Abstract
We describe the case of a patient with AIDS who had persistent infection with a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Delta variant for >80 days. The variant contained mutations that were not present in other Delta viruses in our hospital. Prolonged infection in immunosuppressed individuals may lead to evolution of SARS-CoV-2 lineages.
Keywords: COVID-19; SARS-CoV2, PLWH, HIV
A 45-year-old partially vaccinated man with a history of untreated human immunodeficiency virus (HIV) infection presented to an outside hospital in December 2021 with 3 days of fever, chills, nonproductive cough, dyspnea, left-sided chest pain, nausea, and emesis. He was afebrile and mildly tachycardic with normal oxygen saturation. He had received a single dose of the Pfizer BNT162b messenger RNA severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine 8 months earlier. Upon hospital presentation, SARS-CoV-2 polymerase chain reaction (PCR) was positive. Chest radiography was unremarkable. He was not given antiviral therapy or monoclonal antibodies in the emergency department and was discharged with instructions to isolate. His symptoms improved over the course of a few days, but repeat SARS-CoV-2 testing was not performed.
He was transferred to our tertiary care center for treatment of orbital cellulitis in February 2022. A SARS-CoV-2 PCR test was positive with a cycle threshold (Ct) value of 21.8, 72 days after the initial positive test. His symptoms included severe frontal headache and throbbing periorbital pain, but he denied other symptoms of coronavirus disease 2019 (COVID-19) infection. His oxygen saturation was normal and his chest radiograph was unremarkable. He was admitted to a negative-pressure room for treatment of orbital cellulitis.
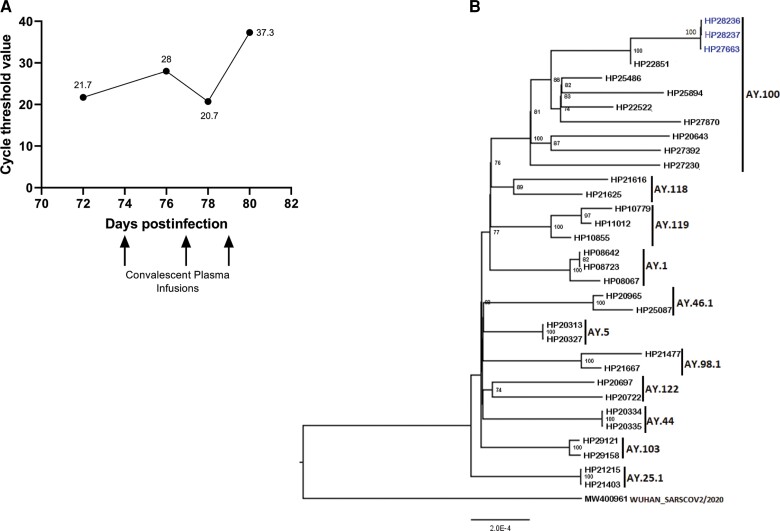
CD4+ T-cell count and HIV viral load were found to be 2 cells/µL and 56 200 copies RNA/mL, respectively. SARS-CoV-2 immunoglobulin G and immunoglobulin A levels were below the limit of detection. Viral sequencing was performed on the SARS-CoV-2 isolates from February 2022 and was consistent with the Delta variant, which was no longer circulating in February 2022 in Baltimore [1]. He had not traveled outside the city since his initial infection, making it likely that he had persistent viral infection since December 2021. He received 3 transfusions of high-titer convalescent plasma, which was collected in August and September 2021 in Florida (One Blood). SARS-CoV-2 PCR testing with Ct determination was performed and the Ct value was 37.3 (Figure 1A). He was started on antiretroviral therapy and was discharged to an isolation unit. He did not follow through with repeat testing after 20 days of isolation.
Figure 1.
A, Trend of severe acute respiratory syndrome coronavirus 2 cycle threshold values with infusions of convalescent plasma. B, Whole-genome sequence analysis of the virus isolated from the patient. The phylogenetic tree was generated using a maximum likelihood algorithm with the GTR + F nucleotide substitution model using IQ-TREE 2 to estimate the evolutionary distances. The statistical significance was tested by 1000 bootstrapping replicates. Bootstrap values >70% are shown at the branch nodes. The whole-genome sequence of isolates from our patient is colored blue and the other Delta complete genome from the laboratory is colored black. The tree is rooted by the Wuhan strain genome.
This case is an example of prolonged viral replication in a patient with advanced HIV infection. The low Ct value at the time of his admission in February suggests that substantial viral replication was occurring >2 months after his initial diagnosis. Phylogenetic analysis revealed that his complete genome sequences belonged to clade 21J (Delta) and lineage AY.100 (Figure 1B). Additionally, the phylogenetic tree showed that this strain was more evolved than other strains of the AY.100 lineage sequenced at Johns Hopkins Hospital during the same period. This observation is in line with a prior study in which phylogenetic analysis confirmed a persistent infection with accelerated viral evolution, with the majority of mutations occurring in the spike protein and receptor-binding domain (RBD) [2]. In addition, infrequently encountered amino acid changes such as V3G, L18F, H245P, and E484K were observed in the spike of our strain compared to other Delta variant isolates from our hospital (Supplementary Figures 1 and 2). The E484K mutation, which improves the affinity of the spike protein RBD for angiotensin-converting enzyme 2 [3], is rarely seen in the Delta variant [4] and the V3G mutation is now commonly seen in the Omicron BA.4 sublineage [5]. Thus, these mutations could be a sign of viral evolution in this patient. Interestingly, he did not seroconvert despite partial vaccination and prolonged infection. This is probably due to his advanced HIV infection, as partial vaccination has been shown to induce detectable antibody responses in healthy donors [6, 7]. A recent small case series reported that treatment with convalescent plasma was not effective in people living with HIV (PLWH) [8]. However, the convalescent plasma given to our patient, obtained from patients who were probably infected during the Delta variant surge, was effective at inhibiting SARS-CoV-2 replication.
REVIEW OF THE LITERATURE
Persistence of SARS-CoV-2 in immunosuppressed patients has been reported. For example, an early case report by Choi et al described an immunosuppressed individual with severe anti-phospholipid syndrome complicated by diffuse alveolar hemorrhage with persistent SARS-CoV-2 who ultimately died on day 154 of infection [2]. Similar reports of prolonged SARS-CoV-2 shedding have been described in patients with hematologic malignancies [9, 10]. There have been several reports of persistent SARS-CoV-2 infection in PLWH [11–27]. One cohort study in China found the median duration of SARS-CoV-2 shedding in PLWH was 30 days [11]. The majority of PLWH with prolonged shedding described in case reports have advanced HIV infection. In 13 cases where clinical information was available, the patients had a median CD4 count of 6 cells/µL and median viral load of 378 000 copies/mL (Table 1). This is consistent with the findings in a cohort in South Africa where prolonged shedding of high levels of virus (defined as Ct value <30) in PLWH was associated with low CD4+ T-cell counts (median duration of 27 days in patients with CD4 counts <200 cells/µL vs 7 days in patients with CD4 counts >200 cells/µL) and uncontrolled viral replication (median duration of 26 days in patients with viral loads >400 copies/mL vs 6 days in patients with viral loads <400 copies/mL) [26].
Table 1.
Cases of Persistent Severe Acute Respiratory Syndrome Coronavirus 2 Shedding in the Literature
| Time of SARS-CoV-2 RNA Positivity | Age | Sex | CD4 Count, Cells/µL | Viral Load, Copies/mL | ART Regimen at Symptom Onset | Treatment | Author |
|---|---|---|---|---|---|---|---|
| 9 mo | 22 | F | 91 | 5.07 log10 | None | ART | Maponga et al [12] |
| 147 d | 54 | M | 25 | 930 000 | None | Remdesivir, ART | Giubelan et al [13] |
| 92 d | 30 | M | 49 | <20 | Lamivudine, dolutegravir | Prednisone, remdesivir, convalescent plasma, sotrovimab | Montejano et al [14] |
| 66 d | 28 | M | 3 | 563 000 | None | ART | Álvarez et al [15] |
| 98 d | 30 | M | 5 | 109 859 | None | NA | Wenlock et al [16] |
| 85 d | 28 | M | 3 | NA | None | ART | Yousaf et al [17] |
| 216 d | Late 30s | F | 6 | 34 151 | Tenofovir, emtricitabine, and efavirenz | Dexamethasone, new ART regimen | Karim et al [18] |
| 109 d | NA | NA | 3 | 558 000 | None | ART, Pfizer vaccine, bamlanivimab, and etesevimab | Quaranta et al [19] |
| 164 d | 28 | F | NA | NA | Regimen not specified | ART | Maan et al [20] |
| 3 mo | 38 | M | <1 | 980 000 | None | ART, convalescent plasma | Ketels et al [21] |
| 232 d | 38 | M | 663 | <20 | Regimen not specified | None | Cunha et al [22] |
| 107 d | 26 | F | 2 | 198 000 | None | Dexamethasone, ART, convalescent plasma, bamlanivimab, and etesevimab | Spinicci et al [23] |
| 34 d | 49 | F | >600 | <20 | Lamivudine, zidovudine, efavirenz | Interferon, ribavirin, abidol | Menghua et al [24] |
| 2 mo | 40s | NA | 19 | 975 000 | None | ART | Ridell et al [25] |
| 3 mo | 40s | NA | NA | NA | Regimen not specified | New ART regimen | Ridell et al [25] |
| 8.5 mo | 30s | NA | NA | NA | Regimen not specified | New ART regimen, remdesivir | Ridell et al [25] |
Abbreviations: ART, antiretroviral therapy; F, female; M, male; NA, information not available in manuscript; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Three patients described in case reports were diagnosed with Kaposi sarcoma [13, 21, 23], and 2 patients had diffuse large B-cell lymphoma with 1 receiving chemotherapy [14, 20]. Opportunistic infections were common, with Pneumocystis jirovecii diagnosed in 2 cases [15, 16, 21] and suspected in another 4 [16, 17, 18, 25]. There were also 2 cases of Mycobacterium avium-intracellulare infection [15, 25] as well as oral and esophageal candidiasis [13, 23], central nervous system toxoplasmosis [23], cytomegalovirus reactivation [23], and cryptosporidiosis [25]. However, in addition to these severely immunocompromised patients, there have been reports of patients with high CD4+ T-cell count and undetectable HIV viral loads with prolonged SARS-CoV-2 shedding [22, 24].
Antiretroviral therapy was initiated in most patients (Table 1), and several patients received antivirals [13, 14, 24, 25]. Passive antibody therapy likely assisted in lowering the viral quantity in some cases. Convalescent plasma with high titers of neutralizing antibodies is most effective when provided early in the disease course [28]. It was effective in our patient and was associated with an in increase in Ct values over a 7-week course in another patient [21] but was ineffective in 2 other cases [14, 23]. The combination of bamlanivimab and etesevimab was given to 2 patients [19, 23] and in 1 case was associated with clearance 3 weeks later [23]. Sotrovimab was associated with clearance over a 2-week period in a case where remdesivir and convalescent plasma had been ineffective [14].
It has been proposed that highly mutated variants evolving in immunocompromised persons could be a key factor in the emergence of new variants of concern [29]. There have been several reports of the accumulation of SARS-CoV-2 mutations in PLWH with prolonged shedding [12, 19, 22, 25]. Karim et al documented persistent SARS-CoV-2 infection confirmed by phylogenetic analysis of whole genomes at 7 time points across 6 months in a patient with AIDS in South Africa [18]. The ancestral virus found in the patient accumulated mutations present in the Omicron variant and eventually developed resistance to neutralization by vaccine-elicited antibodies [27]. These cases illustrate how persistent infection can lead to immune escape and potentially to new variants.
CONCLUSIONS
This phenomenon of persistent SARS-CoV-2 infection has important public health implications including the need to vaccinate PLWH. COVID vaccination results in robust antibody responses [30–33] and a relatively low rate of breakthrough infections [34] in PLWH on suppressive ART regimens. Patients with advanced HIV disease are more likely to have persistent infection; thus, efforts should be made to minimize transmission of SARS-CoV-2 from these individuals. There is a need for additional research on therapeutic strategies to inhibit persistent viral replication.
Supplementary Material
Contributor Information
Jillian L Peters, Department of Medicine, Johns Hopkins Medicine, Baltimore, Maryland, USA.
Amary Fall, Department of Pathology, Johns Hopkins Medicine, Baltimore, Maryland, USA.
Steven D Langerman, Department of Medicine, Johns Hopkins Medicine, Baltimore, Maryland, USA.
Margueritta El Asmar, Department of Medicine, Johns Hopkins Medicine, Baltimore, Maryland, USA.
Mari Nakazawa, Department of Medicine, Johns Hopkins Medicine, Baltimore, Maryland, USA.
Aishat Mustapha, Department of Medicine, Johns Hopkins Medicine, Baltimore, Maryland, USA.
Aaron A R Tobian, Department of Pathology, Johns Hopkins Medicine, Baltimore, Maryland, USA.
Heba H Mostafa, Department of Pathology, Johns Hopkins Medicine, Baltimore, Maryland, USA.
Joel N Blankson, Department of Medicine, Johns Hopkins Medicine, Baltimore, Maryland, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Patient consent. Informed consent was obtained from the patient. The study protocol was approved by the Johns Hopkins University Institutional Review Board.
Financial support. This work was supported by the Center of Excellence in Influenza Research and Surveillance, National Institute of Allergy and Infectious Diseases, National Institutes of Health (contract number HHS N2772201400007C); National Institute of Allergy and Infectious Diseases, National Institutes of Health (R21AI167705), Johns Hopkins University, Maryland Department of Health, Centers for Disease Control and Prevention (contract number 75D30121C11061); the Johns Hopkins COVID-19 Vaccine-Related Research Fund; and the Johns Hopkins Center for AIDS Research.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention . COVID data tracker. https://covid.cdc.gov/covid-data-tracker/#variant-proportions. Accessed 9 June 2022.
- 2. Choi B, Choudhary MC, Reagan J, et al. . Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med 2020; 383:2291–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barton MI, MacGowan SA, Kutuzov MA, et al. . Effects of common mutations in the SARS-CoV-2 spike RBD and its ligand, the human ACE2 receptor on binding affinity and kinetics. Elife 2021; 10:e70658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baj A, Novazzi F, Pasciuta R, et al. . Breakthrough infections of E484K-harboring SARS-CoV-2 Delta variant, Lombardy, Italy. Emerg Infect Dis 2021; 27:3180–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caputo E, Mandrich L. Structural and phylogenetic analysis of SARS-CoV-2 spike glycoprotein from the most widespread variants. Life (Basel) 2022; 12:1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krammer F, Srivastava K, Alshammary H, et al. . Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA 2021; 325:1467–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krammer F, Srivastava K, Alshammary H, et al. . Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med 2021; 384:1372–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silvera RJ, Lin H, Rahman F, et al. . Hospitalized patients with HIV and COVID-19 receiving convalescent plasma: a case series. World Acad Sci J 2022; 4:25. [Google Scholar]
- 9. Laracy JC, Kamboj M, Vardhana SA. Long and persistent COVID-19 in patients with hematologic malignancies: from bench to bedside. Curr Opin Infect Dis 2022; 35:271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dioverti MV, Gaston DC, Morris CP, et al. . Combination therapy with casirivimab/imdevimab and remdesivir for protracted SARS-CoV-2 infection in B-cell-depleted patients. Open Forum Infect Dis 2022; 9:ofac064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang J, Xie N, Hu X, et al. . Epidemiological, virological and serological features of coronavirus disease 2019 (COVID-19) cases in people living with human immunodeficiency virus in Wuhan: a population-based cohort study. Clin Infect Dis 2021; 73:e2086–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maponga TG, Jeffries M, Tegally H, et al. . Persistent SARS-CoV-2 infection with accumulation of mutations in a patient with poorly controlled HIV infection [manuscript published online ahead of print 6 July 2022]. Clin Infect Dis 2022. doi: 10.1093/cid/ciac548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giubelan L, Stanciu I, Ilie C, Pădureanu V. Persistent RNA SARS-CoV-2 detection in a HIV-infected patient. Healthcare (Basel) 2022; 10:982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Montejano R, Marcelo C, Falces-Romero I, et al. . Efficacy of sotrovimab for persistent coronavirus disease-2019 in a severely immunocompromised person living with HIV. AIDS 2022; 36:751–3. [DOI] [PubMed] [Google Scholar]
- 15. Álvarez H, Ruiz-Mateos E, Juiz-González PM, et al. . SARS-CoV-2 evolution and spike-specific CD4+ T-cell response in persistent COVID-19 with severe HIV immune suppression. Microorganisms 2022; 10:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wenlock RD, Brown CS, Iwuji C, Vera JH. Can I go back to work? A case of persistent SARS-CoV-2 with advanced untreated HIV infection. Int J STD AIDS 2022; 33:209–11. [DOI] [PubMed] [Google Scholar]
- 17. Yousaf M, Hameed M, Alsoub H, et al. . COVID-19: prolonged viral shedding in an HIV patient with literature review of risk factors for prolonged viral shedding and its implications for isolation strategies. Clin Case Rep 2021; 9:1397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karim F, Moosa MYS, Gosnell BI, et al. . Persistent SARS-CoV-2 infection and intra-host evolution in association with advanced HIV infection. medRxiv [Preprint]. Posted online 4 June2021. doi: 10.1101/2021.06.03.21258228 [DOI] [Google Scholar]
- 19. Quaranta EG, Fusaro A, Giussani E, et al. . SARS-CoV-2 intra-host evolution during prolonged infection in an immunocompromised patient. Int J Infect Dis 2022; 122:444–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maan I, Paraskevopoulou SM, Cwynarski K, et al. . Prolonged SARS-CoV-2 shedding in a person living with advanced HIV and diffuse large B-cell lymphoma: a case report. Infect Dis (Lond) 2022; 54:529–33. [DOI] [PubMed] [Google Scholar]
- 21. Ketels T, Gisolf J, Claassen M, et al. . Short communication: prolonged COVID-19 infection in a patient with newly diagnosed HIV/AIDS. AIDS Res Hum Retroviruses 2022; 38:399–400. [DOI] [PubMed] [Google Scholar]
- 22. Cunha MDP, Vilela APP, Molina CV, et al. . Atypical prolonged viral shedding with intra-host SARS-CoV-2 evolution in a mildly affected symptomatic patient. Front Med (Lausanne) 2021; 8:760170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spinicci M, Mazzoni A, Borchi B, et al. . AIDS patient with severe T cell depletion achieved control but not clearance of SARS-CoV-2 infection. Eur J Immunol 2022; 52:352–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Menghua W, Xin Z, Jianwei L, Yu Z, Qinwei Y. Case report: one case of coronavirus disease 2019 (COVID-19) in a patient co-infected by HIV with a normal CD4+ T cell count. AIDS Res Ther 2020; 17:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Riddell AC, Kele B, Harris K, et al. . Generation of novel SARS-CoV-2 variants on B.1.1.7 lineage in three patients with advanced HIV disease [manuscript published online ahead of print 25 May 2022]. Clin Infect Dis 2022. doi: 10.1093/cid/ciac409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meiring S, Tempia S, Bhiman JN, et al. . Prolonged shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at high viral loads among hospitalized immunocompromised persons living with human immunodeficiency virus (HIV), South Africa. Clin Infect Dis 2022; 75:e144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cele S, Karim F, Lustig G, et al. . SARS-CoV-2 prolonged infection during advanced HIV disease evolves extensive immune escape. Cell Host Microbe 2022; 30:154–62.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tobian AAR, Cohn CS, Shaz BH. COVID-19 convalescent plasma. Blood 2022; 140:196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kemp SA, Collier DA, Datir RP, et al. . SARS-CoV-2 evolution during treatment of chronic infection. Nature 2021; 592:277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frater J, Ewer KJ, Ogbe A, et al. . Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV 2021; 8:e474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Levy I, Wieder-Finesod A, Litchevsky V, et al. . Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clin Microbiol Infect 2021; 27:1851–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Woldemeskel BA, Karaba AH, Garliss CC, et al. . The BNT162b2 mRNA vaccine elicits robust humoral and cellular immune responses in people living with human immunodeficiency virus (HIV). Clin Infect Dis 2022; 74:1268–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Woldemeskel BA, Karaba AH, Garliss CC, et al. . Decay of coronavirus disease 2019 mRNA vaccine-induced immunity in people with HIV. AIDS 2022; 36:1315–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Coburn SB, Humes E, Lang R, et al. . Analysis of postvaccination breakthrough COVID-19 infections among adults with HIV in the United States. JAMA Netw Open 2022; 5:e2215934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.