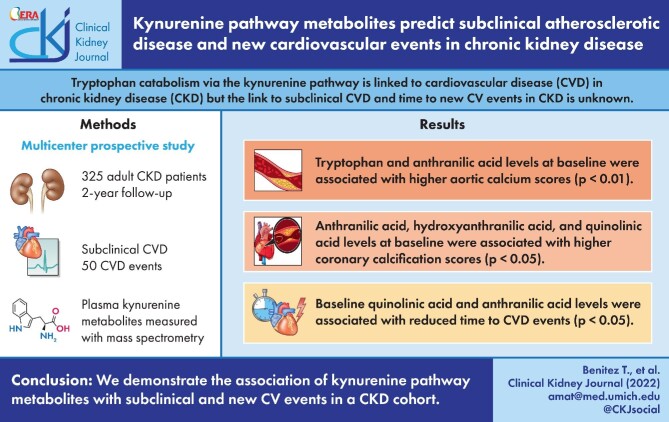
ABSTRACT
Introduction
Inflammation and oxidative stress contribute to the disproportionate burden of cardiovascular disease (CVD) in chronic kidney disease (CKD). Disordered catabolism of tryptophan via the kynurenine and indole pathways is linked to CVD in both CKD and dialysis patients. However, the association between specific kynurenine and indole metabolites with subclinical CVD and time to new cardiovascular (CV) events in CKD has not been studied.
Methods
We measured kynurenine and indole pathway metabolites using targeted mass spectrometry in a cohort of 325 patients with moderate to severe CKD and a median follow-up of 2 years. Multiple linear regression and Cox regression analyses were used to assess the relationship between these tryptophan metabolites and subclinical CVD, including calcium scores, carotid intima-media thickness and time to new cardiovascular (CV) events.
Results
Elevated quinolinic and anthranilic acids were independently associated with reduced time to new CVD [hazard ratio (HR) 1.28, P = .01 and HR 1.02, P = .02, respectively). Low tryptophan levels were associated with reduced time to new CV events when adjusting for demographics and CVD history (HR 0.30, P = .03). Low tryptophan levels were also associated with aortic calcification in a fully adjusted linear regression model (β = −1983, P = .006). Similarly, high levels of several kynurenine pathway metabolites predicted increased coronary, aortic and composite calcification scores.
Conclusions
We demonstrate the association of kynurenine pathway metabolites, and not indole derivatives, with subclinical and new CV events in an advanced CKD cohort. Our findings support a possible role for altered tryptophan immune metabolism in the pathogenesis of CKD-associated atherosclerosis.
Keywords: anthranilic acid, calcium scores, 3-hydroxy anthranilic acid, inflammation, metabolomics, tryptophan, quinolinic acid
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Chronic kidney disease (CKD) is prevalent in 15% of the US population and ∼650 000 Americans undergo renal replacement therapies annually [1]. Cardiovascular (CV) risk in the CKD population is 10 to 40-fold higher than the general population; cardiovascular disease (CVD) remains the primary cause of mortality and morbidity in the CKD population [2], adding nearly US$120 billion in CKD-related healthcare costs to the Medicare budget in 2017 [1]. While therapies that target traditional modifiable risk factors for CVD, such as hypertension, diabetes, smoking and hyperlipidemia, reduce risk in the general population, mitigating these factors in CKD patients still leaves a high residual risk [3, 4]. Moreover, several studies have failed to demonstrate the benefits of low-density lipoprotein (LDL)-lowering therapies in decreasing the incidence of nonfatal myocardial infarctions, strokes or CV mortality in dialysis patients [5–7].
Non-traditional CV risk factors such as uremia, inflammation, oxidative stress and endothelial dysfunction drive the CVD burden among those with CKD [8]. Accumulating toxins induce oxidative stress that propagates vascular endothelial damage even in early CKD, while they exacerbate macrophage-mediated vascular inflammation in patients on dialysis [8–10]. In fact, inhibiting macrophage-derived proinflammatory cytokine interleukin-1β (IL-1β) in patients with moderate CKD significantly decreased major adverse CV event rates and mortality much more than in the general population without significant change to LDL cholesterol levels [11, 12]. Therefore inflammation and oxidative stress play a disproportionate role in the development of atherosclerosis in CKD patients.
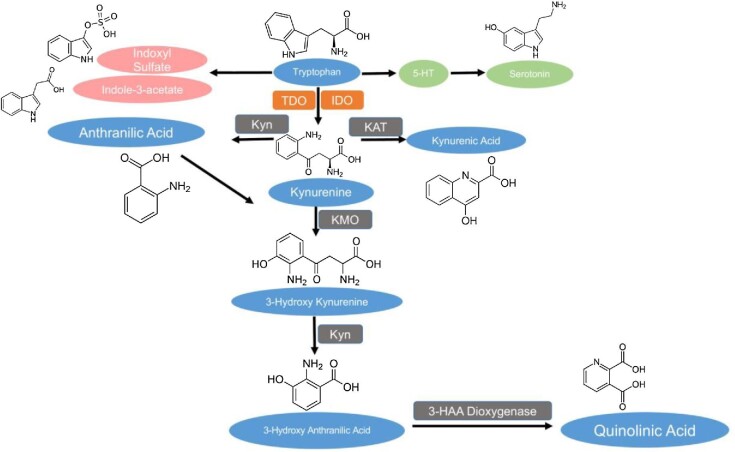
Elevated levels of the catabolites of the amino acid tryptophan are associated with decreased renal function in experimental models and CKD cohorts [13, 14]. Tryptophan is catabolized through the kynurenine pathway (KP) within the liver and extrahepatic tissues by the enzymes tryptophan dioxygenase and indoleamine dioxygenase 1 (IDO1), respectively (Fig. 1) [15, 16]. In inflammatory states like CKD, IDO1 is upregulated, promoting extrahepatic flux into the KP. Tryptophan catabolites also accumulate in CKD because of the decreased renal excretion [16–19]. Prior research has implicated several tryptophan metabolites with prevalent CVD and inflammation in CKD cohorts [14, 19–22]. In CKD, alteration of the intestinal microbiome generates increased indole metabolites, notably indoxyl sulfate and indole-3-acetate from dietary tryptophan, which contributes to systemic inflammation and CVD [23]. However, the kynurenine and indole pathways have never been compared against each other in their ability to predict CVD in a CKD cohort, and the potential contribution of indole metabolites to predict subclinical and new CV events in a western CKD cohort has never been explored.
FIGURE 1:
Tryptophan catabolism via the KP. Metabolites measured by LC-MS are depicted in the blue ovals. Rate-limiting enzymes of the KP are depicted in the red rectangles and include tryptophan 2,3-dioxygenase (TDO) and indoleamine 2,3-dioxygenase (IDO). Other downstream enzymes of the KP are depicted in the dark gray rectangles and include kynureninase (Kyn), kynurenine aminotransferase (KAT), kynurenine monooxygenase (KMO) and 3-hydroxy anthranilic acid dioxygenase (3-HAA dioxygenase). Indole derivatives are depicted in the pink ovals and intermediates of the serotonin pathway, including 5-hydroxy tryptamine (5-HT), are depicted in the green ovals.
Prior research links KP activation in patients on dialysis with increased carotid intima-media thickness (CIMT), a subclinical marker of atherosclerosis that can independently predict CV events and mortality within CKD and dialysis [24, 25]. However, the relationship between KP activation and subclinical vascular calcification has not been explored. We previously found low tryptophan associates with incident CVD in CKD [14]. We hypothesized that altered tryptophan catabolism is associated with subclinical CVD and can predict the time to the first CV event in a cohort of advanced CKD patients. In this cohort of 325 patients with Stage 3 b–4 CKD with well-defined atherosclerotic CV events, we independently assessed the association of all kynurenine metabolites in addition to key indole derivatives to new CV events and vascular calcification in a western cohort (Fig. 1).
MATERIALS AND METHODS
Study design
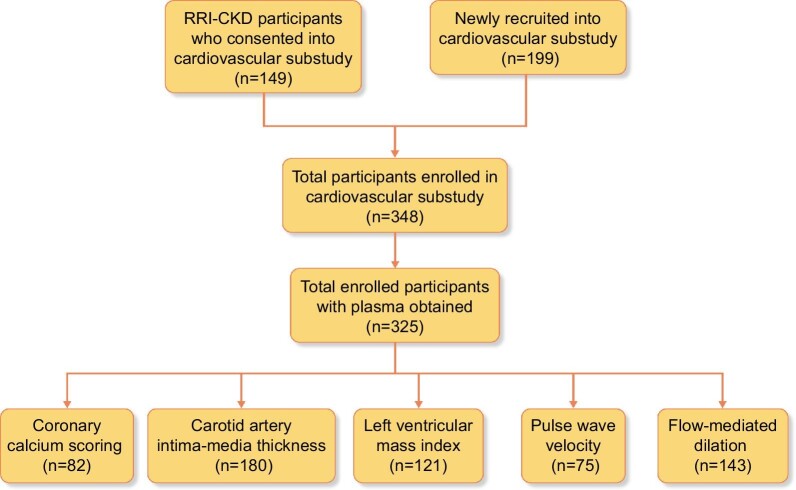
The Renal Research Institute Chronic Kidney Disease (RRI-CKD) study was a four-center, prospective, observational cohort study involving adult patients with moderate–severe CKD, not on dialysis, enrolled between June 2000 and February 2006 [25]. Eligibility criteria included age ≥18 years and estimated glomerular filtration rate (eGFR) ≤50 mL/min/1.73 m2 by the Cockcroft–Gault formula on two separate occasions. Data on demographic characteristics, anthropometric measures, cause of CKD, symptoms, laboratory values and medications were collected at enrollment and follow-up visits. The RRI-CKD cardiac substudy included a subset of participants from the RRI-CKD cohort (n = 149) who consented to undergo blood, urine and non-invasive CV studies. In addition, 199 participants were newly recruited into the cardiac substudy. Of the 348 patients in the cardiac substudy, 325 plasma samples were available for use in this study; while some patients opted for all the subclinical CV studies, others declined or were unable to participate due to a lack of resources at their enrollment center (Fig. 2) [25]. The institutional review boards of the participating centers approved the protocol and written informed consent was obtained.
FIGURE 2:
Flow chart displaying prior and newly recruited participants who consented to enrollment into the RRI-CKD CV substudy. The bottom row gives the sample size for each subclinical CVD assessment.
Clinical and lab measures
Baseline variables collected at the time of enrollment included demographic characteristics, clinical measurements, clinical comorbidities and laboratory parameters. Blood pressure was measured in duplicate after 5 min of rest in the seated position using an automated oscillometer (model HEM-412C; Omron Healthcare, Kyoto, Japan), and the results were averaged. Blood specimens were acquired after a 12-h fast. Diabetes was defined as fasting glucose ≥126 mg/dL or the use of insulin or another medication for glycemic control. Hypertension was defined as a systolic blood pressure (SBP) ≥140 mmHg or the use of antihypertensive agents. Smoking status was obtained by self-report.
Quantification of kynurenine and indole pathway metabolites
Plasma samples were obtained at baseline (n = 325), stored at −80°C and analyzed at 15–18 years post-enrollment depending on the enrollment date. We quantified free plasma levels of downstream metabolites of the KP, tryptophan, kynurenine, 3-hydroxykynurenine, kynurenic acid, anthranilic acid, 3-hydroxyanthranilic acid and quinolinic acid, in addition to indole metabolites indoxyl sulfate and indole-3-acetate (Fig. 1), using a targeted liquid chromatography-mass spectrometry (LC-MS) platform. Tryptophan 15N2, kynurenic acid D5, anthranilic acid 13C6, indoxyl sulfate 13C6, indoxyl acetate D5 and all authentic standards were purchased from Sigma Aldrich (St. Louis, MO, USA). A total of 50 µL of plasma was extracted with 200 µL of chilled acetonitrile spiked with internal standards, vortexed and centrifuged. The supernatant was transferred to glass vials that were vacufuged. The dried samples were reconstituted in 50 µL of water with 0.1% formic acid. The samples were then analyzed with a 6490 QQQ mass spectrometer with a 1290 Liquid Chromatography machine attached (Agilent, Santa Clara, CA, USA) [14].
Measures of subclinical CVD
Subsets of participants underwent specific noninvasive CV studies that revealed their atherosclerotic burden and could independently predict CVD risk over traditional risk factors in CKD [26]. These studies included computed tomography (CT)-based Agatston calcium scores, CIMT, left ventricular mass index (LVMI), flow-mediated dilation (FMD) and pulse wave velocity (PWV).
Coronary calcium was measured using a 4-slice LightSpeed QXi CT scanner without contrast administration and scored at baseline using coronary artery calcium (CAC) scoring software (SmartScore: both from GE Healthcare, Chicago, IL, USA) by an expert investigator unaware of the subjects’ identities. Clusters of calcified lesions were automatically highlighted in color based on a threshold of 130 HU. After manual confirmation of each cluster of calcium, total values for the Agatston score and the volumetric score were obtained. Because the Agatston and volumetric scores were highly correlated at all levels (r = 0.99, P < .0001), only the Agatston score was included in subsequent analyses. Calcium scores were separately obtained at the levels of the coronary arteries and thoracic aorta. Each final score was the sum of the values obtained in every single axial image of the scan [27].
CIMT
Longitudinal images of the right and left common carotid arteries, carotid bulbs and internal carotid arteries were acquired with a high-resolution B-mode ultrasound transducer and recorded for subsequent analysis at the core laboratory. Using electronic calipers, CIMT was measured as described previously [25]. To ensure uniformity of the technique, study coordinators and sonographers at each site were trained in procedures for carotid artery ultrasound imaging at the University of Michigan data coordinating center. Images were analyzed at the core vascular laboratory at the University of Michigan.
Other measures of subclinical CVD
Left ventricular mass was calculated from echography measurements that assessed end-diastolic internal dimension and wall thickness as previously described by Devereux et al. [28]. LVMI was derived by correcting left ventricular mass for body surface area (LVMI = left ventricular mass/body surface area). Arterial stiffness was quantified as the carotid–femoral PWV using an ATCOR (version 7.0; Naperville, IL, USA) device, as described previously [29]. FMD was determined by measuring the change in arterial diameter induced by reactive hyperemia and calculated as the percentage change from the participant baseline [30]. End-diastole arterial diameter was measured using customized software (Brachial Tools, Medical Imaging Applications, Coralville, IA, USA).
Clinical follow-up
Participant follow-up concluded on 31 December 2006; of a total of 325 participants, 295 were followed for at least 10 months. The median follow-up time was 2.16 years. All outcomes were ascertained on an ongoing basis by study coordinators based on regular review of electronic health records, direct patient contact in the clinic and telephonic communication. Coordinators had no knowledge of subclinical CV study results. CV endpoints included coronary events (myocardial infarction, coronary revascularization procedures), cerebrovascular events (stroke or transient ischemic attack), new-onset heart failure, sudden cardiac death or development of peripheral vascular disease requiring revascularization or amputation. We considered new CV events to include coronary artery disease (acute myocardial infarction, coronary artery bypass grafting, coronary artery stent), cerebrovascular disease (carotid endarterectomy, stroke), peripheral vascular disease (arterial bypass, peripheral artery disease, claudication, chronic extremity ulceration, cellulitis/gangrene) and other CVDs (abdominal artery aneurysm, aortic or mitral valve replacement or repair, cardiac arrest, congestive heart failure; Supplementary data, Table S1).
Statistical analysis
Descriptive statistics were reported as the mean and standard deviation (SD) for continuous variables and counts and percentages for categorical variables. Group differences were assessed by t-test for continuous variables and chi-squared or Fisher's exact test for categorical variables. Metabolite distributions were assessed by Shapiro–Wilk normality tests and quantile-quantile (Q-Q) plots. All metabolites were winsorized at the 99th percentile except tryptophan, which was log-transformed. Pearson's correlation was used to assess the relationships between metabolites and CVD markers. P-values from the correlation matrix were corrected for multiple comparisons using the false discovery rate method [31]. Ordinary least squares (OLS) regression models and multiple linear regression (MLR) models were used to assess the association between metabolites and CVD markers. Cox proportional hazards models were applied to evaluate associations between metabolites and to the new CV events. Univariate analyses were conducted to assess the association between each metabolite and individual subclinical CVD outcomes or time to the first CV event. For further exploration in multivariable models, we selected associations with raw P-values < .05. Since these metabolites are derived from a single metabolic pathway and are highly correlated, we created principal components (PCs) that were then used to construct multivariable models controlling for well-established risk factors for CVD within CKD [32–34]. Data management and statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Baseline patient characteristics
Baseline demographic characteristics, medications, comorbidities, serum level of metabolites and measures of subclinical CVD by a history of established CVD at baseline (n = 127) and those without a CVD history (n = 198) are presented in Table 1. We also examined two different groups at baseline: those who experienced new CV events during the study period (n = 50) and those who did not (n = 275) (Table 2). The distribution of demographics and clinical characteristics for subgroups that underwent additional subclinical testing are presented in Supplementary data, Tables S2–6.
Table 1.
Baseline demographics and laboratory characteristics by prior history of CVD
| Variables | Patients without a prior history of CVD (n = 198) | Patients with prior a history of CVD (n = 127) | P-value |
|---|---|---|---|
| Age (years), mean ± SD | 57 ± 14.4 | 65.5 ± 13.9 | <.001 |
| Female, n (%) | 105 (53.0) | 48 (37.8) | .01 |
| Race, n (%) | |||
| Black | 34 (17.2) | 22 (17.3) | .97 |
| White | 154 (77.8) | 102 (80.3) | .59 |
| Others | 10 (0.05) | 3 (0.02) | |
| Etiology of CKD, n (%) | |||
| Diabetes | 42 (21.3) | 49 (38.6) | <.001 |
| Hypertension | 79 (40.1) | 77 (60.6) | <.001 |
| Glomerulonephritis | 72 (36.5) | 18 (14.2) | <.001 |
| Interstitial renal disease | 24 (12.2) | 9 (7.1) | .14 |
| Polycystic kidney disease | 17 (8.6) | 3 (2.4) | .02 |
| Other | 22 (11.2) | 23 (18.1) | .08 |
| Clinical and lab characteristics, mean ± SD | |||
| Weight (kg) | 83 ± 18.7 | 87.5 ± 21.4 | .05 |
| BMI (kg/m2) | 29.2 ± 6.3 | 30.4 ± 7.1 | .11 |
| SBP (mmHg) | 135.4 ± 21.1 | 145.8 ± 24.3 | <.001 |
| DBP (mmHg) | 79.7 ± 11.8 | 76.1 ± 12.9 | .01 |
| Heart rate (bpm) | 66.5 ± 10.2 | 64.6 ± 10.8 | .12 |
| Total cholesterol (mg/dL) | 200 ± 50.6 | 174.7 ± 48 | <.001 |
| HDL (mg/dL) | 43.7 ± 14.8 | 39.1 ± 12.2 | .01 |
| Triglycerides (mg/dL) | 154.9 ± 89.7 | 137.9 ± 75.7 | .08 |
| Serum albumin (g/dL) | 4.07 ± 0.45 | 3.93 ± 0.44 | .01 |
| CRP (mg/dL) | 4.57 ± 7.02 | 6.39 ± 8.97 | .04 |
| Serum calcium (mg/dL) | 9.22 ± 0.64 | 9.01 ± 0.78 | .01 |
| Serum phosphorus (mg/dL) | 3.71 ± 0.85 | 3.74 ± 0.86 | .79 |
| Intact parathyroid hormone (pg/mL) | 155.4 ± 163 | 181.7 ± 176.1 | .17 |
| Hematocrit (%) | 36.9 ± 4.6 | 36.3 ± 4.8 | .25 |
| Serum creatinine (mg/dL) | 2.66 ± 1.14 | 2.61 ± 1.4 | .73 |
| eGFR (mL/min/1.73 m2; CKD-EPI) | 27 ± 12 | 28 ± 10 | .68 |
| UPCR (g/g creatinine) | 1.17 ± 2.01 | 1.28 ± 1.84 | .87 |
| Medications, n (%) | |||
| Statin | 78 (39.4) | 78 (61.4) | <.001 |
| Diuretic | 82 (41.4) | 83 (65.4) | <.001 |
| Calcium channel blocker | 72 (36.4) | 72 (56.7) | <.001 |
| Beta blocker | 74 (37.4) | 95 (74.8) | <.001 |
| ACEI/ARB/renin inhibitor | 145 (87.9) | 75 (86.2) | .71 |
| Acetylsalicylic acid | 49 (24.7) | 72 (56.7) | <.001 |
| CKD stage, n (%) | .38 | ||
| Stage 2 | 1 (0.5) | 2 (1.6) | |
| Stage 3 | 78 (39.6) | 60 (47.2) | |
| Stage 4 | 96 (48.7) | 53 (41.7) | |
| Stage 5 | 22 (11.2) | 12 (9.4) | |
| Current tobacco use, n (%) | 24 (12.1) | 12 (9.4) | .45 |
| Tobacco use history, n (%) | .01 | ||
| Current tobacco use | 24 (12.1) | 12 (9.4) | |
| Former tobacco use | 56 (28.3) | 57 (44.9) | |
| Never | 113 (57.1) | 58 (45.7) | |
| New CV event during follow-up, n (%) | 12 (6.1) | 38 (29.9) | <.001 |
| Tryptophan metabolites (µM), mean ± SD | |||
| Tryptophan | 26.70 ± 6.85 | 25.92 ± 6.95 | .32 |
| Kynurenine | 4.11 ± 2.11 | 4.14 ± 1.82 | .89 |
| Kynurenine:tryptophan ratio, mean ± SD | .16 ± 0.07 | .16 ± 0.06 | .34 |
| Hydroxykynurenine (nM) | 108.49 ± 27.41 | 110.45 ± 27.32 | .53 |
| Kynurenic acid (nM) | 51.01 ± 41.23 | 47.92 ± 39.17 | .50 |
| Anthranilic acid (nM) | 33 ± 34 | 34 ± 17 | .79 |
| Hydroxyanthranilic acid, nM | 77.64 ± 19.52 | 77.22 ± 19.43 | .85 |
| Quinolinic acid | 1.51 ± 1.95 | 1.61 ± 1.99 | .66 |
| Indole metabolites (µM) | |||
| Indoxyl sulfate | 9.82 ± 12.98 | 8.53 ± 10.22 | .34 |
| Indole-3-acetate | 1.46 ± 1.63 | 1.49 ± 1.87 | .89 |
| Subclinical CVD, mean ± SD | |||
| Maximum CIMT (mm) | 1.2 ± 0.7 | 1.9 ± 0.9 | <.001 |
| Aorta calcium score | 289 ± 1089 | 1337 ± 2034 | .003 |
| Coronary calcium score | 240 ± 684 | 1109 ± 1631 | .001 |
| Agatston CT score | 214 ± 538 | 820 ± 1358 | <.001 |
BMI, body mass index; HDL, high-density lipoprotein; CRP, C-reactive protein; DBP, diastolic blood pressure; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker. P-values <.05 are in bold.
Table 2.
Baseline demographics and laboratory characteristics by development of new CV events during the study
| Variables | No CV event (n = 275) | New CV event (n = 50) | P-value |
|---|---|---|---|
| Age (years), mean ± SD | 59.2 ± 14.9 | 66.9 ± 12.3 | <.001 |
| Female, n (%) | 136 (49.5) | 17 (34) | .04 |
| Race, n (%) | |||
| Black | 49 (17.8) | 7 (14) | .51 |
| White | 214 (77.8) | 42 (84) | .33 |
| Others | 12 (4.4) | 1 (2) | .43 |
| Etiology of CKD, n (%) | |||
| Diabetes | 67 (24.5) | 24 (48) | <.001 |
| Hypertension | 128 (46.7) | 28 (56) | .23 |
| Glomerulonephritis | 81 (29.6) | 9 (18) | .09 |
| Interstitial renal disease | 31 (11.3) | 2 (4) | .12 |
| Polycystic kidney disease | 20 (7.3) | 0 (0) | .05 |
| Other | 36 (13.1) | 9 (18) | .36 |
| History of prior CVD, n (%) | 12 (24) | 38 (76) | <.001 |
| Clinical and lab characteristics, mean ± SD | |||
| Weight (kg) | 84.0 ± 19.9 | 89.2 ± 19.2 | .09 |
| BMI (kg/m2) | 29.6 ± 6.6 | 30.1 ± 6.8 | .61 |
| SBP (mmHg) | 139.3 ± 22.3 | 140.3 ± 26.6 | .77 |
| DBP (mmHg) | 79.1 ± 12.0 | 74.2 ± 13.5 | .01 |
| Heart rate (bpm) | 65.6 ± 10.2 | 66.5 ± 12.2 | .57 |
| Total cholesterol (mg/dL) | 192.7 ± 50.0 | 175.9 ± 54.6 | .03 |
| HDL (mg/dL) | 41.5 ± 13.0 | 44.2 ± 18.4 | .21 |
| Triglycerides (mg/dL) | 149.4 ± 82.9 | 142.0 ± 95.3 | .57 |
| Serum albumin (g/dL) | 4.05 ± 0.44 | 3.82 ± 0.46 | <.001 |
| CRP (mg/dL) | 4.82 ± 7.47 | 7.80 ± 9.52 | .02 |
| Serum calcium (mg/dL) | 9.18 ± 0.69 | 8.90 ± 0.73 | .01 |
| Serum phosphorus (mg/dL) | 3.66 ± 0.81 | 4.03 ± 1.03 | .01 |
| Intact parathyroid hormone (pg/mL) | 161.2 ± 169.3 | 189.9 ± 163.1 | .27 |
| Hematocrit | 36.9 ± 4.8 | 35.6 ± 4.1 | .09 |
| Serum creatinine (mg/dL) | 2.59 ± 1.18 | 2.93 ± 1.55 | .07 |
| eGFR (ml/min/1.73 m2; CKD-EPI) | 28 ± 11 | 24 ± 11 | .08 |
| UPCR (g/g creatinine) | 0.98 ± 1.44 | 2.47 ± 3.59 | .08 |
| Medications, n (%) | |||
| Statin | 128 (46.5) | 28 (56) | .22 |
| Diuretic | 133 (48.4) | 32 (64) | .04 |
| Calcium channel blocker | 119 (43.3) | 25 (50) | .38 |
| Beta blocker | 139 (50.5) | 30 (60) | .22 |
| ACEI/ARB/renin inhibitor | 183 (86.7) | 37 (90.2) | .54 |
| Acetylsalicylic acid | 97 (35.3) | 24 (48) | .09 |
| CKD stage, n (%) | .56 | ||
| Stage 2 | 3 (1.1) | 0 (0) | |
| Stage 3 | 120 (43.8) | 18 (36) | |
| Stage 4 | 124 (45.3) | 25 (50) | |
| Stage 5 | 27 (9.9) | 7 (14) | |
| Current tobacco use, n (%) | 32 (11.6) | 4 (8) | .45 |
| Tobacco use history, n (%) | .09 | ||
| Current tobacco use | 32 (11.6) | 4 (8) | |
| Former tobacco use | 88 (32) | 25 (50) | |
| Never | 1150 (54.5) | 21 (42) | |
| Tryptophan metabolites (µM), mean ± SD | |||
| Tryptophan | 26.62 ± 6.78 | 25.13 ± 7.41 | .16 |
| Kynurenine | 4.11 ± 1.96 | 4.23 ± 2.23 | .68 |
| Kynurenine:tryptophan ratio, mean ± SD | 0.16 ± 0.06 | 0.17 ± 0.08 | .11 |
| Kynurenic acid (nM) | 48.81 ± 38.07 | 55.24 ± 51.51 | .30 |
| Anthranilic acid (nM) | 33 ± 30 | 37 ± 20 | .36 |
| Quinolinic acid | 1.44 ± 1.62 | 2.13 ± 3.22 | .02 |
| Hydroxykynurenine (nM) | 108.50 ± 28.86 | 113.42 ± 29.81 | .24 |
| Hydroxyanthranilic acid (nM) | 77.05 ± 19.01 | 79.81 ± 21.84 | .36 |
| Indole metabolites (µM, mean ± SD | |||
| Indoxyl sulfate | 9.18 ± 11.51 | 10.08 ± 14.40 | .63 |
| Indole-3-acetate | 1.46 ± 1.58 | 1.53 ± 2.39 | .80 |
| Subclinical CVD, mean ± SD | |||
| Maximum CIMT (mm) | 1.3 ± 0.7 | 2.1 ± 1.0 | <.001 |
| Aorta calcium score | 475 ± 1320 | 1295 ± 2117 | .04 |
| Coronary calcium score | 355 ± 751 | 1187 ± 1912 | .01 |
| Agatston CT score | 295 ± 590 | 989 ± 1719 | <.001 |
BMI, body mass index; HDL, high-density lipoprotein; CRP, C-reactive protein; DBP, diastolic blood pressure; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker. P-values <.05 are in bold.
Association of metabolites with subclinical atherosclerosis markers
A correlation matrix between tryptophan metabolites and subclinical markers of CVD is summarized in Table 3. Linear regression models assessed the relationship between metabolite PC's and individual subclinical markers with stepwise additions of demographic and clinical characteristic covariates across five models (Table 4).
Table 3.
Relationship of kynurenine and indole pathway metabolites with subclinical markers of CVD
| Aorta calcium score | Coronary calcium score | Agatston CT score | CIMT | LVMI | PWV | FMD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolitesa | r | P-value | r | P-value | r | P-value | r | P-value | r | P-value | r | P-value | r | P-value | |
| TRP | −0.23 | .03 | −0.04 | .71 | <0.01 | .99 | 0.02 | .76 | −0.1 | .17 | −0.15 | .02 | −0.02 | .77 | |
| Kynurenine pathway | KYN | 0.00 | .97 | 0.19 | .08 | 0.18 | .04 | 0.11 | .13 | 0.00 | .96 | −0.01 | .91 | 0.62 | .41 |
| KTR | 0.06 | .59 | 0.21 | .05 | 0.19 | .03 | 0.12 | .12 | 0.02 | .74 | 0.02 | .73 | 0.08 | .33 | |
| HK | 0.07 | .51 | 0.09 | .42 | 0.03 | .70 | 0.12 | .10 | 0.07 | .35 | 0.04 | .48 | 0.05 | .48 | |
| KA | 0.03 | .79 | 0.22 | .05 | 0.18 | .04 | 0.07 | .34 | 0.11 | .13 | 0.01 | .87 | 0.06 | .44 | |
| AA | 0.28 | <.01 | 0.35 | <.01 | 0.20 | .02 | 0.14 | .06 | 0.23 | <.01 | 0.19 | <.01 | 0.01 | .87 | |
| HAA | 0.17 | .12 | 0.30 | <.01 | 0.27 | <.01 | 0.17 | .02 | 0.05 | .47 | 0.03 | .58 | 0.04 | .62 | |
| QA | 0.18 | .10 | 0.32 | <.01 | 0.22 | .01 | 0.22 | <.01 | 0.16 | .03 | 0.09 | .16 | 0.09 | .22 | |
| Indole pathway | IS | −0.06 | .62 | 0.20 | .07 | 0.11 | .22 | −0.07 | .33 | 0.13 | .07 | −0.03 | .64 | −0.01 | .91 |
| IA | −0.01 | .91 | 0.12 | .27 | 0.08 | .34 | 0.03 | .65 | 0.11 | .10 | −0.02 | .72 | −0.05 | .49 | |
TRP, tryptophan; KYN, kynurenine; KTR, kynurenine: tryptophan ratio; KA, kynurenic acid; AA, anthranilic acid; HAA, hydroxyanthranilic acid; QA, quinolinic acid; IS, indoxyl sulfate; IA, indole-3-acetate. Correlation coefficients (r) and corresponding raw P-values are given; significant values are given in italics. P-values <.05 by the false discovery rate are given in italics and bold.
Represents log-transformed metabolites.
Table 4.
Kynurenine pathway metabolites can predict subclinical CVD markers as demonstrated by multiple linear regression
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Principal components (% variance explained) | Metabolite(s) only | + Age, gender, race, prior CVD history | + SBP, DM | + eGFR, UPCR | + CRP, serum albumin | ||||||
| β (95% CI) | P-value | β (95% CI) | P-value | β (95% CI) | P-value | β (95% CI) | P-value | β (95% CI) | P-value | ||
| Aorta calcium score (n = 82) | TRP:AA | −57 | <.01 | −54 | <.01 | −46 | <.01 | −45 | <.01 | −45 | <.01 |
| (53.6%) | (−92 to −22) | (−85 to −22) | (−79 to −13) | (−79 to −10) | (−79 to −10) | ||||||
| Coronary calcium score (n = 82) | QA:HAA:AA | 32 | <.01 | 28 | <.01 | 23 | <.01 | 24 | .01 | 23 | .01 |
| (60.2%) | (16–47) | (13–42) | (6.6–39) | (5.5–42) | (5.1–42) | ||||||
| Agatston CT score (n = 82) | KYN:KA:QA:HAA:AA | 9.4 | <.01 | 8.5 | <.01 | 6.7 | .03 | 8.6 | .03 | 7.6 | .07 |
| (58.3%) | (3.4–15.4) | (2.9–14.1) | (0.8–12.6) | (0.7–16.4) | (−0.5–15.7) | ||||||
| CIMT (n = 180) | QA:HAA | 0.012 | .02 | 0.007 | .07 | 0.005 | .18 | 0.005 | .26 | 0.006 | .20 |
| (71.9%) | (0.002–0.021) | (-0.001–0.015) | (-0.002–0.013) | (-0.004–0.014) | (-0.003–0.015) | ||||||
| LVMI (n = 121) | QA:AA | 0.593 | <.01 | 0.395 | .05 | 0.395 | .04 | 0.293 | .13 | 0.319 | .08 |
| (69.4%) | (0.163–1.023) | (0.007–0.783) | (0.025–0.765) | (−0.088–0.673) | (−0.041–0.679) | ||||||
| PWV (n = 75) | TRP:AA | −0.050 | <.01 | −0.038 | .02 | −0.031 | .04 | −0.019 | .25 | −0.018 | .28 |
| (53.6%) | (−0.086 to −0.014) | (−0.071 to −0.006) | (−0.062 to −0.001) | (−0.051–0.013) | (−0.050–0.015) | ||||||
DM, diabetes mellitus; CRP, C-reactive protein; TRP, tryptophan; AA, anthranilic acid; QA, quinolinic acid; HAA, hydroxyanthranilic acid; KYN, kynurenine; KA, kynurenic acid. Parameter estimates (β), 95% CIs and P-values are displayed. Statistically significant (P < .05) parameter estimates and P-values are in bold.
PC (Trp: AA), constructed with tryptophan and anthranilic acid, was inversely associated with aortic calcification across all models (Model 5, β = −45, P < .01). PC (QA: HAA: AA), constructed with quinolinic, hydroxyanthranilic and anthranilic acid demonstrated a positive association with coronary calcium across all models, including the fully adjusted model with demographics, history of CVD, SBP, diabetes status, eGFR, urine protein:creatinine ratio (UPCR), serum albumin and C-reactive protein (CRP) (Model 5, β = 23, P = .01). PC (Kyn: KA: QA: HAA: AA), which included kynurenine, kynurenic acid, quinolinic acid, hydroxyanthranilic acid and anthranilic acid, was positively associated with Agatston CT score across Models 1–4 (Model 4, β = 8.6, P = .03). PC (Trp: AA) was inversely associated with PWV across Models 1–3 (Model 3, β = −0.031, P = .04). PC (QA: HAA), with quinolinic and hydroxy anthranilic acid, independently predicted increased CIMT (Model 1, β = 0.012, P = .02), while the PC involving quinolinic and anthranilic acids positively associated with increased LVMI across Models 1–3, (Model 3, β = 0.395, P = .04; Table 4).
We also performed linear regression of individual metabolites with subclinical markers of CVD (Supplementary data, Table S7). A lower level of tryptophan at baseline was associated with higher aortic calcification in all models (Model 5, β = −1983, P < .01) and increased PWV in Models 1–4 (Model 4, β = −1.60, P = .02). Anthranilic and quinolinic acids are associated with higher aortic (β = 40, P < .01) and higher coronary calcium (Model 5, β = 328, P = .02) across all adjusted models respectively, while hydroxyanthranilic acid is associated with the Agatston CT score in all models (Model 5, β = 12, P = .03).
Association of metabolites with CV outcomes
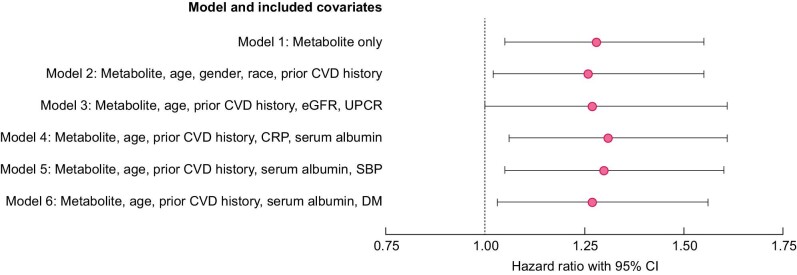
Cox proportional hazards models were used to predict the time to the first new CV event based on baseline serum tryptophan and metabolite levels (Table 5, Fig. 3). Quinolinic and anthranilic acids were associated with a reduced time to a CVD event. In the unadjusted model including all patients, a 1-µmol increase in quinolinic acid level at baseline was associated with a 28% higher risk of a new CV event {Model 1, HR 1.28 [95% (CI) 1.05–1.55], P = .01}. The direction and significance of these outcomes were represented in all models. Each nanomole increase in baseline anthranilic acid levels was associated with a 2% higher risk for a new CV event [Model 1, HR = 1.02 (95% CI 1.00–1.03), P = .02]. Low tryptophan was associated with a lower risk of time to the first CV event in all patients when adjusting for demographics and CVD history (Model 2). A one-unit increase of log-transformed tryptophan was associated with a 70% lower risk of new CV event [Model 2, HR 0.30 (95% CI 0.10–0.90), P = .03; Table 5].
Table 5.
Cox proportional hazards model for time to new CV event by significant serum metabolites
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model Covariates | Metabolite only | Metabolite, age, gender, race, prior CVD history | Metabolite, age, prior CVD history, eGFR, UPCR | Metabolite, age, prior CVD history, CRP, serum albumin | Metabolite, age, prior CVD history, serum albumin, SBP | Metabolite, age, prior CVD history, serum albumin, DM | ||||||
| Metabolites | HR | P-value | HR | P-value | HR | P-value | HR | P-value | HR | P-value | HR | P-value |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |||||||
| TRP | 0.38 | .07 | 0.30 | .03 | 0.40 | .1 | 0.21 | .21 | 0.46 | .16 | 0.49 | .2 |
| (0.14–1.07) | (0.10–0.90) | (0.13–1.18) | (0.16–1.50) | (0.15–1.38) | (0.16–1.47) | |||||||
| AA | 1.02 | .02 | 1.01 | .07 | 1.02 | .06 | 1.02 | .03 | 1.02 | .03 | 1.01 | .07 |
| (1.00–1.03) | (1.00–1.03) | (1.00–1.03) | (1.00–1.03) | (1.00–1.03) | (1.00–1.03) | |||||||
| QA | 1.28 | .01 | 1.26 | .03 | 1.27 | .05 | 1.31 | .01 | 1.3 | .01 | 1.27 | .03 |
| (1.05–1.55) | (1.02–1.55) | (1.00–1.61) | (1.06–1.61) | (1.05–1.60) | (1.03–1.56) | |||||||
CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; UPCR, urine protein to creatinine ratio; CRP, C-reactive protein; SBP, systolic blood pressure; DM, diabetes mellitus; CI, confidence interval
TRP, tryptophan; AA, anthranilic acid; QA, quinolinic acid. HRs, 95% CIs and P-values are displayed. Statistically significant (P < .05) HRs and P-values are given in bold.
FIGURE 3:
Forest plot showing higher baseline serum quinolinic acid associates with an increased risk of new CV events in CKD. For each model (1–6), the pink dot represents the HR and the black line spans the 95% CI. CRP, C-reactive protein; DM, diabetes mellitus.
DISCUSSION
To our knowledge, this is the first study to use LC-MS to simultaneously assess the relationship between metabolites from the kynurenine and indole pathways in new and subclinical CVD in patients with moderate–severe CKD. We found that several downstream KP metabolites—most notably, quinolinic acid, anthranilic acid and hydroxyanthranilic acid—associate with vascular calcification and do not find a similar association with indole derivatives. We also found increased quinolinic acid levels at baseline associated with an increased risk for new CV events among CKD patients. Conversely, we found that lower levels of tryptophan were associated with a shorter time to the first CVD event when controlling for demographic factors and prior CVD history and predicted greater aortic calcification in a fully adjusted model. Altogether, these findings strongly support the hypothesis that altered tryptophan metabolism via the KP, and not indole derivatives, uniquely drive the higher CVD burden within the CKD population beyond traditional risk factors.
Mounting evidence supports the role of inflammation in the pathogenesis of atherosclerosis and CVD independent of traditional risk factors [11]. CKD is a well-known chronic inflammatory state marked by increased circulating cytokines and biomarkers such as interferon-γ (IFN-γ), IL-1β and CRP [35]. IFN-γ has been shown to induce IDO1, accelerating extrahepatic flux through the KP and generation of downstream metabolites [17, 36]. Serum IDO1 levels and activity are enhanced with worsening CKD and in those on dialysis [17, 37, 38]. In turn, CKD progression results in an accumulation of the kynurenine metabolites via induction of IDO1 and reduced renal elimination, resulting in high levels within the circulation and peripheral tissues [13, 19, 39].
Many kynurenine derivatives have a significant role in inflammation, oxidative stress, endothelial dysfunction and coagulation defects, contributing to the CVD burden in the CKD population [40]. KP metabolites—kynurenine, kynurenic acid, anthranilic acid and quinolinic acid—are associated with markers of endothelial dysfunction, including thrombomodulin, von Willebrand factor, soluble intercellular adhesion molecule-1 (sICAM-1) and soluble vascular adhesion molecule-1 (sVCAM-1) in patients with CKD [20]. Kaminski et al. [41] reported that anthranilic acid associated with tissue plasminogen activator positively in early CKD and negatively correlated with the tissue plasminogen activator during severe and end-stage CKD. Similarly, 3-hydroxyanthranilic acid is associated with monocyte chemoattractant protein-1 (CCL2) and macrophage inflammatory protein 1beta (CCL4) in patients with CKD [22]. A positive correlation between kynurenine, hydroxykynurenine, anthranilic acid and quinolinic acid with crucial factors associated with the development of atherosclerosis such as von Willebrand factor, thrombomodulin and prothrombin fragments F(1 + 2) concentration as well as sICAM-1 and sVCAM-1 levels was also observed in patients on dialysis [42, 43]. Kynurenine and quinolinic acid accumulation was independently related to CIMT and associated with inflammation and oxidative stress in dialysis patients [24]. Pawlak et al. [42] documented that kynurenine and hydroxykynurenine were positively associated with inflammation and oxidative stress markers in uremic patients. Therefore, multiple studies support the existence of a link between KP activation, inflammation and the progression of atherosclerosis. However, the kynurenine and indole metabolites measured in this study did not associate with any measures of FMD.
Aryl hydrocarbon receptor (AhR) is a cytosolic receptor involved in the transcriptional signaling of many uremic toxins influencing multiple CV functions [44]. Many tryptophan metabolites, including kynurenine, kynurenic acid, indoxyl sulfate and indoxyl-3-acetate, are known activators of AhR [45]. Watanabe et al. [46] showed that overexpression of IDO1 in coronary atherosclerotic plaques via increased oxidative stress level and AhR pathway stimulation enhanced tissue factor (TF) expression in activated macrophages. Similarly, the AhR activation within endothelial cells and vascular smooth muscle cells leads to increased stenosis and increased expression of TF, resulting in increased thrombotic events [47, 48]. In fact, both animal and clinical models demonstrate higher kynurenine levels, but not indoxyl sulfate, enhanced thrombosis following vascular injury and angioplasty in an AhR-dependent manner [48]. AhR activation leading to increased stenosis and thrombosis contributes to CV morbidity and mortality observed in CKD. This may explain the development of CV events in our study.
Prior epidemiologic studies have found that increased flux through the KP can predict the risk of atherosclerosis and acute coronary events within the general population [36, 49–51]. Pedersen et al. [36] demonstrated increased IDO1 activity at baseline correlated with significantly increased risk for major CV events and all-cause mortality in patients with established CVD. Several KP metabolites—kynurenic acid, hydroxykynurenine and anthranilic acid—were significantly associated with an increased risk for acute myocardial infarction in those with prior history of CVD [49] and kynurenine and hydroxykynurenine with a risk for acute coronary events despite clinical factors [50]. Conversely, a higher tryptophan concentration is associated with a lower risk of new CV events [51]. In CKD, inflammatory modulation of tryptophan metabolism, in addition to decreased clearance, multiplies the CV risk beyond that observed in the general population. Pawlak et al. [21] documented that in a cross-sectional cohort of 146 end-stage renal disease (ESRD) patients, hydroxykynurenine was positively associated with the history of CVD. This study was restricted to dialysis patients and prevalent CVD. Our current study looked at moderate–severe CKD and found significantly elevated quinolinic acid levels in those with a history of CVD as compared with patients without a prior history of CVD. In an earlier study, we demonstrated that lower levels of tryptophan and kynurenine in an independent cohort of mild CKD patients were associated with incident CVD events in those with no prior history of CVD [14]. The addition of tryptophan to models significantly improved the classification of the incident CVD group beyond baseline clinical characteristics [14]. However, CV events in this cohort included nonatherosclerotic disease–based events like arrhythmia and lacked information on the temporal association between KP metabolites and new CV events. Our current study involves moderate–severe CKD patients, uses a stringent definition of atherosclerotic events and followed patients for new CV events for a median time of >2 years.
Few studies have linked specific KP metabolites to subclinical markers of atherosclerosis within CKD. CIMT is a widely used marker of subclinical CVD and a predictor of new CV events [52]. Prior research has found an association between quinolinic acid with CIMT among 106 patients with CKD [20]. A cross-sectional study of 124 patients (42 CKD, 82 dialysis) showed a progressive increase in CIMT values with decreasing renal function. Kynurenine and quinolinic acid accumulation were independently related to CIMT values in the dialysis patients [24]. Our study found significant correlations between baseline levels of quinolinic and hydroxyanthranilic acid and CIMT. We also found that quinolinic acid was associated with CIMT, adjusting for demographic factors and prior history of CVD. However, the lack of significance in fully adjusted models is likely due to our study design, which excluded patients with ESRD on dialysis. Prior studies found plasma levels of anthranilic and hydroxyanthranilic acid to be nearly 5- to 10-fold higher in CKD compared with controls with normal renal function [22, 53]. These KP metabolites associate with CC-chemokines CCL2 and CCL4, which in turn recruit macrophages into atherosclerotic plaques [22, 54]. Specifically, hydroxyanthranilic acid associates with these chemokines independent of CRP in CKD patients [22]. This study is the first to find that both anthranilic and hydroxyanthranilic acid associate with vascular calcium, suggesting their role in the pathogenesis of subclinical atherosclerosis.
The predominantly gut-derived uremic toxins of tryptophan metabolism, indoxyl sulfate and, to a lesser extent, indole-3-acetate, have been implicated as inflammatory mediators that contribute to CVD within CKD [23, 55–57]. While the indoles represent a small portion of overall tryptophan degradation (4–6%) [58], some hypothesize these toxins contribute to CVD [55, 58]. Experimental studies demonstrate that indole toxins induce oxidative stress while reducing antioxidant defenses in endothelial cells [59, 60]. Indoxyl sulfate caused the loss of endothelial cells and enhanced the expression of adhesive molecules (ICAM-1, VCAM-1) in CKD mice [61]. Furthermore, patients with impaired renal function demonstrated elevated indoxyl sulfate levels, markers of vascular inflammation and endothelial dysfunction such as soluble Fas, soluble VCAM-1 and monocyte chemoattractant protein-1 [62]. Despite this premise, clinical studies demonstrating the association of serum indole metabolites with CV morbidity or mortality within CKD have been less robust. Several studies have investigated the relationship of serum indoxyl sulfate to CV events within CKD [56, 57, 63, 64]. These have involved small cohorts of non-Western patients with moderate CKD and found elevated indoxyl sulfate increased the risk for CV morbidity [56, 57, 63]. Barreto et al. [56] found indoxyl sulfate levels correlated with aortic calcification and PWV. However, more recent studies produced ambiguous results, especially within more advanced CKD. Lin et al. [64] found no association between serum indoxyl sulfate and all-cause mortality or adverse cardiac events. Similarly, the Hemodialysis (HEMO) Study, which involved a large sample of hemodialysis patients (n = 1273), found no association of indoxyl sulfate and CV outcomes [65]. As this trial was previously the only study to investigate indoxyl sulfate and CVD within Western CKD patients, the paucity of findings could be attributed to differences in regional diets. Variations in dietary preferences significantly influence the microbiome and, consequently, indoxyl sulfate levels [66, 67], which may explain the negative findings in both the HEMO Study and our current work. We found no association of indole metabolites with new CV events, nor a correlation with any subclinical markers of atherosclerosis, including vascular calcification and PWV. While previous studies used reverse-phase high-performance liquid chromatography, our current study employed an LC-MS platform to quantify both kynurenine and indole metabolites and a more advanced methodology to measure aortic calcification. However, our study did not include dialysis participants, which may partially explain our lack of similar findings.
Our findings strengthen the hypothesis that altered tryptophan catabolism via the KP contributes to atherosclerosis in CKD for several reasons. First, we expanded tryptophan derivative profiling to include major indole metabolites, indoxyl sulfate and indole-3-acetate, a major limiting factor of our previous study [14]. While serotonin, indole and kynurenine derivatives are derived from tryptophan, only kynurenine and indole products have been linked to CVD within CKD [14, 23, 24]. While there are multiple indole derivatives, only indoxyl sulfate and indole acetate are linked with CVD and CKD [23]. Nicotinamide adenine dinucleotide (NAD) is derived de novo from tryptophan via quinolinic acid, however, much of the available NAD is generated from salvage pathways, not directly from dietary tryptophan [68, 69]. Moreover, NAD is not linked to CVD in CKD. Our work measures all directly derived tryptophan derivatives linked to CVD within CKD and is the first to compare indole and kynurenine derivatives with subclinical and new CV events. While prior studies have previously investigated indole metabolites and CVD in CKD, this has not involved concurrent profiling of KP derivatives [56, 57, 63–65].
Another strength of the current work is the specificity of our primary outcome, new CV events, to inflammation and atherosclerosis, whereas our prior study included nonvascular pathology (e.g, arrhythmia) as CVD outcomes [14]. Prior studies have only explored prevalent CVD in CKD either by history or CIMT measures [20, 21, 24]. We also examined several measures of subclinical atherosclerosis and found a significant association with several metabolites, even in fully adjusted models, suggesting a role of the KP in the pathogenesis of CVD. The current study is also the first to link vascular calcification to KP metabolites in CKD. Moreover, the tryptophan metabolites were not linked to FMD, a marker of early CVD and endothelial dysfunction, indicating the specificity of these KP metabolites in the pathogenesis of established atherosclerosis in this cohort with advanced CKD. The inclusion criteria for our current study required an eGFR ≤50 mL/min/1.73 m2, which, compared with our prior cohort, resulted in a cohort with more advanced CKD. Despite more impaired clearance, we find tryptophan was inversely related to new CV events and subclinical CVD. We also included time-to-event analysis with Cox proportional hazards models to assess the relationship to time to the first CVD event. This was not possible in our prior independent cohort. Lastly, whereas our previous methodology involved a priori matching of demographic and clinical comorbidities to define an incident CVD group, in our current work we examine the predictive capability of tryptophan equivocally in all patients with and without a CVD history while controlling for prior CVD.
Our current study has several limitations. As a prospective observational study, we cannot ascribe a causal link between metabolites and the development of CVD within the CKD population. However, the temporal association of tryptophan levels to CV events and its relation to subclinical atherosclerosis suggests early involvement of the KP in the pathogenesis of CKD atherosclerosis. Changes to tryptophan levels parallel changes in inflammatory markers such as CRP and serum albumin. However, we find alterations in KP and its association with subclinical CVD suggest a unique role of these metabolites in atherosclerosis. More experimental studies are needed to elucidate the mechanistic role of the KP in this. Although we captured 50 CV events, only 12 of these occurred in patients without a prior history of CVD, thus we were not adequately powered to perform subgroup analyses to test the association of metabolites with new CV events in those with or without a prior history. The samples used for the analysis of tryptophan metabolites were stored for 15–18 years at −80°C, possibly introducing storage artifacts [70]. However, the uniform handling of plasma samples makes potential changes in metabolite levels due to storage similar across all individuals [71]. Another limitation of our work is the sole evaluation of metabolites at enrollment, which prevented time-varying risk association with CVD in our population. A larger study with longer follow-up and regular metabolite sampling will better investigate biomarker prediction of subclinical atherosclerosis and CVD within the CKD population. The number of patients undergoing each subclinical testing depended on testing availability at the centers and the number of patients who consented to further subclinical testing. These factors resulted in a nonuniform number of patients undergoing different cardiac tests, which might bias our interpretation of the statistical tests.
Our study proves the indoles are not altered in CKD patients with CV events, though previously linked to CVD, while specific KP metabolites are linked to CV events when controlling for renal function.
CONCLUSION
In conclusion, we demonstrate the role of KP but not indole metabolites in subclinical atherosclerotic burden and new CV events in advanced CKD. Our findings support a possible role for altered tryptophan immune metabolism in the pathogenesis of CKD-associated atherosclerosis.
Supplementary Material
ACKNOWLEDGEMENTS
T.B. performed the experiments, analyzed the data and drafted the manuscript. E.V. performed the experiments and drafted the manuscript. Y.H. and B.G. performed statistical analyses and revised the manuscript. J.B. performed the mass spectrometry experiments, assisted with data collection and revised the manuscript. V.C.K performed the experiments and revised the manuscript. R.S. designed the RRI-CKD study, analyzed the data, and revised the manuscript. A.V.M. designed and conducted the experiments, analyzed the data, provided funding and drafted and revised the manuscript. All authors contributed to the intellectual content of the work and gave final approval of the manuscript.
Contributor Information
Trista Benitez, University of Michigan Medical School, Ann Arbor, MI, USA.
Elizabeth VanDerWoude, University of Michigan, Ann Arbor, MI, USA.
Yun Han, Department of Internal Medicine, Division of Nephrology, University of Michigan, Ann Arbor, MI, USA.
Jaeman Byun, Department of Internal Medicine, Division of Nephrology, University of Michigan, Ann Arbor, MI, USA.
Vetalise Cheofor Konje, Department of Internal Medicine, Division of Nephrology, University of Michigan, Ann Arbor, MI, USA.
Brenda W Gillespie, University of Michigan School of Public Health, Ann Arbor, MI, USA.
Rajiv Saran, Department of Internal Medicine, Division of Nephrology, University of Michigan, Ann Arbor, MI, USA.
Anna V Mathew, Department of Internal Medicine, Division of Nephrology, University of Michigan, Ann Arbor, MI, USA.
FUNDING
This work is supported in part by grants from the National Institutes of Health (HL130944 and UL1TR002240; Michigan Institute for Clinical and Health Research) to A.V.M. The RRI-CKD Study (principal investigator R.S.) was supported by the Renal Research Institute, New York, NY.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Saran R, Robinson B, Abbott KCet al. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2020; 75: A6–A7 [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Chertow GM, Fan Det al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305 [DOI] [PubMed] [Google Scholar]
- 3. Park SH, Stenvinkel P, Lindholm B. Cardiovascular biomarkers in chronic kidney disease. J Ren Nutr 2012; 22: 120–127 [DOI] [PubMed] [Google Scholar]
- 4. Sun J, Axelsson J, Machowska Aet al. Biomarkers of cardiovascular disease and mortality risk in patients with advanced CKD. Clin J Am Soc Nephrol 2016; 11: 1163–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wanner C, Krane V, März Wet al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005; 353: 238–248 [DOI] [PubMed] [Google Scholar]
- 6. Fellstrom BC, Jardine AG, Schmieder REet al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009; 360: 1395–1407 [DOI] [PubMed] [Google Scholar]
- 7. Baigent C, Landray MJ, Reith Cet al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377: 2181–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kwiatkowska I, Hermanowicz JM, Mysliwiec Met al. Oxidative storm induced by tryptophan metabolites: missing link between atherosclerosis and chronic kidney disease. Oxid Med Cell Longev 2020; 2020: 6656033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakano T, Katsuki S, Chen Met al. Uremic toxin indoxyl sulfate promotes proinflammatory macrophage activation via the interplay of OATP2B1 and Dll4-notch signaling. Circulation 2019; 139: 78–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang M, Wei R, Wang Yet al. The uremic toxin hippurate promotes endothelial dysfunction via the activation of Drp1-mediated mitochondrial fission. Redox Biol 2018; 16: 303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ridker PM, Everett BM, Thuren Tet al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377: 1119–1131 [DOI] [PubMed] [Google Scholar]
- 12. Ridker PM, MacFadyen JG, Glynn RJet al. Inhibition of Interleukin-1β by canakinumab and cardiovascular outcomes in patients with chronic kidney disease. J Am Coll Cardiol 2018; 71: 2405–2414 [DOI] [PubMed] [Google Scholar]
- 13. Pawlak D, Tankiewicz A, Mysliwiec Pet al. Tryptophan metabolism via the kynurenine pathway in experimental chronic renal failure. Nephron 2002; 90: 328–335 [DOI] [PubMed] [Google Scholar]
- 14. Konje VC, Rajendiran TM, Bellovich Ket al. Tryptophan levels associate with incident cardiovascular disease in chronic kidney disease. Clin Kidney J 2021; 14: 1097–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Palego L, Betti L, Rossi Aet al. Tryptophan biochemistry: structural, nutritional, metabolic, and medical aspects in humans. J Amino Acids 2016; 2016: 8952520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Savitz J. The kynurenine pathway: a finger in every pie. Mol Psychiatry 2020; 25: 131–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schefold JC, Zeden JP, Fotopoulou Cet al. Increased indoleamine 2,3-dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: a possible link between chronic inflammation and uraemic symptoms. Nephrol Dial Transplant 2009; 24: 1901–1908 [DOI] [PubMed] [Google Scholar]
- 18. Saito K, Fujigaki S, Heyes MPet al. Mechanism of increases in l-kynurenine and quinolinic acid in renal insufficiency. Am J Physiol Renal Physiol 2000; 279: F565–F572 [DOI] [PubMed] [Google Scholar]
- 19. Pawlak D, Pawlak K, Malyszko Jet al. Accumulation of toxic products degradation of kynurenine in hemodialyzed patients. Int Urol Nephrol 2001; 33: 399–404 [DOI] [PubMed] [Google Scholar]
- 20. Pawlak K, Mysliwiec M, Pawlak Det al. Kynurenine pathway — a new link between endothelial dysfunction and carotid atherosclerosis in chronic kidney disease patients. Adv Med Sci 2010; 55: 196–203 [DOI] [PubMed] [Google Scholar]
- 21. Pawlak K, Domaniewski T, Mysliwiec Met al. The kynurenines are associated with oxidative stress, inflammation and the prevalence of cardiovascular disease in patients with end-stage renal disease. Atherosclerosis 2009; 204: 309–314 [DOI] [PubMed] [Google Scholar]
- 22. Pawlak K, Kowalewska A, Mysliwiec Met al. 3-hydroxyanthranilic acid is independently associated with monocyte chemoattractant protein-1 (CCL2) and macrophage inflammatory protein-1beta (CCL4) in patients with chronic kidney disease. Clin Biochem 2010; 43: 1101–1106 [DOI] [PubMed] [Google Scholar]
- 23. Hung SC, Kuo KL, Wu CCet al. Indoxyl sulfate: a novel cardiovascular risk factor in chronic kidney disease. J Am Heart Assoc 2017; 6: e005022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pawlak K, Brzosko S, Mysliwiec Met al. Kynurenine, quinolinic acid—the new factors linked to carotid atherosclerosis in patients with end-stage renal disease. Atherosclerosis 2009; 204: 561–566 [DOI] [PubMed] [Google Scholar]
- 25. Hinderliter A, Padilla RL, Gillespie BWet al. Association of carotid intima-media thickness with cardiovascular risk factors and patient outcomes in advanced chronic kidney disease: the RRI-CKD study. Clin Nephrol 2015; 84: 10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Criqui MH, Denenberg JO, Ix JHet al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA 2014; 311: 271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dellegrottaglie S, Saran R, Gillespie Bet al. Prevalence and predictors of cardiovascular calcium in chronic kidney disease (from the Prospective Longitudinal RRI-CKD Study). Am J Cardiol 2006; 98: 571–576 [DOI] [PubMed] [Google Scholar]
- 28. Devereux RB, Alonso DR, Lutas EMet al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986; 57: 450–458 [DOI] [PubMed] [Google Scholar]
- 29. Sengstock D, Sands RL, Gillespie BWet al. Dominance of traditional cardiovascular risk factors over renal function in predicting arterial stiffness in subjects with chronic kidney disease. Nephrol Dial Transplant 2010; 25: 853–861 [DOI] [PubMed] [Google Scholar]
- 30. Rajagopalan S, Brook R, Rubenfire Met al. Abnormal brachial artery flow-mediated vasodilation in young adults with major depression. Am J Cardiol 2001; 88: 196–198 [DOI] [PubMed] [Google Scholar]
- 31. McDonald J. Multiple comparisons. Biological Statistics. Baltimore: Sparky House, 2009 [Google Scholar]
- 32. Goicoechea M, de Vinuesa SG, Gómez-Campderá Fet al. Predictive cardiovascular risk factors in patients with chronic kidney disease (CKD). Kidney Int Suppl 2005; 67(Suppl 93); S35–S38 [DOI] [PubMed] [Google Scholar]
- 33. Kim JY, Park JT, Kim HWet al. Inflammation alters relationship between high-density lipoprotein cholesterol and cardiovascular risk in patients with chronic kidney disease: results from KNOW-CKD. J Am Heart Assoc 2021; 10: e021731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Major RW, Cheng MRI, Grant RAet al. Cardiovascular disease risk factors in chronic kidney disease: a systematic review and meta-analysis. PLoS One 2018; 13: e0192895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dai L, Golembiewska E, Lindholm Bet al. End-stage renal disease, inflammation and cardiovascular outcomes. Contrib Nephrol 2017; 191: 32–43 [DOI] [PubMed] [Google Scholar]
- 36. Pedersen ER, Midttun Ø, Ueland PMet al. Systemic markers of interferon-γ-mediated immune activation and long-term prognosis in patients with stable coronary artery disease. Arterioscler Thromb Vasc Biol 2011; 31: 698–704 [DOI] [PubMed] [Google Scholar]
- 37. Debnath S, Velagapudi C, Redus Let al. Tryptophan metabolism in patients with chronic kidney disease secondary to type 2 diabetes: relationship to inflammatory markers. Int J Tryptophan Res 2017; 10: 1178646917694600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haverkamp GL, Loosman WL, Franssen CFet al. The role of tryptophan degradation in the association between inflammatory markers and depressive symptoms in chronic dialysis patients. Nephrol Dial Transplant 2017; 32: 1040–1047 [DOI] [PubMed] [Google Scholar]
- 39. Pawlak D, Tankiewicz A, Buczko W. Kynurenine and its metabolites in the rat with experimental renal insufficiency. J Physiol Pharmacol 2001; 52: 755–766 [PubMed] [Google Scholar]
- 40. Wang Q, Liu D, Song P et al.Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front Biosci (Landmark Ed) 2015; 20: 1116–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaminski TW, Pawlak K, Karbowska Met al. Association between uremic toxin-anthranilic acid and fibrinolytic system activity in predialysis patients at different stages of chronic kidney disease. Int Urol Nephrol 2018; 50: 127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pawlak K, Domaniewski T, Mysliwiec Met al. Kynurenines and oxidative status are independently associated with thrombomodulin and von Willebrand factor levels in patients with end-stage renal disease. Thromb Res 2009; 124: 452–457 [DOI] [PubMed] [Google Scholar]
- 43. Pawlak K, Kowalewska A, Pawlak Det al. Kynurenine and its metabolites—kynurenic acid and anthranilic acid are associated with soluble endothelial adhesion molecules and oxidative status in patients with chronic kidney disease. Am J Med Sci 2009; 338: 293–300 [DOI] [PubMed] [Google Scholar]
- 44. Dou L, Poitevin S, Sallee Met al. Aryl hydrocarbon receptor is activated in patients and mice with chronic kidney disease. Kidney Int 2018; 93: 986–999 [DOI] [PubMed] [Google Scholar]
- 45. Schroeder JC, Dinatale BC, Murray IAet al. The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry 2010; 49: 393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Watanabe Y, Koyama S, Yamashita Aet al. Indoleamine 2,3-dioxygenase 1 in coronary atherosclerotic plaque enhances tissue factor expression in activated macrophages. Res Pract Thromb Haemost 2018; 2: 726–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ravid JD, Kamel MH, Chitalia VCet al. Uraemic solutes as therapeutic targets in CKD-associated cardiovascular disease. Nat Rev Nephrol 2021; 17: 402–416 [DOI] [PubMed] [Google Scholar]
- 48. Kolachalama VB, Shashar M, Alousi Fet al. Uremic solute-aryl hydrocarbon receptor-tissue factor axis associates with thrombosis after vascular injury in humans. J Am Soc Nephrol 2018; 29: 1063–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pedersen ER, Tuseth N, Eussen SJet al. Associations of plasma kynurenines with risk of acute myocardial infarction in patients with stable angina pectoris. Arterioscler Thromb Vasc Biol 2015; 35: 455–462 [DOI] [PubMed] [Google Scholar]
- 50. Eussen SJ, Ueland PM, Vollset SEet al. Kynurenines as predictors of acute coronary events in the Hordaland Health Study. Int J Cardiol 2015; 189: 18–24 [DOI] [PubMed] [Google Scholar]
- 51. Yu E, Ruiz-Canela M, Guasch-Ferré Met al. Increases in plasma tryptophan are inversely associated with incident cardiovascular disease in the Prevención con Dieta Mediterránea (PREDIMED) Study. J Nutr 2017; 147: 314–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nezu T, Hosomi N, Aoki Set al. Carotid intima-media thickness for atherosclerosis. J Atheroscler Thromb 2016; 23: 18–31 [DOI] [PubMed] [Google Scholar]
- 53. Tankiewicz A, Pawlak D, Pawlak Ket al. Anthranilic acid-uraemic toxin damaged red cell's membrane. Int Urol Nephrol 2005; 37: 621–627 [DOI] [PubMed] [Google Scholar]
- 54. Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 2006; 354: 610–621 [DOI] [PubMed] [Google Scholar]
- 55. Dou L, Sallee M, Cerini Cet al. The cardiovascular effect of the uremic solute indole-3 acetic acid. J Am Soc Nephrol 2015; 26: 876–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Barreto FC, Barreto DV, Liabeuf Set al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 2009; 4: 1551–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lin CJ, Liu HL, Pan CFet al. Indoxyl sulfate predicts cardiovascular disease and renal function deterioration in advanced chronic kidney disease. Arch Med Res 2012; 43: 451–456 [DOI] [PubMed] [Google Scholar]
- 58. Gao J, Xu K, Liu Het al. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol 2018; 8: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dou L, Jourde-Chiche N, Faure Vet al. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost 2007; 5: 1302–1308 [DOI] [PubMed] [Google Scholar]
- 60. Tumur Z, Niwa T. Indoxyl sulfate inhibits nitric oxide production and cell viability by inducing oxidative stress in vascular endothelial cells. Am J Nephrol 2009; 29: 551–557 [DOI] [PubMed] [Google Scholar]
- 61. Six I, Gross P, Rémond MCet al. Deleterious vascular effects of indoxyl sulfate and reversal by oral adsorbent AST-120. Atherosclerosis 2015; 243: 248–256 [DOI] [PubMed] [Google Scholar]
- 62. Claro LM, Moreno-Amaral AN, Gadotti ACet al. The impact of uremic toxicity induced inflammatory response on the cardiovascular burden in chronic kidney disease. Toxins 2018; 10: 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fan P-C, Chang C-H, Lin C-Net al. Serum indoxyl sulfate predicts adverse cardiovascular events in patients with chronic kidney disease. J Formos Med Assoc 2019; 118: 1099–1106 [DOI] [PubMed] [Google Scholar]
- 64. Lin TY, Chou HH, Huang HLet al. Indoxyl sulfate and incident peripheral artery disease in hemodialysis patients. Toxins 2020; 12: 696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shafi T, Sirich TL, Meyer TWet al. Results of the HEMO Study suggest that p-cresol sulfate and indoxyl sulfate are not associated with cardiovascular outcomes. Kidney Int 2017; 92: 1484–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Di Iorio BR, Rocchetti MT, De Angelis Met al. Nutritional therapy modulates intestinal microbiota and reduces serum levels of total and free indoxyl sulfate and p-cresyl sulfate in chronic kidney disease (Medika Study). J Clin Med 2019; 8: 1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Marzocco S, Dal Piaz F, Di Micco Let al. Very low protein diet reduces indoxyl sulfate levels in chronic kidney disease. Blood Purif 2013; 35: 196–201 [DOI] [PubMed] [Google Scholar]
- 68. Liu L, Su X, Quinn WJet al. Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab 2018; 27: 1067–1080.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mori V, Amici A, Mazzola Fet al. Metabolic profiling of alternative NAD biosynthetic routes in mouse tissues. PLoS One 2014; 9: e113939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hustad S, Eussen S, Midttun Øet al. Kinetic modeling of storage effects on biomarkers related to b vitamin status and one-carbon metabolism. Clin Chem 2012; 58: 402–410 [DOI] [PubMed] [Google Scholar]
- 71. Zeleznik O, Wittenbecher C, Deik Aet al. Intrapersonal stability of plasma metabolomic profiles over 10 years among women. Metabolites 2022; 12: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.