Abstract
Background
During the coronavirus disease 2019 pandemic, a nucleic acid test is frequently conducted to identify positive cases. Compared with a hospital-based strategy, whole-community nucleic acid testing displays a unique advantage in rapid screening of a massive population. Yet a management plan to ensure ample and contamination-free sample collection is lacking.
The objective of the current study was to establish an efficient operational mode of whole-community nucleic acid testing by management of a sample collection team and to provide a reference for joint prevention work to contain the spread of severe acute respiratory syndrome coronavirus 2.
Methods
The efficient operation of nucleic acid testing within the community was implemented by urgent setting up of sample collection teams, efficient allocation of medical supplies, optimization of management procedures and coordination among multiple working departments.
Results
A total of 21 585 nucleic acid samples were collected within 3 d, while no one was missed or experienced a cross infection. No falls, heatstroke, disputes or other adverse events occurred.
Conclusions
Under the emergency setting of nucleic acid testing of a large population, a management system with orderly organization, clear division of responsibilities and standardized operational procedures should be formulated.
Keywords: management system, nucleic acid test, sample collection
Introduction
The Delta variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Delta, B.1.617.2), which circulates in over 130 countries and regions, constitutes the major strain of the current coronavirus disease 2019 (COVID-19) pandemic.1 The Delta variant exhibits the characteristics of enhanced transmissibility, shortened latency or passage interval and high viral load. Upon infection, the probability of patients progressing to severe cases is higher, accompanying an earlier disease onset.2,3 Because of the large numbers of people affected and the various source areas of imported cases, epidemic prevention and control have become much more difficult and complicated.4
Nucleic acid testing is essential to achieve the goal of ‘early detection, early isolation, early diagnosis and early treatment’, thereby preventing the virus from widespread dissemination. Noteworthy, the current operational procedures proposed by the WHO SARS-CoV-2 guidelines and others mainly focus on how to carry out nucleic testing in professional medical institutes (e.g. hospitals). However, because of the limited number of medical institutions, it is necessary for medical staff to go to different communities for nucleic acid collection, and for a qualified third-party company to undertake the testing. Wuhan responded quickly and conducted whole-society nucleic acid testing when facing up to the new round of infectious events.5 On the frontline of the testing task, emergency sample collection teams need to be informationized, possess strong mobility, be equipped with basic medical modules and receive the necessary logistical support.6 With the authorization of the Wuhan Municipal Health Commission, emergency sample collection teams can also be dispatched into underdeveloped districts where the medical resources are insufficient.7 In such a context, a management plan to ensure efficient and contamination-free sample collection is required. To this end, we summarize the detailed operational mode of our sample collection team under the setting of whole-community SARS-CoV-2 nucleic acid testing.
Materials and Methods
General information
On 3 August 2021, Wuhan Municipal Government and the Health Commission requested that the emergency sample collection team of Union Hospital take charge of the nucleic acid sample collection work in Jiang-han District. The focus of collection was residents living in Hua An-li Community (Han-xing Street) who could cooperate with the collection of specimens from the upper respiratory tract. Nucleic acid screening of the entire community was completed within 3 d. A total of 4 collection sites, 9 collection stations, 53 nurses (6 males and 47 females) and 5 management personnel were engaged. The nurses included 1 director nurse, 1 deputy director nurse, 15 supervisor nurses, 21 senior nurses and 20 nurses, all with bachelor degrees. The ages of the nurses ranged from 23 to 54 (28.88±6.35) y. All the team members accepted vaccination against COVID-19 and were experienced in nucleic acid collection.
Setting up the nucleic acid sample collection team
When new Delta variant-related COVID-19 cases emerged, Wuhan Union Hospital responded quickly by establishing an emergency group that was composed of staff members from the medical, nursing, infectious disease management, human resources, medical apparatus and general affairs departments and the computer network center. Team members had a clear division of labor, performed their respective tasks and kept in close contact with each other, so as to fulfill the purpose of efficient cooperation.8 Specifically, the medical department was responsible for communicating with the government and making the overall arrangements; the nursing department took comprehensive charge of the sample collection work, which included the training of operators and personnel transfer; the infectious disease management department was responsible for technical guidance and inspection of potential infection risk; the computer network center was responsible for the information platform; and the department of medical apparatus department and general affairs department were responsible for the necessary logistics support.
On the other hand, reserve personnel were essential to guarantee high-quality implementation of sample collection work.9 Our emergency sample collection team consisted of 64 people, including 58 clinical nurses, 2 doctors, 2 ambulance and 2 carrier vehicle drivers. All team members were voluntarily engaged and qualified as reserve personnel after carrying out an educational program and passing the examination.10 Meanwhile, attention was paid to the psychological state of team members and psychological support was provided to relieve any anxieties or worries they may have been experiencing.11 The emergency team were on 24-h standby ready for departure at any time.
Allocation of materials
With the coordination of all relevant departments, we actively formulated a list of material reserves, sought sources of supply and adjusted the quantity of material reserves in a timely manner to meet the varying demands. In the context of whole-society nucleic acid testing, the ‘green channel’ was opened and medical supplies were dynamically managed on our constructed information platform. Protective masks, goggles, isolation suits and other urgently needed protective supplies were strictly classified and applied in different conditions,12 which not only ensured the regular use of supplies but also avoided material waste. The vehicles involved included a negative-pressure emergency isolation ambulance and a communication command vehicle, as well as others transporting disinfectant, medical and daily supplies. The materials required consisted of (1) basic items such as tents, tables, chairs, power supplies and other necessities; (2) medical supplies, that is, protective masks, surgical masks, isolation masks, disposable surgical caps, protective clothing, disposable shoe covers, medical waste buckets, medical garbage bags and rubber gloves; special sampling swabs, collection tubes, tongue depressors, test tube racks, cold-storage incubators and biological specimen bags; hand sanitizer, disinfectant paper towels, forehead temperature guns, sealing tape and quick hand disinfectant; (3) an information collection system incorporating a computer with an ID card reader or electronic two-dimensional code reader; (4) daily supplies including paper towels, plastic bags and wiring boards; and (5) office supplies such as flashlights, batteries, marker, tape, scissors and long tail folders, etc.
Standards for protective equipment usage
According to the documentation for COVID-19 prevention and control issued by the National Health Commission,13–15 the protection levels and standards for medical staff in different positions were strictly defined.16,17 Thus the protective equipment for sampling personnel consisted of medical masks, face shields and protective clothing, as well as latex and film gloves. The film gloves had to be replaced after each sampling and hands thoroughly disinfected. The protective equipment required for registration personnel included medical masks, latex gloves, surgical caps and working clothes. For specimen-collection personnel, the protective equipment consisted of disposable caps, medical protective masks (N95), gloves (in double layers when necessary), goggles, face shields, isolation clothing and shoe covers (when necessary). The protective equipment for specimen-transport personnel consisted of medical protective masks, latex gloves, working clothes and medical protective caps, while that for sample-detection personnel consisted of medical protective masks (N95), single- or double-layer latex gloves, face shields, goggles, protective clothing and single- or double-layer protective caps.
Personnel allocation and management
A material support team of three members (i.e. one leader and two team members) was responsible for the supply and distribution of materials, equipment and basic facilities. The information assurance team (consisting of seven members: a leader and six team members) was responsible for statistical analysis and providing information support for the sample collection team. The infectious disease prevention and control team consisted of five members, who were responsible for the supervision, personnel training and other related work. The comprehensive support group was made up of four people responsible for team communication and personnel care work. The emergency sample collection team was responsible for nucleic acid sample collection, sample packaging and recording relevant information to ensure that all samples met the quality requirements. Each sampling site scientifically assigned the numbers and rotation of personnel according to the workload. In principle, rotation and rest needed to take place once every 2–4 h. The sample collection team was divided into three groups (groups A, B and C, each containing 17 people) to ensure the implementation of day (07:00–17:00 h) and night (17:00–01:00 h [the next day]) shifts. Medical staff were placed under closed-loop management and were not allowed to gather together. They were required to stay in single rooms and perform hand hygiene after returning to the isolated hotel. Scientific shift arrangement, daily monitoring of fever, cough and diarrhea and the potential occurrence of occupational exposure were carried out.
Formulation of the emergency plan
According to the relevant policy documents,14,18,19 procedures and schemes for the onsite disposal of emergency events were drafted to deal with different possible scenarios.
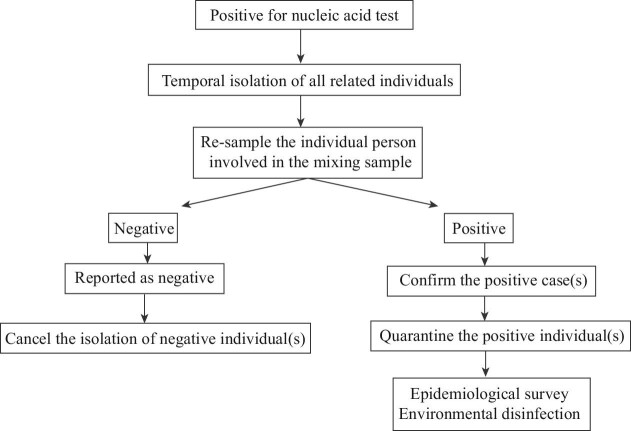
(1) Emergent response to positive nucleic acid test results
If nucleic acid test results were positive → resample the individual person involved in the mixing sample → report to Chinese Center for Disease Control and Prevention to temporarily isolate the related individuals → report to the infectious disease network within 2 h → recheck each single nucleic acid test for the corresponding individual → confirm the positive individual(s) and cancel the isolation for those testing negative → transport the positive case(s) to a designated hospital by negative-pressure ambulance → carry out an epidemiological investigation, local environmental disinfection and track all those people who had close contact with the positive individual(s) (Figure 1).
Figure 1.
Flowchart for the emergent response to positive nucleic acid test results.
(2) Emergency plan for respiratory exposure
If occupational respiratory exposure of medical staff occurs → take measures to protect the respiratory tract (put their hands over the face mask after the implementation of hand hygiene or add another layer of mask) → evacuate from the polluted area → take off the protective equipment → disinfect the oral and/or nasal cavity with 0.1% hydrogen peroxide solution, povidone iodine solution or clean water → leave the sampling site after wearing a surgical mask → report to the hospital managing department in time → organize experts to conduct a risk assessment (i.e. the need for isolated medical observation, preventive medication and psychological counseling, etc.) → high-risk individuals should be treated as contact personnel and placed under medical observation for 14 d (Figure 2).
Figure 2.
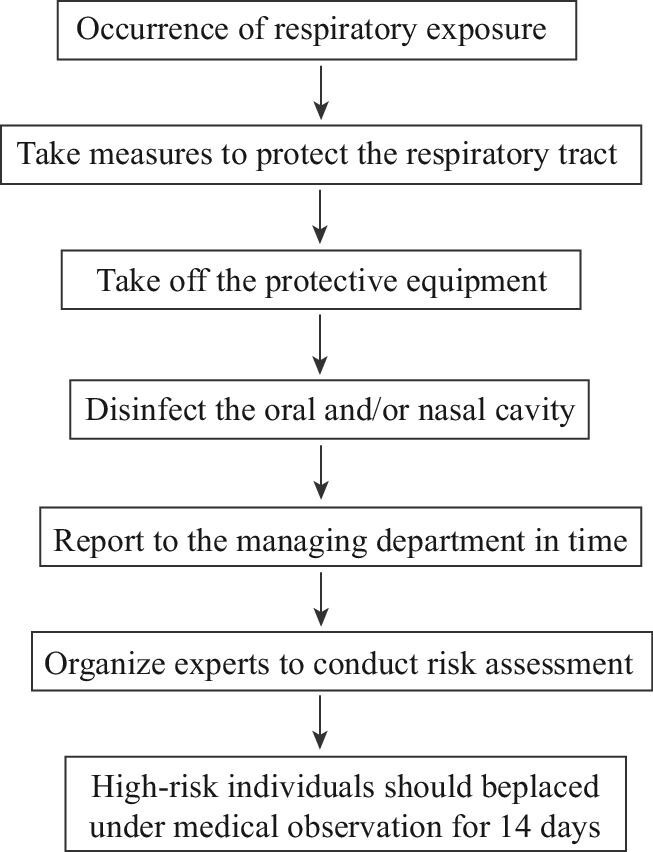
Flowchart for the emergency plan for respiratory exposure.
(3) Emergency plan for a physical accident
If a physical accident occurs→ immediately contact the emergency doctors → assist the injured staff to remove protective clothing and other isolation equipment → transfer the injured staff member to the rest area for observation → if there is no abnormality after the rest period, send the staff member back to the hotel; if discomfort is experienced during the rest period, send the injured staff member to hospital for medical treatment (Figure 3).
Figure 3.
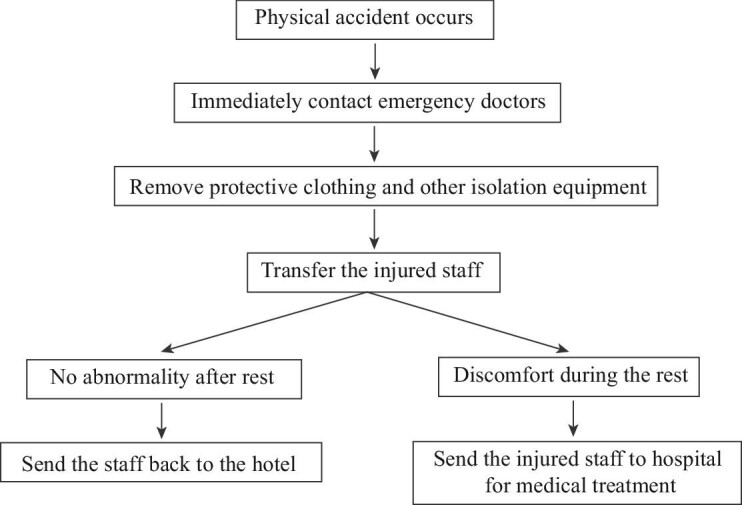
Flowchart for the emergency plan for a physical accident.
Medical waste management
The collection, packaging, handover, transportation, temporary storage and harmless disposal of medical waste were strictly carried out. When the content of medical waste reached three-quarters of the capacity of the packaging bag, the medical waste was contained in double-layered yellow garbage bags, sealed with a gooseneck-knot and sprayed with 1000 mg/L chlorine-containing disinfectant. A label was completed and pasted onto the seal. The label content included details of the producing department, the production date and waste category. Medical waste was handled by local medical institutions with qualified certification. A medical waste management ledger system was established to ensure that the packaging of medical waste was free from damage or leakage and that no medical waste was omitted.
Management of sample collection sites
Nucleic acid sample collection sites were set up following the principles of safety and convenience. Communities were responsible for determining the quantity and distribution of residents, scientifically designing the layout of sample collection sites and creating the timetable and route map for crowd flow. In general, open and well-ventilated sites were selected as the central collection sites for a large-scale population. Each site was divided into a waiting area, a collection area, a buffer zone and a temporary isolation area,14 where first-aid equipment was on standby. According to the weather conditions, facilities were also equipped with items for keeping warm and cool, for providing shade from the sun or shelter from rain. Older people, the disabled, children and pregnant women were given priority during sample collection.
Collection area: tents, fans, desks and chairs were used to ensure that medical staff could work in a relatively comfortable environment. Paper towels, vomit bags, heatstroke medicine and masks, etc., were also prepared.
Buffer zone: this area was closed off, so that sample-collection personnel could place protective equipment, disinfection supplies, swabs, sample collection tubes and outdoor disinfection equipment, etc.
Temporary isolation area: this area was used to temporarily isolate suspected patients or high-risk groups identified during collection.
Management of medical supplies
An adequate supply of sample tubes, throat swabs, consumables, protective equipment and other materials for large-scale nucleic acid testing was ensured. Consumables were stored according to the population of the community. Medical supplies were supplemented by personnel in a timely fashion. Emergency supplies were placed at fixed points, divided, classified and layered with clear identification and convenient access. The consumption of protective equipment and disinfectant and handover of medical waste were carefully recorded to guarantee nucleic acid collection and that medical support operated at capacity.
Establishment of a multidepartment cooperation model
The epidemic prevention and control work required a joint response, solidarity and cooperation across multiple departments. Government departments were responsible for unified arrangement. Hospital, community and the third-party sample-testing institutions fulfilled their respective responsibilities, improved their cooperation and allocated human resources in a scientific and reasonable way.20 Each of the hospital, government, community and third-party testing institutions assigned a single person to the contacting work. Community staff and volunteers were responsible for inputting information before the sample collection started and for ensuring that residents were grouped and tested in an orderly manner. The staff would arrange 10 subjects into 1 group, collect and register the relevant information of each group member and number the collection tubes based on group prior to sample collection. The third-party testing institution was responsible for specimen transport, nucleic acid detection and for ensuring the traceability of sample information.
Results
During the first round of detection, a ‘10 mixed in 1’ (10:1) strategy was adopted to quickly screen out potentially infected people. The emergency sample-collection team from the hospital cooperated with multiple departments, deployed 58 working personnel and collected samples from 21 585 people within 3 d (8 h of working time per day). Based on our experience, one site could be set up for 2000–2500 people, which indicates a maximal detection capacity of around 10 000 people per day by a team of 58. No resident was omitted from sample collection; no medical staff experienced occupational exposure; no falls, heatstroke, disputes or other adverse events occurred. The nucleic acid test results for all medical staff were negative. Our work was well appreciated by the community and serves as an example, showing the unique advantage of community-based nucleic acid testing compared with that conducted in a hospital.
Discussion
Attention should be paid to the differences between sample collection within or outside of a hospital. Nucleic acid collection in the community is characterized by a large collection area, varying environment and complex personnel cooperation.21 Upon receipt of the sampling task, our hospital immediately organized a preparation meeting and drafted an emergency plan to specify the management of human resources and medical supplies. At the same time, we contacted the third-party detection institution and the community to determine the quantity of material reserves, regional sampling sites, sample delivery personnel and vehicle requirements. We also made a comprehensive survey of community members with the aim of formulating a clear timetable and roadmap to complete nucleic acid testing in an efficient manner. On the first day of sample collection, the information input took a long time and the problems related to this were solved by adding information-gathering equipment over time. Therefore, an information platform for sampling, testing, reporting results and sample tracking is essential for sample collection with a large number of people.
Cross infection was another critical issue that had to be addressed. To this end, regulations, workflow and emergency plans for nucleic acid sample collection were formulated to meet practical needs.22 First of all, the training of medical staff was strengthened to reduce occupational exposure and infection risk. The training mainly included hand hygiene, nucleic acid sample collection and the correct wearing of protective clothing, and everyone involved had to pass the test. Also, strictly following the standards for medical protective equipment ensured the safety of the collection personnel; the ratio of sampling staff, supporting personnel and people awaiting sample collection was set at around 1:4:100.14 It was strictly forbidden to have two or more people with their masks off at the same operating table, which could have resulted in cross infection. Sampling was carried out in the upwind direction of the individual being tested. Also, independent waiting areas were set up to ensure a one-way flow of people; a ‘1m line’ interval requirement was implemented and personnel density was strictly controlled. To shorten queuing times, an ID card reader or an electronic two-dimensional code was used to record information. No more than 10 people were allowed to enter each sampling unit in the sampling area at one time to effectively avoid cross infection during the collection process.
Finally, multidepartment cooperation holds the key to the success of sample-collection work. For such a purpose, sample collection should be placed under unified leadership to establish a coordination mechanism with the government and the third-party detection institution. Specifically, ensure that samples are transported every 2 h and delivered to the laboratory within 4 h after collection; also, ensure the accuracy of sample handover, testing and result tracking, and establish an internal coordination mechanism to provide powerful logistical support for working staff members.
Conclusions
Nucleic acid testing for the whole of society is an effective measure for the prevention and control of COVID-19 during a pandemic. A well-organized emergency management system with clear responsibilities, standardized procedures and a scientific mode of operation based on the local situation can help to quickly and efficiently control the spread of virus. Although community sample collection cannot match that conducted in a medical institution, during this unique period of time it has played an important role in epidemic control and provides a valuable reference for nucleic acid sample collection outside of a hospital.
Acknowledgements
We would like to thank all team members for their contributions to development of this study.
Contributor Information
Yong-li Lyu, Department of Stomatology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China; Nursing Department, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China.
Shan-jie Rong, The Center for Biomedical Research, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China.
Fei Sun, The Center for Biomedical Research, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China.
Can Xiang, Office of Academic Research, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China.
Jun-yi Li, Department of Integrated Traditional Chinese and Western Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China.
Authors’ contributions
JL, CX and YL drafted the manuscript; SR and FS made critical revisions; CX, YL and JL designed and supervised the entire study. All the authors participated in the study and approved the final version of the manuscript.
Funding
Funding was provided by the Natural Science Foundation of Hubei Province [grant no. 2021CFB589] and the National Natural Science Foundation of China [no. 82104488].
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
Not required.
Data availability
All data needed to evaluate the conclusions in this article are included in the paper and/or its supplementary information. Additional data related to this paper may be requested from the authors.
References
- 1. European Centre for Disease Prevention and Control . Threat assessment brief: emergence of SARS-CoV-2 B.1.617 variants in India and situation in the EU/EEA. 2021. Available at https://www.ecdc.europa.eu/en/publications-data/threat - assessment - emergence - sars -cov- 2 - b1617 - variants. [Google Scholar]
- 2. CHU B SH. Experts answer the latest epidemic prevention issues. Fujian Daily. 2021-08-06(004). [Google Scholar]
- 3. The Guangxi Zhuang autonomous region health committee. Guangxi issued “ten strictly” beware of the delta mutant strains invade spread. [EB/OL] (2021-07-15) [2021-8-8]. Available at http://wsjkw.gxzf.gov.cn/ztbd_49627/sszt/xxgzbdfyyqfk/fkgz/t9495739.shtml [accessed 8 August 2021]. [Google Scholar]
- 4. National Health Commission. Existing disease prevention and control measures for delta variants are still valid. [EB/OL]. (2021-8-5) [2021-8-8]. Available at http://www.legaldaily.com.cn/index/content/2021/08/05/content_8573909.html [accessed 5 August 2021]. [Google Scholar]
- 5. Hubei daily commentator. Total nucleic acid testing witness universal force. Hubei Daily, 2021-08-09 (001). [Google Scholar]
- 6. Huang TH, Wang GW, Zhu Y. Thinking of the construction of the equipment of the municipal health emergency team. Chongqing Med. 2012;24:2566–7. [Google Scholar]
- 7. Huang M, Liu DCH, Wu XH. Research on the present situation and countermeasures of national maritime emergency medical rescue team construction in Guangxi. Chin J Emerge Resuscitation Disaster Med. 2018;13:86–9. [Google Scholar]
- 8. Yao HM, Ding F, Gao JYet al. Practice of the hospital emergency management in fighting against the outbreak of COVID-19 from the aspects of man, machine, material, method, environment and monitoring. J Xi'an Jiaotong Univ Med Sci. 2021;42:381–8. [Google Scholar]
- 9. Xu ZW. Analysis of human resources management in public hospitals under the normalization of epidemic prevention and control. China Economist. 2021:259–64. [Google Scholar]
- 10. Xiu H, Wei LL, Zhang WY, Song YK, Liu GF, Wang JY. Construction and effective analysis of emergency team. Chin Nurs Manag. 2018;18:1507–11. [Google Scholar]
- 11. Wang R, Gu ZJ, Lin ZH. Establishment and management of nursing emergency personnel reserve bank in general hospitals during epidemic of coronavirus disease 2019. Chin J Nurs. 2020;55:1020–4. [Google Scholar]
- 12. Wen J, Zeng R, Xu CG. Ten measures to fight against the 2019-nCoV pneumonia in West China Hospital. Chin J Evid Based Med. 2020;20:365–8. [Google Scholar]
- 13. National Health Commission. Guidelines for the use of common medical protective equipment in the prevention and control of novel coronavirus's pneumonia. [EB/OL]. (2020-01-26) [2021-8-8]. Available at http://www.Nhc.gov.cn/yzygj/s7659/202001/e71c5de925a64eafbe1ce790debab5c6.Shtml [accessed 26 January 2020]. [Google Scholar]
- 14. National Health Commission. Notice on printing and distributing technical guidelines for prevention and control of novel coronavirus infection in medical institutions (second edition): National Health Commission. 2021. No. 169. [EB/OL. (2020-04-13) [2021-8-8]. Available at http://www.nhc.gov.cn/yzygj/s7659/202104/f82ac450858243e598747f99c719d917.shtml [accessed 13 April 2021]. [Google Scholar]
- 15. National Health Commission. Notice on printing and distributing the technical specifications for 10-in-1 mixed detection of nucleic acids in Covid-19: Joint prevention and control mechanism. 2020. No. 352 [EB/OL. (2020-08-19) [2021-8-8]. Available at http://www.nhc.gov.cn/yzygj/s7659/202008/fa5057afe4314ef8a9172edd6c65380e.shtml [accessed 19 August 2020]. [Google Scholar]
- 16. Yao HW, Suo JJ, Du MM. Difficulties and countermeasures for control of healthcare-associated infection during epidemic of COVID-19. Chin J Nosocomiology. 2020;30:806–10. [Google Scholar]
- 17. Wu An-H, Huang X, Li CHH.. Novel coronavirus (2019-nCoV) pneumonia in medical institutions: problems in prevention and control. Chin J Infect Control. 2020;19:99–104. [Google Scholar]
- 18. Comprehensi ve Group of Joint Prevention and Control Mechanism in response to COVID-19 epidemic situation of the State Council. Notice on issuing the implementation Guide of novel coronavirus Nucleic Acid testing Organization (second Edition): Comprehensive development of joint prevention and control mechanism. 2021. No. 97 [EB/OL]. (2021-09-13) [2021-9-24]. National Health Commission of China. http://www.nhc.gov.cn/yzygj/s7659/202109/a84fe1eccb414418aa5ebb21b4369c8b.shtml [Google Scholar]
- 19. Gao XL, Shen JH, Wang ZY, et al. Nursing management strategy for coronavirus disease 2019 patients admitted to square hospital. Chin J Nurs. 2020;S1:62–5. [Google Scholar]
- 20. Ji HT. Hospital human resource allocation and mobilization management strategy under epidemic prevention and control emergency. Employment Security. 2021;8:181–2. [Google Scholar]
- 21. He HY, Yang Q, Li JL. Organization and nursing management of mass nucleic acid testing of COVID-19. J Nurs Chin. 2021;14:52–5. [Google Scholar]
- 22. Xi XX, Wang H, Mao J. Difficulties and coping strategies of safety management for inpatients with coronavirus disease 2019 at cabin hospital. Chin J Nurs. 2021;14:52–5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data needed to evaluate the conclusions in this article are included in the paper and/or its supplementary information. Additional data related to this paper may be requested from the authors.