Abstract
Background
Some patients with inflammatory bowel disease (IBD) on immunosuppressive therapies may have a blunted response to certain vaccines, including the messenger RNA (mRNA) coronavirus disease 2019 (COVID-19) vaccines. However, few studies have evaluated the cell-mediated immune response (CMIR), which is critical to host defense after COVID-19 infection. The aim of this study was to evaluate the humoral immune response and CMIR after mRNA COVID-19 vaccination in patients with IBD.
Methods
This prospective study (HERCULES [HumoRal and CellULar initial and Sustained immunogenicity in patients with IBD] study) evaluated humoral immune response and CMIR after completion of 2 doses of mRNA COVID-19 vaccines in 158 IBD patients and 20 healthy control (HC) subjects. The primary outcome was the CMIR to mRNA COVID-19 vaccines in patients with IBD. The secondary outcomes were a comparison of (1) the CMIR in patients with IBD and HC subjects, (2) CMIR and humoral immune response in all participants, and (3) correlation between CMIR and humoral immune response.
Results
The majority (89%) of patients with IBD developed a CMIR, which was not different vs HC subjects (94%) (P = .6667). There was no significant difference (P = .5488) in CMIR between immunocompetent (median 255 [interquartile range, 146-958] spike T cells per million peripheral blood mononuclear cells) and immunosuppressed patients (median 377 [interquartile range, 123-1440]). There was no correlation between humoral and cell-mediated immunity after vaccination (P = .5215). In univariable analysis, anti-tumor necrosis factor therapy was associated with a higher CMIRs (P = .02) and confirmed in a multivariable model (P = .02). No other variables were associated with CMIR.
Conclusions
Most patients with IBD achieved CMIR to a COVID-19 vaccine. Future studies are needed evaluating sustained CMIR and clinical outcomes.
Keywords: Crohn’s disease, ulcerative colitis, immune response
Key Messages.
What is already known?
Most patients with inflammatory bowel disease (IBD) will have an antibody response to coronavirus disease 2019 (COVID-19) vaccines despite being on immune-modifying therapies.
What is new here?
Most patients with IBD will produce a cell-mediated immune response (CMIR) to COVID-19 vaccines. Immune-modifying therapies do not appear to blunt CMIR, and those on anti-tumor necrosis factor therapy will have a stronger CMIR.
How can this study help patient care?
Our study should reassure providers that immune-modifying therapies used to treat IBD do not appear to affect the CMIR to COVID-19 vaccine, unlike other immunosuppressed populations.
Introduction
Two messenger RNA (mRNA) coronavirus disease 2019 (COVID-19) vaccines, mRNA-1273 (Moderna) and BNT162b2 (Pfizer-BioNTech), are highly effective in the general population.1 However, the pivotal trials that evaluated the efficacy of these vaccines excluded patients with inflammatory bowel disease (IBD) and other immunosuppressed populations, who may have a lower immune response to selected vaccines.2-4 These vaccines have been found to be safe in patients with IBD with similar rates of localized and systemic adverse events as found in the general population. Additionally, rates of IBD flares following vaccination are low (2%).5,6 Among immunosuppressed solid organ transplant recipients, seroconversion after COVID-19 vaccines is suboptimal. For example, among 658 transplant recipients, only 54% mounted a humoral immune response after vaccination.7 In contrast, 95% to 99% of patients with IBD have measurable antibody responses after the 2-dose mRNA vaccine series.8-10 However, selected patients have an impaired immune response to the COVID-19 vaccine. The Partnership to Report Effectiveness of Vaccination in populations Excluded from iNitial Trials of COVID (PREVENT-COVID) trial observed that lower seroconversion was associated with old age, the BNT162b2 vaccine, and combination therapy with an anti-tumor necrosis factor α (TNF) inhibitor and an immunomodulator.8 Similarly, a multicenter prospective study from the United Kingdom found that lower antibody concentrations were associated with age and use of infliximab or tofacitinib but not with use of ustekinumab, thiopurines, or vedolizumab.11 The ImpaCt of bioLogic therApy on saRs-cov-2 Infection and immuniTY (CLARITY)-IBD study is a large multicenter study in the United Kingdom evaluating the impact of infliximab and vedolizumab on humoral immunogenicity of 2 doses of COVID-19 vaccines found similar rates of seroconversion regardless of treatment (94% [infliximab] and 98% [vedolizumab]). They found that treatment with infliximab, age >60 years, a diagnosis of Crohn’s disease, and vaccination with a viral vector vaccine ChAdOx1 was associated with lower antibody concentrations.12 Other studies have also found that patients with IBD who were vaccinated with non-mRNA vaccines may have lower rates of seroconversion and lower antibody concentrations.11-14
The HERCULES (HumoRal and CellULar initial and Sustained immunogenicity in patients with IBD) study observed a lower serological response after COVID-19 vaccination in patients with IBD than in healthy control (HC) subjects.10 However, the clinical relevance of these differences is unknown. It has been shown that antibody concentrations wane with time after vaccination, but cellular immunity may persist.15 Additionally, many viral variants of concern may evade humoral immunity, but cellular responses induced by vaccines show strong protection against these variants.16 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–specific cellular immune responses are important for viral clearance, provide robust memory, and mediate recognition of viral variants.15 Few studies of immune responses to vaccine in patients with IBD have focused on evaluating vaccine-induced cell-mediated immune response (CMIR), an important component for protection against viruses such as SARS-CoV-2. The aim of this study was to evaluate the CMIR of COVID-19 vaccine patients with IBD and determine if different immune-modifying therapies may impact CMIR.
Methods
This, prospective, nonrandomized study (HERCULES) enrolled 158 IBD patients and 20 HC subjects.10 Participants with IBD were enrolled at the University of Wisconsin–Madison (UW) and Mayo Clinic Florida (MAYOFL). HC subjects were only enrolled at MAYOFL. Patients with IBD were 18 to 85 years of age and on a stable medication regimen in the maintenance phase for at least 2 months, and had completed an mRNA vaccine series.10 Patients with IBD were categorized into 2 groups. The first group was on nonsystemic immunosuppressive therapy, which included being on no therapy, aminosalicylate monotherapy, or vedolizumab monotherapy. Vedolizumab was considered in this group because previous studies have shown that it does not appear to impact vaccine responses.4,17 The second group was the immunosuppressed group, which consisted of being in 1 of the following treatment groups: the thiopurine therapy group (on azathioprine at least 2 mg/kg or 6-mercaptopurine 1 mg/kg), the anti-TNF therapy group (on maintenance infliximab [at least every 8 weeks], golimumab [at least monthly], adalimumab [at least every 2 weeks], or certolizumab [at least monthly]), the anti-TNF combination therapy group (on anti-TNF therapy as described previously along with either 15 mg of methotrexate or azathioprine at least 1 mg/kg or 6-mercaptopurine 0.5 mg/kg), the ustekinumab therapy group (on either ustekinumab monotherapy or combination therapy with methotrexate or azathioprine), the tofacitinib therapy group (on tofacitinib at least 5 mg twice daily); or the corticosteroid therapy group (on any 1 of the systemic immunosuppressive groups and any dose of corticosteroids). The anti-TNF therapy group was analyzed together regardless of dosing schedule (standard or accelerated) or type of dose (subcutaneous or intravenous), given that previous studies have not shown that they type of therapy impacts vaccine response or if vaccine response was impacted by drug dosing.18,19;
Patients with IBD were excluded if they had a previous known diagnosis of COVID-19 infection or had serological evidence of asymptomatic infection. HC subjects were eligible if they were not on immunosuppressive therapy and had documentation that they completed an mRNA vaccine series. Completion of an mRNA vaccines series was confirmed by review of the Wisconsin Immunization Registry (WIR) for those recruited at UW and via electronic health records for those recruited at MAYOFL. Similar to the original COVID-19 immunogenicity clinical trials, the humoral immune response and CMIR were measured at 28 to 35 days after the 2-dose mRNA series in patients with IBD and at approximately 30 days in HC subjects.20
Wisconsin Immunization Registry
The WIR is a statewide database maintained by the Department of Health and Family Services of the State of Wisconsin in which vaccine data for each Wisconsin resident are stored. The WIR captures 97% of vaccines administered in the state, and 98.5% of Wisconsin residents have an active WIR record. The WIR does not capture vaccines administered outside the state, and all Wisconsin vaccine providers are required to enter record of COVID-19 vaccine administration into the registry.21 The WIR has been previously been used to evaluate COVID-19 vaccine uptake in patients with IBD.22
Outcomes
The primary outcome was the CMIR to mRNA COVID-19 vaccines in patients with IBD. The secondary outcomes were a comparison of the (1) CMIR in patients with IBD and HC subjects, (2) CMIR and humoral immune response in all participants, and (3) correlation between CMIR and humoral immune response.
Humoral immune response measurements
Nucleocapsid and spike protein S1 receptor binding domain–specific IgG antibodies were measured in sera at 28 to 35 days postcompletion of the 2-dose mRNA series in patients with IBD and at approximately 30 days in HC subjects, similar to COVID-19 immunogenicity clinical trials.23
LabCorp’s Cov2Quant immunoglobulin G (IgG) assay uses electrochemiluminescence immunoassay technology for the quantitative measurement of IgG antibodies to SARS-CoV-2. This assay was used to measure the levels of IgG antibodies against S1 receptor binding domain of SARS-CoV-2 (the target of COVID-19 vaccines). Anti-nucleocapsid (indicative of a prior infection) antibodies were measured in all patients with IBD and HC subjects. Anti-nucleocapsid method is qualitative electrochemiluminescence immunoassay by Roche Elecsys platform (Roche Diagnostics). Patients with prior COVID-19 infection (as assessed with a nucleocapsid antibody test) were excluded. The sensitivity and correlation to neutralizing antibodies has been previously described.10
Fluorospot Analysis
Fluorospot assays were performed to quantitate antigen-specific T cells capable of secreting interferon (IFN)-γ with use of the human IFN- γ FluorospotPlus kit (Mabtech). Cryopreserved peripheral blood mononuclear cells were thawed at 37 °C and washed twice with RPMI media with 10% AB serum (Gemini Bio-Products), and their viability was determined by trypan blue exclusion using the Cellometer Vision (Nexcelom Bioscience). Only samples with >85% viability were used in the assay. PMBCs were plated at 2.5 × 105 per well in triplicate in 96-well round bottom plates and incubated at 37 °C, 5% CO2 for 24 hours with complete medium alone, spike protein peptide pools 1 + 2 (1 µg/mL; STEMCELL Technologies ), or phytohemagglutinin (PHA) (7.5 µg/mL, positive control). The SARS-CoV spike protein peptides were in separate 2 pools that consisted of 158 peptides each and consisted of 15-mer peptides with 11 amino acid overlaps that spanned amino acids 1 to 1273 of the spike protein. After 24 hours, cells were transferred to fluorospot plates precoated with anti-IFN- γ and that were blocked for 2 hours with complete media at 37 °C. Plates were incubated for additional 24 hours, washed, and incubated with biotinylated anti-IFN- γ and streptavidin-550 conjugates with washes between each step. After the final wash, plates were incubated for 15 minutes with fluorescence enhancer-II, and after its removal, dried under a hood blower for 15 minutes. Plates were read on an AID ELISpot reader using the Cy3 filter. AID Spot parameters were as follows: intensity (minimum 14, maximum 250), size (minimum 43, maximum 5000), emphasis (small), and algorithm C. Antigen-specific T cells were defined as the average number of spots elicited by the antigen of interest minus the average number of spots elicited with culture medium alone. For each patient, the number of spike-specific T cells was calculated by summing the individual responses to pools 1 and 2. For samples where spots were too numerous to count, spot number was set to 6400. All spot numbers were multiplied by 4 to achieve a standardized spots per million cells. Six patients with IBD and 2 HC subjects were excluded in the final analysis due to lack of PHA response. Although the lack of a PHA could indicate profound therapy-induced immune suppression, it could also indicate poor cell quality or lost sample; thus, the results were not included. One IBD patient was excluded due to prevaccine positive COVID nucleocapsid response.
Data analysis and statistical design
Categorical variables were reported as frequency and percentage and continuous variables were reported as median (interquartile range [IQR]). The Mann-Whitney test was used to compare continuous variables between groups and the Fisher exact test was used to compare categorical variables. Spearman’s test was used to evaluate for correlations between antibody and T cell responses. Univariable linear regression analysis was conducted to assess the association of CMIR with age, sex, and IBD therapy. Multivariable regression was performed to estimate the relationship between age, anti-TNF therapy, vedolizumab, vaccine type, and the CMIR. All tests were 2 sided, with a P value <.05 considered statistically significant. All analysis were performed using R Studio version 4.1.2.
Ethical considerations
The study received Institutional Review Board approval at the UW and MAYOFL.
Results
A greater proportion of HC subjects than patients with IBD (85% vs 54%) received the Pfizer vaccine (Table 1). Most patients with IBD had a diagnosis of Crohn’s disease (n = 106, 67%), were on stable medication regimens (mean 62 months), and were on immunosuppressive therapy (n = 105, 66%).
Table 1.
Baseline Demographics
| IBD Patients (n = 158) | Healthy Control Subjects (n = 20) | P Value | |
|---|---|---|---|
| Age, y | 42 (35-57) | 50 (42-58) | .2462 |
| Male | 79 (50) | 9 (45) | .8133 |
| Vaccine manufacturer | |||
| Moderna | 72 (46) | 3 (15) | .0086 |
| Pfizer | 86 (54) | 17 (85) | |
| Type of IBD | |||
| Crohn’s disease | 106 (67) | — | — |
| Ulcerative colitis | 52 (33) | — | — |
| IBD treatmenta | |||
| Mesalamine monotherapy or no IBD therapy | 18 (11) | — | — |
| Vedolizumab monotherapy | 25 (16) | — | — |
| Thiopurine | 9 (6) | — | — |
| Anti-TNF monotherapy | 61 (39) | — | — |
| Adalimumab | 33 (10)a | — | — |
| Infliximab | 28 (11)a | — | — |
| Anti-TNF combination | 13 (8) | — | — |
| Infliximab | 7 (1)a | — | — |
| Adalimumab | 6 (2)a | — | — |
| Ustekinumab monotherapy or combination | 16 (10) | — | — |
| Tofacitinib | 6 (4) | — | — |
| Corticosteroid therapy (2.5-40 mg/d) | 10 (6) | — | — |
| Duration of immunosuppression | 62.2 ± 56.7 | — | — |
| Postvaccine immune summary | |||
| Postvaccine spike antibody concentration, µg/mL | 34 (17-67), n = 152 evaluable | — | N/A |
| Postvaccine spike antibody concentration, U/mL | — | 2500 (1534-2500), n = 20 evaluable | N/A |
| Postvaccine spike T cell levels (per million PBMCs) | 357 (14-1285), n = 151 evaluable | 576 (112-1717), n = 18 evaluable | .3288 |
| Antibody response | 147 (97) | 18 (100) | 1.000 |
| Cell-mediated immune response ≥50 spots | 130 (89) | 17 (94) | .6997 |
Values are median (interquartile range), n (%), or mean ± SD, unless otherwise indicated.
Abbreviations: IBD, inflammatory bowel disease; N/A, not applicable; PBMC, peripheral blood mononuclear cell; TNF, tumor necrosis factor α.
Dosing of anti-TNF therapy in intensified schedule (eg, adalimumab more frequent than every 14 days or infliximab more frequent than every 8 weeks).
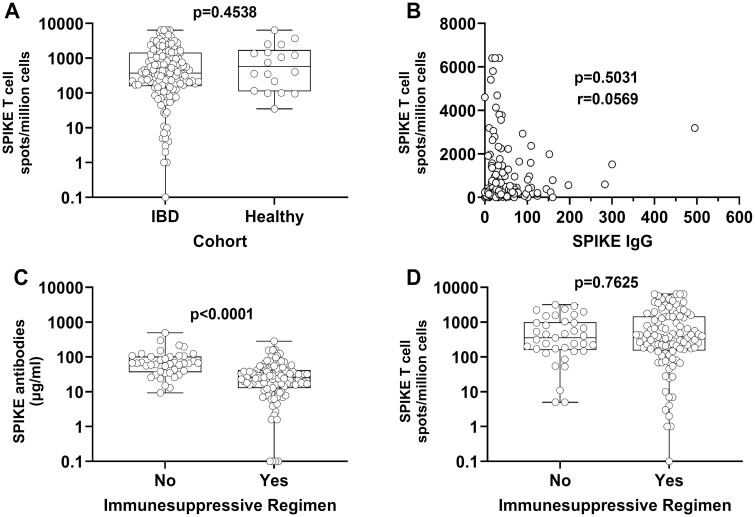
The spike antibody levels were evaluable in 152 patients with IBD and in 18 HC subjects. A humoral immune response was observed in 97% of patients with IBD vs 100% of HC subjects. Thus, the numbers of T cells responsive to spike antigens were evaluable in 151 patients with IBD and in 18 HC subjects. Seventeen (97%) HC subjects and 135 (89%) patients with IBD had a CMIR (Table 1, Figure 1A). Three of 4 participants with no measurable antibodies did have a CMIR (76, 232, and 4600 spike T cells per million peripheral blood mononuclear cells, respectively). There was no association between levels of antibodies and CMIR (Figure 1B). Among patients with IBD, the humoral immune response but not CMIR was lower in patients taking vs not taking immunosuppressive medication(s) (Figures 1C, 1D). Additionally, no difference in spike T cell responses was found between those on anti-TNF therapy or JAK inhibitors compared with other therapies (Table 2).
Figure 1.
Humoral and cell mediated immune responses in inflammatory bowel disease (IBD) patients and normal healthy individuals following vaccination. A, Box-and-whisker plot comparing spike-specific T cell levels (per million peripheral blood mononuclear cells [PBMCs]) in all IBD patients and normal healthy control subjects. The P value was calculated using the Mann-Whitney test. B, Correlation plot comparing paired antibody (µg/mL serum) and spike-specific T cell (per million PBMCs) levels in patients with IBD. The P value and r correlation coefficient were calculated using the Spearman correlation test. C and D, Box-and-whisker plot comparing spike-specific antibody levels (µg/mL serum) and spike-specific T cell (per million PBMCs) levels in IBD patients treated with either nonimmunosuppressive or immunosuppressive regimens. The P values were calculated using the Mann-Whitney test. Each symbol represents a unique patient or healthy donor.
Table 2.
Humoral and Cellular Vaccine Immune Responses by IBD Therapy or Vaccine Type
| Not Immunosuppresseda | Immunosuppressedb | P Value (Mann-Whitney U) | |
|---|---|---|---|
| Postvaccine spike antibody concentration, µg/mL | 66 (37-103), n = 41 evaluable | 27 (14-48), n = 111 evaluable | <.0001 |
| Postvaccine spike T cell level (per million PBMCs) | 255 (146-958), n = 41 evaluable | 377 (123-1440), n = 110 evaluable | .5488 |
| No Anti-TNF or Tofacitinib | Anti-TNF or Tofacitinib | P Value (Mann-Whitney U) | |
|---|---|---|---|
| Postvaccine spike antibody concentration, µg/mL | 59 (28-100), n = 74 evaluable | 78 (14-38), n = 78 evaluable | <.0001 |
| Postvaccine spike T cell level (per million PBMCs) | 314 (96-975), n = 72 evaluable | 401 (172-1572), n = 79 evaluable | .1137 |
| Pfizer | Moderna | P Value (Mann-Whitney U) | |
|---|---|---|---|
| Postvaccine spike antibody concentrations, µg/mL | 31 (12-56), n = 81 | 38 (24-78), n = 71 | .0060 |
| Postvaccine spike T cell levels (per million PBMCs) | 380 (146-1377), n = 82 | 352 (120-1008), n = 69 | .6718 |
Values are median (interquartile range).
Abbreviations: IBD, inflammatory bowel disease; PBMC, peripheral blood mononuclear cell; TNF, tumor necrosis factor α.
Not immunosuppressed = no treatment, mesalamine, budesonide, vedolizumab.
Immunosuppressed includes all patients treated with thiopurines, anti-TNF, ustekinumab, tofacitinib, or corticosteroids.
In univariable analysis, anti-TNF therapy was the only variable associated with a higher CMIR (beta coefficient = 594.5; P = .02). Age, mRNA vaccine type, and other IBD therapies were not associated with CMIR. In our multivariable model, we confirmed that anti-TNF therapy was associated with higher CMIR (beta coefficient = 665; P = .02). Age, vedolizumab, and mRNA vaccine type were not associated with CMIR.
Discussion
In this study, essentially all patients with IBD, even those on immunosuppressant medications, mounted a CMIR to the COVID-19 vaccine. By contrast to earlier studies, which observed a lower antibody response after COVID-19 vaccination in immunosuppressed patients with IBD, the CMIR was not significantly different between patients who were vs were not taking immunosuppressants medications. We did not find a correlation between vaccine-induced antibody levels and CMIR, similar to what has been seen in HC subjects.16 We did find that anti-TNF therapy was associated with a higher CMIR, as was seen in a previous study.24
Our findings are in contrast with the impaired cell-mediated and humoral responses after COVID-19 vaccination observed in other immunosuppressed populations. For example, a CMIR was observed in 36% to 46% of solid organ transplant recipients, in 58% of patients on B cell–depleting therapy, and in 62% to 74% of patients with psoriasis on biological therapy or an immunomodulator.25-28 The humoral immune response after a primary mRNA series was also impaired in solid organ transplant recipients and in rituximab-treated patients.7,26 Studies that have evaluated the CMIR in patients with IBD have found mixed results. In the CLARITY IBD study, the CMIR after the first or second dose of mRNA COVID-19 vaccine was not different between 211 infliximab-treated and 71 vedolizumab-treated patients; up to one-fifth of patients did not have a CMIR. They also found a modest positive correlation between T cell responses and antibody concentrations for those who received an mRNA vaccine but no association between T cell responses and antibody concentration in those immunized with ChAdOx1 COVID-19 vaccine (viral vector vaccine).12 There was no difference observed in T cell response between mRNA and ChAdOx1n COVID-19 vaccines. Among 60 patients with IBD from the Czech Republic, the CMIR, measured 26 weeks after the second dose with the IFN- γ–released assay response, was absent in 18% of patients, who were more likely to be on anti-TNF therapy.29 Similar to the CLARITY IBD study, the study found an agreement between CMIR and antibody concentrations. In contrast, 2 other studies observed that most patients with IBD had a measurable CMIR. A small study evaluating CMRI 2 weeks postimmunization in 29 patients with IBD found that they had similar frequencies of spike-specific CD4+ and CD8+ T cells, irrespective of their therapy.30 In the Coronavirus Risk Associations and Longitudinal Evaluation in IBD (CORALE IBD) study, the T cell clonal response was observed in all 303 patients with IBD. Compared with those with no treatment, there were no significant effects by ustekinumab, vedolizumab, tofacitinib, or steroids. Those on anti-TNF therapy had an augmented response compared with those on no therapy.24 These differences among studies may be at least partly explained by differences in the COVID-19 vaccine preparations and the immunization schedules among studies. For example, UK health authorities allowed for an extended dosing interval at the beginning of the pandemic so that the second dose of a COVID-19 vaccine could be administered up to 12 weeks later instead of 3 to 4 weeks after the first dose.
A correlation between humoral antibody concentrations and CMIR could be an important finding because unlike antibody tests, evaluating CMIR is an expensive, time-consuming process that is not readily available. The mixed results in the previous studies suggest that a strong correlation between antibody and CMIR does not exist in patients with IBD. Given the important role of CMIR in viral clearance, immunologic memory, and recognition of viral variants, CMIR is a critical component of COVID-19 vaccine–induced protection.15
Our results and those of previous studies evaluating antibody responses to COVID-19 vaccines in patients with IBD suggest that most patients with IBD have a vaccine-induced immune response after an mRNA COVID-19 primary series, similar to HC subjects. An additional dose to the primary series was recommended by the Advisory Committee on Immunization Practices and other international societies for those who are moderately to severely immunocompromised, which included those on anti-TNF therapy, systemic corticosteroids, or thiopurines.31 This recommendation was largely based on evidence that solid organ transplant recipients had a suboptimal rate of seroconversion (56%) after the primary series, and these data were extrapolated to other similarly immunosuppressed populations.7 This additional dose to the primary series is appropriate for persons who did not mount an adequate initial humoral immune response.31 Whether an inadequate CMIR also warrants an additional dose to the primary series for most patients with IBD is unknown.12,29 After primary immunization, boosters should be administered as recommended for the general population. In fact, studies have shown robust antibody responses after 3 doses of COVID-19 vaccines in patients with IBD, with antibody concentrations being higher after the third dose than after the 2-dose primary series.32,33 Such booster doses, preferentially mRNA vaccines, should be given to persons 12 years of age and older, 5 months after their primary series for the general population and 3 months in moderately to severely immunosuppressed patients. In late March 2022, the Food and Drug Administration authorized a second booster dose of mRNA-1273 and BNT162b2 for older people and certain immunocompromised individuals at least 4 months after receipt of a first booster. They defined immunocompromised individuals as those who have undergone solid organ transplantation or who have an equivalent immunocompromised condition.34 The treatment regimens of most patients with IBD are not equivalent to those of a solid organ transplant recipient. Studies evaluating humoral immunogenicity have found that those on anti-TNF therapy, who are on corticosteroids, and who are older are more likely to have lower antibody concentrations. The clinical relevance of lower antibody concentrations is not known because many of these studies did not have a control group, and it is unknown whether a lower concentration warrants additional doses of COVID-19 vaccines. Based on the Food and Drug Administration most recent guidance, any patients with IBD on anti-TNF therapy, thiopurines, and >20 mg of prednisone would be eligible for up to 5 doses of mRNA COVID-19 vaccines.34
There are many things we have learned about the use of COVID-19 vaccines such as that immune-modifying therapies other than corticosteroids do not increase the risk of severe COVID-19 disease even prior to widespread vaccination programs.35 COVID-19 vaccines are safe and not associated with IBD disease flares, and most patients are able to mount a humoral immune response similar to that seen in HC subjects.5 Additionally, a large population-based study showed that COVID-19 vaccines are equally effective at preventing infection in patients with IBD compared with non-IBD control subjects.36 The goal of COVID-19 vaccines since their inception has been to prevent severe disease that may result in hospitalization, intensive care unit stay, or death.1 This data suggest that most patients with IBD may follow COVID-19 immunization guidelines for the general population, rather than for solid organ transplant recipients. Potentially, older patients who are on anti-TNF therapy or those with risk factors for severe COVID-19 may benefit from 5 mRNA vaccine doses to prevent symptomatic disease. Similarly, while monoclonal antibodies and small molecules are now available to treat COVID-19 disease, most patients with IBD without underlying risk factors for severe disease may not need these therapies.37
Our study has several strengths. We evaluated patients on stable treatment regimens. The CMIR was measured with an established assay, the results of which have been associated with protection from disease.38 We also evaluated CMIR at similar time points of the original COVID-19 vaccine immunogenicity clinical trials. However, there were only 20 control subjects, and a small number of patients with IBD treated with tofacitinib and ustekinumab. We only evaluated 1 component of the CMIR and did not differentiate between CD4 and CD8 cells. We also only evaluated CMIR after 2 doses of mRNA vaccines.
Conclusions
In summary, we found that almost all patients with IBD were able to mount a CMIR after a 2-dose series of an mRNA vaccine, which did not correlate with the humoral antibody response. Further studies are needed to evaluate sustained CMIR, the impact of booster doses on CMIR, and long-term antibody concentrations and CMIR in patients with IBD.
Acknowledgements
The authors thank all the subjects who participated in the study, the specialty pharmacists at UW Health for their help, and all the staff at the Office of Clinical Trials at the University of Wisconsin–Madison for all their work in completion of the study. The authors acknowledge Edward Famularo at Mayo Florida; Sue McCrone and Kayla Dillon, both at the University of Wisconsin–Madison, for assisting with sample processing at Mayo Florida; and Zhou Li for statistical advice.
Contributor Information
Freddy Caldera, Division of Gastroenterology and Hepatology, Department of Medicine, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA.
Francis A Farraye, Inflammatory Bowel Disease Center, Department of Gastroenterology and Hepatology, Mayo Clinic, Jacksonville, FL, USA.
Brian M Necela, Department of Immunology, Mayo Clinic, Jacksonville, FL, USA.
Davitte Cogen, Department of Immunology, Mayo Clinic, Jacksonville, FL, USA.
Sumona Saha, Division of Gastroenterology and Hepatology, Department of Medicine, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA.
Arnold Wald, Division of Gastroenterology and Hepatology, Department of Medicine, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA.
Nader D Daoud, Inflammatory Bowel Disease Center, Department of Gastroenterology and Hepatology, Mayo Clinic, Jacksonville, FL, USA.
Kelly Chun, R&D and Specialty Medicine, LabCorp, Calabasas, CA, USA.
Ian Grimes, Division of Gastroenterology and Hepatology, Department of Medicine, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA.
Megan Lutz, Division of Gastroenterology and Hepatology, Department of Medicine, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA.
Sean R Van Helden, School of Pharmacy, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA.
Melanie D Swift, Division of Public Health, Infectious Diseases and Occupational Medicine, Mayo Clinic, Rochester, MN, USA.
Abinash Virk, Division of Infectious Diseases, Mayo Clinic, Rochester, MN, USA.
Adil E Bharucha, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, USA.
Tushar C Patel, Division of Gastroenterology and Hepatology, Mayo Clinic, Jacksonville, FL, USA.
Gregory J Gores, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, USA.
Saranya Chumsri, Division of Hematology and Medical Oncology, Mayo Clinic, Jacksonville, FL, USA.
Mary S Hayney, School of Pharmacy, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA.
Keith L Knutson, Department of Immunology, Mayo Clinic, Jacksonville, FL, USA.
Author Contribution
F.C. contributed to study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript. K.L.K. contributed to acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript. B.M.N. contributed to acquisition of data and drafting of the manuscript. D.C. contributed to acquisition of data. S.S. contributed to critical revision of the manuscript. A.W. contributed to critical revision of the manuscript. H.S.P. contributed to acquisition of data, critical revision of the manuscript, K.C. contributed to acquisition of data, critical revision of the manuscript, analysis and interpretation of data, I.G. contributed to critical revision of the manuscript, M.L. contributed to critical revision of the manuscript, M.D.S. contributed to data acquisition and critical revision of the manuscript. A.V. contributed to data acquisition and critical revision of the manuscript. A.E.B. contributed to data acquisition and critical revision of the manuscript. T.C.P. contributed to data acquisition and critical review of the manuscript. G.J.G. contributed to data acquisition and critical review of the manuscript. M.S.H. contributed to study concept and design, acquisition of data, analysis, and interpretation of data, drafting of the manuscript, and critical revision of the manuscript. F.A.F. contributed to study concept and design, analysis and interpretation of data and critical revision of the manuscript. N.D.D. contributed to acquisition of data.
Funding
This work was supported by Takeda Pharmaceuticals, American College of Gastroenterology, and the Mayo Clinic.
Conflicts of Interest
F.C. has received research support from Takeda Pharmaceuticals and served as a consultant for Takeda, Arena Pharmaceuticals, GSK, and Celgene. F.A.F. has served a consultant for Arena, BMS, Braintree Labs, Gilead, GSK, Innovation Pharmaceuticals, Iterative Scopes, Janssen, Pfizer, and Sebela; and served on the DSMB for Bacainn Pharmaceuticals, Lilly, and Theravance. M.S.H. has served as a consultant for GSK Vaccines and Seqirus and has received research support from Takeda Pharmaceuticals, Dynavax, and Sanofi. K.C. is an employee of LabCorp. M.D.S. has received research support from Pfizer.
References
- 1. Rolak S, Hayney MS, Farraye FA, Temte JL, Caldera F.. What gastroenterologists should know about COVID-19 vaccines. Clin Gastroenterol Hepatol. 2021;19(4):657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chumsri S, Advani PP, Pai TS, et al. Humoral responses after SARS-CoV-2 mRNA vaccination and breakthrough infection in cancer patients. Mayo Clin Proc Innov Qual Outcomes. 2022;6(2):120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caldera F, Ley D, Hayney MS, Farraye FA.. Optimizing immunization strategies in patients with IBD. Inflamm Bowel Dis. 2021;27(1):123–133. [DOI] [PubMed] [Google Scholar]
- 4. Caldera F, Hillman L, Saha S, et al. Immunogenicity of high dose influenza vaccine for patients with inflammatory bowel disease on anti-TNF monotherapy: a randomized clinical trial. Inflamm Bowel Dis. 2020;26(4):593–602. [DOI] [PubMed] [Google Scholar]
- 5. Weaver KN, Zhang X, Dai X, et al. Impact of SARS-CoV-2 vaccination on inflammatory bowel disease activity and development of vaccine-related adverse events: results from PREVENT-COVID. Inflamm Bowel Dis. Published online December 6, 2021. doi: 10.1093/ibd/izab302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Botwin GJ, Li D, Figueiredo J, et al. Adverse events after SARS-CoV-2 mRNA vaccination among patients with inflammatory bowel disease. Am J Gastroenterol. 2021;116(8):1746–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kappelman MD, Weaver KN, Zhang X, et al. Factors affecting initial humoral immune response to SARS-Cov-2 vaccines among patients with inflammatory bowel diseases. Am J Gastroenterol. 2022;117(3):462–469. [DOI] [PubMed] [Google Scholar]
- 9. Melmed GY, Botwin GJ, Sobhani K, et al. Antibody responses after SARS-CoV-2 mRNA vaccination in adults with inflammatory bowel disease. Ann Intern Med. 2021;174(12):1768–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caldera F, Knutson KL, Saha S, et al. Humoral immunogenicity of mRNA COVID-19 vaccines among patients with inflammatory bowel disease and healthy controls. Am J Gastroenterol. 2022;117(1):176–179. [DOI] [PubMed] [Google Scholar]
- 11. Alexander JL, Kennedy NA, Ibraheim H, et al. COVID-19 vaccine-induced antibody responses in immunosuppressed patients with inflammatory bowel disease (VIP): a multicentre, prospective, case-control study. Lancet Gastroenterol Hepatol. 2022;7(4):342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin S, Kennedy NA, Saifuddin A, et al. Antibody decay, T cell immunity and breakthrough infections following two SARS-CoV-2 vaccine doses in inflammatory bowel disease patients treated with infliximab and vedolizumab. Nat Commun. 2022;13(1):1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lutz M, Hayney MS, Caldera F.. We should not forget about patients with inflammatory bowel disease who received a COVID-19 viral vector vaccine. Am J Gastroenterol. 2022;117(8):1329. [DOI] [PubMed] [Google Scholar]
- 14. Pozdnyakova V, Botwin GJ, Sobhani K, et al. Decreased antibody responses to Ad26.COV2.S relative to SARS-CoV-2 mRNA vaccines in patients with inflammatory bowel disease. Gastroenterology. 2021;161(6):2041–2043.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022;23(2):186–193. [DOI] [PubMed] [Google Scholar]
- 16. Skelly DT, Harding AC, Gilbert-Jaramillo J, et al. Two doses of SARS-CoV-2 vaccination induce robust immune responses to emerging SARS-CoV-2 variants of concern. Nat Commun. 2021;12(1):5061–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wyant T, Leach T, Sankoh S, et al. Vedolizumab affects antibody responses to immunisation selectively in the gastrointestinal tract: randomised controlled trial results. Gut. 2015;64(1):77–83. [DOI] [PubMed] [Google Scholar]
- 18. deBruyn J, Fonseca K, Ghosh S, et al. Immunogenicity of influenza vaccine for patients with inflammatory bowel disease on maintenance infliximab therapy: a randomized trial. Inflamm Bowel Dis. 2016;22(3):638–647. [DOI] [PubMed] [Google Scholar]
- 19. Garcillán B, Salavert M, Regueiro JR, Díaz-Castroverde S.. Response to vaccines in patients with immune-mediated inflammatory diseases: a narrative review. Vaccines (Basel). 2022;10(2):297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith R, Hubers J, Farraye FA, Sampene E, Hayney MS, Caldera F.. Accuracy of self-reported vaccination status in a cohort of patients with inflammatory bowel disease. Dig Dis Sci. 2021;66(9):2935–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schell TL, Richard LJ, Tippins K, Russ RK, Hayney MS, Caldera F.. High but inequitable COVID-19 vaccine uptake among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2022;20(7):1606–1608.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA-Based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li D, Xu A, Mengesha E, et al. The T-cell response to SARS-CoV-2 vaccination in inflammatory bowel disease is augmented with anti-TNF therapy. Inflamm Bowel Dis. 2022;28(7):1130–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mahil SK, Bechman K, Raharja A, et al. Humoral and cellular immunogenicity to a second dose of COVID-19 vaccine BNT162b2 in people receiving methotrexate or targeted immunosuppression: a longitudinal cohort study. Lancet Rheumatol. 2022;4(1):e42–e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mrak D, Tobudic S, Koblischke M, et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann Rheum Dis. 2021;80(10):1345–1350. [DOI] [PubMed] [Google Scholar]
- 27. Ruether DF, Schaub GM, Duengelhoef PM, et al. SARS-CoV2-specific humoral and T-cell immune response after second vaccination in liver cirrhosis and transplant patients. Clin Gastroenterol Hepatol. 2022;20(1):162–172.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hall VG, Ferreira VH, Ierullo M, et al. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am J Transplant. 2021;21(12):3980–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cerna K, Duricova D, Hindos M, et al. Cellular and humoral immune responses to SARS-CoV-2 vaccination in inflammatory bowel disease patients. J Crohns Colitis. Published online March 31, 2022. doi: 10.1093/ecco-jcc/jjac048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boland BS, Goodwin B, Zhang Z, et al. Preserved SARS-CoV-2 vaccine cell-mediated immunogenicity in inflammatory bowel disease patients on immune-modulating therapies. Clin Transl Gastroenterol. 2022;13(4):e00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mbaeyi S, Oliver SE, Collins JP, et al. The advisory committee on immunization practices’ interim recommendations for additional primary and booster doses of COVID-19 Vaccines - United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(44):1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Long MD, Weaver KN, Zhang X, Chun K, Kappelman MD, Group P-CS.. Strong response to SARS-CoV-2 vaccine additional doses among patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2022;20(8):1881–1883.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schell TL, Knutson KL, Saha S, et al. Humoral immunogenicity of 3 COVID-19 messenger RNA vaccine doses in patients with inflammatory bowel disease. Inflamm Bowel Dis. Published online April 9, 2022. doi: 10.1093/ibd/izac082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Centers for Disease Control and Prevention. Use of COVID-19 Vaccines Currently Approved or Authorized in the United States. Interim Clinical Considerations. Accessed April 25, 2022. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html
- 35. Ungaro RC, Brenner EJ, Agrawal M, Zhang X, Kappelman MD, Colombel JF.. Impact of medications on COVID-19 outcomes in inflammatory bowel disease: analysis of more than 6000 patients from an international registry. Gastroenterology. 2022;162(1):316–319.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lev-Tzion R, Focht G, Lujan R, et al. COVID-19 vaccine is effective in inflammatory bowel disease patients and is not associated with disease exacerbation. Clin Gastroenterol Hepatol. 2022;20(6):e1263–e1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2021;386(6):509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wyllie D, Jones HE, Mulchandani R, et al. SARS-CoV-2 responsive T cell numbers and anti-Spike IgG levels are both associated with protection from COVID-19: a prospective cohort study in keyworkers. medRxiv. doi: 10.1101/2020.11.02.20222778, May 2, 2021, not peer reviewed. [DOI] [Google Scholar]