Abstract
Background
Inflammation is at the core of many chronic conditions and exacerbates infectious conditions, including the severity of coronavirus disease 2019 (COVID-19) infections.
Objectives
This study aimed to examine the effects of a novel food supplement, palmitoylethanolamide (PEA), specifically Levagen+, as compared with a placebo on proinflammatory biomarkers in adults recently diagnosed with COVID-19 who were unvaccinated and nonhospitalized.
Methods
This study was a double-blind randomized placebo-controlled trial conducted October 2020–March 2021 (clinicaltrials.gov: NCT04912921). Participants aged 19–53 y were unvaccinated and recently infected with COVID-19 as indicated by a positive test result per RT-PCR or antigen test, and they reported to the test site following diagnosis as allowed by the CDC’s return-to-work policy. Participants were stratified by age, sex, and BMI and randomly assigned by coin toss to receive 600 mg Levagen+ twice daily (LEV) or placebo tablets twice daily (CON) for 4 wk. At baseline and week 4, participants completed health histories, 24-h dietary recalls, anthropometrics, and nonfasting blood sampling. The primary outcomes were the 4-wk change between groups for IL-6, C-reactive protein, ferritin, intercellular adhesion molecule 1, soluble P-selectin (sP-selectin), and neutrophil/lymphocyte ratio. Multiple linear regression models were utilized to assess treatment effects on outcomes, adjusting for covariates.
Results
A total of 60 participants completed the study (LEV: n = 30; CON: n = 30). After 4 wk of supplementation, sP-selectin (β = −11.5; 95% CI: −19.8, −3.15; P = 0.0078), IL-1β (β = −22.9; 95% CI: −42.4, −3.40; P = 0.0222), and IL-2 (β = −1.73; 95% CI: −3.45, −0.065; P = 0.0492) concentrations were significantly reduced in the LEV group compared with the CON group.
Conclusions
Inflammatory mechanisms are crucial to optimal resolution of infectious conditions, yet unchecked secretion of inflammatory mediators can promote the dysregulated immune response implicated in COVID-19 complications. Overall, PEA supplementation produced anti-inflammatory effects in individuals recently diagnosed with COVID-19 who were nonhospitalized.
Keywords: palmitoylethanolamide; COVID-19, SARS-CoV-2; inflammation; dietary supplements; cytokines; adhesion molecules
Introduction
Inflammation is at the core of many chronic and infectious conditions, including the severity of coronavirus disease 2019 (COVID-19) infection (1, 2). The SARS-CoV-2 virus, responsible for COVID-19, is classified in the β genus of the Coronaviridae family (3). This family of enveloped viruses has a positive-strand RNA genome capable of encoding critical structural proteins, including the spike surface glycoprotein and the membrane, envelope, and nucleocapsid proteins, which play a pivotal role in the pathogenic outcomes of the virus (4). Upon exposure to SARS-CoV-2, largely through transmission by respiratory droplets, the viral spike protein interacts with angiotensin-converting enzyme 2 receptors on host cells, triggering subsequent changes in spike protein conformation and endosomal formation, events that facilitate viral entry into host cells (5). Once inside a cell, the virus releases its RNA and replicates, and high numbers of formed virus leave the cell to invade other cells, furthering infection (6).
Derangements in angiotensin-converting enzyme 2 activity caused by viral attachment in individuals infected with SARS-CoV-2 are supported by observed increases in plasma angiotensin II (7). Overactive immune responses may be tied to this loss in angiotensin-converting enzyme 2 activity and therefore inhibition of its protective mechanisms related to inflammation, such as its conversion of angiotensin II to angiotensin 1–7 and its inhibitory effects on macrophage cytokine expression (8). Furthermore, signaling mechanisms associated with the innate immune response against viral infection lead to the activation of transcription factors, such as NF-κB, which induce production of a diverse array of proinflammatory cytokines and chemokines (9). COVID-19 has also been reported to induce lymphopenia among dendritic cells, T cells, and NK cells and to upregulate other immune cells, such as neutrophils and monocytes (10, 11). This overproduction of cytokines leads to the enhanced expression of adhesion molecules on the surface of endothelial cells, further expanding the inflammatory sequelae. For example, elevated circulating P-selectin has been demonstrated to predict thrombosis in patients with COVID-19 (12). Excessive cytokine production, termed “cytokine storm,” can potentiate negative downstream consequences, including a variety of pulmonary and extrapulmonary complications, which are associated with severity of COVID-19 outcomes (13). Thus, investigations have utilized proinflammatory biomarkers to characterize disease severity and risk for death. Consequently, strategies to control inflammation are considered a key approach for slowing the progression of disease and tissue pathology.
Whereas the heightened release of inflammatory mediators is typically observed in the acute stage of COVID-19 (14), some patients develop symptoms lasting beyond 12 wk, often occurring in those with mild-moderate infection severity. Recently, in a study of patients with mild-moderate SARS-CoV-2 infection, those experiencing persistent symptoms had increased immune cell activation and exhibited elevations in proinflammatory mediators 8 mo after infection (15). Hence, approaches to reduce inflammatory mediators in those with even mild-moderate symptoms could be of key importance to mitigating the acute and long-term impacts of COVID-19.
Palmitoylethanolamide (PEA)—a fatty acid amide first isolated from lipid fractions of egg yolks, peanuts, and soybeans—has been documented as being beneficial for reducing inflammation (16). PEA is also endogenously produced in human tissues and functions as a lipid mediator targeting ion channels, nuclear hormone receptors, and G protein–coupled receptors, with wide-reaching effects on metabolism (17). One of the direct targets for PEA has been identified as nuclear receptor peroxisome proliferator–activated receptor α (PPAR-α), which is expressed in several cells, including immune cells, and supports PEA’s ability for use in modulating inflammatory responses (18, 19). PEA’s interaction with PPAR-α has been shown to mediate its anti-inflammatory effects, and PEA’s binding to PPAR-α on immune cells leads to reductions in proinflammatory and pain signals (20). Furthermore, PEA exerts its action on G protein–coupled receptor 55, a cannabinoid-like receptor, and indirectly influences activity of cannabinoid receptors 1 and 2 via inhibition of cannabinoid degradation, termed the “entourage effect” (21). Importantly, research has demonstrated that PEA inhibits the migration and degranulation of mast cells by direct and indirect mechanisms (17). Given the emerging evidence that COVID-19 infection activates mast cells via toll-like receptor 4, the ability of PEA to lessen immune cell–induced inflammation via toll-like receptor 4–dependent PPAR-α activation may have important physiologic relevance (22). PEA has been utilized in several trials to treat influenza and the common cold and was shown to be effective in treating upper respiratory infections (16).
Given the parallels between COVID-19 and the mechanisms by which PEA has been successful as an immunomodulator in conditions such as influenza, it may be a viable adjunctive treatment for COVID-19. Notably, PEA is endogenously produced and has not been associated with adverse side effects. Although PEA has been put forth as a possible adjunctive treatment strategy for COVID-19, there is a dearth of evidence on its use in patients with COVID-19 (23). In this context, the aim of this research is to expand this literature related to PEA in a novel manner and to evaluate the efficacy of 4-wk PEA supplementation to reduce the mediators of inflammation in adults who recently tested positive for COVID-19 but were not hospitalized, a group likely to experience elevations in inflammatory mediators following infection. We hypothesized that 4 wk of PEA supplementation would result in a favorable modulation in inflammatory mediators as compared with placebo supplementation. Given that inflammatory mediators would be expected to return toward homeostatic levels following infection, changes in inflammatory mediators are expected to improve to a larger degree in the treatment group as compared with the placebo group, all else being equal.
Methods
Participants
Healthy adults between the ages of 18–65 y who recently tested positive for COVID-19 and were not hospitalized for their illness were recruited via online advertisements, local news outlets, e-mail lists, and word of mouth to participate in this study. Inclusion in the study required a recent positive COVID-19 test result per PCR in asymptomatic/symptomatic individuals, although positive antigen test results were also accepted upon symptomatic infection consistent with COVID-19 per the symptoms outlined by the CDC. The exclusion criteria included the following: any unstable or serious illness; serious mood disorders; neurologic disorders, such as multiple sclerosis or cognitive damage; active smoking and/or nicotine or drug abuse; active regular marijuana or other cannabinoid use; other street/recreational drug use; chronic past and/or current alcohol use (>14 alcoholic drinks/wk); allergies to any of the ingredients in the active or placebo formula, including peanuts and eggs; pregnancy or lactation; medical prescription of drugs that affect immune and/or inflammatory responses; malignancy treatment in the last 5 y; chronic use of steroids; and a BMI > 40 kg/m2. Eligible respondents were sent an electronic consent form and scheduled to visit the test site based on CDC return-to-work guidance. All CDC guidance for workplace safety was followed at the test site, including hand hygiene practices, environmental infection control, and personal protective equipment. Study recruitment was conducted October 2020–March 2021, and all participants were unvaccinated for COVID-19. The Arizona State University Institutional Review Board approved this study (No. 00,012,406), and all participants provided written consent. The study is registered at clinicaltrials.gov as NCT04912921.
Study protocol
The study followed a placebo-controlled randomized parallel-arm study design. Participants were stratified by age, sex, and BMI and randomly assigned by coin toss to receive the active ingredient or placebo treatment for 4 wk. Randomization was performed by a research team member not involved in data collection or analysis. Participants were screened for eligibility using an online questionnaire. Those who met the inclusion criteria were interviewed by phone for a secondary screening to confirm eligibility and to reduce physical contact with investigators during infectious periods. Eligible individuals were scheduled to visit the test site (Arizona Biomedical Collaborative Laboratory Building) based on CDC return-to-work guidance: 1) at least 10 d since symptoms first appeared or from positive test result in asymptomatic individuals, 2) at least 24 h since last fever without the use of fever-reducing medications, and 3) improvement of symptoms (e.g., cough, shortness of breath).
Participants attended two in-person study visits and completed a health history questionnaire and 24-h dietary recall. Anthropometrics were collected during study visits, including height, weight, and BMI. At study visits, venous blood samples were collected by a certified staff phlebotomist into appropriate collection tubes for subsequent processing and analyses of inflammatory mediators. At study baseline, participants were provided with the assigned supplements; a calendar to track supplement intake; and directions on supplement ingestion, symptom reporting, and over-the-counter medication tracking. The final posttrial visit was scheduled after 4 wk, and the baseline assessments and blood draw protocol were repeated. Nutrient intakes obtained from 24-h dietary recalls at baseline and posttrial were assessed using Food Processor Nutrient Analysis Software (ESHA Research).
Supplementation
Participants in the active treatment group ingested 600 mg Levagen+ twice daily (LEV) for 4 wk. A 2013 review (16) reported on the role of PEA as an anti-inflammatory and therapeutic agent for influenza and the common cold. In six clinical trials in nearly 4000 volunteers, PEA demonstrated effectiveness and safety for the treatment of these indications. The effective dose range has consistently been 10–30 mg PEA/kg bodyweight. Levagen + also contains LipiSperse: a novel delivery system designed to increase the dispersion of lipophilic agents in aqueous environments and indicated to enhance PEA bioavailability by nearly 75% (24). As such, each pill contains 350 mg Levagen + with the label claim “Not less than 300 mg palmitoylethanolamide.” Ten percent of the 350 mg is LipiSperse; therefore, each pill contains 315 mg PEA. This is per USP regulations for finished products such that Levagen + contains 270–330 mg PEA (i.e., 90%–110% of the label’s claimed ingredients). Those in the placebo group (CON) ingested placebo capsules (maltodextrin) twice daily for 4 wk. LEV and CON supplements were identical in appearance, and supplement blinding was completed by an investigator not involved in data collection or analyses. For adherence checking, participants were asked to record pill ingestion daily and to return unemptied pill bottles. Participants recorded any physical symptoms and/or over-the-counter medication use daily during the trial. Participants also received weekly prompts via e-mail from investigators, which contained reminders related to the study protocol.
Blood processing and analyses
During baseline and posttrial visits, participant blood samples were collected from the antecubital vein by standard phlebotomy techniques, with blood collection sets in 4-mL dipotassium EDTA tubes and 8.5-mL serum separator tubes. After centrifugation of serum separator tubes, serum samples were treated with 1% Triton X-100 to inactivate SARS-CoV-2 virus (25). Whole blood collected in EDTA tubes was immediately lysed in Trizol RNA extraction buffer (TRIzol LS; Thermo Fisher Scientific) and prepared for qRT-PCR for gene expression analysis. Whole blood collected in EDTA tubes was transported and analyzed for complete blood count with differentials, specifically utilized for calculation of neutrophil/lymphocyte ratios by Sonora Quest Laboratories. Serum samples were analyzed for high-sensitivity C-reactive protein and ferritin by Sonora Quest Laboratories. Samples were stored at −80 °C until time of analyses. Serum cytokines and chemokines were analyzed by human focused 15-plex discovery assay (Eve Technologies Corporation): IL-6, IL-1β, IL-1Ra, IL-2, IL-4, IL-5, IL-8, IL-10, IL-12p40, IL-12p70, IL-13, monocyte chemoattractant protein 1, IFN-γ, granulocyte-macrophage colony-stimulating factor, and TNF-α. Serum soluble P-selectin (sP-selectin) and intercellular adhesion molecule 1 were analyzed via commercially available ELISA methods (Invitrogen; Thermo Fisher Scientific).
RNA isolation and qRT-PCR
The expression of NF-κB, IL-6, and CD177 was quantified from whole blood samples. Trizol-treated whole blood was sonicated for 1 min and then centrifuged to remove cell debris. RNA was purified using the Direct-zol RNA MicroPrep Kit (R2062; Zymo Research), and cDNA was synthesized from total RNA using the cDNA Reverse Transcriptase Kit (4,374,966; Thermo Fisher Scientific) according to the manufacturers’ instructions. Amplification of NF-κB, IL-6, CD177, and GAPDH genes was accomplished using SYBR Green Master Mix (A46012; Thermo Fisher Scientific) according to the following primers: NF-κB2 (F–GAAGACAAGGAAGAGGTGCAG, R–CACCTCCTCCAGCTCCT), IL-6 (F–TGAGAAAGGAGACATGTAACAAGAG, R–CAAGTCTCCTCATTGAATCCAGA), CD177 (F–GAGGAGGCATCTTCTCCAATC, R–CCCGATGACAGGTCAGAAA), and GAPDH (F–GAAGGTGAAGGTCGGAGTC, R–GAAGATGGTGATGGGATTTC). The RNA levels of each gene were normalized with GAPDH and expressed as ΔCt.
Statistical analysis
The sample size (N = 60) was informed by a meta-analysis reporting 11 randomized controlled trials that investigated the impact of coenzyme Q10 on reducing IL-6 in chronic inflammatory diseases. In these trials, the sample size averaged 44 (range: 26–60), and power analyses based on several of these trials indicated that a sample size of 30–63 was adequate for 80% power at an alpha of 0.05 to detect a significant change in IL-6 (26). We examined histograms of input variables and performed log transformations as needed for analyses to reduce skewness. The primary outcomes included the change in circulating levels of IL-6, C-reactive protein, ferritin, intercellular adhesion molecule 1, sP-selectin, and neutrophil/lymphocyte ratio between groups. Secondary analyses were conducted on the changes in a panel of serum cytokines and chemokines and expression of NF-κB, IL-6, and CD177 in whole blood between groups. A series of linear regression models was built to test if the postchange as compared with the prechange in each inflammatory marker was associated with the treatment. In these models, the response variable was the postchange as compared with the prechange; the independent variable was the treatment group; and the covariates were age, sex, BMI, and existing conditions (binary). An overall P value <0.05 indicated statistical significance. The Benjamini-Hochberg procedure (27) was employed to account for multiple comparisons in analyses of primary outcomes with a false discovery rate of 10%. Data were analyzed using open source R software.
Results
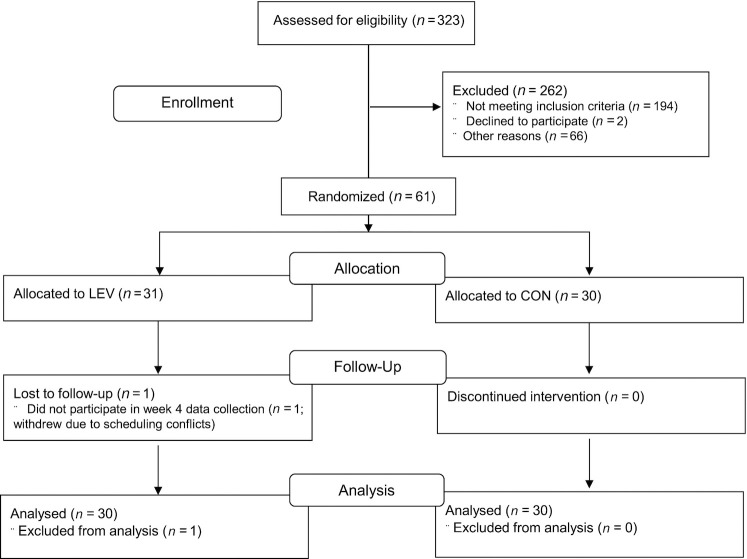
From the 323 study respondents, 61 participants were enrolled in the trial. One LEV participant withdrew due to scheduling conflicts, and 60 participants (n = 30/group) completed the trial ( Figure 1). Average compliance to supplement ingestion was high such that 87% and 91% of the tablets were taken during the study in the CON and LEV groups, respectively. Baseline characteristics of participants are shown in Table 1. Baseline characteristics of participants were comparable between study arms. Additionally, of the 60 participants who completed the trial, 2 in the CON group and 1 in the LEV group reported asymptomatic infection. Types of COVID-19 symptoms reported by participants were also comparable between groups (Supplemental Figure 1).
FIGURE 1.
Study participant flow. CON, placebo; LEV, 600 mg Levagen + twice daily.
TABLE 1.
Baseline characteristics of study participants (N = 60) in the LEV and CON groups1
| CON (n = 30) | LEV (n = 30) | |
|---|---|---|
| Demographics | ||
| Sex: male, % | 25 | 50 |
| Age, y | 26.3 ± 7.8 | 25.2 ± 7.4 |
| Anthropometrics | ||
| Height, cm | 167 ± 7.6 | 170 ± 6.7 |
| Weight, kg | 73.8 ± 16.3 | 75.0 ± 18.1 |
| BMI, kg/m2 | 26.5 ± 6.0 | 25.9 ± 5.6 |
| COVID-19: asymptomatic | 2 (7) | 1 (3) |
| Reported existing condition | ||
| High blood pressure | 2 (7) | 0 (0) |
| Thyroid conditions | 3 (10) | 4 (13) |
| Anemia | 2 (7) | 1 (3) |
| Asthma | 1 (3) | 3 (10) |
| Depression | 3 (10) | 4 (13) |
| Heart murmur | 0 (0) | 1 (3) |
Data are presented as n (%) of participants or mean ± SD unless noted otherwise. CON, placebo; COVID-19, coronavirus disease 2019; LEV, 600 mg Levagen + twice daily.
Based on the 24-h dietary records obtained throughout the trial, no significant baseline or posttrial differences were seen between the LEV and CON groups in dietary intakes of energy, carbohydrates, proteins, fats, saturated fatty acids, sugar, total dietary fiber, vitamin C, vitamin D, calcium, iron, sodium, and zinc (Supplemental Table 1).
There were no significant differences between groups in the reductions of IL-6, C-reactive protein, ferritin, intercellular adhesion molecule 1, or neutrophil/lymphocyte ratio ( Table 2). However, the reduction in sP-selectin from baseline was significantly associated with LEV treatment compared with CON, which saw increases in these markers after adjustment for covariates and remained significant with a Benjamini-Hochberg false discovery rate ≤ 0.10 (β = −11.5; 95% CI: −19.8, −3.15; P = 0.0078). sP-selectin fell 8% in the experimental group (mean ± SD: –6.8 ± 17.3) and rose 5% in the control group (3.4 ± 15.3).
TABLE 2.
Status of primary outcome serum inflammatory biomarkers at baseline and after the 4-wk intervention and linear regression analyses on change from baseline scores in adults recently diagnosed with COVID-19 in the LEV and CON groups (n = 30/group)1
| Change in outcome measures associated with LEV treatment2 | ||||
|---|---|---|---|---|
| Measure: treatment | Baseline | Week 4 | Estimate (95% CI) | P value |
| IL-6, pg/mL | 0.01 (−0.23, 0.26) | 0.91 | ||
| CON | 2.15 ± 3.79 | 1.60 ± 1.95 | ||
| LEV | 1.24 ± 0.84 | 2.26 ± 3.41 | ||
| Ferritin, ng/mL | 5.06 (−22.7, 32.8) | 0.72 | ||
| CON | 135 ± 177 | 94.6 ± 157 | ||
| LEV | 149 ± 162 | 98.6 ± 102 | ||
| CRP, mg/L | 0.05 (−0.10, 0.20) | 0.52 | ||
| CON | 4.99 ± 10.58 | 3.39 ± 6.96 | ||
| LEV | 1.47 ± 1.30 | 1.53 ± 1.51 | ||
| sP-selectin, ng/mL | −11.5 (−19.8, −3.15) | 0.0078* | ||
| CON | 74.2 ± 28.3 | 77.6 ± 31.0 | ||
| LEV | 81.3 ± 24.8 | 74.5 ± 23.9 | ||
| ICAM-1, ng/mL | 1.49 (−21.4, 18.4) | 0.88 | ||
| CON | 417 ± 75.1 | 407 ± 70.5 | ||
| LEV | 405 ± 73.8 | 394 ± 74.8 | ||
| N/L | 0.12 (−0.46, 0.70) | 0.69 | ||
| CON | 1.93 ± 0.74 | 2.09 ± 0.75 | ||
| LEV | 1.84 ± 0.54 | 2.10 ± 1.49 | ||
Data are presented as mean ± SD. Data were analyzed by multiple linear regression models with the change from baseline as the response variable regressed on the treatment group, adjusting for age, sex, BMI, and existing conditions. CON, placebo; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; ICAM-1, intercellular adhesion molecule 1; LEV, 600 mg Levagen + twice daily; N/L, neutrophil/lymphocyte ratio; sP-selectin, soluble P-selectin.
Data are presented as the regression estimate (95% CI) of the estimate for the group assignment variable from each model.
*Significance is retained after correction for multiple comparisons: Benjamini-Hochberg false discovery rate ≤ 0.10.
The changes in circulating cytokines and chemokines and whole blood expression of inflammatory markers after 4 wk of supplementation are presented in Table 3. Our analyses did not reveal significant associations between the treatment group and the change from baseline in most serum inflammatory factors or whole blood expression of NF-κB, IL-6, or CD177. However, significant reductions from baseline in IL-1β (β = −22.9; 95% CI: −42.4, −3.40; P = 0.0222) and IL-2 (β = −1.73; 95% CI: −3.45, –0.065; P = 0.0492) were associated with LEV treatment compared with CON (Table 3).
TABLE 3.
Status of inflammatory cytokines and chemokines in serum and RNA levels of inflammatory markers in whole blood determined by qRT-PCR and linear regression analyses on change from baseline scores in adults recently diagnosed with COVID-19 in the LEV and CON groups (n = 30/group)1
| Change in outcome measures associated with LEV treatment2 | ||||
|---|---|---|---|---|
| Measure: treatment | Baseline | Week 4 | Estimate (95% CI) | P value |
| GM-CSF, pg/mL | −0.12 (–0.51, 0.27) | 0.55 | ||
| CON | 17.7 ± 39.5 | 29.18 ± 51.9 | ||
| LEV | 11.4 ± 21.8 | 17.72 ± 35.3 | ||
| IFN-γ, pg/mL | −0.15 (–0.40, 0.10) | 0.24 | ||
| CON | 2.98 ± 3.34 | 3.92 ± 4.33 | ||
| LEV | 3.27 ± 4.00 | 3.43 ± 3.84 | ||
| IL-1β, pg/mL | −22.9 (−42.4, −3.40) | 0.0222 | ||
| CON | 51.5 ± 98.2 | 59.42 ± 105.5 | ||
| LEV | 46.4 ± 76.8 | 35.08 ± 50.4 | ||
| IL-1Ra, pg/mL | −0.12 (–0.30, 0.05) | 0.16 | ||
| CON | 20.0 ± 30.6 | 15.88 ± 22.3 | ||
| LEV | 13.7 ± 13.9 | 9.88 ± 8.64 | ||
| IL-2, pg/mL | −1.73 (−3.45, –0.065) | 0.0492 | ||
| CON | 2.04 ± 4.05 | 2.62 ± 4.57 | ||
| LEV | 2.98 ± 6.37 | 2.21 ± 3.73 | ||
| IL-4, pg/mL | −0.13 (–0.78, 0.52) | 0.69 | ||
| CON | 1.52 ± 2.20 | 2.03 ± 2.85 | ||
| LEV | 0.90 ± 0.82 | 1.17 ± 1.21 | ||
| IL-5, pg/mL | 0.05 (–0.10, 0.21) | 0.50 | ||
| CON | 4.01 ± 3.28 | 4.60 ± 4.05 | ||
| LEV | 3.72 ± 2.74 | 4.87 ± 3.55 | ||
| IL-8, pg/mL | 0.01 (–0.12, 0.15) | 0.86 | ||
| CON | 7.48 ± 3.46 | 6.72 ± 3.42 | ||
| LEV | 7.83 ± 5.49 | 7.57 ± 4.51 | ||
| IL-10, pg/mL | −0.03 (–0.56, 0.51) | 0.92 | ||
| CON | 2.02 ± 5.39 | 2.55 ± 5.10 | ||
| LEV | 2.03 ± 7.47 | 1.12 ± 1.72 | ||
| IL-12p40, pg/mL | −0.07 (–0.21, 0.07) | 0.31 | ||
| CON | 112 ± 74.9 | 126 ± 85.6 | ||
| LEV | 136 ± 152 | 129 ± 103 | ||
| IL-12p70, pg/mL | −0.11 (–0.62, 0.41) | 0.68 | ||
| CON | 11.7 ± 16.6 | 13.9 ± 16.6 | ||
| LEV | 8.7 ± 12.0 | 12.3 ± 17.1 | ||
| IL-13, pg/mL | −0.06 (–0.33, 0.21) | 0.65 | ||
| CON | 71.0 ± 71.8 | 84.0 ± 86.6 | ||
| LEV | 55.3 ± 42.2 | 67.2 ± 55.9 | ||
| MCP-1, pg/mL | 0.04 (–0.08, 0.16) | 0.54 | ||
| CON | 313 ± 104 | 258 ± 115 | ||
| LEV | 354 ± 132 | 316 ± 144 | ||
| TNF-α, pg/mL | −0.98 (−18.6, 16.6) | 0.91 | ||
| CON | 59.8 ± 38.5 | 59.9 ± 49.20 | ||
| LEV | 49.7 ± 23.7 | 52.6 ± 34.03 | ||
| RNA level | ||||
| NF-κB | −0.04 (–0.35, 0.28) | 0.82 | ||
| CON | 10.4 ± 0.92 | 10.4 ± 0.72 | ||
| LEV | 10.3 ± 0.92 | 10.3 ± 0.81 | ||
| IL-6 | −0.07 (–0.31, 0.17) | 0.57 | ||
| CON | 11.8 ± 0.62 | 11.9 ± 0.57 | ||
| LEV | 11.80 ± 0.74 | 11.7 ± 0.80 | ||
| CD177 | −0.10 (–0.88, 0.67) | 0.79 | ||
| CON | 8.26 ± 1.54 | 8.26 ± 1.78 | ||
| LEV | 8.72 ± 1.83 | 8.66 ± 1.74 | ||
Data are presented as mean ± SD. Data were analyzed by multiple linear regression models with the change from baseline as the response variable regressed on the treatment group, adjusting for age, sex, BMI, and existing conditions. CON, placebo; COVID-19, coronavirus disease 2019; GM-CSF, granulocyte-macrophage colony-stimulating factor; LEV, 600 mg Levagen + twice daily; MCP-1, monocyte chemoattractant protein 1.
Data are presented as regression estimate (95% CI) of the estimate for the group assignment variable from each model. Secondary outcome measures were not adjusted for multiplicity.
Discussion
These data suggest that daily PEA supplementation reduced sP-selectin concentrations in adults recently diagnosed with COVID-19. Specifically, sP-selectin fell 8% in the LEV experimental group and rose 5% in the CON group, a difference that was statistically significant after false discovery rate adjustment using the Benjamini-Hochberg procedure (P = 0.0078). Furthermore, LEV treatment was associated with significant reductions from baseline in IL-2 (P = 0.0492) and IL-1β (P = 0.0222) compared with CON. P-selectin, a key thromboinflammatory marker, is stored in the α-granules of platelets and Weibel-Palade bodies of endothelial cells and is translocated to the surface of the cell upon stimulation (28). This membrane glycoprotein is expressed on activated platelets and endothelial cells and contributes to the localized inflammation that ultimately eliminates pathogens and clears cell debris. P-selectin is involved in the initial attachment of platelets and endothelial cells to leukocytes and the rolling of immune cells to injured regions (29). In addition, P-selectin on activated platelets has been shown to stimulate exposure of tissue factor on monocytes, which may promote intravascular hemostasis and thrombosis (30). Although P-selectin is essential for optimal immune responses and the rapid elimination of infectious agents and foreign particles, under conditions of unresolved disease or chronic insult, P-selectin is related to the propagation and amplification of the inflammatory response. Importantly, P-selectin has also been linked to COVID-19 complications.
Authors have reported 6%–12% declines in sP-selectin following pharmaceutical interventions in patient populations. Nomura et al. (31) noted a 6% reduction in sP-selectin following administration of efonidipine to patients with hypertension and diabetes and suggested that this may prevent the development of cardiovascular complications caused by cell adhesion molecules or activated platelets and monocytes. Riondino et al. (32) noted a 12% reduction in sP-selectin following drug-induced normalization of blood pressure in older adults who were hypertensive. Thus, the 8% reduction in sP-selectin noted herein is within the ranges cited in other intervention trials, suggesting its clinical relevance.
Procoagulant responses and thrombosis are augmented in patients with COVID-19 (33). These derangements in hemostasis persist in moderate and severe COVID-19 (34) and are associated with the signature inflammatory imbalances of the cytokine storm and severity of disease outcomes (11, 35). P-selectin expression on the surface of platelets was recently shown to be significantly elevated in hospitalized patients with COVID-19, regardless of severity, compared with healthy donors (36). Furthermore, P-selectin is a key receptor for formation of platelet-leukocyte aggregation, which is increased in those infected with COVID-19 (36), and a marker of in vivo platelet activation during viral illness (37). Moreover, serum sP-selectin levels were found to be higher in moderate and severe cases of COVID-19 than in a healthy control group, suggesting the potential of sP-selectin as a prognostic marker for COVID-19 disease (38). It has been noted that sP-selectin, the form in circulation, is a useful biomarker and potential contributor to vascular complications (39). Additionally, as patients with COVID-19 have highly stimulated circulating platelets, it has been suggested that P-selectin can be utilized as a marker of platelet activation in COVID-19 infection (40). Studies have further reported that crizanlizumab, a monoclonal antibody that targets sP-selectin, can decrease inflammation by binding to it and blocking leucocyte and platelet adherence to the vessel wall (41). Consistent with these findings, blocking of sP-selectin has been demonstrated to attenuate the hyperinflammatory and hyperthrombotic state characteristic of COVID infections (42). Given the prothrombotic consequences of increases in P-selectin on COVID-19 immunothrombosis, interventions targeting P-selectin may be favorable in dually targeting platelet and endothelial cell mechanisms of this disease. In the present study, ingestion of LEV for 4 wk induced a significant reduction in sP-selectin from baseline in adults recently diagnosed with COVID-19, as opposed to an increase in this marker in the CON group.
Additionally, it has been noted that whereas inflammatory mediators play a crucial role in viral defense, disproportionate immune responses are associated with the severity of COVID-19. Several proinflammatory cytokines, chemokines, and markers of infection have been indicated to be upregulated in COVID-19: C-reactive protein, IL-6, IL-1β, IL-2, TNF-α, monocyte chemoattractant protein 1, granulocyte-macrophage colony-stimulating factor, and IFN-γ (43). Of note, several of these factors are upregulated by increases in NF-κB expression. Therefore, it has been suggested that the NF-κB pathway could be a beneficial therapeutic target for mitigating COVID-19 severity (44). In the present study, we also noted significant mean decreases in IL-1β (P = 0.0222) and IL-2 (P = 0.0492) associated with LEV treatment.
Evidence from a 2016 study suggested that PEA administration reduces P-selectin, neutrophil infiltration, cytokine production (TNF-α and IL-1β), and NF-κB expression in rats following myocardial ischemia reperfusion injury (45). It also suggested, in support of the PEA mechanisms previously reported, that the effect of NF-κB may be associated with PPAR-α activation and expression. This finding agrees with a prior study demonstrating that the anti-inflammatory effects of PEA are in part mediated by PPAR-α activation (46). Importantly, NF-κB and mast cell activation are linked to production of inflammatory cytokines, which can induce the expression of adhesion molecules on the endothelium (45). Regarding the results of the present study, although systemic reductions in sP-selectin, IL-2, and IL-1β were observed in the experimental group (LEV), we did not observe reductions in expression of NF-κB, IL-6, CD177, and other circulating inflammatory factors. However, it is possible that these markers would be modestly upregulated in those with mild-moderate COVID-19 infections, as participants in this study were not hospitalized for COVID-19 illness. It is also plausible that whereas sP-selectin may be a simple diagnostic tool related to COVID-19 diagnosis and severity, alterations in markers associated with sP-selectin reduction at the level of specific target tissues (e.g., the lungs) may not be revealed by our analysis.
Importantly, a recent investigation suggested that murine alveolar macrophages challenged with the SARS-CoV-2 spike viral protein in vitro exhibited significant increases in proinflammatory markers, including NF-κB, IL-6, TNF-α, and IL-1β, and inflammasome expression, with this response being reduced significantly by treatment with PEA (47). It was additionally demonstrated that this response was mediated by PPAR-α. Moreover, in a study of LPS-induced acute lung injury in rats, PEA administration reduced markers of neutrophil infiltration, immune cells quantity, and mast cell degranulation in the lungs and inhibited bronchoalveolar lavage fluid levels of the proinflammatory cytokines IL-6, TNF-α, IL-1β, and IL-18. Furthermore, PEA was suggested to block inflammatory cytokine production through inhibition of NF-κB activation in lung tissue (48). It is also possible that the reductions in sP-selectin and cytokines found in this study could be related to implications of PEA in the entourage effect. It has been postulated that PEA can inhibit degradation of endocannabinoids, thereby potentiating the effects at their targets. In line with this potential contributory mechanism, Zhao et al. (49) demonstrated that activation of CB2 receptors can inhibit the expression of P-selectin in an animal model of atherosclerosis, which was associated with reductions in macrophage infiltration. It has also been demonstrated that PEA may enhance the release of 2-arachidonoyl glycerol, an endocannabinoid that has been shown to reduce IL-2 secretion (50, 51, 52).
Although the mechanisms attributable to PEA that are associated with reductions in P-selectin and inflammation have not been fully elucidated, the findings of this study support a growing body of evidence that PEA may be beneficial in modulating thromboinflammatory markers in various models and disease states. Our findings within the current context are especially promising, as P-selectin has recently been associated with COVID-19 coagulopathy in both the short term and the long term (i.e., “long COVID”) (38, 53).
A limitation to this study was that our participants were allowed to the test site following CDC return-to-work guidance; therefore, we were unable to capture responses immediately following viral infection. Yet, it is important to note that the LEV and CON groups were comparable in terms of the types of COVID-19 symptoms reported and the total number of symptoms reported. Furthermore, the CDC stated that inflammatory reactions and health problems related to COVID-19 can be experienced weeks after viral infection, even in those who experienced no or minimal symptoms during infection. With all of this noted, secondary outcome data were not adjusted for multiplicity, and caution should be exercised in interpreting findings of secondary outcome analysis. Finally, it is a limitation of this trial that plasma concentrations of PEA were not measured; however, a previous report documented the pharmacokinetics and bioavailability of the Levagen + brand of PEA as well as the standard PEA formulation in humans (24). Elevations in plasma PEA were evident at 30 min postdose and remained elevated for at least 4 h postdose for both preparations, and bioavailability was elevated nearly 75% for the Levagen + PEA formulation compared with the standard PEA formulation (24).
Though inflammatory mechanisms are crucial to an optimal immune response, unchecked secretion of cytokines and thromboinflammatory markers can promote the development of the inflammatory response in unresolved disease states and is implicated in COVID-19 complications. Therefore, the reduction in inflammatory markers noted herein suggests that PEA may exert anti-inflammatory actions and possibly reduce the severity of COVID-19. P-selectin and inflammatory cytokines are also elevated in many chronic conditions linked to inflammation, including obesity, atherosclerosis, asthma, and cancer; hence, Levagen + administration may offer a degree of relief from inflammatory symptoms for many chronic conditions.
Acknowledgments
The authors’ responsibilities were as follows—LL, YC, CSJ: designed research; SNF, TY: conducted research; SNF, LL: analyzed data; SNF, CSJ: wrote the paper; SNF: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Footnotes
This research was funded by Gencor Pacific Ltd.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figure 1 and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn.
SUPPORTING INFORMATION
nxac154_Supplemental_File
References
- 1.Zhao Y, Forst CV, Sayegh CE, Wang IM, Yang X, Zhang B. Molecular and genetic inflammation networks in major human diseases. Mol Biosyst. 2016;12(8):2318–2341. doi: 10.1039/c6mb00240d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amaral-Machado L, Oliveira WN, Rodrigues VM, Albuquerque NA, Alencar ÉN, Egito EST. Could natural products modulate early inflammatory responses, preventing acute respiratory distress syndrome in COVID-19-confirmed patients? Biomed Pharmacother. 2021;134:111143. doi: 10.1016/j.biopha.2020.111143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nilea SH, Nilea A, Qiua J, Lib L, Jiac X, Kaia G. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;54:66–70. doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Q, Xiang R, Huo S, Zhou Y, Jiang S, Wang Q, et al. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal Transduct Target Ther. 2021;6(1):233. doi: 10.1038/s41392-021-00653-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. 2022;23(1):3–20. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banu N, Panikar SS, Leal LR, Leal AR. Protective role of ACE2 and its downregulation in SARS-CoV-2 infection leading to macrophage activation syndrome: therapeutic implications. Life Sci. 2020;256:117905. doi: 10.1016/j.lfs.2020.117905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee C, Choi WJ. Overview of COVID-19 inflammatory pathogenesis from the therapeutic perspective. Arch Pharmacal Res. 2021;44(1):99–116. doi: 10.1007/s12272-020-01301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sallenave JM, Guillot L. Innate immune signaling and proteolytic pathways in the resolution or exacerbation of SARS-CoV-2 in COVID-19: key therapeutic targets? Front Immunol. 2020;11:1229. doi: 10.3389/fimmu.2020.01229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenyves BG, Mehta A, MGH COVID-19 Collection & Processing Team. Kays KR, Beakes C, Margolin J, Goldberg MB, et al. Plasma P-selectin is an early marker of thromboembolism in COVID-19. Am J Hematol. 2021;96(12):E468–E471. doi: 10.1002/ajh.26372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 14.Carvalho T, Krammer F, Iwasaki A. The first 12 months of COVID-19: a timeline of immunological insights. Nat Rev Immunol. 2021;21(4):245–256. doi: 10.1038/s41577-021-00522-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phetsouphanh C, Darley DR, Wilson DB, Howe A, Munier CML, Patel SK, et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol. 2022;23(2):210–216. doi: 10.1038/s41590-021-01113-x. [DOI] [PubMed] [Google Scholar]
- 16.Keppel Hesselink JM, De Boer T, Witkamp RF. Palmitoylethanolamide: a natural body-own anti-inflammatory agent, effective and safe against influenza and common cold. Int J Inflam. 2013;2013:1–8. doi: 10.1155/2013/151028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrosino S, Di Marzo V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br J Pharmacol. 2017;174(11):1349–1365. doi: 10.1111/bph.13580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, et al. The nuclear receptor peroxisome proliferator-activated receptor-α mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005;67(1):15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- 19.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2(10):748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 20.Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, et al. The nuclear receptor peroxisome proliferator-activated receptor-α mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005;67(1):15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- 21.Rankin L, Fowler CJ. The basal pharmacology of palmitoylethanolamide. Int J Mol Sci. 2020;21(21):7942. doi: 10.3390/ijms21217942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gigante A, Aquili A, Farinelli L, Caraffa A, Ronconi G, Enrica Gallenga C, et al. Sodium chromo-glycate and palmitoylethanolamide: a possible strategy to treat mast cell-induced lung inflammation in COVID-19. Med Hypotheses. 2020;143:109856. doi: 10.1016/j.mehy.2020.109856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noce A, Albanese M, Marrone G, Di Lauro M, Zaitseva AP, Palazzetti D, et al. Ultramicronized palmitoylethanolamide (um-PEA): a new possible adjuvant treatment in COVID-19 patients. Pharmaceuticals. 2021;14(4):336. doi: 10.3390/ph14040336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Briskey D, Mallard AR, Rao A. Increased absorption of palmitoylethanolamide using a novel dispersion technology system (LipiSperse) study design and procedures. J nutraceuticals Food Sci. 2020;5(2):3. [Google Scholar]
- 25.Remy MM, Alfter M, Chiem MN, Barbani MT, Engler OB, Suter-Riniker F. Effective chemical virus inactivation of patient serum compatible with accurate serodiagnosis of infections. Clin Microbiol Infect. 2019;25(7):907.e7–907.e12. doi: 10.1016/j.cmi.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farsi F, Heshmati J, Keshtkar A, Irandoost P, Alamdari NM, Akbari A, et al. Can coenzyme Q10 supplementation effectively reduce human tumor necrosis factor-α and interleukin-6 levels in chronic inflammatory diseases? A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2019;148:104290. doi: 10.1016/j.phrs.2019.104290. [DOI] [PubMed] [Google Scholar]
- 27.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- 28.Wang HB, Wang JT, Zhang L, Geng ZH, Xu WL, Xu T, et al. P-selectin primes leukocyte integrin activation during inflammation. Nat Immunol. 2007;8(8):882–892. doi: 10.1038/ni1491. [DOI] [PubMed] [Google Scholar]
- 29.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74(3):541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 30.Ivanov II, Apta BHR, Bonna AM, Harper MT. Platelet P-selectin triggers rapid surface exposure of tissue factor in monocytes. Sci Rep. 2019;9(1):13397. doi: 10.1038/s41598-019-49635-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nomura S, Kanazawa S, Fukuhara S. Effects of efonidipine on platelet and monocyte activation markers in hypertensive patients with and without type 2 diabetes mellitus. J Hum Hypertens. 2002;16(8):539–547. doi: 10.1038/sj.jhh.1001447. [DOI] [PubMed] [Google Scholar]
- 32.Riondino S, Pignatelli P, Pulcinelli FM, Lenti L, Di Veroli C, Marigliano V, et al. Platelet hyperactivity in hypertensive older patients is controlled by lowering blood pressure. J Am Geriatr Soc. 1999;47(8):943–947. doi: 10.1111/j.1532-5415.1999.tb01288.x. [DOI] [PubMed] [Google Scholar]
- 33.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Litvinov RI, Evtugina NG, Peshkova AD, Safiullina SI, Andrianova IA, Khabirova AI, et al. Altered platelet and coagulation function in moderate-to-severe COVID-19. Sci Rep. 2021;11(1):16290. doi: 10.1038/s41598-021-95397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy JH, Iba T, Gardiner EE. Endothelial injury in COVID-19 and acute infections: putting the pieces of the puzzle together. Arterioscler Thromb Vasc Biol. 2021;41(5):1774–1776. doi: 10.1161/ATVBAHA.121.316101. [DOI] [PubMed] [Google Scholar]
- 36.Manne BK, Denorme F, Middleton EA, Portier I, Rowley JW, Stubben C, et al. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136(11):1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finsterbusch M, Schrottmaier WC, Kral-Pointner JB, Salzmann M, Assinger A. Measuring and interpreting platelet-leukocyte aggregates. Platelets. 2018;29(7):677–685. doi: 10.1080/09537104.2018.1430358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karsli E, Sabirli R, Altintas E, Canacik O, Sabirli GT, Kaymaz B, et al. Soluble P-selectin as a potential diagnostic and prognostic biomarker for COVID-19 disease: a case-control study. Life Sci. 2021;277:119634. doi: 10.1016/j.lfs.2021.119634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furie B, Furie BC. Role of platelet P-selectin and microparticle PSGL-1 in thrombus formation. Trends Mol Med. 2004;10(4):171–178. doi: 10.1016/j.molmed.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Petito E, Falcinelli E, Paliani U, Cesari E, Vaudo G, Sebastiano M, et al. Association of neutrophil activation, more than platelet activation, with thrombotic complications in coronavirus disease 2019. J Infect Dis. 2021;223(6):933–944. doi: 10.1093/infdis/jiaa756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Man Y, Goreke U, Kucukal E, Hill A, An R, Liu S, et al. Leukocyte adhesion to P-selectin and the inhibitory role of crizanlizumab in sickle cell disease: a standardized microfluidic assessment. Blood Cells Mol Dis. 2020;83:102424. doi: 10.1016/j.bcmd.2020.102424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Xiang D, Gao F, Yao H, Ye Q, Wang Y. The inhibition of P-Selectin reduced severe acute lung injury in immunocompromised mice. Oxid Med Cell Long. 2020;2020:1–13. doi: 10.1155/2020/8430465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Perlman S. COVID-19: inflammatory profile. Annu Rev Med. 2022;73(1):65–80. doi: 10.1146/annurev-med-042220-012417. [DOI] [PubMed] [Google Scholar]
- 44.Hariharan A, Hakeem AR, Radhakrishnan S, Reddy MS, Rela M. The role and therapeutic potential of NF-kappa-B pathway in severe COVID-19 patients. Inflammopharmacology. 2021;29(1):91–100. doi: 10.1007/s10787-020-00773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Paola R, Cordaro M, Crupi R, Siracusa R, Campolo M, Bruschetta G, et al. Protective effects of ultramicronized palmitoylethanolamide (PEA-um) in myocardial ischaemia and reperfusion injury in vivo. Shock. 2016;46(2):202–213. doi: 10.1097/SHK.0000000000000578. [DOI] [PubMed] [Google Scholar]
- 46.Di Paola R, Impellizzeri D, Mondello P, Velardi E, Aloisi C, Cappellani A, et al. Palmitoylethanolamide reduces early renal dysfunction and injury caused by experimental ischemia and reperfusion in mice. Shock. 2012;38(4):356–366. doi: 10.1097/SHK.0b013e318267bbb9. [DOI] [PubMed] [Google Scholar]
- 47.Del Re A, Corpetti C, Pesce M, Seguella L, Steardo L, Palenca I, et al. Ultramicronized palmitoylethanolamide inhibits NLRP3 inflammasome expression and pro-inflammatory response activated by SARS-CoV-2 spike protein in cultured murine alveolar macrophages. Metabolites. 2021;11(9):592. doi: 10.3390/metabo11090592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peritore AF, D’amico R, Siracusa R, Cordaro M, Fusco R, Gugliandolo E, et al. Management of acute lung injury: palmitoylethanolamide as a new approach. Int J Mol Sci. 2021;22(11):5533. doi: 10.3390/ijms22115533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao Y, Yuan Z, Liu Y, Xue J, Tian Y, Liu W, et al. Activation of cannabinoid CB2 receptor ameliorates atherosclerosis associated with suppression of adhesion molecules. J Cardiovasc Pharmacol. 2010;55(3):292–298. doi: 10.1097/FJC.0b013e3181d2644d. [DOI] [PubMed] [Google Scholar]
- 50.Musella A, Fresegna D, Rizzo FR, Gentile A, Bullitta S, De Vito F, et al. A novel crosstalk within the endocannabinoid system controls GABA transmission in the striatum. Sci Rep. 2017;7(1):7363. doi: 10.1038/s41598-017-07519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rockwell CE, Snider NT, Thompson JT, Vanden Heuvel JP, Kaminski NE. Interleukin-2 suppression by 2-arachidonyl glycerol is mediated through peroxisome proliferator-activated receptor γ independently of cannabinoid receptors 1 and 2. Mol Pharmacol. 2006;70(1):101–111. doi: 10.1124/mol.105.019117. [DOI] [PubMed] [Google Scholar]
- 52.Petrosino S, Schiano Moriello A, Cerrato S, Fusco M, Puigdemont A, De Petrocellis L, et al. The anti-inflammatory mediator palmitoylethanolamide enhances the levels of 2-arachidonoyl-glycerol and potentiates its actions at TRPV1 cation channels. Br J Pharmacol. 2016;173(7):1154–1162. doi: 10.1111/bph.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pretorius E, Vlok M, Venter C, Bezuidenhout JA, Laubscher GJ, Steenkamp J, et al. Persistent clotting protein pathology in long COVID/post-acute sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol. 2021;20(1):172. doi: 10.1186/s12933-021-01359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
nxac154_Supplemental_File