Abstract
Background
Oral polio vaccine (OPV) may improve resistance to non-polio-infections. We tested whether OPV reduced the risk of illness and mortality before coronavirus disease 2019 (COVID-19) vaccines were available.
Methods
During the early COVID-19 pandemic, houses in urban Guinea-Bissau were randomized 1:1 to intervention or control. Residents aged 50+ years were invited to participate. Participants received bivalent OPV (single dose) or nothing. Rates of mortality, admissions, and consultation for infections (primary composite outcome) during 6 months of follow-up were compared in Cox proportional hazards models adjusted for age and residential area. Secondary outcomes included mortality, admissions, consultations, and symptoms of infection.
Results
We followed 3726 participants (OPV, 1580; control, 2146) and registered 66 deaths, 97 admissions, and 298 consultations for infections. OPV did not reduce the risk of the composite outcome overall (hazard ratio [HR] = 0.97; 95% confidence interval [CI], .79–1.18). OPV reduced the risk in males (HR = 0.71; 95% CI, .51–.98) but not in females (HR = 1.18; 95% CI, .91–1.52) (P for same effect = .02). OPV also reduced the risk in Bacillus Calmette-Guérin scar-positive (HR = 0.70; 95% CI, .49–.99) but not in scar-negative participants (HR = 1.13; 95% CI, .89–1.45) (P = .03). OPV had no overall significant effect on mortality (HR = 0.96; 95% CI, .59–1.55), admissions (HR = 0.76; 95% CI, .49–1.17) or recorded consultations (HR = 0.99; 95% CI, .79–1.25), but the OPV group reported more episodes with symptoms of infection (6050 episodes; HR = 1.10 [95% CI, 1.03–1.17]).
Conclusions
In line with previous studies, OPV had beneficial nonspecific effects in males.
Keywords: oral polio vaccine, morbidity, mortality, nonspecific (heterologous) effects of vaccines
In a cluster-randomised trial among adults aged 50+ in Guinea-Bissau, oral polio vaccine (OPV) had no overall effect on morbidity/mortality (composite outcome). OPV effects differed significantly by sex and BCG scar status: ∼30% reduction for males and BCG scar-positive individuals.
When coronavirus disease 2019 (COVID-19) was first spreading, readily available tools to mitigate the impact of severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) infections were urgently needed. Evidence indicates that some of the vaccines used for decades, besides their effects on the targeted pathogen, alter the susceptibility to other infections [1]. Live vaccines, including oral polio vaccine (OPV), seem to increase resistance to unrelated infections, that is, to have beneficial nonspecific effects (NSEs).
OPV has been associated with lower mortality in observational studies among children in Guinea-Bissau [2, 3], Ghana [4], and Bangladesh [5] and with lower risk of hospital admissions in Burkina Faso [6] and Denmark [7]. Studies from Bangladesh [8] and Denmark [7] indicate stronger reductions for respiratory infections.
In randomized trials of OPV given at birth in Guinea-Bissau, OPV reduced early child mortality by 32% [9, 10]. In a randomized trial comparing OPV with inactivated polio vaccine (IPV) in Bangladesh, OPV lowered the duration and severity of diarrhea [11]. In Finland, OPV, compared with IPV, was associated with a lower risk of upper airway infections [12]. OPV's NSEs seem to be stronger for boys than for girls in most [3, 9, 11], but not all [5], studies.
OPV administered to adults has been less frequently studied. In 1970, a large Russian study found that OPV reduced the risk of respiratory infections: 40 678 factory employees given OPV had approximately 50% lower incidence than 18 880 nonrecipients [13]. Effects were not reported by gender.
In early 2020, before the availability of vaccines against SARS-CoV-2, it was suggested that OPV could mitigate the impact of the of SARS-CoV-2 infections [14]. The Global Polio Eradication Initiative issued a statement supporting that studies on the effect of OPV against COVID-19 were important [15].
We designed a cluster-randomized trial in Guinea-Bissau to investigate whether OPV reduced the risk of illness and mortality among adults >50 years who were at higher risk of severe outcomes if infected with SARS-CoV2 [16]. We assessed the effect on the primary composite outcome, death, hospitalization, or consultation for infection during 6 months of follow up. Secondary outcomes were the separate components of the primary outcome and self-reported illness episodes. For primary and secondary outcomes, we investigated potential effect modifiers.
METHODS
Setting and Study Population
Guinea-Bissau is a West African country with a population of ∼1.9 million with an estimated life expectancy at birth of 58 years [17]. Bandim Health Project (BHP) monitors health and survival of 100 000 individuals through a Health and Demographic Surveillance System (HDSS) in 6 suburbs of the capital, Bissau. The population aged >50 years constitutes 8% of the registered population, and the median life expectancy of 50-year-olds in 2005–2013 was 19 years [18].
The HDSS area is situated 2 km outside the city center, which houses the National Hospital. Three public health centers in the study area provide outpatient consultations, and one admits patients. Health worker strikes occurred throughout the trial period and were frequent in 2021 (Supplementary Methods).
The first SARS-CoV2 infection in Guinea-Bissau was identified on March 25, 2020. By November 2020, 18% of the BHP HDSS staff had serologic evidence of prior infection [19]. We used the number of weekly detected cases to define 3 periods with higher transmission (Supplementary Figure 1). Testing intensity was low (Supplementary Methods).
OPV has been used in the Guinean vaccination program since 1981 [20] and provided in numerous vaccination campaigns since 1998 [2, 21]. Thus, the age group targeted in our trial has been exposed to natural poliovirus in childhood, and they have been repeatedly exposed to polioviruses excreted from vaccinated children [22]. The Guinean health authorities started COVID-19 vaccinations in April 2021. During our trial, the target groups for COVID-19 vaccinations were healthcare workers and people with pre-existing chronic illness [23] (Supplementary Methods).
Participant Consent
The invitation for participation took place during a home visit: a nurse and a field assistant visited the household and brought preprinted enrollment forms that displayed the randomization group. After receiving information about the trial (Supplementary material), individuals interested in participating signed or fingerprinted a consent form. Before implementing the trial, stories of experimental COVID-19 vaccines to be tested in Africa circulated on social media. Some perceived the trial OPV as an experimental vaccine, which resulted in high refusal rates (Supplementary Figure 2).
The trial was approved by Comité Nacional de Ética na Saúde, Guinea-Bissau on May 21, 2020 (Ref: 077/CNES/INASA/2020) and received consultative approval from The National Committee on Health Research Ethics, Denmark, on June 16, 2020 (Ref: 2008258). We registered the trial at www.clinicaltrials.gov (ClinicalTrials.gov Identifier NCT04445428) on June 22, 2020.
Randomization and Enrollment
Details on the procedures for randomizations, consent, and enrollment are provided in the Supplementary material. In brief, houses in the HDSS area were randomized 1:1 to the intervention or control group stratified by zone (n = 37). The trial was unblinded. After the consent process, consenting individuals were interviewed about past and current illness. We excluded potential participants with acute infections, signs of immune suppression, previous confirmed COVID-19, or past paralyses suspected to be caused by thromboembolic complications.
To avoid physical contact, nutritional status, and the presence or absence of Bacillus Calmette-Guérin (BCG) and smallpox vaccination scars, which are important predictors of survival [24–26], were visually assessed from a distance. Nutritional status was assessed using a visual scoring system, based on the field assistant's perception of the participant's body weight [27].
Participants received a study card with identifiers, which they were asked to bring if they sought consultations at 1 of the 2 participating health centers. Participants in the intervention group subsequently received 2 drops of bivalent OPV (serotype 1 + 3). Two different OPV types were used: (1) GSK, Batch AOP4A633AB (before March 2021) and (2) Bharat Biotech, Batch 68D20015A. OPV was administered on a sugar lump in a single-use spoon.
Follow-Up and Outcomes
Participant follow up was conducted by (1) telephone calls every 4 weeks until 6 months after enrollment and (2) registration of consultations at the 2 health centers in the study area where BHP covered the cost of consultations. Furthermore, all individuals are also followed for survival through the HDSS. All follow-up information was collected by interviewers blind to the group allocation. (1) Telephone interviews were conducted with the trial participant or in his/her absence with a relative in the same household. The interviewers asked whether participants, since enrollment/last call, had experienced any of the following symptoms: common cold, cough, fever, breathlessness, vomiting, diarrhea, loss of sense of smell, loss of sense of taste, headache, sore throat, body aches, extreme tiredness, or other symptoms. All participants were asked whether they had a COVID-19 test performed, whether they had lost weight, and whether they had sought consultation (“self-reported consultations”) or been admitted to hospital during the interval. If yes, they were asked when, where, and why and whether they had received a diagnosis. (2) The BHP collected information on consultations at 2 health centers in the study area (“recorded consultations”). The BHP staff extracted information of date of consultation, symptoms, diagnostic tests, and diagnosis and prescribed treatment from the health centers consultation books. (3) Information on vital status and date of death was reconciled between data from the telephone interviews and the HDSS data.
When the trial was initiated, the level of morbidity in the age group >50 was largely unknown. To ensure sufficient power, we combined 3 health outcomes. Hence, the primary outcome was a composite outcome of the first of death, hospitalization for infection, or recorded consultation for infection at the health center within the follow-up period of 6 months. We aimed to enroll 1700 participants in each trial arm, because we had 1700 doses of OPV available. We estimated that this sample size would give us 80% power to detect a difference if the real effect was a 28% reduction (anticipated control group rate, 10%).
As secondary outcomes, we assessed the subcomponents of the main outcome, thus considering all-cause mortality and hospital admission for infectious disease and recorded consultation for infectious disease as separate outcomes. Further secondary outcomes were other measures of infectious disease morbidity: episodes with self-reported infectious disease symptoms, and self-reported infectious disease morbidity suspected to be COVID-19 (Supplementary Methods). During data collection, we also added self-reported consultations for infectious diseases to the list of secondary outcomes.
Statistical Analyses
Baseline characteristics are presented as proportions for categorical values and medians with interquartile ranges for continuous variables. Distribution by group is compared by χ2 and rank-sum tests.
We compared outcome rates by randomization arm using Cox proportional hazards models with time since randomization as the underlying time scale. Estimates presented are adjusted for age (5-year age bands from 50–74 years and >75 years) and zone (based on which the randomization was stratified). Because the randomization unit was the house, we adjusted for within-house clustering using cluster-robust standard error. Although each person could only contribute 1 event in the main and mortality analyses, all other outcomes were analyzed as repeated events, in which the person re-entered the at-risk population 2 weeks after an event.
We investigated whether the refusal rates could have confounded our results by comparing the estimated HRs with and without adjustment for potential confounders: (1) sex and (2) indicators of education and health status. We furthermore conducted an inverse probability treatment weight (IPTW) analysis using the same background factors to calculate a propensity score [28] (Supplementary Methods).
In robustness analyses, we used narrower outcome definitions and limited the analyses to (1) areas with lower refusal rates, (2) interviews in which the participant was the respondent, and (3) interviews conducted by specific interviewers.
Prior studies have indicated that the NSEs of OPV may be stronger in males [3, 9, 11] and may vary by season [3]. Furthermore, the risk of mortality and morbidity may differ by background factors and by exposures that change during follow up. We used Wald tests to compare the estimates in the strata defined by the potential effect modifiers, both those that were constant for each participants (sex, presence of a BCG and/or smallpox vaccination scar, age at enrollment, visual body weight score, chronic illness, and dry versus rainy season of enrollment) and those that varied during follow up (first versus last 3 months of follow up, season of follow up, periods with many versus few detected COVID-19 cases, and periods with strikes among health staff).
Further details on the statistical analyses can be found in the Supplementary material, and the full analysis plan is available at https://clinicaltrials.gov/ct2/show/NCT04445428. Stata/BE 17.0 (StataCorp, College Station, TX) and R were used for analyses.
RESULTS
Study Population
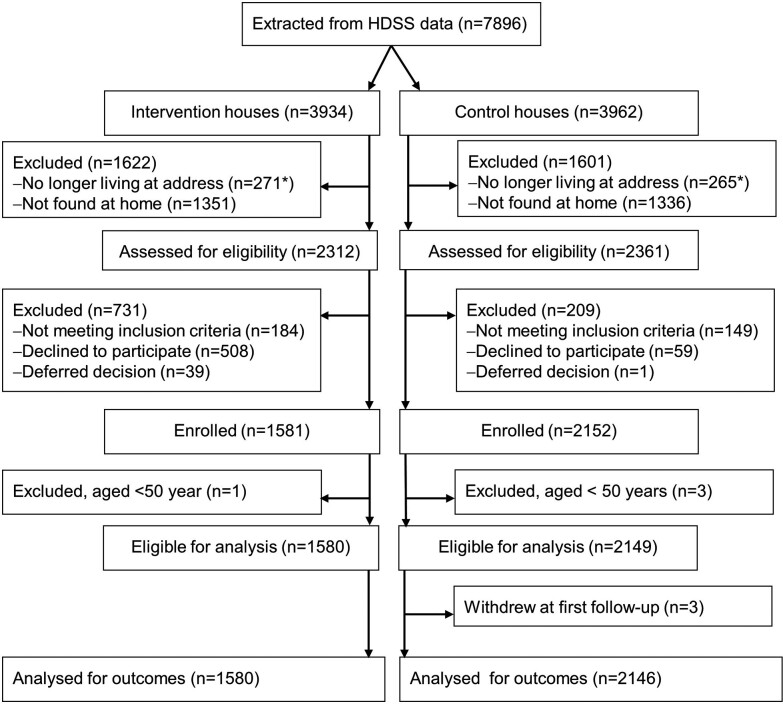
Between July 15, 2020 and April 20, 2021, we contacted 59% (4673 of 7896) of the persons extracted from the HDSS database and enrolled 3729 (80%) (Figure 1 and Supplementary Results). The risk of refusals was 8.78 (95% CI = 6.51–11.85) times greater in the intervention than the control group with particularly high refusal rates in 2 suburbs (Supplementary Figure 2 and Supplementary Table 1).
Figure 1.
Flowchart of potential trial participants. *“No longer living at address” included 187 persons (89 in intervention houses, 98 in control houses) who had died before we could ask them to participate.
Participants had a median age of 59 years (interquartile range [IQR], 55–66 years) and 40% were male. Fifty-nine percent had attended school (Table 1). Baseline characteristics were balanced for most parameters but showed statistically significant imbalances for suburb (P = .008). The intervention group was slightly thinner (median visual body weight score, 4; IQR, 3–6) than the control group (median visual body weight score, 5; IQR, 3–6) (P = .03) and fewer had chronic illness (49% vs 52%). The proportion with a BCG scar was lower in the OPV group (39%) than in the control group (45%; P = .001) (Table 1). The classification of nutritional status and scar varied by data collector (Supplementary Figure 3, Supplementary Tables 2 and 3).
Table 1.
Baseline Characteristics According to Group Allocation
| Characteristics | OPV (n, %) | No OPV (n, %) | P Value, Test of Same Distribution |
|---|---|---|---|
| Number | 1580 (42) | 2149 (58) | |
| Males | 643 (41) | 863 (40) | .74 |
| Age (years), median (IQR) | 59 (54–66) | 60 (55–66) | .64 |
| Suburb | … | … | .008 |
| Bandim-1 | 500 (32) | 657 (31) | |
| Bandim-2 | 216 (14) | 344 (16) | |
| Belem | 88 (6) | 159 (7) | |
| Mindara | 99 (6) | 122 (6) | |
| Cuntum-1 | 448 (28) | 524 (24) | |
| Cuntum-2 | 229 (14) | 343 (16) | |
| Educationa | … | … | .41 |
| None | 634 (42) | 823 (40) | |
| 1–4 years | 307 (20) | 418 (20) | |
| 5–9 years | 262 (17) | 341 (16) | |
| 10+ years | 324 (21) | 486 (24) | |
| Signed consent formb | 887 (56) | 1280 (60) | .06 |
| Health Status | |||
| Visual classification of weight, median (IQR)c | 4 (3–6) | 5 (3–6) | .03 |
| Any chronic illnessd | 759 (49) | 1106 (52) | .03 |
| Hypertension | 622 (82) | 919 (83) | .52 |
| Diabetes | 116 (15) | 152 (14) | .35 |
| Medicine intake last monthe | 749 (48) | 998 (47) | .63 |
| Admissions during prior 3 monthsf | 20 (1) | 25 (1) | .78 |
| Other Background Factors | |||
| Vaccinia scarg | 910 (58) | 1251 (59) | .65 |
| BCG scarh | 618 (39) | 955 (45) | .001 |
| Enrollment in rainy season | 694 (44) | 1038 (48) | .008 |
Abbreviations: BCG, Bacillus Calmette-Guérin; IQR, interquartile range; OPV, oral polio vaccine.
One hundred thirty-four with missing information on education.
For participants unable to sign the consent form, consent was documented with a fingerprint and the form was signed by an independent witness.
Twenty-nine with missing information on visual classification of weight.
Forty-six with missing information on chronic illness.
Twenty-nine with missing information on medicine intake last month.
Three with missing information on admissions during prior 3 months.
Twenty-three with missing information on vaccinia scar.
Twenty-nine with missing information on BCG scar.
Main Outcomes
During follow up, we registered 66 deaths, 97 hospital admissions with reported diagnosis/symptoms of infectious disease (in 91 persons), and 298 outpatient contacts for infections (in 286 persons) (Supplementary Figure 4). The analysis of the primary outcome included a total of 408 events. Rates did not differ between groups: 22.7 events/100 person-years (PYRS) in the OPV group and 23.8/100 PYRS in the control group (hazard ratio [HR] = 0.97, 95% CI = .79–1.18, adjusted for zone and age) (Table 2). Adjusting for the prespecified background factors had little effect on effect estimates (<2%; Supplementary Table 4). The IPTW analysis also did not alter the results (HR = 0.95; 95% CI, .78–1.16). Hence, conclusions are based on the zone- and age-adjusted estimates.
Table 2.
Effect of OPV on the Rates of Morbidity and Mortality Among Participants in the OPV-COVID Trial in Urban Guinea-Bissau
| Outcome | Rate/100 PYRS (Events/PYRS) | HR (95% CI)a | |
|---|---|---|---|
| OPV | No OPV | ||
| Primary (composite) outcome | 22.7 (169/746) | 23.8 (239/1006) | 0.97 (.79–1.18) |
| Secondary Outcomes | |||
| Mortalityb | 3.6 (28/786) | 3.6 (38/1065) | 0.96 (.59–1.55) |
| Mortality first 3 months | 2.3 (9/394) | 3.4 (18/535) | 0.65 (.29–1.45) |
| Mortality last 3 months | 4.8 (19/392) | 3.8 (20/530) | 1.23 (.66–2.30) |
| Hospital admissions for infection | 4.5 (35/783) | 5.9 (62/1057) | 0.76 (.49–1.17) |
| Recorded consultations for infections | 15.9 (124/782) | 16.4 (174/1058) | 0.99 (.79–1.25) |
| Reported symptoms of infections | 391.3 (2724/696) | 351.7 (3326/946) | 1.10 (1.03–1.17) |
| Reported symptoms of COVID-19 | 20.9 (162/774) | 17.9 (187/1042) | 1.14 (.89–1.47) |
| Reported consultations for infectionsb | 57.6 (442/767) | 50.9 (528/1038) | 1.13 (.99–1.31) |
| Reported consultations for infections first 3 months | 50.3 (194/386) | 51.1 (267/523) | 0.99 (.81–1.20) |
| Reported consultations for infections last 3 months | 65.1 (248/381) | 50.7 (261/515) | 1.29 (1.06–1.55) |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; HR, hazard ratio; OPV, oral polio vaccine; PYRS, person-years.
Hazard ratio compared in Cox proportional hazards models with time since enrollment as underlying timescale. Adjusted for age and zone of residence.
Proportional hazards could not be confirmed for the main analysis.
Secondary Outcomes
The effects were different for the separate components of the composite outcomes. The annual mortality was 3.6% in both groups, and the estimated effect of OPV on all-cause mortality was HR = 0.96 (95% CI, .59–1.55), but the proportional hazards assumption was violated. After splitting the follow-up time after the first 3 months, the problem was resolved and revealed opposite associations during the 2 periods: HR = 0.65 (95% CI, .29–1.45) during the first 3 months of follow up and HR = 1.23 (95% CI, .66–2.30) during the last 3 months. The rate of hospital admissions for infections tended to be lower in the OPV group (4.5/100 PYRS) than in the control group (5.9/100 PYRS; HR = 0.76; 95% CI, .49–1.17), whereas the thrice as frequent outcome consultations did not differ by trial arm (HR = 0.99; 95% CI, .79–1.25). None of the consultations were recorded as COVID-19.
For the secondary outcomes reported during the telephone interviews, OPV increased the risk of reported symptoms of infections (HR = 1.10; 95% CI, 1.03–1.17) and tended to increase the reported consultations with infectious symptoms (HR = 1.13; 95% CI, .99–1.31). Reported rates of consultation and “reported symptoms of COVID-19” also tended to be higher in the intervention group (Table 2 and Supplementary Results).
The robustness analyses restricting the event criterion to episodes with weight loss and the analyses to information reported by the participant, or to suburbs with lower refusal rates, yielded similar results (Supplementary Results, Supplementary Tables 5 and 6). Excluding interviews performed by an interviewer who captured fewer events than the 3 others had little effect on the effect estimates (Supplementary Results, Supplementary Figures 5 and 6, Supplementary Table 7 and 8).
Potential Effect Modification
Table 3 displays the planned analyses of potential effect modifiers for the composite outcome. The effect of OPV differed by sex. Men benefitted from OPV (HR = 0.71; 95% CI, .51–.98) whereas women did not (HR = 1.18, 95% CI = .91–1.52, and P = .02 for interaction between sex and OPV) (Figure 2). Beneficial effects for men but not for women were also observed across the separate components of the composite outcome (mortality, P for same effect in males and females = 0.04; infectious admissions, P = .12; and recorded consultations, P = .11). Differences were smaller for the self-reported secondary outcomes (Figure 3). We found no indication that the sex-differential effects were caused by confounding (Supplementary Results).
Table 3.
Effect of OPV on the Main Composite Outcome (Mortality, Hospital Admissions, and Consultations for Infections) in Strata Defined by the Potential Effect Modifiers
| Strata | Rate/100 PYRS (Events/PYRS) | HR (95% CI)a | P Value, Test of No Differenceb | |
|---|---|---|---|---|
| OPV | No OPV | |||
| Sex | ||||
| Male | 17.8 (55/309) | 25.3 (102/403) | 0.71 (.51–.98) | .02 |
| Female | 26.1 (114/436) | 22.7 (137/604) | 1.18 (.91–1.52) | |
| BCG Scar | ||||
| Yes | 15.8 (47/298) | 22.6 (101/447) | 0.70 (.49–.99) | .03 |
| No | 27.4 (121/441) | 25.0 (138/551) | 1.13 (.88–1.45) | |
| Smallpox Scar | ||||
| Yes | 22.8 (98/430) | 24.1 (141/586) | 1.00 (.77–1.31) | .64 |
| No | 22.4 (70/312) | 23.5 (97/413) | 0.91 (.67–1.24) | |
| Age | ||||
| Below median | 18.2 (70/384) | 21.3 (107/502) | 0.89 (.65–1.20) | .47 |
| Above median | 27.3 (99/362) | 26.2 (132/504) | 1.03 (.79–1.35) | |
| Visual Classification of Weight | ||||
| Below median | 24.6 (126/512) | 26.9 (178/663) | 0.91 (.72–1.15) | .42 |
| Above median | 18.0 (41/228) | 17.6 (59/335) | 1.10 (.74–1.63) | |
| Season of Enrollment | ||||
| Rainy | 26.8 (86/320) | 31.0 (147/474) | 0.85 (.65–1.12) | .13 |
| Dry | 19.5 (83/425) | 17.3 (92/532) | 1.16 (.86–1.58) | |
| Chronic Illness | ||||
| Yes | 26.1 (93/356) | 26.3 (135/514) | 1.03 (.79–1.36) | .65 |
| No | 19.6 (75/382) | 20.7 (99/478) | 0.94 (.69–1.28) | |
| Follow-up Time | ||||
| First 3 months | 24.7 (94/381) | 27.7 (143/516) | 0.90 (.70–1.17) | .42 |
| After 3 months | 20.6 (75/365) | 19.6 (96/491) | 1.06 (.78–1.44) | |
| Season of Follow-up | ||||
| Rainy season | 30.6 (94/307) | 29.5 (121/410) | 1.06 (.81–1.40) | .30 |
| Dry season | 17.1 (75/439) | 19.8 (118/596) | 0.86 (.64–1.16) | |
| COVID-19 Transmission | ||||
| Low transmission | 19.2 (72/376) | 21.3 (108/508) | 0.91 (.68–1.23) | .62 |
| High transmission | 26.2 (97/370) | 26.3 (131/498) | 1.01 (.77–1.32) | |
| Health Worker Strikes | ||||
| Few | 24.7 (134/542) | 26.8 (195/728) | 0.94 (.75–1.17) | .56 |
| Continuous | 17.2 (35/203) | 15.8 (44/278) | 1.09 (.69–1.70) | |
Abbreviations: BCG, Bacillus Calmette-Guérin; CI, confidence interval; COVID-19, coronavirus disease 2019; HR, hazard ratio; OPV, oral polio vaccine; PYRS, person-years.
Hazard ratio compared in Cox proportional hazards models with time since enrollment as underlying timescale. Adjusted for age and zone of residence.
Wald test of same effect across the strata.
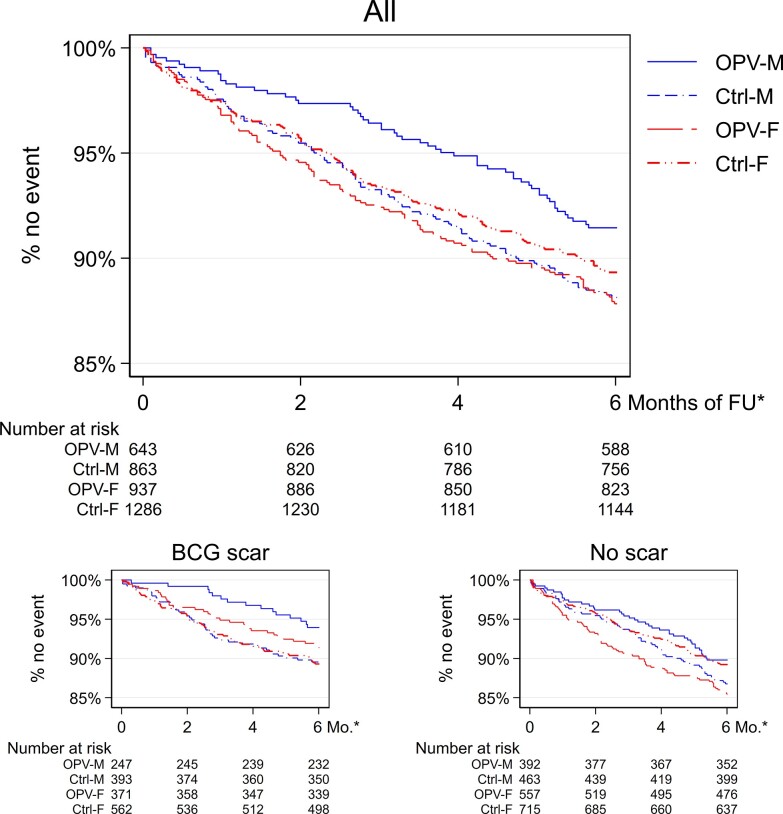
Figure 2.
Kaplan-Maier estimates of event-free observation time (main, composite outcome) by sex and intervention group overall and stratified by Bacillus Calmette-Guérin (BCG) scar status. *Months of follow up (FU). Ctrl, control group; F, females; M, males; OPV, intervention group.
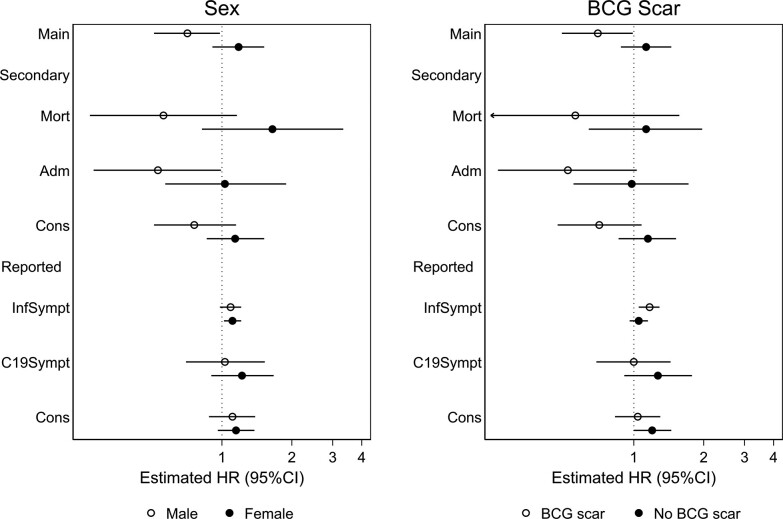
Figure 3.
Hazard ratios (HR) in strata defined by sex and Bacillus Calmette-Guérin (BCG) scar (potential effect modifiers) for main and secondary outcomes. Main outcome - primary, composite outcome: first of mortality, admission, and recorded health center consultation for infection. Secondary outcomes - subcomponents of the composite outcome: mortality (Mort), hospital admission for infection (Adm), and recorded consultation for infection at a health center. Reported, secondary outcomes: symptoms of infection (InfSympt). Symptoms of coronavirus disease 2019 infections (C19Sympt) and reported consultations at any health facility. Secondary outcomes, except for mortality, were analyzed as repeated outcomes.
Individuals with a BCG scar had overall lower rates of the composite outcome (Table 3 and Supplementary Result) and OPV reduced the rate. The HR was 0.70 (95% CI, .49–.99) among BCG scar-positive individuals but 1.13 (95% CI, .88–1.45) among those without a scar (P for same effect = .03). The benefit of OPV among scar-positive individuals was observed across all subcomponents of the composite outcome, but we observed no difference in effect for the self-reported symptoms (Figure 3). Robustness analyses did not alter conclusions, and OPV tended to benefit both men and women with a BCG scar (Supplementary Results, Figure 2, and Supplementary Table 9). The effect of OPV on the primary outcome did not differ by the other potential effect modifiers (Table 3, Supplementary Results, Supplementary Table 9, Supplementary Figures 7 and 8).
DISCUSSION
We detected no overall effect of OPV on the risk of the composite outcome, but as observed in previous studies, OPV had beneficial effects in males; these were not seen in females. Having a BCG scar has been associated with reduced all-cause mortality, and we observed that indeed BCG scar was associated with lower mortality and morbidity, but this was particularly seen in those who received OPV. To our knowledge, this is the first study to assess potential interaction between these 2 live vaccines in adults. The sex- and BCG scar-differential effects were consistent across the main (composite) outcome and its separate constituents. In contrast to the overall effects estimated for the severe morbidity outcomes, the reported rates of symptoms of infection were higher in the OPV than in the control group, which may reflect a more active immune system.
Strengths and Weaknesses
We performed the first randomized trial of OPV as a potential tool to mitigate the risk of illness and mortality during the COVID-19 pandemic. Although the trial was randomized, the high and unevenly distributed refusal rate posed a challenge. However, because adjusting for background factors had no impact, we consider the estimated effect as a valid measure of the effect of OPV. Fecal shedding of OPV is common in infants but is less so in adults [22]. Spillover between trial arms was reduced by allocating participants in the same house to the same trial arm.
Because we used a composite outcome, we may have impaired our ability to detect important OPV effects. However, the consistent sex- and BCG-differential effects of OPV observed across the 3 subcomponents of the composite outcome are reassuring. The outcome information was collected using structured interviews by interviewers blind to group allocation. Nevertheless, the lack of blinding may potentially explain an increased likelihood to report symptoms in the OPV group. However, if a differential reporting pattern should have caused the results, we would expect stronger differences when limiting the analysis to information provided by the trial participant. This did not markedly increase the estimated HRs (Supplementary Table 5). More importantly, our main outcome was based on objective endpoints, less susceptible to an impact of (not) blinding.
Because we had a 4-week-long recall period for interviews, we likely underestimated the incidence of symptoms. Reports of childhood illness episodes during a 2-week recall are fewer with 7–13 days recall than with 0–6 days recall [29]. Provided that the same patterns are present in adults, we will have underestimated the rates. However, the effect of the recall period does not differ by group allocation, and we anticipate less impact for more severe episodes.
Several of our measures are observer dependent. Although interviewers are trained in scar reading, the registered prevalence of vaccination scars differed by team. The reporting rates of outcomes also differed by interviewer. However, our results were robust to excluding enrollments performed by the team with fewer BCG scars and excluding interviews performed by the interviewer with outlying event rates.
When we planned the trial, we had anticipated that COVID-19 would be a major contributor to the disease burden. However, the diagnosis “COVID-19” was not registered for any of the consultations in the health facilities, and only 2 of the 590 trial participants who had been tested reported a positive test. Stigma relating to the COVID-19 diagnosis may have led to underreporting of positive tests. Furthermore, it is also likely that there are many undetected cases, both due to (1) low testing intensity among people with symptoms and (2) asymptomatic cases [30]. Among the tested BHP employees, among whom 18% had serological indication of past COVID-19, only half with past COVID-19 reported an illness episode, and only 12% reported having had a positive PCR test [19].
The COVID-19 vaccines became available in Guinea-Bissau during the last weeks of our trial enrollments. Because the number of vaccines available before the end of the trial was low, and because healthcare personnel were prioritized, we expect that few have received the vaccine before the end of follow up. We did not collect the information on COVID-19 vaccines, and therefore we cannot assess potential interactions.
Consistency With Other Studies
When we initiated the trial, only 1 prior study had investigated the effects of OPV in adults [13]. Very recently, a trial among 1115 adult volunteers randomized to OPV or placebo in Russia demonstrated a significantly lower number of PCR-confirmed COVID-19 cases during 3 months of follow up, but it found no effect on the more frequent self-reported airway infections [31]. Observational studies have also investigated whether OPV confers protection against COVID-19. In Iran, women indirectly exposed to OPV (through their vaccinated infants) had lower risk of COVID-19 than their age, parity, and residency matched controls [32]. In an ecological study, researchers found lower incidence of COVID-19 in countries using OPV [33], although residual confounding may (partly) explain the effects.
In line with our study, prior studies have indicated sex-differential effects of OPV in children with more pronounced benefits in males than in females [3, 9, 11]. Sex-differential effects of OPV on morbidity in adults have not been assessed, but a recent study has identified a potential protective effect of measles-mumps-rubella vaccines against COVID-19 limited to men [34].
Prior trials have demonstrated that BCG vaccination may affect the response to other unrelated viruses [35] and vaccines [36] through epigenetic pathways. In a recent study, researchers showed that the epigenetic methylation patterns induced by BCG remain detectable for >1 year after vaccination [37]. Epidemiological studies have shown that the effect of BCG vaccination may persist for decades [24].
Interpretation
Ample evidence before the pandemic supports that OPV has beneficial NSEs. Most studies were conducted in children. All studies conducted during the COVID-19 pandemic, although they were of various designs, corroborated that OPV had beneficial nonspecific effects. OPV was associated with an increased rate of reported symptoms of infections. Although OPV may slightly increase symptomatology, it seems to specifically reduce the risk of more severe outcomes. The increased symptomatology may reflect a more active immune system. Only our study reported data by gender and BCG scar status; hopefully, the results of the present study will inspire additional analyses in other data.
The duration of OPV's effect is unknown. Because the effect on the more severe outcomes (mortality and admissions) tended to be stronger during the first 3 months (Supplementary Figure 8), an effect may have been diluted with 6 months of follow up.
CONCLUSIONS
In this cluster-randomized trial, we found no overall positive effect of OPV on severe morbidity in adults aged >50 years during the COVID-19 pandemic. However, OPV seemed to benefit men and BCG scar-positive participants. Whether the observed increased rates of reported symptoms of infections is an indication that OPV makes recipients more reactogenic, but potentially able to clear infections better, compels further studies. Our findings furthermore stress the importance of (1) investigating and understanding health interventions separately for men and women and (2) considering interactions between health interventions with effects on the immune system. The result of the present and previous studies during the pandemic supports that OPV may have a role as a stop-gap vaccine during pandemics.
Supplementary Material
Acknowledgments
We sincerely thank all trial participants, collaborators at health centers, and the community health program for contribution to the trial. We are indebted to Christine S. Benn and Peter Aaby for their roles in conceptualizing the trial and commenting on the results. We thank the Bandim Health Project data collectors and nurses for their efforts in collecting the data.
Author contributions. A. B. F., J. S. D. M., L. M. N., A. M. J., S. N., C. L. M., and A. R. contributed to the conceptualization, study design, and methods. A. B. F., J. S. D. M., L. M. N., A. M. J., and E. J. C. C. contributed to the acquisition of data. A. B. F. and J. S. D. M. accessed and verified enrollment data, and A. B. F. and S.N. accessed and verified follow-up data. A. B. F. analyzed the data and wrote the first draft of the report with input from J. S. D. M., A. M. J., and S. N. All authors contributed to the review and editing of the manuscript.
Financial support. This work was funded by the University of Southern Denmark. A. B. F. was supported by an Ascending Investigator Grant (R313-2019-635) from Lundbeck Foundation and a Sapere Aude Grant (9060-00018B) from Independent Research Fund Denmark.
Contributor Information
Ane B Fisker, Bandim Health Project, INDEPTH Network, Bissau, Guinea-Bissau; Bandim Health Project, OPEN, Odense Patient data Explorative Network, Institute of Clinical Research, Odense University Hospital/University of Southern Denmark, Odense, Denmark.
Justiniano S D Martins, Bandim Health Project, INDEPTH Network, Bissau, Guinea-Bissau.
Line M Nanque, Bandim Health Project, INDEPTH Network, Bissau, Guinea-Bissau; Bandim Health Project, OPEN, Odense Patient data Explorative Network, Institute of Clinical Research, Odense University Hospital/University of Southern Denmark, Odense, Denmark.
Andreas M Jensen, Bandim Health Project, INDEPTH Network, Bissau, Guinea-Bissau; Bandim Health Project, OPEN, Odense Patient data Explorative Network, Institute of Clinical Research, Odense University Hospital/University of Southern Denmark, Odense, Denmark.
Elsi J C Ca, Bandim Health Project, INDEPTH Network, Bissau, Guinea-Bissau.
Sebastian Nielsen, Bandim Health Project, INDEPTH Network, Bissau, Guinea-Bissau; Bandim Health Project, OPEN, Odense Patient data Explorative Network, Institute of Clinical Research, Odense University Hospital/University of Southern Denmark, Odense, Denmark.
Cesario L Martins, Bandim Health Project, INDEPTH Network, Bissau, Guinea-Bissau.
Amabelia Rodrigues, Bandim Health Project, INDEPTH Network, Bissau, Guinea-Bissau.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Benn CS, Fisker AB, Rieckmann A, Sorup S, Aaby P. Vaccinology: time to change the paradigm? Lancet Infect Dis 2020; 20:e274–83. [DOI] [PubMed] [Google Scholar]
- 2. Andersen A, Fisker AB, Nielsen S, Rodrigues A, Benn CS, Aaby P. National immunization campaigns with oral polio vaccine may reduce all-cause mortality: an analysis of 13 years of demographic surveillance data from an urban African area. Clin Infect Dis 2021; 72:e596–603. [DOI] [PubMed] [Google Scholar]
- 3. Andersen A, Fisker AB, Rodrigues A, et al. National immunization campaigns with oral polio vaccine reduce all-cause mortality: a natural experiment within seven randomized trials. Front Public Health 2018; 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Welaga P, Oduro A, Debpuur C, et al. Fewer out-of-sequence vaccinations and reduction of child mortality in Northern Ghana. Vaccine 2017; 35:2496–503. [DOI] [PubMed] [Google Scholar]
- 5. Nielsen S, Khalek MA, Benn CS, Aaby P, Hanifi SMA. National immunisation campaigns with oral polio vaccine may reduce all-cause mortality: analysis of 2004–2019 demographic surveillance data in rural Bangladesh. EClinicalMedicine 2021; 36:100886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schoeps A, Nebie E, Fisker AB, et al. No effect of an additional early dose of measles vaccine on hospitalization or mortality in children: a randomized controlled trial. Vaccine 2018; 36:1965–71. [DOI] [PubMed] [Google Scholar]
- 7. Sorup S, Stensballe LG, Krause TG, Aaby P, Benn CS, Ravn H. Oral polio vaccination and hospital admissions with non-polio infections in Denmark: nationwide retrospective cohort study. Open Forum Infect Dis 2016; 3:ofv204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nielsen S, Sujan HM, Benn CS, Aaby P, Hanifi SMA. Oral polio vaccine campaigns may reduce the risk of death from respiratory infections. Vaccines (Basel) 2021; 9:1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lund N, Andersen A, Hansen AS, et al. The effect of oral polio vaccine at birth on infant mortality: a randomized trial. Clin Infect Dis 2015; 61:1504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lund N, Biering-Sorensen S, Andersen A, et al. Neonatal vitamin A supplementation associated with a cluster of deaths and poor early growth in a randomised trial among low-birth-weight boys of vitamin A versus oral polio vaccine at birth. BMC Pediatr 2014; 14:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Upfill-Brown A, Taniuchi M, Platts-Mills JA, et al. Nonspecific effects of oral polio vaccine on diarrheal burden and etiology among Bangladeshi infants. Clin Infect Dis 2017; 65:414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seppala E, Viskari H, Hoppu S, et al. Viral interference induced by live attenuated virus vaccine (OPV) can prevent otitis media. Vaccine 2011; 29:8615–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Voroshilova MK. Potential use of nonpathogenic enteroviruses for control of human disease. Prog Med Virol 1989; 36:191–202. [PubMed] [Google Scholar]
- 14. Chumakov K, Benn CS, Aaby P, Kottilil S, Gallo R. Can existing live vaccines prevent COVID-19? Science 2020; 368:1187–8. [DOI] [PubMed] [Google Scholar]
- 15. Global Polio Eradication Initiative . The use of oral polio vaccine (OPV) to prevent SARS-CoV2. Available at: http://polioeradication.org/wp-content/uploads/2020/03/Use-of-OPV-and-COVID-20200421.pdf. Accessed 12 March 2022.
- 16. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Bank . Country profile. Available at: https://data.worldbank.org/country/guinea-bissau?view=chart. Accessed 20 March 2022.
- 18. Engell-Sorensen T, Rieckmann A, Medina C, et al. Life expectancy of HIV-infected patients followed at the largest hospital in Guinea-Bissau is one-fourth of life expectancy of the background population. Infection 2021; 49:631–43. [DOI] [PubMed] [Google Scholar]
- 19. Benn CS, Salinha A, Mendes S, et al. SARS-CoV-2 serosurvey among adults involved in healthcare and health research in Guinea-Bissau, West Africa. Public Health 2022; 203:19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mogensen SW, Andersen A, Rodrigues A, Benn CS, Aaby P. The introduction of diphtheria-tetanus-pertussis and oral polio vaccine among young infants in an urban African community: a natural experiment. EBioMedicine 2017; 17:192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aaby P, Hedegaard K, Sodemann M, et al. Childhood mortality after oral polio immunisation campaign in Guinea-Bissau. Vaccine 2005; 23:1746–51. [DOI] [PubMed] [Google Scholar]
- 22. Holubar M, Troy SB, Nathoo K, et al. Shedding of oral poliovirus vaccine (OPV) by HIV-infected and -uninfected mothers of OPV-vaccinated Zimbabwean infants. J Pediatric Infect Dis Soc 2017; 6:105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. UN News . Após ser vacinado, presidente da Guiné-Bissau pede que todos sejam imunizados. Available at: https://news.un.org/pt/story/2021/04/1746852. Accessed 3 April 2022.
- 24. Rieckmann A, Villumsen M, Sorup S, et al. Vaccinations against smallpox and tuberculosis are associated with better long-term survival: a Danish case-cohort study 1971–2010. Int J Epidemiol 2017; 46:695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jensen ML, Dave S, van der Loeff MS, et al. Vaccinia scars associated with improved survival among adults in rural Guinea-Bissau. PLoS One 2006; 1:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aaby P, Gustafson P, Roth A, et al. Vaccinia scars associated with better survival for adults. An observational study from Guinea-Bissau. Vaccine 2006; 24:5718–25. [DOI] [PubMed] [Google Scholar]
- 27. Cohen E, Bernard JY, Ponty A, et al. Development and validation of the body size scale for assessing body weight perception in African populations. PLoS One 2015; 10:e0138983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williamson E, Morley R, Lucas A, Carpenter J. Propensity scores: from naive enthusiasm to intuitive understanding. Stat Methods Med Res 2012; 21:273–93. [DOI] [PubMed] [Google Scholar]
- 29. Feikin DR, Audi A, Olack B, et al. Evaluation of the optimal recall period for disease symptoms in home-based morbidity surveillance in rural and urban Kenya. Int J Epidemiol 2010; 39:450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sah P, Fitzpatrick MC, Zimmer CF, et al. Asymptomatic SARS-CoV-2 infection: a systematic review and meta-analysis. Proc Natl Acad Sci U S A 2021; 118:e2109229118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yagovkina NV, Zheleznov LM, Subbotina KA, et al. Vaccination with oral polio vaccine reduces COVID-19 incidence. Front Immunol 2022; 13:907341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Habibzadeh F, Sajadi MM, Chumakov K, et al. COVID-19 infection among women in Iran exposed vs unexposed to children who received attenuated poliovirus used in oral polio vaccine. JAMA Netw Open 2021; 4:e2135044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Habibzadeh F, Chumakov K, Sajadi MM, et al. Use of oral polio vaccine and the incidence of COVID-19 in the world. PLoS One 2022; 17:e0265562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lundberg L, Bygdell M, von Feilitzen GS, et al. Recent MMR vaccination in health care workers and COVID-19: a test negative case-control study. Vaccine 2021; 39:4414–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moorlag S, Arts RJW, van Crevel R, Netea MG. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect 2019; 25:1473–8. [DOI] [PubMed] [Google Scholar]
- 36. Arts RJW, Moorlag S, Novakovic B, et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 2018; 23:89–100.e5. [DOI] [PubMed] [Google Scholar]
- 37. Bannister S, Kim B, Dominguez-Andres J, et al. Neonatal BCG vaccination is associated with a long-term DNA methylation signature in circulating monocytes. Sci Adv 2022; 8:eabn4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.