Abstract
Although numerous studies have evaluated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection using cycle threshold (Ct) values as a surrogate of viral ribonucleic acid (RNA) load, few studies have used standardized, quantitative methods. We validated a quantitative SARS-CoV-2 digital polymerase chain reaction assay normalized to World Health Organization International Units and correlated viral RNA load with symptoms and disease severity.
Keywords: COVID-19, digital PCR, international units, SARS-CoV-2, viral load
Studies have evaluated SARS-CoV-2 infection using Cycle Thresholds (Ct) values as a surrogate of viral RNA load. We validated a quantitative SARS-CoV-2 digital PCR assay normalized to WHO International Units and correlated viral RNA load with symptoms and disease severity.
Since the discovery of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in December 2019, more than 500 million individuals have been infected and more than 6 million died worldwide. In the United States alone, more than 80 million infections and more than 1 million deaths have been reported [1]. Despite the development and availability of effective vaccines, rapid viral evolution, vaccine hesitancy, and vaccine inequity have enabled the pandemic to continue [2, 3].
Although our knowledge on SARS-CoV-2 has significantly expanded, the role of viral ribonucleic acid (RNA) load in coronavirus disease 2019 (COVID-19) remains poorly understood, partly due to the widely adopted misuse and faulty interpretation of cycle threshold (Ct) values. Cycle threshold values have been used to predict disease severity, infer transmissibility, and discriminate active viral infection from viral shedding [4–6]. However, real-time reverse-transcription PCR (RT-PCR) assays are not typically calibrated with known RNA standards and are therefore suboptimal tools for accurate measurement of nucleic acid in a sample [7–9]. Accordingly, scientific societies have issued statements advising against reporting and using Ct values as a method to infer viral quantity [10].
Even when using real-time RT-PCR methods calibrated to provide results in copies/mL, assays can vary markedly in their results. Similar to other quantitative viral nucleic acid amplification tests, results among laboratories testing the same material can vary across several log units [11, 12]. In turn, this limits the utility of any quantitative findings to those using the same methodology with the same calibrators. Digital PCR (dPCR) and digital reverse-transcription PCR (RT-dPCR) have been demonstrated to provide reproducible, highly accurate results without the need for quantitative calibrators [13, 14]. Furthermore, the use of international quantitative standards has helped to markedly improve interlaboratory viral nucleic acid load agreement in other settings [11, 12]. In this study, we describe the use of a quantitative SARS-CoV-2 RT-dPCR assay to allow correlation of viral RNA load to clinical course, providing results in a standardized manner that will facilitate widespread applicability and consistent interlaboratory result interpretation.
METHODS
Human Cohort
The SJTRC (ClinicalTrials.gov Identifier NCT04362995) is a prospective, longitudinal cohort study of St. Jude Children's Research Hospital adult (≥18 years old) employees. The St. Jude Institutional Review Board approved the study. For this study, we included 114 individuals who have tested positive for SARS-CoV-2 and in whom a respiratory sample was available between March 2020 and April 2021 at a time in which Wuhan-like and B.1.1.7 variants were the predominant circulating strain locally, and before the Delta surge. Severity of illness was classified as asymptomatic, mild-moderate, severe or critical as previously described [15] (Supplementary Methods).
Patient Consent Statement
All participants consented to the study that was reviewed and approved by The St. Jude Institutional Review Board.
Severe Acute Respiratory Syndrome Coronavirus 2 Reverse-Transcription Digital Polymerase Chain Reaction Assay
The SARS-CoV-2 RT-dPCR Test (Bio-Rad Laboratories, Inc., Hercules, CA) was used for qualitative detection of SARS-CoV-2 RNA authorized for emergency use by US Food and Drug Administration. Viral RNA loads were generated for both N1 and N2 targets and showed similar results. Quantitative results in the manuscript are for N1, whereas N2 results are shown in the Supplementary Documents. Results were normalized to the 20/146 First World Health Organization (WHO) International Standard for SARS-CoV-2 RNA (product code 20/146; National Institute for Biological Standards and Control (NIBSC), Potters Bar, Hertfordshire, UK) to produce data in log10 IU/mL (Supplementary Methods).
Statistical Analysis
Descriptive statistics of the sample were presented as (1) frequencies and percentages for categorical variables and (2) mean (standard deviation) or median (25th to 75th percentiles) based on data distribution for continuous variables. Values of positive controls reported in copies/mL were regressed against corresponding nominal values in IU/mL with simple linear regression. The slope and intercept derived from linear regression was used to calculate viral RNA load in copies/mL from patient samples into IU/mL units, with the regression recalibration result approximately showing 5 copies/mL equaled 1 IU/mL across the quantitative range of the assay. To assess the value of viral RNA load in predicting outcome, receiver operating characteristic (ROC) curves were built to estimate the area under curve (AUC). Optimal cut-point levels were derived using the highest Youden indices (J statistic). All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC) and R 4.2.0 (R Core Team, 2020; R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria) (https://www.R-project.org/) (Supplementary Methods).
RESULTS
The RT-dPCR assay was confirmed to have a limit of detection (LOD) of 3.84 log10 IU/mL for N1 and 3.82 log10 IU/mL for N2, matching the manufacturer's LOD. The quantitative linearity was demonstrated across a wide dynamic range, with lower limits of quantitation (LOQ) of 3.84 log10 IU/mL and 3.82 log10 IU/mL for N1 and N2, respectively, and upper LOQ of 7.86 log10 IU/mL and 7.88 log10 IU/mL for both N1 and N2, respectively. The assay showed a high degree of reproducibility (coefficient of variation% = 8% and 7% for N1 and N2); and when WHO standards were tested, regression of results against nominal values showed a slope of 1.0060 and a y-intercept of (1.0199) for N1 and a slope of 1.0131 and a y-intercept of (0.9878) for N2.
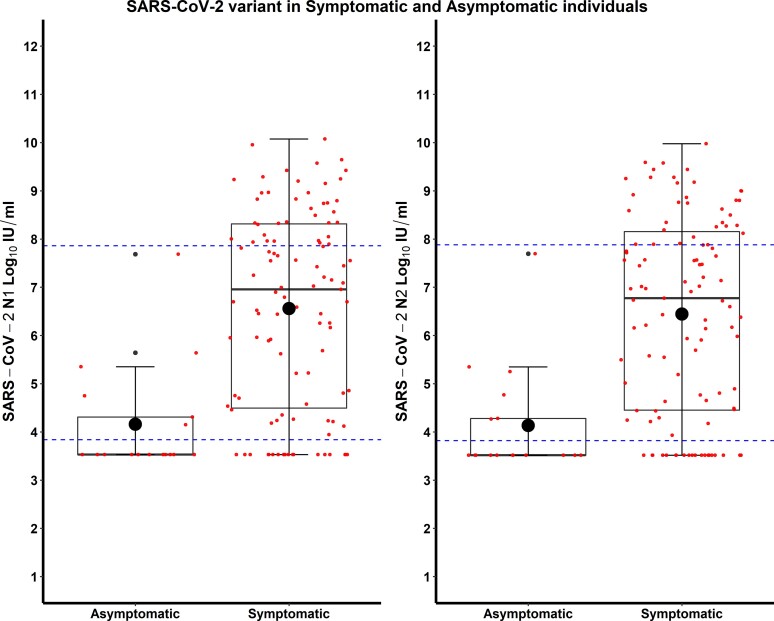
During the study period, 114 individuals tested positive for SARS-CoV-2 and contributed 125 samples. The median age was 42 years (interquartile range [IQR], 33–53), 76.3% were female, and most were non-Hispanic and White. Fourteen (12.3%) were asymptomatic at the time of positive test and never developed symptoms. Among those who had symptoms, fever, headaches, rhinorrhea, and loss of smell and/or taste were most commonly reported (Supplementary Table 1). Most cases were mild, with 26 (22.8%) seeking medical care and only 2 individuals needing hospital admission. The median SARS-CoV-2 viral RNA load was 6.45 log10 IU/mL (IQR, 34.13–7.98). Median viral RNA load in symptomatic individuals (6.96 log10 IU/mL; IQR, 4.46–8.32) was higher when compared to those without symptoms (3.53 log10 IU/mL; IQR, 3.53–4.31) with P < .001 (Figure 1, Supplementary Table 2), and viral RNA load significantly correlated with the severity of symptoms (correlation coefficient 0.33, P < .001). However, viral RNA load upon symptom onset did not significantly correlate with duration of symptoms among those who reported being sick.
Figure 1.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load (N1 and N2 log10 IU/mL) in asymptomatic and symptomatic individuals. Dotted lines represent the demonstrated upper and lower limits of the assay analytical measurement range.
Viral RNA load from the initial sample discriminated and predicted individuals with and without symptoms (odds ratio [OR] = 2.26; 95% confidence interval [CI], 1.41–3.61) in univariate analysis (Supplementary Table 3). These results did not change in a multivariate stepwise logistic regression model adjusted for age, gender, and race (OR = 2.26; 95% CI, 1.41–3.61) (Supplementary Table 4). An ROC curve analysis determined that a viral RNA load of 5.68 log10 IU/mL discriminated asymptomatic from symptomatic infection with a sensitivity of 71.7% and specificity of 92.9% (AUC = 0.84) (Supplementary Table 5, Supplementary Figure 1). In addition, viral RNA load predicted development of symptoms in those individuals who were asymptomatic at the time of diagnosis irrespective of age, gender, and race (OR = 2.33; 95% CI, 1.22–4.44) (Supplementary Tables 6 and 7). A similar ROC curve analysis showed that a viral RNA load of 4.86 log10 IU/mL upon initially asymptomatic presentation predicted subsequent development of symptoms with a sensitivity of 70.6% and specificity of 85.7% (AUC = 0.81) (Supplementary Table 8, Supplementary Figure 2).
DISCUSSION
Although much work has been done in correlating viral RNA load with disease severity, most studies have used Ct values, with relatively few using quantitative methods and international quantitative standards. In this study, we provided details and results of a standardized quantitative SARS-CoV-2 RT-dPCR assay, normalized to the first WHO International Standard for SARS-CoV-2 RNA.
As reported by others, we observed a correlation between RNA quantity and presence of symptoms. Although we also noted an increase in viral RNA load in those with more severe symptoms, few participants had critical illness to be able to correlate viral RNA load with poor clinical outcomes. Only those 14 cases who never developed symptoms were considered “asymptomatic” for all models. Although we were able to distinguish individuals who develop symptoms from those who remained asymptomatic, results should be taken with caution given the small numbers in our cohort. If this is confirmed by others, initial viral RNA load could be considered an additional variable when prioritizing access to early treatment to prevent progression of COVID-19. This emphasizes the need to establish reliable quantitative methods that are standardized and can be replicated, potentially allowing the development of consensus thresholds for treatment.
There are many limitations in using RT-PCR Ct values as a surrogate of RNA concentration. First, most Ct values are not normalized to standardized controls of known concentration. In addition, such assays may not have a linear relationship between the Ct values and the amount of nucleic acid across the full range of detected RNA loads. This is particularly important when working with specimens with high or low viral load [7]. In general, Ct values have not been well evaluated for analytical measurement range (linear range of quantitation), within- or between-run reproducibility, or collinearity using differing viral strains. The Ct values cannot be compared across different platforms or laboratories, making the generalization or portability of results unfeasible. In fact, the College of American Pathologists found variability of up to 4000-fold across different emergency use authorization platforms using same controls and up to 10-fold differences in the reproducibility of the control using the same instrument [8]. Although reporting viral quantity is clearly more useful than Ct values, in the absence of normalization to a common quantitative standard, results remain hard to interpret and replicate, and establishment of consensus treatment thresholds remains elusive.
This study has several limitations. The samples are limited to infections with ancestral strain virus and B.1.1.7. Viral RNA load among different variants and stratified by vaccination status could not be performed. In addition, the study was underpowered to evaluate an association between viral RNA load and disease severity, and very few individuals had more than 1 sample to be able to perform those analysis. Although it is difficult to guarantee that sample quality had no impact on results, a wide variety of clinical samples were tested during assay validation to demonstrate consistent performance across patients. In addition, all tested samples included an internal positive control that had to be detectable at a certain level to demonstrate lack of assay inhibition. Finally, staff underwent training on proper sample collection techniques and competency assessment before collecting samples for testing.
CONCLUSIONS
This work was performed with commercially available reagents, on a widely available digital PCR platform. Such testing would therefore likely be feasible for implementation in any laboratory running high-complexity molecular testing for clinical purposes. This may largely limit the initial use of such methods to referral laboratories and academic centers. Nonetheless, this work represents a substantial advance in providing a correlation between clinical course and standardized quantitative results. Our results will facilitate reproducibility of these data in other settings, comparison to results in other centers, and open the door to the establishment of common interpretive thresholds. These potentially marked advantages suggest that this work can serve as a guide for future work related to quantitative detection of SARS-CoV-2 and of other pathogens of clinical and public health interest.
Supplementary Material
Acknowledgments
We thank Ashleigh Gowen, Jamie Russell-Bell, Pam Merritt, Jamie Russell-Bell, David Wittman, Matthew Lear, and Charles Mullighan, in The St. Jude Biorepository, and Ben McKinley, Austin Springer, Nancy Kornegay, Jason Hodges, Tamanna Shamrin, Rishi Kodela, Bertha Davis, and Gail Fortner.
Author contributions. L. T. had full access to all the data and takes responsibility for the integrity of the data and accuracy of the data analysis. All authors contributed to concept and design and acquisition, analysis, or interpretation of data. D. R. H. contributed to drafting of the manuscript. All authors contributed to critical revision of the manuscript for important intellectual content. L. T. and H. D. contributed to statistical analysis. All authors contributed to administrative, technical, or material support. D. R. H. and R. T. H. contributed to supervision.
Disclaimer. The sponsors had no role in design or conduct of the study; the collection, management, analysis, or interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Financial support. This study was funded by the American Lebanese Syrian Associated Charities and St. Jude Children’s Research Hospital, the National Institute of Allergy and Infectious Diseases (NIAID) for the Center of Excellence for Influenza Research and Surveillance (CEIRS contract HHSN27220140006C) and NIAID Collaborative Influenza Vaccine Innovation Centers Contract 75N93019C00052, NIAID Grant 3U01AI144616-02S1.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
Diego R Hijano, Departments of Infectious Diseases, St. Jude Children's Research Hospital, Memphis, Tennessee, USA.
Zhengming Gu, Departments of Pathology, St. Jude Children's Research Hospital, Memphis, Tennessee, USA.
Jessica Brazelton, Departments of Pathology, St. Jude Children's Research Hospital, Memphis, Tennessee, USA.
Haiqing Zhu, Departments of Pathology, St. Jude Children's Research Hospital, Memphis, Tennessee, USA.
Sri Suganda, Departments of Pathology, St. Jude Children's Research Hospital, Memphis, Tennessee, USA.
Heather L Glasgow, Departments of Pathology, St. Jude Children's Research Hospital, Memphis, Tennessee, USA.
Himani Darji, Departments of Biostatistics, St. Jude Children's Research Hospital, Memphis, Tennessee, USA.
Li Tang, Departments of Biostatistics, St. Jude Children's Research Hospital, Memphis, Tennessee, USA.
Thomas P Fabrizio, Departments of Infectious Diseases, St. Jude Children's Research Hospital, Memphis, Tennessee, USA.
Kim J Allison, Departments of Infectious Diseases, St. Jude Children's Research Hospital, Memphis, Tennessee, USA.
E Kaitlynn Allen, Departments of Immunology, St. Jude Children's Research Hospital, Memphis, Tennessee, USA.
Aditya H Gaur, Departments of Infectious Diseases, St. Jude Children's Research Hospital, Memphis, Tennessee, USA.
Joshua Wolf, Departments of Infectious Diseases, St. Jude Children's Research Hospital, Memphis, Tennessee, USA.
Paul G Thomas, Departments of Immunology, St. Jude Children's Research Hospital, Memphis, Tennessee, USA.
Richard J Webby, Departments of Infectious Diseases, St. Jude Children's Research Hospital, Memphis, Tennessee, USA.
Randall T Hayden, Departments of Pathology, St. Jude Children's Research Hospital, Memphis, Tennessee, USA.
SJTRC Study Team:
Jeremie H Estepp, Maureen A McGargill, Motomi Mori, Stacey Schultz-Cherry, Hana Hakim, and Elaine I Tuomanen
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
SJTRC Study Team
Department of Pharmaceutical Sciences, St. Jude Children's Research Hospital, Memphis, Tennessee, USA: James Hoffman; Department of Hematology, St. Jude Children's Research Hospital, Memphis, Tennessee, USA: Jeremie H. Estepp; Department of Immunology, St. Jude Children's Research Hospital, Memphis, Tennessee, USA: Maureen A. McGargill; Department of Biostatistics, St. Jude Children's Research Hospital, Memphis, Tennessee, USA: Motomi Mori; Department of Infectious Diseases, St. Jude Children's Research Hospital, Memphis, Tennessee, USA: Stacey Schultz-Cherry, Hana Hakim, Elaine I. Tuomanen.
References
- 1. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/map.html. Accessed 28 June 2022.
- 2. McAlister FA, Nabipoor M, Chu A, Lee DS, Saxinger L, Bakal JA. The impact of shifting demographics, variants of concern and vaccination on outcomes during the first 3 COVID-19 waves in Alberta and Ontario: a retrospective cohort study. CMAJ Open 2022; 10:e400–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bruns R, Hosangadi D, Trotochaud M, Kirk Sell T. COVID-19 vaccine misinformation and disinformation costs an estimated $50 to $300 million each day. Available at: https://www.centerforhealthsecurity.org/our-work/pubs_archive/pubs-pdfs/2021/20211020-misinformation-disinformation-cost.pdf. Accessed 25 May 2022.
- 4. Bellon M, Baggio S, Jacquerioz Bausch F, et al. . Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load kinetics in symptomatic children, adolescents, and adults. Clin Infect Dis 2021; 73:e1384–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shenoy S. SARS-CoV-2 (COVID-19), viral load and clinical outcomes; lessons learned one year into the pandemic: a systematic review. World J Crit Care Med 2021; 10:132–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Binnicker MJ. Can testing predict SARS-CoV-2 infectivity? The potential for certain methods to be surrogates for replication-competent virus. J Clin Microbiol 2021; 59:e0046921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schnuriger A, Perrier M, Marinho V, et al. . Caution in interpretation of SARS-CoV-2 quantification based on RT-PCR cycle threshold value. Diagn Microbiol Infect Dis 2021; 100:115366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rhoads D, Peaper DR, She RC, et al. . College of American Pathologists (CAP) microbiology committee perspective: caution must be used in interpreting the cycle threshold (Ct) value. Clin Infect Dis 2021; 72:e685–6. [DOI] [PubMed] [Google Scholar]
- 9. Poon KS, Wen-Sim Tee N. Caveats of reporting cycle threshold values from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) qualitative polymerase chain reaction assays: a molecular diagnostic laboratory perspective. Clin Infect Dis 2021; 73:e2851–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. IDSA and AMP joint statement on the use of SARS-CoV-2 PCR cycle threshold (Ct) values for clinical decision-making. Available at: https://www.idsociety.org/globalassets/idsa/public-health/covid-19/idsa-amp-statement.pdf. Accessed 4 April 2022.
- 11. Hayden RT, Sun Y, Tang L, et al. . Progress in quantitative viral load testing: variability and impact of the WHO quantitative international standards. J Clin Microbiol 2017; 55:423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayden RT, Tang L, Su Y, et al. . Impact of fragmentation on commutability of Epstein-Barr virus and cytomegalovirus quantitative standards. J Clin Microbiol 2019; 58:e00888-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kojabad AA, Farzanehpour M, Galeh HEG, et al. . Droplet digital PCR of viral DNA/RNA, current progress, challenges, and future perspectives. J Med Virol 2021; 93:4182–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Milosevic D, Moyer AM, Majumdar R, Kipp BR, Yao JD. A reverse-transcription droplet digital PCR assay to detect and quantify SARS-CoV-2 RNA in upper respiratory tract specimens. J Clin Virol 2022; 153:105216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tang L, Cherry S, Tuomanen EI, et al. . Host predictors of broadly cross-reactive antibodies against SARS-CoV-2 variants of concern differ between infection and vaccination. Clin Infect Dis 2022; 75:e705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.