicine 2008816. ( 10.1186/1471-2466-8-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alshayeb HM, Showkat A, Babar F, Mangold T, Wall BM. Severe hypernatremia correction rate and mortality in hospitalized patients. American Journal of the Medical Sciences 2011341356–360. ( 10.1097/MAJ.0b013e31820a3a90) [DOI] [PubMed] [Google Scholar]
- 18. O'Shea PM, Lee GR, Griffin TP, Tormey V, Hayat A, Costelloe SJ, Griffin DG, Srinivasan S, O'Kane M, Burke CMet al. COVID-19 in adults: test menu for hospital blood science laboratories. Irish Journal of Medical Science 20201891147–1152. ( 10.1007/s11845-020-02252-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JPSTROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. International Journal of Surgery 2014121495–1499. ( 10.1016/j.ijsu.2014.07.013) [DOI] [PubMed] [Google Scholar]
- 20. Smith GB, Redfern OC, Pimentel MA, Gerry S, Collins GS, Malycha J, Prytherch D, Schmidt PE, Watkinson PJ. The national early warning score 2 (NEWS2). Clinical Medicine 201919260. ( 10.7861/clinmedicine.19-3-260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. R Core T. R: A Language and Environment for Statistical Computing 2018. [Google Scholar]
- 22. Frontera JA, Valdes E, Huang J, Lewis A, Lord AS, Zhou T, Kahn DE, Melmed K, Czeisler BM, Yaghi Set al. Prevalence and impact of hyponatremia in patients with coronavirus disease 2019 in New York City. Critical Care Medicine 202048e1211–e1217. ( 10.1097/CCM.0000000000004605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tezcan ME, Dogan Gokce G, Sen N, Zorlutuna Kaymak N, Ozer RS. Baseline electrolyte abnormalities would be related to poor prognosis in hospitalized coronavirus disease 2019 patients. New Microbes and New Infections 202037100753. ( 10.1016/j.nmni.2020.100753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D, Decaux G, Fenske W, Hoorn EJ, Ichai Cet al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. European Journal of Endocrinology 2014170G1–G47. ( 10.1530/EJE-13-1020) [DOI] [PubMed] [Google Scholar]
- 25. Verbalis JG, Goldsmith SR, Greenberg A, Korzelius C, Schrier RW, Sterns RH, Thompson CJ. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. American Journal of Medicine 2013126(Supplement 1) S1–S. ( 10.1016/j.amjmed.2013.07.006) [DOI] [PubMed] [Google Scholar]
- 26. Fiumefreddo R, Zaborsky R, Haeuptle J, Christ-Crain M, Trampuz A, Steffen I, Frei R, Müller B, Schuetz P. Clinical predictors for Legionella in patients presenting with community-acquired pneumonia to the emergency department. BMC Pulmonary Medicine 200994. ( 10.1186/1471-2466-9-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ruscitti P, Berardicurti O, Iagnocco A, Giacomelli R. Cytokine storm syndrome in severe COVID-19. Autoimmunity Reviews 202019102562. ( 10.1016/j.autrev.2020.102562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Henry BM, Oliveira MHS de, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clinical Chemistry and Laboratory Medicine 2020581021–1028. ( 10.1515/cclm-2020-0369) [DOI] [PubMed] [Google Scholar]
- 29. Hodax JK, Bialo SR, Yalcindag A. SIADH in systemic JIA resolving after treatment with an IL-6 inhibitor. Pediatrics 2018141. ( 10.1542/peds.2016-4174) [DOI] [PubMed] [Google Scholar]
- 30. Yousaf Z, Al-Shokri SD, Al-Soub H, Mohamed MFH. COVID-19-associated SIADH: a clue in the times of pandemic! American Journal of Physiology. Endocrinology and Metabolism 2020. 318 E882–E885. ( 10.1152/ajpendo.00178.2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berni A, Malandrino D, Parenti G, Maggi M, Poggesi L, Peri A. Hyponatremia, IL-6, and SARS-CoV-2 (COVID-19) infection: may all fit together? Journal of Endocrinological Investigation 2020. 43 1137–1139. ( 10.1007/s40618-020-01301-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovascular Research 20201161097–1100. ( 10.1093/cvr/cvaa078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Post A, Dullaart RPF, Bakker SJL. Sodium status and kidney involvement during COVID-19 infection. Virus Research 2020286198034. ( 10.1016/j.virusres.2020.198034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hu J, Wang Y, Geng X, Chen R, Zhang P, Lin J, Teng J, Zhang X, Ding X. Dysnatremia is an independent indicator of mortality in hospitalized patients. Medical Science Monitor 2017232408–2425. ( 10.12659/msm.902032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nigro N, Winzeler B, Suter-Widmer I, Schuetz P, Arici B, Bally M, Refardt J, Betz M, Gashi G, Urwyler SAet al. Copeptin levels and commonly used laboratory parameters in hospitalised patients with severe hypernatraemia—the "Co-MED study". Critical Care 20182233. ( 10.1186/s13054-018-1955-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oude Lansink-Hartgring A, Hessels L, Weigel J, Smet AMGA de, Gommers D, Panday PVN, Hoorn EJ, Nijsten MW. Long-term changes in dysnatremia incidence in the ICU: a shift from hyponatremia to hypernatremia. Annals of Intensive Care 2016622. ( 10.1186/s13613-016-0124-x). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morley JE Dehydration, hypernatremia, and hyponatremia. Clinics in Geriatric Medicine 201531389–399. ( 10.1016/j.cger.2015.04.007) [DOI] [PubMed] [Google Scholar]
- 38. Li Li C, Oi Yan T, Ming Chit Arthur K, Hoi Ping S, King Chung Kenny C, Wing Wa Y. Insensible water loss through adult extracorporeal membrane oxygenation circuit: an in vitro study. ASAIO Journal 201460508–512. ( 10.1097/MAT.0000000000000098) [DOI] [PubMed] [Google Scholar]
- 39. Chawla A, Sterns RH, Nigwekar SU, Cappuccio JD. Mortality and serum sodium: do patients die from or with hyponatremia? Clinical Journal of the American Society of Nephrology 2011. 6 960–965. ( 10.2215/CJN.10101110) [DOI] [PMC free article] [PubMed] [Google Scholar]