Abstract
Vascular endothelial growth factor (VEGF) serves a critical role in vasculogenesis, angiogenesis, tumor, inflammatory angiogenesis and lymphangiogenesis. Since 2004, bevacizumab (Avastin), a humanized anti-VEGFA monoclonal antibody, has been approved for the treatment of non-small cell lung, breast, kidney and ovarian cancer in combination with standard chemotherapy. VEGF has been demonstrated to be important in the clinic as a therapeutic target in the anti-angiogenic approach to cancer therapy. The targeting of VEGF, together with immunotherapy, has been reported to be able to reverse the immunosuppressive effects of VEGF. A positive correlation between VEGF expression and the reduced survival rates of patients with cancer has also been demonstrated. Furthermore, increased VEGF expression can lead to immune suppression via the inhibition of dendritic cell maturation, the reduction of T-cell tumor infiltration and the promotion of inhibitory cell types in the tumor microenvironment.
Keywords: anti-angiogenesis, dendritic cell, immunosuppression, T-cell, vascular endothelial growth factor
1. Introduction
The vascular endothelial growth factor (VEGF) family consists of five mammalian factors, VEGFA, VEGFB, VEGFC, VEGFD and placenta growth factor (PlGF). Genetic inactivation of VEGFA and VEGFC in mice results in embryonic death due to defects in the development of blood and lymphatic vessels, respectively. The VEGFs bind to three different but structurally related tyrosine kinase receptors, named VEGFR-1, VEGFR-2 and VEGFR-3 (Fig. 1). VEGFR-2 is preferentially expressed on blood vascular endothelial cells, whereas VEGFR-3 is primarily expressed on lymphatic endothelial cells. Moreover, VEGFR-1 is expressed on a range of non-endothelial cells and is essential for the regulation of leukocyte motility (1–3). All VEGFRs contain seven immunoglobulin (Ig) homology domains, which comprise the ligand-binding site, and an intracellular region endowed with tyrosine kinase (TK) activity, which transduces the signal. The downstream signaling includes activation of phospholipase Cγ1, MAPK pathway via Ras/Raf1 activation and PI3K/Akt pathway. Phospholipase Cγ1 regulates the concentration of intracellular Ca+2 ions and formation of endothelial nitric oxide synthase (Fig. 1). The effect of all the cascades provides a balance of pro- and anti-angiogenic signals that maintain the vasculature and/or result in sprouting of new blood vessels, cell proliferation and cell migration. The VEGF/VEGFR signaling pathway is upregulated in many types of cancers, contributing to uncontrolled angiogenesis and metastatic spreading.
Figure 1.
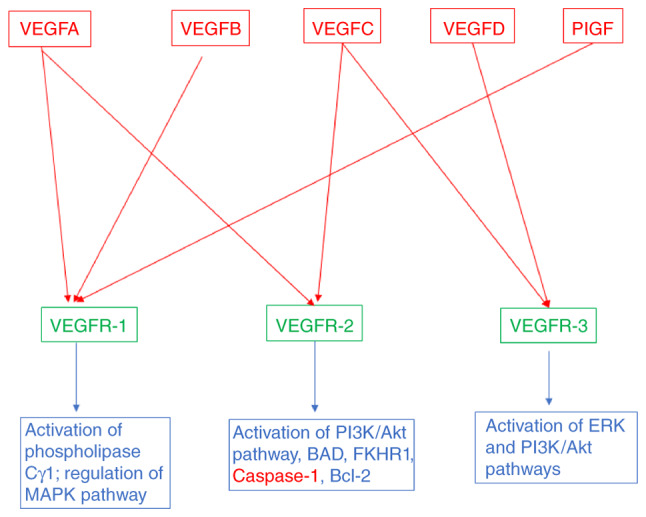
Schematic illustrating the VEGF family members and their respective receptors, and intracellular signaling pathways (pro-angiogenic in blue and anti-angiogenic in red) that are activated by VEGFRs. VEGF, vascular endothelial growth factor; PIGF, placental growth factor; FKHR1, forkhead transcription factor R1.
VEGFA serves a critical role in vasculogenesis, angiogenesis, tumors, inflammatory angiogenesis and lymphangiogenesis. VEGFA was initially discovered as a vascular permeability factor with a potency 50,000 times that of histamine (1) and extravascular fluid accumulation is prominent in tumor growth in body cavities, such as the peritoneum. A striking positive correlation between VEGFA expression levels, tumor progression and consequently reduced patient survival, has also been demonstrated (2).
2. Immunosuppressive effects of VEGF
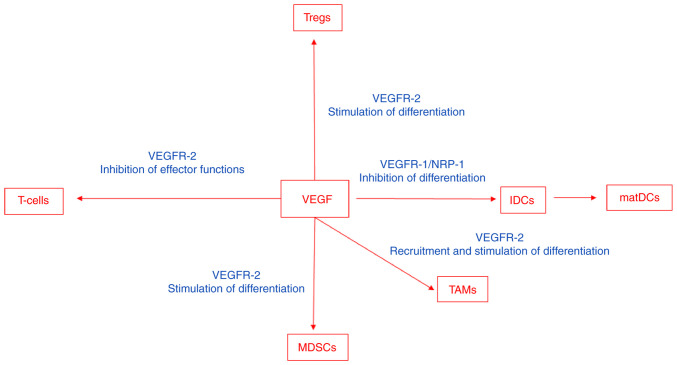
VEGF modulates the function of T-cells, suppressive immune cells and the stroma in the tumor microenvironment, which results in an immunosuppressive state (Fig. 2). VEGF impairs interactions between leukocytes and endothelial cells. It achieves this via the downregulation of the expression of adhesion molecules, including vascular cell adhesion molecule-1 (VCAM-1) and intercellular cell adhesion molecule-1 (ICAM-1), or via the inhibition of their clustering, which impairs the ability of immune cells to adhere and migrate across the endothelium into the tumor (3–7). The anergic phenotype of tumor endothelial cells can be reversed via antiangiogenic therapy, which upregulates the expression of endothelial adhesion molecules in the tumor vasculature (8).
Figure 2.
VEGF modulates the function of T-cells, suppressive immune cells and stroma in the tumor microenvironment, which results in an immunosuppressive state. VEGF, vascular endothelial growth factor; MDSC, myeloid-derived suppressor cell; IDC, immature dendritic cell; matDC, mature dendritic cell; TAM, tumor-associated macrophage; Treg, regulatory T-cell; NRP-1, neuropilin-1.
The number of tumors infiltrating lymphocytes has been demonstrated to be markedly increased in animal tumor models and in humans following VEGF inhibition (4,9,10). VEGF signaling directly affects T-cell development, homing and cytotoxic functions (7). Moreover, VEGF negatively impacts T-cell migration from the lymph nodes into the tumor bed via the stimulation of abnormal tumor vasculature formation (11). VEGF/VEGFR-2 induces the production of pro-inflammatory molecules, including interferon-γ and interleukin-2, and stimulates the migratory response in memory CD4+ T-cells (12). Furthermore, VEGF induces regulatory T-cells (T-regs) (13). VEGF also impedes the differentiation of hematopoietic progenitor cells in the thymus to CD8+ and CD4+ T-cells (14).
VEGF/VEGFR-2 also suppresses CD3+ T-cells and cytotoxic effects (15,16). Moreover, VEGFA contributes to CD8+ T-cell exhaustion, which is characterized via the expression of negative immune checkpoints, such as programmed cell death protein-1 (PD-1) receptors (17), in a VEGFR-2 and nuclear factor of activated T-cells-dependent manner. In this context, VEGFA promotes the expression of check point molecules, including PD-1, cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), T-cell immunoglobulin mucin receptor 3 and lymphocyte activation gene 3 protein (18). VEGF can suppress effector T-cell functions via the induction of FAS-ligand expression on endothelial cells and by causing the apoptosis of infiltrating CD8+ T-cells (19).
Dendritic cells are antigen-presenting cells that serve a critical role in T-cell priming and activation. VEGF inhibits dendritic maturation and antigen presentation (20). High levels of VEGF expression in human cancers have been associated with defective dendritic cell function and number (21–23). VEGFR-1 and neuropilin-1 are involved in the VEGF-inhibition of dendritic cell maturation (24,25). VEGF upregulates programmed death-ligand 1 (PD-L1) in dendritic cells, which results in the inhibition of T-cell expansion and function (26). Furthermore, it inhibits the function of human mature dendritic cells to stimulate T-cells, mediated by VEGFR-2 (27), via the inhibition of NF-κB activation (28).
Myeloid-derived suppressor cells (MDSCs) are a mixed cell population consisting mainly of neutrophils but also of macrophages and dendritic cells with immunosuppressive and tumor-promoting capacities (2,29). VEGF induces the differentiation of myeloid cells into immunosuppressive MDSCs (30) and the VEGF concentration has been demonstrated to be associated with the presence of MDSCs (31). In tumors resistant to anti-VEGF treatment, the increased mobilization and infiltration of MDSCs is distinguishable when compared with treatment-sensitive tumors (32).
Tumor-associated macrophages (TAMs) differentiate into anti-inflammatory M1 macrophages or pro-inflammatory and tumor promoting M2 macrophages, which is dependent on the local cues provided within the tumor microenvironment (33). M2 macrophages secrete immunosuppressive cytokines, including IL-10, chemokine (C-C) ligand (CCL)-7 and CCL-22 and angiogenic and tissue remodeling factors, including VEGF, PlGF and matrix metalloproteinase-9 (33). VEGF recruits TAMs and therefore contributes to the establishment of an immunosuppressive microenvironment (34) and induces the maturation of myeloid cells into the M2-like phenotype (35). A reduced recruitment of TAMs or the reprogramming of M2-like TAMs contributes towards anti-cancer M1 phenotype reversed immunosuppression (36).
The increased recruitment of neutrophils during anti-VEGF treatment promotes tumor progression and treatment resistance (32). Cancer-associated fibroblasts (CAFs) secrete several angiogenic factors, including epidermal growth factor, hepatocyte growth factor, insulin-like growth factor and fibroblast growth factor-2 (37). CAFs that are resistant to anti-VEGF therapy promote tumor growth in anti-VEGF treatment sensitive tumors (29).
VEGF may also contribute to the prevention of an uncontrolled, detrimental immune response to the microbiota in the lung. In this context, VEGF signaling in the alveolar microenvironment is responsible for endothelial cell-mediated tolerance to airborne pathogens and toxins via the interaction of VEGF released by alveolar type I cells and secretory epithelial cells with capillary endothelial cells (38). Moreover, VEGF is involved in fibrotic lung disease (39). Transfection of anti-VEGF gene therapy, in the form of the sFlt-1, resulted in the attenuation of pulmonary fibrosis with a reduction in lung collagen deposition and additional anti-inflammatory and anti-angiogenic effects.
3. Anti-angiogenic therapies reverse the immunosuppressive effects of VEGF
VEGF has served an important role in the clinic as a therapeutic target in the anti-angiogenic approach to tumor therapy. Bevacizumab binds VEGFA, which prevents VEGFA from interacting with VEGFR-2 on vascular endothelial cells, endothelial progenitor cells and megakaryocytes. Bevacizumab treatment in patients with metastatic colorectal cancer induces an increase in B- and T-cells (40). Furthermore, the addition of bevacizumab to chemotherapy for patients with non-small cell lung cancer results in improved dendritic cell activation and T-cell cytotoxicity (41). Bevacizumab also reverses the VEGF-induced inhibition of monocyte differentiation into dendritic cells (42) and restores peripheral blood dendritic cell numbers in patients with cancer (43). In renal cell carcinoma, bevacizumab reduces the number of MDSCs (44). Furthermore, VEGF induces T-reg proliferation via VEGFR-2 activation. Bevacizumab also inhibits T-reg accumulation in the peripheral blood of patients with metastatic colorectal cancer (45). Moreover, ramucirumab (Cyramaza) is a distinct monoclonal anti-VEGFR-2 antibody approved for the treatment of non-small cell lung cancer, colorectal cancer, hepatocellular carcinoma and gastric cancer (46).
Since VEGFRs possess a tyrosine kinase domain, several small ATP mimetics that can inhibit the activity of tyrosine kinase receptors involved in angiogenesis have been developed. Sorafenib and sunitinib were the first multi-kinase inhibitors approved for the treatment of metastatic renal cell and hepatocellular carcinomas. Sunitinib, a multi-tyrosine kinase inhibitor has been demonstrated to induce the upregulation of ICAM-1 and VCAM-1 adhesion molecules on endothelial cells in tumor-bearing mice (47). These mice displayed increased Th1 responses via the reduction of inhibitory molecule expression, including transforming growth factor-β, IL-10, forkhead box protein-3, PD-1 and CTLA-4 (48). Sunitinib reduces the immunosuppressive activity of MDSCs (49) and in human renal cell carcinoma increases tumor infiltrating lymphocytes and reduces MDSCs (50,51). Moreover, sunitinib in combination with CD40 immuno-stimulating immunotherapy induces dendritic cell activation, reduces MDSCs and enhances cytotoxic T-cell recruitment (47).
4. Immunotherapies reverse the immunosuppressive effects of VEGF
PD-1, its ligand PD-L1 and CTLA-4 are negative regulators of T-cell immune function. Following ligand binding, PD-1 attenuates T-cell activation via recruiting SH2 domain-containing protein tyrosine phosphatase-2 and reducing cytokine production and T-cell proliferation (52). PD-L1 is constitutively expressed via T-cells, B-cells, dendritic cells, macrophages and non-hematopoietic cells, including endothelial cells, epithelial cells, hepatocytes and astrocytes (53). CTLA-4 is a cell surface receptor expressed on activated T-cells. Following T-cell receptor interactions with its cognate peptide/major histocompatibility complex, and co-stimulation via CD28, CTLA-4 suppresses this co-stimulation (54).
Numerous trials with anti-CTLA-4 and anti-PD-1/PD-L1 antibodies, which re-activate the anti-tumor processes of the human immune system, have resulted in durable responses in patients with cancer. In a previous study, VEGF expression decreased in patients with metastatic melanoma responding to anti-CTLA-4 and anti-PD-1 therapy, but increased in non-responders, which indicated therapeutic resistance (55).
Anti-angiogenic agents improve the effectiveness of immunotherapy by transiently restoring the abnormal tumor vasculature and increasing the infiltration of immune effector cells into the tumor microenvironment (56). This induces the formation of high endothelial venules that improve lymphocyte infiltration and improve anti-tumor immunity (57). A previous study demonstrated that patients with renal cell cancer or non-small cell lung cancer with a high dendritic cell number before treatment, exhibited an improved response to PD-L1 inhibition with atezolizumab (58).
5. Conclusion
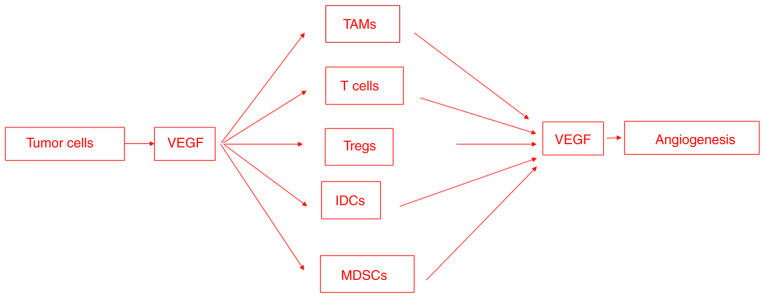
Angiogenesis and immunosuppression are closely associated and occur simultaneously in response to different stimuli (Fig. 3). The VEGF/VEGFR signaling pathway is recognized as the master regulator of tumor angiogenesis. VEGFRs may be targeted through monoclonal antibodies to inhibit VEGF binding to the extracellular domain of the receptor or, alternatively, using different small molecule tyrosine kinase inhibitors blocking the ATP binding in the kinase domain and phosphorylation of tyrosine residues, in clinical trials for the treatment of renal cell carcinoma, hepatocellular carcinoma, metastatic colorectal cancer and gastrointestinal stromal tumors. Another strategy concerns the use of small-molecule inhibitors targeting cell signaling pathways activated by VEGFRs, approved for the treatment of metastatic melanoma and metastatic renal cell carcinoma.
Figure 3.
VEGF is involved in both angiogenesis and immunosuppression. Tumor cells secrete VEGF in order to recruit immunosuppressive cells to the tumor site. These cells, including TAMs, T cells, Treg cells, IDCs and MDSCs, secrete VEGF, which promotes endothelial cell proliferation and migration. VEGF, vascular endothelial growth factor; TAM, tumor-associated macrophage; Treg, regulatory T-cell; IDC, immature dendritic cell; MDSC, myeloid-derived suppressor cell.
Tumor cells secrete soluble factors, such as VEGFA, that recruit immunosuppressive cells, including TAMs, neutrophils, MDSCs, dendritic cells, T-regs and natural killer cells. The immunosuppressive cells, in turn, secrete VEGF, which promotes endothelial cell proliferation and migration and/or induces the release of MMPs. VEGFA exerts different immunosuppressive effects, including the inhibition of dendritic cell maturation, the induction of inhibitory molecule expression, such as PD-L1, on dendritic cells, and the activation of T-regs. The present review has demonstrated that VEGF can simultaneously promote angiogenesis and mediate immunosuppression. These overlapping activities may potentially explain the efficacy of anti-angiogenic and immunotherapies in reversing the immunosuppressive effects of VEGF.
Acknowledgements
Not applicable.
Funding Statement
This research was supported by a grant from European Union Seventh Framework Programme (FP7/2007-2013; grant no. 278570).
Availability of data and materials
Not applicable.
Authors' contributions
DR wrote, edited and revised the manuscript. Data authentication is not applicable. The author read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The author declares that they have no competing interests.
References
- 1.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: Therapeutic implications. Semin Oncol. 2002;29:10–14. doi: 10.1016/S0093-7754(02)70064-X. [DOI] [PubMed] [Google Scholar]
- 3.Bouzin C, Brouet A, De Vriese J, DeWever J, Feron O. Effects of vascular endothelial growth factor on the lymphocyte-endothelium interactions: Identification of Caveolin-1 and nitric oxide as control points of endothelial cell anergy. J Immunol. 2007;178:1505–1511. doi: 10.4049/jimmunol.178.3.1505. [DOI] [PubMed] [Google Scholar]
- 4.Dirkx AE, Oude Egbrink MG, Castermans K, van der Schaft DW, Thijssen VL, Dings RP, Kwee L, Mayo KH, Wagstaff J, Bouma-ter Steege JC, Griffioen AW. Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. FASEB J. 2006;20:621–630. doi: 10.1096/fj.05-4493com. [DOI] [PubMed] [Google Scholar]
- 5.Munn LL, Jain RK. Vascular regulation of antitumor immunity. Science. 2019;365:544–545. doi: 10.1126/science.aaw7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tromp SC, oude Egbrink MG, Dings RP, van Velzen S, Slaaf DW, Hillen HF, Tangelder GJ, Reneman RS, Griffioen AW. Tumor angiogenesis factors reduce leukocyte adhesion in vivo. Int Immunol. 2000;12:671–676. doi: 10.1093/intimm/12.5.671. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol. 2018;9:978–978. doi: 10.3389/fimmu.2018.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffioen AW, Damen CA, Mayo KH, Barendsz-Janson AF, Martinotti S, Blijham GH, Groenewegen G. Angiogenesis inhibitors overcome tumor induced endothelial cell anergy. Int J Cancer. 1999;80:315–319. doi: 10.1002/(SICI)1097-0215(19990118)80:2<315::AID-IJC23>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 9.Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171–6180. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S, Hernandez G, Mier J, He X, Hodi FS, et al. Atezolizumab in combination with bevacizumab enhances migration of antigen-specific T-cells in metastatic renal cell carcinoma. Nat Commun. 2016;7:12624. doi: 10.1038/ncomms12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2018;52:117–124. doi: 10.1016/j.semcancer.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Basu A, Hoerning A, Datta D, Edelbauer M, Stack MP, Calzadilla K, Pal S, Briscoe DM. Cutting edge: Vascular endothelial growth factor-mediated signaling in human CD45RO+ CD4+ T cells promotes Akt and ERK activation and costimulates IFN-gamma production. J Immunol. 2010;184:545–549. doi: 10.4049/jimmunol.0900397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wada J, Suzuki H, Fuchino R, Yamasaki A, Nagai S, Yanai K, Koga K, Nakamura M, Tanaka M, Morisaki T, Katano M. The contribution of vascular endothelial growth factor to the induction of regulatory T-cells in malignant effusions. Anticancer Res. 2009;29:881–888. [PubMed] [Google Scholar]
- 14.Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, Carbone DP. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101:4878–4886. doi: 10.1182/blood-2002-07-1956. [DOI] [PubMed] [Google Scholar]
- 15.Gavalas NG, Tsiatas M, Tsitsilonis O, Politi E, Ioannou K, Ziogas AC, Rodolakis A, Vlahos G, Thomakos N, Haidopoulos D, et al. VEGF directly suppresses activation of T cells from ascites secondary to ovarian cancer via VEGF receptor type 2. Br J Cancer. 2012;107:1869–1875. doi: 10.1038/bjc.2012.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziogas AC, Gavalas NG, Tsiatas M, Tsitsilonis O, Politi E, Terpos E, Rodolakis A, Vlahos G, Thomakos N, Haidopoulos D, et al. VEGF directly suppresses activation of T cells from ovarian cancer patients and healthy individuals via VEGF receptor type 2. Int J Cancer. 2011;130:857–864. doi: 10.1002/ijc.26094. [DOI] [PubMed] [Google Scholar]
- 17.Kim CG, Jang M, Kim Y, Leem G, Kim KH, Lee H, Kim TS, Choi SJ, Kim HD, Han JW, et al. VEGF-A drives TOX-dependent T cell exhaustion in anti-PD-1-resistant microsatellite stable colorectal cancers. Science Immunol. 2019;4:eaay0555. doi: 10.1126/sciimmunol.aay0555. [DOI] [PubMed] [Google Scholar]
- 18.Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, Latreche S, Bergaya S, Benhamouda N, Tanchot C, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212:139–148. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, Lal P, Feldman MD, Benencia F, Coukos G. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20:607–615. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176:1248–1264. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen DS, Hurwitz H. Combinations of Bevacizumab with cancer immunotherapy. Cancer J. 2018;24:193–204. doi: 10.1097/PPO.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 22.Lissoni P, Malugani F, Bonfanti A, Bucovec R, Secondino S, Brivio F, Ferrari-Bravo A, Ferrante R, Vigoré L, Rovelli F, et al. Abnormally enhanced blood concentrations of vascular endothelial growth factor (VEGF) in metastatic cancer patients and their relation to circulating dendritic cells, IL-12 and endothelin-1. J Biol Regul Homeost Agents. 2001;15:140–144. [PubMed] [Google Scholar]
- 23.Boissel N, Rousselot P, Raffoux E, Cayuela JM, Maarek O, Charron D, Degos L, Dombret H, Toubert A, Rea D. Defective blood dendritic cells in chronic myeloid leukemia correlate with high plasmatic VEGF and are not normalized by imatinib mesylate. Leukemia. 2004;18:1656–1661. doi: 10.1038/sj.leu.2403474. [DOI] [PubMed] [Google Scholar]
- 24.Dikov MM, Ohm JE, Ray N, Tchekneva EE, Burlison J, Moghanaki D, Nadaf S, Carbone DP. Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. J Immunol. 2004;174:215–222. doi: 10.4049/jimmunol.174.1.215. [DOI] [PubMed] [Google Scholar]
- 25.Oussa NA, Dahmani A, Gomis M, Richaud M, Andreev E, Navab-Daneshmand AR, Taillefer J, Carli C, Boulet S, Sabbagh L, et al. VEGF Requires the receptor NRP-1 to inhibit lipopolysaccharide-dependent dendritic cell maturation. J Immunol. 2016;197:3927–3935. doi: 10.4049/jimmunol.1601116. [DOI] [PubMed] [Google Scholar]
- 26.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 27.Mimura K, Kono K, Takahashi A, Kawaguchi Y, Fujii H. Vascular endothelial growth factor inhibits the function of human mature dendritic cells mediated by VEGF receptor-2. Cancer Immunol Immunother. 2006;56:761–770. doi: 10.1007/s00262-006-0234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, Gabrilovich DI. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol. 1998;160:1224–1232. [PubMed] [Google Scholar]
- 29.Crawford Y, Ferrara N. Tumor and stromal pathways mediating refractoriness/resistance to anti-angiogenic therapies. Trends Pharmacol Sci. 2009;30:624–630. doi: 10.1016/j.tips.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Huang Y, Chen X, Dikov MM, Novitskiy SV, Mosse CA, Yang L, Carbone DP. Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood. 2007;110:624–631. doi: 10.1182/blood-2007-01-065714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karakhanova S, Link J, Heinrich M, Shevchenko I, Yang Y, Hassenpflug M, Bunge H, von Ahn K, Brecht R, Mathes A, et al. Characterization of myeloid leukocytes and soluble mediators in pancreatic cancer: Importance of myeloid-derived suppressor cells. Oncoimmunology. 2015;4:e998519. doi: 10.1080/2162402X.2014.998519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 33.Mantovani A, Locati M. Tumor-associated macrophages as a paradigm of macrophage plasticity, diversity, and polarization: Lessons and open questions. Arterioscler Thromb Vasc Biol. 2013;33:1478–1483. doi: 10.1161/ATVBAHA.113.300168. [DOI] [PubMed] [Google Scholar]
- 34.Linde N, Lederle W, Depner S, van Rooijen N, Gutschalk CM, Mueller MM. Vascular endothelial growth factor-induced skin carcinogenesis depends on recruitment and alternative activation of macrophages. J Pathol. 2012;227:17–28. doi: 10.1002/path.3989. [DOI] [PubMed] [Google Scholar]
- 35.Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, Mack M, Pipeleers D, In't Veld P, De Baetselier P, Van Ginderachter JA. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 36.Gabrusiewicz K, Liu D, Cortes-Santiago N, Hossain MB, Conrad CA, Aldape KD, Fuller GN, Marini FC, Alonso MM, Idoate MA, et al. Anti-vascular endothelial growth factor therapy-induced glioma invasion is associated with accumulation of Tie2-expressing monocytes. Oncotarget. 2014;5:2208–2220. doi: 10.18632/oncotarget.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raredon MSB, Adams TS, Suhail Y, Schupp JC, Poli S, Neumark N, Leiby KL, Greaney AM, Yuan Y, Horien C, et al. Single-cell connectomic analysis of adult mammalian lungs. Sci Adv. 2019;5:eaaw3851. doi: 10.1126/sciadv.aaw3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barratt SL, Flower VA, Pauling JD, Millar AB. VEGF (vascular endothelial growth factor) and fibrotic lung disease. Int J Mol Med. 2018;19:1269. doi: 10.3390/ijms19051269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manzoni M, Rovati B, Ronzoni M, Loupakis F, Mariucci S, Ricci V, Gattoni E, Salvatore L, Tinelli C, Villa E, Danova M. Immunological effects of Bevacizumab-based treatment in metastatic colorectal cancer. Oncology. 2010;79:187–196. doi: 10.1159/000320609. [DOI] [PubMed] [Google Scholar]
- 41.Martino EC, Misso G, Pastina P, Costantini S, Vanni F, Gandolfo C, Botta C, Capone F, Lombardi A, Pirtoli L, et al. Immune-modulating effects of bevacizumab in metastatic non-small-cell lung cancer patients. Cell Death Discov. 2016;2:16025–16025. doi: 10.1038/cddiscovery.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alfaro C, Suarez N, Gonzalez A, Solano S, Erro L, Dubrot J, Palazon A, Hervas-Stubbs S, Gurpide A, Lopez-Picazo JM, et al. Influence of bevacizumab, sunitinib and sorafenib as single agents or in combination on the inhibitory effects of VEGF on human dendritic cell differentiation from monocytes. Br J Cancer. 2009;100:1111–1119. doi: 10.1038/sj.bjc.6604965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osada T, Chong G, Tansik R, Hong T, Spector N, Kumar R, Hurwitz HI, Dev I, Nixon AB, Lyerly HK, et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother. 2008;57:1115–1124. doi: 10.1007/s00262-007-0441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kusmartsev S, Eruslanov E, Kübler H, Tseng T, Sakai Y, Su Z, Kaliberov S, Heiser A, Rosser C, Dahm P, et al. Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J Immunol. 2008;181:346–353. doi: 10.4049/jimmunol.181.1.346. [DOI] [PubMed] [Google Scholar]
- 45.Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, Dubreuil O, Carpentier AF, Tartour E, Taieb J. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2012;73:539–549. doi: 10.1158/0008-5472.CAN-12-2325. [DOI] [PubMed] [Google Scholar]
- 46.Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, Park K, Gorbunova V, Kowalyszyn RD, Pikiel J, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665–673. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 47.van Hooren L, Georganaki M, Huang H, Mangsbo SM, Dimberg A. Sunitinib enhances the antitumor responses of agonistic CD40-antibody by reducing MDSCs and synergistically improving endothelial activation and T-cell recruitment. Oncotarget. 2016;7:50277–50289. doi: 10.18632/oncotarget.10364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, Schwartz M, Divino CM, Pan PY, Chen SH, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–2522. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 50.Finke J, Ko J, Rini B, Rayman P, Ireland J, Cohen P. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol. 2011;11:856–861. doi: 10.1016/j.intimp.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guislain A, Gadiot J, Kaiser A, Jordanova ES, Broeks A, Sanders J, van Boven H, de Gruijl TD, Haanen JB, Bex A, Blank CU. Sunitinib pretreatment improves tumor-infiltrating lymphocyte expansion by reduction in intratumoral content of myeloid-derived suppressor cells in human renal cell carcinoma. Cancer Immunol Immunother. 2015;64:1241–1250. doi: 10.1007/s00262-015-1735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marasco M, Berteotti A, Weyershaeuser J, Thorausch N, Sikorska J, Krausze J, Brandt HJ, Kirkpatrick J, Rios P, Schamel WW, et al. Molecular mechanism of SHP2 activation by PD-1 stimulation. Sci Adv. 2020;6:eaay4458. doi: 10.1126/sciadv.aay4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 54.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 55.Chen PL, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, Miller JP, Bassett RL, Gopalakrishnan V, Wani K, et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov. 2016;6:827–837. doi: 10.1158/2159-8290.CD-15-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, Santosuosso M, Martin JD, Martin MR, Vianello F, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci USA. 2012;109:17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allen E, Jabouille A, Rivera LB, Lodewijckx I, Missiaen R, Steri V, Feyen K, Tawney J, Hanahan D, Michael IP, Bergers G. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med. 2017;9:eaak9679. doi: 10.1126/scitranslmed.aak9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mayoux M, Roller A, Pulko V, Sammicheli S, Chen S, Sum E, Jost C, Fransen MF, Buser RB, Kowanetz M, et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci Transl Med. 2020;12:534. doi: 10.1126/scitranslmed.aav7431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.