Abstract
Longitudinal virological and serological surveillance is essential for understanding severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) transmission among children but requires increased test capacity. We assessed the uptake of serial at-home testing in children (2–17 years) via mailed SARS-CoV-2 antibody and molecular tests. Completion rates demonstrated the feasibility and sustainability of at-home testing across age groups.
Keywords: COVID-19, SARS-CoV-2, feasibility, serological surveillance, virological surveillance
As the coronavirus disease 2019 (COVID-19) pandemic evolves, population-based longitudinal virological and serological surveillance is critical to understanding transmission among children and informing public health measures. Although virological surveillance is needed for detection of acute severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) infection, serological surveillance provides a more accurate estimate of cumulative incidence by tracking infections over time and capturing missed infections. The latter is especially important in children, a substantial portion of whom are mildly symptomatic or asymptomatic and may not undergo virological testing [1].
Pediatric seroprevalence studies are advancing our understanding of the dynamics of SARS-CoV-2 infections, but longitudinal data are lacking due to cross-sectional design and reliance on residual serum samples [2, 3]. Increasing test capacity to capture such data can be accomplished by at-home testing, with its advantages of convenience, reduced health care costs, and lower infection prevention risks. To our knowledge, the feasibility of serial at-home testing in children, including parents’ willingness and ability to obtain specimens, has not been thoroughly examined.
We describe serial at-home virological and serological testing in children conducted as part of a syndromic surveillance study. Our objective is to demonstrate the acceptability and feasibility of such testing to support future efforts at longitudinal surveillance of SARS-CoV-2 in children.
METHODS
The COVID-19 Community Research Partnership (CCRP) is a multisite prospective study combining electronic symptom surveillance with at-home testing in adults and children [4]. This analysis includes children 2–17 years old enrolled at a large North Carolina health care system from April 2 to June 24, 2021. The study was approved by a centralized institutional review board.
Parents/guardians of participants consented to symptom surveillance alone or combined with at-home serological and virological testing; adolescents 13 years and older provided assent. Daily electronic surveys administered by Oracle Corporation (Redwood, CA, USA) solicited symptoms of COVID-19-like illness, infection with or exposure to SARS-CoV-2, and receipt of COVID-19 vaccines.
Participants were shipped up to 8 buccal swab viral tests with written and video instructions. Specimens were collected monthly or with illness or exposure to SARS-CoV-2 and mailed for testing by quantitative reverse transcription polymerase chain reaction (PCR; Integrated DNA Technologies, Coralville, IA, USA) targeting SARS-CoV-2 N1, RNase P, and ORF1ab (IP2). On the accompanying requisition, parents/guardians indicated whether the child was symptomatic at the time of collection. Cycle thresholds <35 were classified as positive. The sensitivity and specificity for N1 were 96.5%/100%, 100%/100% for RNase P, and 100%/100% for IP2 in clinical samples. Specimens were considered invalid if they were damaged during shipping or if the control target was negative, and participants were instructed to submit another specimen. Participants were shipped at least 4 serology tests with written instructions. Participants downloaded a smartphone application for video instructions on use of the immunoglobulin (Ig)M/IgG rapid lateral flow immunoassay (Innovita Biological Technology, Beijing, China) detecting both (but not differentiating between) nucleocapsid and spike proteins. Finger-prick blood was placed into a test cassette, and an image of the result was uploaded to the app. Sensitivity and specificity (IgG) were 84.5% and 99.0%, respectively. Results were considered invalid if the internal control line was absent or there was sample streaking on the test strip, and participants were instructed to complete another test. Participants received results via email or app within 2–5 days for viral results and 24–48 hours for antibody results. Serology testing was completed by October 31, virology testing by November 30, and syndromic surveillance by December 31, 2021.
Testing retention was supported by email reminders to participants who did not submit monthly tests. Participants had e-mail and telephone access to a call center for troubleshooting. Retention was also encouraged by inviting participants to periodic virtual townhalls to share data and solicit feedback.
As the serologic test used did not differentiate between antispike and antinucleocapsid antibodies, we analyzed seroprevalence in unvaccinated participants only. Seropositive was defined as a positive IgG on the most recent test result available for a participant unvaccinated at the time of testing. We calculated descriptive statistics for the sample and utilized the Fisher exact test to assess proportional differences in number of at-home tests returned by age and vaccination status with Bonferroni post hoc tests to adjust for multiple comparisons. P values <.05 were considered statistically significant; all tests were run in SPSS, version 27.
RESULTS
Of 1501 children enrolled in syndromic surveillance, 1412 (94.1%) consented to testing; 918 (65%) returned ≥1 valid virology specimen, and 745 (52.8%) returned ≥1 valid antibody test (Table 1). Among 628 children participating in both types of testing, 237 completed ≥1 of each test, 136 completed ≥2, 113 completed ≥3, and 142 completed ≥4 of each type of test. Of 2962 virology tests and 2038 serology tests submitted, only 38 (1.3%) and 26 (1.3%), respectively, were invalid; all but 2 virology and 4 serology tests were repeated.
Table 1.
Frequencies of SARS-CoV-2 Serology and Virology Tests Completed
| … | Serology Tests | Virology Tests | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tests Completed | Tests Completed | |||||||||
| No. | 1, No. (%) | 2, No. (%) | 3, No. (%) | ≥4, No. (%) | No. | 1, No. (%) | 2, No. (%) | 3, No. (%) | ≥4, No. (%) | |
| Total | 745 | 246 (33.0) | 139 (18.7) | 116 (15.6) | 244 (32.8) | 918 | 189 (20.6) | 179 (19.5) | 182 (19.8) | 368 (40.1) |
| Age | ||||||||||
| 2–4 y | 100 | 39 (39.0) | 14 (14.0) | 16 (16.0) | 31 (31.0) | 145 | 34 (23.5) | 34 (23.5) | 19 (13.1) | 58 (40.0) |
| 5–11 y | 332 | 116 (34.9) | 60 (18.1) | 49 (14.8) | 107 (32.2) | 414 | 74 (17.9) | 77 (18.6) | 94 (22.7) | 169 (40.8) |
| 12–17 y | 313 | 91 (29.1) | 65 (20.8) | 51 (16.3) | 106 (33.9) | 359 | 81 (22.6) | 68 (18.9) | 69 (19.2) | 141 (39.3) |
| Sex at birth | ||||||||||
| Female | 385 | 122 (31.7) | 62 (16.1) | 69 (17.9) | 132 (34.3) | 475 | 100 (21.1) | 86 (18.1) | 105 (22.1) | 184 (38.7) |
| Race | ||||||||||
| White | 611 | 190 (31.1) | 113 (18.5) | 96 (15.7) | 212 (34.7) | 757 | 149 (19.7) | 143 (18.9) | 153 (20.2) | 312 (41.2) |
| Black | 59 | 24 (40.7) | 14 (23.7) | 8 (13.6) | 13 (22.0) | 60 | 13 (21.7) | 14 (23.3) | 10 (16.7) | 23 (38.3) |
| Native Hawaiian/Pacific Islander | 3 | 0 (0.0) | 1 (33.3) | 0 (0.0) | 2 (66.8) | 3 | 0 (0.0) | 0 (0.0) | 1 (33.3) | 2 (66.7) |
| American Indian/Alaska Native | 2 | 0 (0.0) | 1 (50.0) | 1 (50.0) | 0 (0.0) | 3 | 2 (66.7) | 1 (33.3) | 0 (0.0) | 0 (0.0) |
| Asian | 25 | 11 (44.0) | 2 (8.0) | 6 (24.0) | 6 (24.0) | 36 | 9 (25.0) | 9 (25.0) | 4 (11.1) | 14 (38.9) |
| Other | 29 | 11 (37.9) | 6 (20.7) | 5 (17.3) | 7 (24.1) | 39 | 11 (28.2) | 8 (20.5) | 7 (18.0) | 13 (33.3) |
| Ethnicity | ||||||||||
| Hispanic/Latino | 49 | 19 (38.8) | 6 (12.2) | 11 (22.5) | 13 (26.5) | 51 | 9 (17.7) | 7 (13.7) | 13 (25.5) | 22 (43.1) |
| Not Hispanic/Latino | 679 | 221 (32.5) | 129 (19.0) | 105 (15.5) | 224 (33.0) | 852 | 178 (20.9) | 168 (19.7) | 163 (19.1) | 343 (40.3) |
No. refers to the total number of participants completing either serology or virology tests; No. (%) refers to the number of participants who completed either 1, 2, 3, or ≥4 tests. All column proportion differences were nonsignificant.
Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The median age (interquartile range) of participants completing ≥1 test (viral and/or antibody) was 10 (7–14) years. The majority of participants completing either a virology (82.5%) or serology (82.0%) test were non-Hispanic White. The proportion of those returning ≥1 test who were 2 to 4 years of age was significantly lower than the proportion of those age 5–11 years or 12–17 years (P < .01) (Table 1). The proportions completing multiple viral or antibody tests were not significantly different than those completing only 1, regardless of age category, race, or ethnicity. Of 947 participants providing household demographics, 56.3% reported living with a health care worker. The proportion of participants completing tests did not differ by presence of a health care worker in the household, regardless of age group.
Only 5 of 918 participants submitting virology specimens tested positive by PCR. Of the requisitions submitted with 2924 valid virology specimens, only 204 (7%) indicated symptomatology at the time of collection; 1433 (49%) indicated no symptoms, and 1286 (44%) remained uncompleted. As of June 15, a total of 46 (18.6%) of 248 unvaccinated children were seropositive. Stratified by age, 10.8% (4/37) of 2–4-year-olds, 11.4% (16/141) of 5–11-year-olds, and 37.1% (26/70) of 12–17-year-olds were seropositive. By November 15, when serology testing was completed, a total of 120 (22.9%) of 523 unvaccinated children were seropositive (18.0% [18/100] of 2–4-year-olds, 14.4% [46/319] of 5–11-year-olds, and 53.8% [56/104] of 12–17-year-olds).
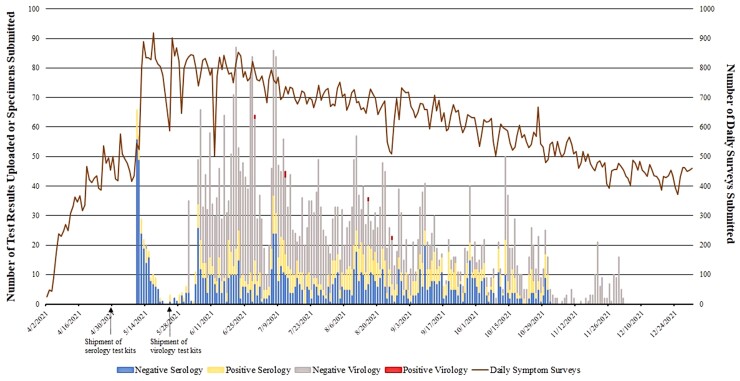
Figure 1 demonstrates the cadence of virology and serology tests submitted during the study. The median percent participation for syndromic surveillance was 48.0%, with peak engagement at 70%; 49 (3.3%) participants withdrew from the study.
Figure 1.
Participation in symptom surveillance and SARS-CoV-2 serology and virology testing over time. Blue bars represent number of negative serology results. Yellow bars represent number of positive serology results. Gray bars represent number of negative virology results. Red bars represent number of positive virology results. The solid brown line represents number of daily symptom surveys completed. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
DISCUSSION
To our knowledge, this is the first study to describe successful uptake of serial at-home virological and serological testing in children and adolescents for the purposes of surveillance. We enrolled >1500 children age 2–17 years, a high proportion of whom remained engaged in syndromic surveillance for the 9-month duration. Of those consenting to testing, 65% participated in virology and 53% in serology testing. Children age 2–4 years were less likely to participate in testing than older children, but once engaged in testing, the proportion submitting multiple virology or serology tests was similar to the proportion submitting only 1, regardless of age group, race, or ethnicity (Table 1). The findings demonstrate the feasibility and sustainability of home testing by parents/guardians using not only swabs for saliva collection but also finger-prick blood collection in children as young as 2 years of age. The ability to conduct such serial testing will facilitate longitudinal surveillance during the ongoing COVID-19 pandemic.
Prior studies utilizing viral testing to understand SARS-CoV-2 transmission in children have relied on specimen collection by health care personnel, which necessitates resources and infection prevention measures [5, 6]. With the US Food and Drug Administration's (FDA’s) Emergency Use Authorization (EUA) of over-the-counter SARS-CoV-2 antigen tests, viral testing is moving into the home and school [7, 8, 9]. Although currently used for risk reduction measures, home testing is being evaluated for surveillance. The “Say Yes! COVID Test” public health intervention, for example, is exploring implementation of large-scale at-home antigen testing in North Carolina [10]. The tests are user-friendly, cost-effective, and safe, but there is a paucity of data on acceptability and uptake, especially in children. Using written and video instructions, we found that at-home saliva collection was easily implemented and sustained. Only a few participants tested positive, which, based on test performance and the low proportion reporting symptoms at the time of collection, likely reflects testing not restricted to symptomatic children.
Seroprevalence surveys highlight the underdetection of SARS-CoV-2 infections in US children based on viral testing alone, with ≥50% of serologically identified infections being asymptomatic [2, 6]. With new variants emerging and vaccinations available to children, longitudinal seroprevalence data are needed to provide accurate estimates of infection rates, immunity, and at-risk populations to better inform public health measures. The Ciao Corona study followed seroprevalence in Swiss schools to identify clustering and evaluate the effects of mitigation measures [11]. Only 2 time points were evaluated, attributed in part to anxiety provoked by venous sampling in schools. Similar anxiety has been described in other studies assessing the feasibility of self-administered finger-prick testing [12, 13] and likely explains why a substantial portion of our participants consenting to testing did not complete a test. However, for those who did participate in testing, the home environment and parental involvement, support provided by study personnel through the call center, and rapid access to results likely sustained participation, which allowed for longitudinal serosurveillance. To our knowledge, the only study to use at-home serological testing in children is the Etude de Seroprevalence (EnCORE), targeting schools and daycares in Montreal [14]. Unlike our study, only a single blood specimen was submitted per participant, with a mean seroprevalence of 5.8%.
There were a few limitations to our study. Minorities were disproportionately underrepresented, underscoring the importance of recruitment strategies to improve representation. However, sustainability of testing was similar regardless of race or ethnicity. Neither the viral test nor the antibody test used had FDA EUA, but no at-home test had EUA for children at the time; thus, test results must be interpreted with caution. However, the focus of this study is not diagnostic accuracy but rather the feasibility of home testing. Presence of a health care worker in the households did not impact testing participation but may have enhanced overall engagement. Lastly, a cost-effectiveness analysis of home testing compared with testing in health care settings was not part of the design of this study but would be valuable in assessing the role of home testing in future longitudinal SARS-CoV-2 surveillance studies.
Our study demonstrates sustained participation in serial at-home testing by children as part of a surveillance study. Families were able to not only collect swabs for viral testing but also blood, the latter supporting serological surveillance to identify the high proportion of missed infections in children. Whether used for research or as a public health intervention, longitudinal surveillance is crucial to understanding the COVID-19 pandemic trajectory in children.
Acknowledgments
We would like to acknowledge Atrium Health Myers Park Pediatric Clinic, Our Lady of Guadalupe Church, Compare Foods, and the participants of the COVID-19 Community Research Partnership. We would also like to acknowledge Michael Dewitt for assistance with data analysis.
Financial support. This work was supported by the Coronavirus Aid, Relief, and Economic Security Act of the US Department of Health and Human Services (HHS; North Carolina Department of Health and Human Services Contract #49927).
Author contributions. Dr. Ahmed contributed to the conceptualization/design, methodology, investigation, supervision, and resources for the study and drafted the initial manuscript. Dr. Dantuluri contributed to the conceptualization/design, investigation, and supervision for the study. W. Rossman contributed to the conceptualization/design, data curation, and resources of the study and drafted the initial manuscript. L. Lu and A. Harris contributed to the conceptualization/design, investigation, supervision, and resources for the study. C. Dunn contributed to the methodology, data curation, and resources of the study. Dr. Priem contributed to the methodology, investigation, and formal analysis of the study and drafted the initial manuscript. A. Porzucek contributed to the conceptualization/design, methodology, investigation, and data curation for the study. Dr. Mores contributed to the methodology, investigation, and resources for the study. T. Hetherington contributed to supervision and data curation for the study. Dr. Castri contributed to the investigation, supervision, and resources for the study. Dr. Lagarde contributed to the conceptualization/design, investigation, supervision, and resources for the study. All authors contributed to review/editing of the manuscript.
Ethical approval. The study was approved by the Atrium Health Wake Forest Baptist Health Institutional Review Board.
Patient consent. Consent was obtained from parents/guardians and assent from participants 13 years of age and older.
Clinical trial registration. The COVID-19 Community Research Partnership is listed on clinicaltrials.gov (NCT04342884).
Contributor Information
Amina Ahmed, Department of Pediatrics (Infectious Diseases) at Levine Children's Hospital, Atrium Health, Charlotte, North Carolina, USA.
Whitney Rossman, Center for Outcomes Research and Evaluation, Atrium Health, Charlotte, North Carolina, USA.
Lauren C Lu, Center for Outcomes Research and Evaluation, Atrium Health, Charlotte, North Carolina, USA.
Connell O Dunn, Department of Emergency Medicine Research, Atrium Health, Charlotte, North Carolina, USA.
Anna M Harris, Center for Outcomes Research and Evaluation, Atrium Health, Charlotte, North Carolina, USA.
Jennifer S Priem, Center for Outcomes Research and Evaluation, Atrium Health, Charlotte, North Carolina, USA.
Timothy C Hetherington, Center for Outcomes Research and Evaluation, Atrium Health, Charlotte, North Carolina, USA.
Abigail J Porzucek, Milken Institute School of Public Health, The George Washington University, Washington, DC, USA.
Christopher N Mores, Milken Institute School of Public Health, The George Washington University, Washington, DC, USA.
Paola Castri, Atrium Health Wake Forest Baptist Health, Winston Salem, North Carolina, USA.
William H Lagarde, Wake Med Health and Hospitals, Raleigh, North Carolina, USA.
Keerti L Dantuluri, Department of Pediatrics (Infectious Diseases) at Levine Children's Hospital, Atrium Health, Charlotte, North Carolina, USA.
References
- 1. Waterfield T, Watson C, Moore R, et al. . Seroprevalence of SARS-CoV-2 antibodies in children: a prospective multicentre cohort study. Arch Dis Child 2021; 106:680–6. [DOI] [PubMed] [Google Scholar]
- 2. Levorson RE, Christian E, Hunter B, et al. . A cross-sectional investigation of SARS-CoV-2 seroprevalence and associated risk factors in children and adolescents in the United States. PLoS One 2021; 16:e0259823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hobbs CV, Drobeniuc J, Kittle T, et al. . Estimated SARS-CoV-2 seroprevalence among persons aged <18 years—Mississippi, May-September 2020. MMWR Morb Mortal Wkly Rep 2021; 70:312–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. COVID-19 Community Research Partnership Study Group . Duration of SARS-CoV-2 sero-positivity in a large longitudinal sero-surveillance cohort: the COVID-19 Community Research Partnership. BMC Infect Dis 2021; 21:889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tönshoff B, Müller B, Elling R, et al. . Prevalence of SARS-CoV-2 infection in children and their parents in Southwest Germany. JAMA Pediatr 2021; 175:586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lugon P, Fuller T, Damasceno L, et al. . SARS-CoV-2 infection dynamics in children and household contacts in a slum in Rio de Janeiro. Pediatrics 2021; 148:e20211050182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rubin R. COVID-19 testing moves out of the clinic and into the home. JAMA 2021; 326:1362–4. [DOI] [PubMed] [Google Scholar]
- 8. Harris-McCoy K, Lee VC, Munna C, Kim AA. Evaluation of a test to stay strategy in transitional kindergarten through grade 12 schools—Los Angeles County, California, August 16-October 31, 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1773–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campbell MM, Benjamin DK, Mann T, et al. . Test-to-stay after exposure to SARS-CoV-2 in K-12 schools. Pediatrics 2022; 149:e2021056045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ciccone EJ, Conserve DF, Dave G, et al. . At-home testing to mitigate community transmission of SARS-CoV-2: protocol for a public health intervention with a nested prospective cohort study. BMC Public Health 2021; 21:2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ulyte A, Radtke T, Abela IA, et al. . Clustering and longitudinal change in SARS-CoV-2 seroprevalence in school children in the canton of Zurich, Switzerland: prospective cohort study of 55 schools. BMJ 2021; 372:n616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pichler J, Zilbauer M, Torrente F, Heuschkel R, Phillips A, Salvestrini C. Feasibility of a finger prick-based self-testing kit in first- and second-degree relatives of children with coeliac disease. World J Gastroenterol 2011; 17:1840–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shlomowitz A, Feher MD. Anxiety associated with self monitoring of capillary blood glucose. Br J Diabetes Vasc Dis 2014; 14:60–3. [Google Scholar]
- 14. Zinszer K, McKinnon B, Bourque N, et al. . Seroprevalence of SARS-CoV-2 antibodies among children in school and day care in Montreal. Canada. JAMA Netw Open 2021; 4:e2135975. [DOI] [PMC free article] [PubMed] [Google Scholar]