Abstract
We investigated long COVID incidence by vaccination status in a random sample of UK adults from April 2020 to November 2021. Persistent symptoms were reported by 9.5% of 3090 breakthrough severe acute respiratory syndrome coronavirus 2 infections and 14.6% of unvaccinated controls (adjusted odds ratio, 0.59 [95% confidence interval, .50–.69]), emphasizing the need for public health initiatives to increase population-level vaccine uptake.
Keywords: coronavirus, COVID-19, long COVID, post-COVID condition, vaccination
Long-term symptoms following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, often referred to as long COVID, postacute coronavirus disease 2019 (COVID-19) syndrome, post-COVID condition, or postacute sequelae of SARS-CoV-2, affect approximately 2% of the population in the United Kingdom (UK), with two-thirds of these individuals experiencing functional impairment [1]. COVID-19 vaccines reduce rates of SARS-CoV-2 infection [2] and transmission [3] and therefore long COVID incidence. However, it is unclear to what extent vaccination reduces the risk of developing long COVID symptoms following breakthrough infection, with mixed evidence to date [4, 5].
To 25 January 2022, 16% of the UK population eligible for a second vaccination was yet to receive it [6], while vaccine coverage was lowest in disadvantaged groups, including ethnic minorities and deprived communities, where rates of infection have been highest [7]. Understanding the role of vaccines in long COVID may therefore aid public health messaging and facilitate informed decision-making regarding vaccine uptake. We investigated whether infection following 2 doses of a COVID-19 vaccine is associated with a reduction in long COVID symptoms after 12 weeks, relative to being unvaccinated when infected, using prospective data from a large, random sample of the UK population with routine testing for SARS-CoV-2.
METHODS
Study Data and Design
The main data source was the UK COVID-19 Infection Survey (CIS, ISRCTN21086382, https://www.ndm.ox.ac.uk/COVID-19/COVID-19-infection-survey/protocol-and-information-sheets), run by the Office for National Statistics (ONS) and comprising a sample of over half a million participants randomly selected from the UK community population (excluding communal establishments such as hospitals, care homes, halls of residence, and prisons). During the pilot phase of the survey from April to August 2020, households were selected from previous respondents to ONS surveys who had consented to participate in future research, achieving an enrollment rate of 51%. From August 2020, sampling was conducted by random selection from national address lists, with the enrollment rate dropping to 12%. Participants were compensated with a £50 voucher at enrollment and a £25 voucher at each follow-up visit.
Ethical approval was obtained from the South Central Berkshire B Research Ethics Committee (20/SC/0195). At enrollment, adult participants provided written consent, including for optional weekly follow-up visits for 1 month followed by at least 12 monthly visits in the majority.
We included CIS participants aged 18–69 years who tested positive for SARS-CoV-2, either by polymerase chain reaction test using swabs obtained at study visits (58.7% of infections) or any swab test in national testing programs (self-reported by study participants), between 26 April 2020 (the start of the CIS) and 30 November 2021 (the latest available data at the time of analysis). We excluded participants who reported suspected COVID-19 or tested positive for antibodies (in the study or elsewhere) >2 weeks before their first positive swab; reported long COVID symptoms at any time before their first positive swab; had never responded to the survey question on long COVID (see “Outcome” below) following its introduction on 3 February 2021; did not have ≥12 weeks of postinfection follow-up by 30 November 2021; or were single-vaccinated when infected.
Exposure
The exposure of interest was receipt of at least 2 doses of a COVID-19 vaccine (Oxford/AstraZeneca ChAdOx1 nCoV-19 [AZD1222], Pfizer/BioNTech BNT162b2, or Moderna mRNA-1273) ≥14 days before the first test-confirmed infection. Vaccination status for participants in England was derived from survey data linked to National Immunisation Management System (NIMS) records, with the latter being prioritized where they conflicted with self-reports. Agreement rates between self-reported CIS data and NIMS records have previously been found to be high for both vaccination type (98%) and date (95% within 1 week) [8]. Administrative data were not available for participants in Wales, Scotland, and Northern Ireland (13.6%); thus, vaccination status was derived solely from self-report. In sensitivity analysis, we restricted the analysis to participants living in England, thereby reducing the risk of exposure misclassification.
Outcome
The primary outcome was long COVID status according to the survey question: “Would you describe yourself as having ‘long COVID,’ that is, you are still experiencing symptoms more than 4 weeks after you first had COVID-19, that are not explained by something else?” Participants were also asked whether their symptoms limited their ability to undertake daily activities. The survey questionnaire was administered by trained study workers during face-to-face interviews conducted at participants’ homes. We considered participants’ first response ≥12 weeks after their first test-confirmed infection. Follow-up time was calculated as the number of days from infection to the first response to the CIS question on long COVID (either positive or negative) ≥12 weeks later.
Statistical Methods
We matched study participants who were double-vaccinated at time of infection to control participants who were unvaccinated when infected and remained so at their first follow-up visit ≥12 weeks later. Double-vaccinated and unvaccinated participants were 1:1 propensity score matched within calipers of 0.1 points of the propensity score on sociodemographic characteristics: single-year of age, sex, ethnicity (White or non-White), country/region of residence, area deprivation quintile group, and preexisting health/disability status. To derive the latter, participants were asked: “Do you have any physical or mental health conditions or illnesses lasting or expected to last 12 months or more (excluding any long-lasting COVID-19 symptoms)?” and “If yes, do any of your conditions or illnesses reduce your ability to carry-out day-to-day activities (a lot, a little, or not at all)?”
Although a “posttreatment” variable, we also included time from infection to follow-up for long COVID in the matching set to avoid evaluating long COVID symptoms in unvaccinated and double-vaccinated participants at different stages of the illness. To assess the robustness of our results to this choice, we performed a sensitivity analysis excluding follow-up time from the matching set.
Continuous variables (age and follow-up time) were modeled as restricted cubic splines, with boundary knots at the 10th and 90th percentiles and an internal knot at the median of the distributions. Large imbalance after matching was identified by absolute standardized differences >10% [9]. We were not able to match on date of infection (a surrogate for SARS-CoV-2 variant); see the Discussion.
We estimated adjusted odds ratios (aORs) for long COVID at ≥12 weeks using logistic regression including all covariates from the matching set, comparing participants who were double-vaccinated to those unvaccinated (reference group) when infected, using robust standard errors to account for matching. We interacted the exposure variable (double-vaccinated vs unvaccinated) with time from infection to follow-up for long COVID (continuous), and with adenovirus vector (Oxford/AstraZeneca) versus messenger RNA (mRNA; Pfizer/BioNTech or Moderna) vaccines, to test for effect modification using a likelihood ratio test. Statistical analyses were performed using R version 3.6 software.
RESULTS
Description of the Study Sample
Of 3333 eligible participants who were double-vaccinated before their first test-confirmed SARS-CoV-2 infection, 3090 (92.7%) were 1:1 matched to participants who were unvaccinated when infected (from a pool of 9854 potential control participants). See Supplementary Figure 1 for details of the study sample selection. Among double-vaccinated participants, 2287 (74.0%), 788 (25.5%), and 15 (0.5%) received Oxford/AstraZeneca, Pfizer/BioNTech, and Moderna vaccines, respectively.
Most double-vaccinated participants (3057 [98.9%]) were infected after 17 May 2021, when the Delta variant dominated in the UK, while nearly all unvaccinated participants (3082 [99.7%]) were infected before this date (Supplementary Figure 2). Median follow-up for long COVID ≥12 weeks after infection among double-vaccinated and unvaccinated participants was 96 (interquartile range [IQR], 90–104) days and 98 (IQR, 89–109) days, respectively (Supplementary Figure 3). After matching, sociodemographic characteristics were generally well balanced for all variables except age (mean, 49 vs 47 years for double-vaccinated vs unvaccinated; absolute standardized difference, 19.6%) (Supplementary Table 1).
Long COVID Symptoms at Follow-up
Long COVID symptoms of any severity were reported by 294 double-vaccinated participants (prevalence, 9.5% [95% confidence interval {CI}, 8.5%–10.6%]) versus 452 unvaccinated participants (14.6% [95% CI, 13.4%–15.9%]), and activity-limiting symptoms by 170 (5.5% [95% CI, 4.8%–6.4%]) and 268 (8.7% [95% CI, 7.7%–9.7%]) participants, respectively.
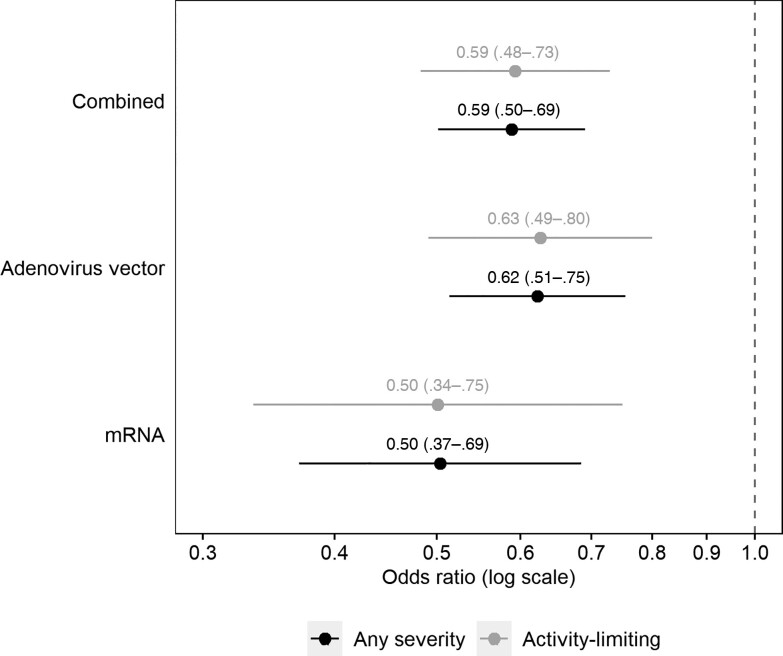
The aORs were 0.59 (95% CI, .50–.69) for long COVID of any severity and 0.59 (95% CI, .48–.73) for activity-limiting symptoms in those infected after double vaccination compared with those who were infected when unvaccinated (Figure 1). There was no evidence of heterogeneity by time from infection to follow-up (P = .65 for symptoms of any severity; P = .68 for activity-limiting symptoms), or between participants receiving adenovirus vector or mRNA vaccines (P = .25 for symptoms of any severity; P = .35 for activity-limiting symptoms).
Figure 1.
Adjusted odds ratios for long COVID symptoms ≥12 weeks after first infection, comparing matched study participants who were double-vaccinated or unvaccinated (reference group) before infection. Odds ratios adjusted for sociodemographic characteristics (age, sex, White or non-White ethnicity, country/region of residence, area deprivation quintile group, and self-reported, preexisting health/disability status) and time from infection to follow-up for long COVID. Confidence intervals are at the 95% level.
Sensitivity analysis demonstrated that the aOR increased when removing time from infection to follow-up for long COVID from the matching set (to 0.68 [95% CI, .56–.81] for the primary outcome), and further increased when it was also omitted from the covariate set in adjusted models (0.73 [95% CI, .62–.85]) (Supplementary Table 2). However, the aOR remained below 1 in all analyses.
The main analysis results were also insensitive to restricting the study sample to the 2311 matched pairs (74.8%) for which both the double-vaccinated and unvaccinated participants lived in England (for whom NIMS data were available for linkage), with an aOR of 0.64 (95% CI, .53–.78) for the primary outcome (Supplementary Table 3), suggesting that exposure misclassification due to self-reporting of vaccination status is unlikely to have substantially impacted the main results.
DISCUSSION
We found that receiving 2 COVID-19 vaccinations at least 2 weeks before SARS-CoV-2 infection was associated with a 41% decrease in the odds of developing long COVID symptoms at least 12 weeks later, relative to not being vaccinated when infected. Our results extend those already published, whereby the risk of long COVID was approximately halved in people who were double-vaccinated when infected compared with those who were unvaccinated, but at 4 rather than 12 weeks postinfection [4]. Conclusions based on healthcare records rather than self-report (as in our study) are less clear, with vaccination associated with reduced rates of only specific symptoms [5] and diagnoses [10], though underpresentation, underdiagnosis, and underrecording are all possible [11].
The main study strength is that the CIS comprises a large sample of participants randomly selected from the population to minimize selection bias. Participants are routinely tested for SARS-CoV-2 at follow-up visits; therefore, our study includes both asymptomatic and symptomatic infections, as well as self-reported tests. We considered participants’ first monthly CIS response that was at least 12 weeks after their positive test for SARS-CoV-2; thus, time from infection to response could have been any duration from 12 weeks upwards. However, recall bias was not a concern because participants were asked about their current long COVID status at the time of the follow-up visit (ie, prospective data collection), and we included time from infection to response in the matching set to ensure balanced follow-up time between double-vaccinated and unvaccinated groups.
Although we adjusted for multiple factors related to vaccination uptake [7] and long-term symptoms [12], some unmeasured confounding may remain. In particular, because the question on long COVID was not introduced until 3 February 2021, shortly after mass COVID-19 vaccination started in the UK on 8 December 2020, a key limitation is that it was not possible to match double-vaccinated and unvaccinated participants on calendar time of infection. Differences in the likelihood of developing long COVID symptoms between exposure groups may therefore partly reflect changes in the dominant COVID-19 variant or other period effects, such as the introduction of National Health Service long COVID assessment and rehabilitation services (though most patients are unlikely to be referred to these inside the first 12 weeks of illness).
Long COVID status was self-reported, so outcome misclassification was possible. Some participants may have been experiencing symptoms because of a health condition unrelated to COVID-19, while others who did have long COVID may not have described themselves as such (for example, due to the perceived stigma attached to the term [13]). Conversely, self-recognition of long COVID (participants’ perception of the change in their own health compared with preinfection) may be more reliable than electronic health records in some respects, for example due to differences in healthcare-seeking behaviors between sociodemographic groups and long COVID diagnoses being underrecorded in primary care [11]. Our key exposure was double vaccination, despite third and booster doses now being available, and the study period was before the Omicron variant became widespread. We were not able to investigate participants who were single-vaccinated when infected because nearly all of these received their second dose within the 12-week follow-up period, confounding any relationship between 1 dose at infection and long COVID symptoms.
There is potential for survivor bias because our study sample did not include people who were infected but subsequently dropped out of the survey before having had the opportunity to respond to the long COVID question after it was introduced on 3 February 2021. This loss to follow-up may be related to the likelihood of developing or reporting long COVID symptoms, for example due to ill health. However, after broadening the study cohort definition by dropping any exclusion criteria dependent on duration of follow-up after infection or response to the long COVID question, just 3% of the resulting 37 145 participants never responded to the long COVID question postinfection. Loss to follow-up is therefore unlikely to have materially impacted our findings.
In conclusion, SARS-CoV-2 infection after double vaccination is associated with a reduced risk of developing long COVID symptoms at least 12 weeks later compared with infection before vaccination, emphasizing the need for public health initiatives to increase population-level vaccine uptake. Studies with longer follow-up are needed to assess the impact of booster doses and the Omicron variant and to evaluate symptom trajectories beyond a single 12-week follow-up visit, particularly given the relapsing nature of long COVID [14]. Further research into possible biological explanations behind our findings, which may inform therapeutic strategies for long COVID, is also required.
Supplementary Material
Contributor Information
Daniel Ayoubkhani, Health Analysis and Life Events Division, Office for National Statistics, Newport, United Kingdom; Leicester Real World Evidence Unit, Diabetes Research Centre, University of Leicester, Leicester, United Kingdom.
Matthew L Bosworth, Health Analysis and Life Events Division, Office for National Statistics, Newport, United Kingdom.
Sasha King, Methodology and Quality Directorate, Office for National Statistics, London, United Kingdom.
Koen B Pouwels, National Institute for Health Research Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance, University of Oxford, Oxford, United Kingdom; Health Economics Research Centre, Nuffield Department of Population Health, University of Oxford, Oxford, United Kingdom.
Myer Glickman, Health Analysis and Life Events Division, Office for National Statistics, Newport, United Kingdom.
Vahé Nafilyan, Health Analysis and Life Events Division, Office for National Statistics, Newport, United Kingdom; Faculty of Public Health, Environment and Society, London School of Hygiene and Tropical Medicine, London, United Kingdom.
Francesco Zaccardi, Leicester Real World Evidence Unit, Diabetes Research Centre, University of Leicester, Leicester, United Kingdom.
Kamlesh Khunti, Leicester Real World Evidence Unit, Diabetes Research Centre, University of Leicester, Leicester, United Kingdom.
Nisreen A Alwan, School of Primary Care, Population Sciences and Medical Education, Faculty of Medicine, University of Southampton, Southampton, United Kingdom; National Institute for Health Research Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton NHS Foundation Trust, Southampton, United Kingdom; National Institute for Health Research Applied Research Collaboration Wessex, Southampton, United Kingdom.
A Sarah Walker, National Institute for Health Research Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance, University of Oxford, Oxford, United Kingdom; Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. All authors contributed to conceptualizing and designing the study. D. A., M. L. B., and S. K. prepared the study data and performed the statistical analysis. All authors contributed to interpretation of the results. D. A., M. L. B., and S. K. were responsible for the first draft of the manuscript. All authors contributed to critical revision of the manuscript. All authors approved the final manuscript.
Data availability. De-identified study data are available to accredited researchers in the ONS Secure Research Service under part 5, chapter 5 of the Digital Economy Act 2017. For further information about accreditation, contact research.support@ons.gov.uk or visit: https://www.ons.gov.uk/aboutus/whatwedo/statistics/requestingstatistics/approvedresearcherscheme.
Ethical approval. Ethical approval for this study was obtained from the National Statistician's Data Ethics Advisory Committee (NSDEC[20]12). The CIS received ethical approval from the South Central Berkshire B Research Ethics Committee (20/SC/0195). All participants provided written consent at enrollment.
Disclaimer. The views expressed are those of the authors and are not necessarily those of the National Health Service, the National Institute for Health Research (NIHR), the Department of Health and Social Care, or the UK Health Security Agency. For the purpose of open access, the authors have applied a Creative Commons Attribution (CC BY) licence to any author-accepted manuscript version arising.
Financial support. D. A., K. K., and F. Z. are supported by the NIHR Applied Research Collaboration East Midlands. K. K. and F. Z. are also supported by the NIHR Leicester Biomedical Research Centre. K. B. P. and A. S. W. are supported by the NIHR Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance (NIHR200915), a partnership between the UK Health Security Agency and the University of Oxford. K. B. P. is also supported by the Huo Family Foundation (501100022111). A. S. W. is also supported by the NIHR Oxford Biomedical Research Centre and is an NIHR Senior Investigator. N. A. A. has lived experience of long COVID and is a coinvestigator on the NIHR-funded STIMULATE-ICP study.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Office for National Statistics . Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK: 6 January 2022. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/6january2022. Accessed 10 May 2022.
- 2. Wei J, Pouwels KB, Stoesser N, et al. SARS-CoV-2 anti-spike IgG antibody responses after second dose of ChAdOx1 or BNT162b2 and correlates of protection in the UK general population. medRxiv [Preprint]. Posted online 14 January 2022. doi: 10.1101/2021.09.13.21263487 [DOI] [Google Scholar]
- 3. Eyre DW, Taylor D, Purver M, et al. The impact of SARS-CoV-2 vaccination on Alpha and Delta variant transmission. medRxiv [Preprint]. Posted online 15 October 2021. doi: 10.1101/2021.09.28.21264260 [DOI] [Google Scholar]
- 4. Antonelli M, Penfold RS, Merino J, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis 2022; 22:43–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taquet M, Dercon Q, Harrison PJ. Six-month sequelae of post-vaccination SARS-CoV-2 infection: a retrospective cohort study of 10,024 breakthrough infections. Brain Behav Immun 2022; 103:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. UK Government . Coronavirus (COVID-19) in the UK. https://coronavirus.data.gov.uk. Accessed 25 January 2022.
- 7. Office for National Statistics . Coronavirus and vaccination rates in people aged 18 years and over by socio-demographic characteristic and occupation, England: 8 December 2020 to 31 December 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthinequalities/bulletins/coronavirusandvaccinationratesinpeopleaged18yearsandoverbysociodemographiccharacteristicandoccupationengland/8december2020to31december2021. Accessed 10 May 2022.
- 8. Pritchard E, Matthews PC, Stoesser N, et al. Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom. Nat Med 2021; 27:1370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28:3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al-Aly Z, Bowe B, Xie Y. Long Covid after breakthrough COVID-19: the post-acute sequelae of breakthrough COVID-19. Research Square [Preprint]. 2021. doi: 10.21203/rs.3.rs-1062160/v1 [DOI] [Google Scholar]
- 11. Walker AJ, MacKenna B, Inglesby P, et al. Clinical coding of long COVID in English primary care: a federated analysis of 58 million patient records in situ using OpenSAFELY. Br J Gen Pract 2021; 71:e806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thompson EJ, Williams DM, Walker AJ, et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat Commun 2022; 13:3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pantelic M, Ziauddeen N, Boyes M, et al. Long Covid stigma: estimating burden and validating scale in a UK-based sample. medRxiv [Preprint]. Posted online 26 May 2022. doi: 10.1101/2022.05.26.22275585 [DOI] [PMC free article] [PubMed]
- 14. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021; 38:101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.