ABSTRACT
The introduction of immune checkpoint inhibitors (ICI) has resulted in significant improvement in cancer care, but has been accompanied by the occurrence of immune-related adverse events (irAEs). Also, kidney irAEs have been reported, and the most frequent one is acute tubulointerstitial disease which impacts renal and overall prognosis. There is an unmet need to stratify renal risk in oncologic patients, to allow individualized monitoring and therefore, early detection of ICI-related acute kidney injury (ICI-AKI). Although risk factors for ICI-AKI have been described in previous case–control studies, where ‘cases’ were ICI-AKI patients and ‘controls’ ICI-treated patients without AKI, there is limited epidemiologic knowledge concerning patients developing different irAEs. In this issue of the Clinical Kidney Journal, Gerard et al. describe five factors that were associated with the development of ICI-AKI: older age, previous chronic kidney disease, and concomitant use of fluindione, non-steroidal anti-inflammatory drugs and proton pump inhibitors. These findings suggest that ICI may be a ‘second hit’ that precipitates AKI caused by a concomitant drug. These results urge an increased focus to prevent the prescription of potential nephrotoxic drugs in ICI-treated patients, avoiding iatrogenic events.
Keywords: acute kidney injury, acute tubulointerstitial nephritis, fluindione, immune checkpoint inhibitors, proton pump inhibitors
INTRODUCTION
The use of immune checkpoint inhibitors (ICI) as anti-cancer treatment is growing exponentially. This rise in clinical use is accompanied by an increase in the occurrence of immune-related adverse events (irAEs). Kidney irAEs can be life-threatening and preclude access of patients to chemotherapy because of impaired renal function [1]. In ˃90% of patients, kidney irAE is an acute tubulointerstitial nephritis (ATIN) defined by an inflammatory infiltration of T cells (potentially resulting in kidney fibrosis, if not reversed by drug withdrawal and/or steroid use). While the incidence of kidney irAEs is expected to rise by 30% in the next years because of a wider prescription of ICI [2], the pathogenesis of ICI-associated ATIN remains unclear. There is an urgent need to stratify renal risk in oncologic patients, to allow individualized monitoring and therefore, early detection of ICI-related acute kidney injury (ICI-AKI).
How can we identify renal risk factors in patients exposed to ICI?
In this issue of Clinical Kidney Journal, Gerard et al. report risk factors associated with ICI-induced AKI by comparing ICI-treated patients with ICI-AKI versus ICI-treated patients with non-renal irAEs [3]. The findings are based on the examination of reports registered in the French Pharmacovigilance Database (PVD). The PVD was queried for reports registered from the last 35 years involving ICI and mentioning either ‘ICI-AKI’ or ‘extrarenal IRAEs’. Clinical data were analysed as well as concomitant drugs known for their propensity to induce ATIN. A total of 167 reports of ICI-AKI were included and compared with 668 reports of extrarenal irAEs, according to a randomly assigned 4:1 ratio [3]. The most frequent malignancies were lung cancers and melanoma (as expected because both were the first cancers to benefit from ICI treatment). A low proportion of patients (6%–7%) received a combination of ICI. The relative share of the different ICI was not significantly different between the two groups [3]. Among ICI-AKI reports, 74 (44.3%) patient files mentioned at least one concomitant extrarenal irAE, most frequent haematologic (7.2%), hepatobiliary (6.6%) and/or cutaneous (6%) irAE. Time to onset for ICI-AKI was 2 months. The present study identified five factors that were significantly and independently associated with ICI-AKI in multivariate analyses: older age, previous chronic kidney disease (CKD) and concomitant use of fluindione [odds ratio (OR) 6.53], non-steroidal anti-inflammatory drug (NSAIDs, OR 3.2) and proton pump inhibitor (PPI, OR 2.2) [3]. This association between comedications and the development of ICI-AKI suggests that ICI may be a ‘second hit’ that precipitates AKI caused by a concomitant drug.
Like in cardiovascular disease, modifiable and non-modifiable risk factors in ICI-AKI are now well established
In the last 2 years, several epidemiologic series on ICI-AKI risk factors have been published, comparing ICI-AKI patients with ICI patients without AKI (138 cases compared with 276 controls in the study by Cortazar et al. [4] and 429 cases compared with 429 controls in the study by Gupta et al. [5]). In the first study, three risk factors were identified: previous chronic kidney disease [OR 1.99 for estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2], PPI use (OR 2.85) and ICI combination (OR 3.88) [4]. In the second study, three factors were identified: previous CKD defined by eGFR <59 mL/min/1.73 m2 (OR 2.23), PPI use (OR 2.4) and prior or concomitant irAEs (OR 2.07) [5]. In contrast, the present study compares patients with ICI-AKI with ICI-treated patients with extrarenal irAEs, and is, therefore, able to identify specific renal irAE risk factors [3]. Its multicentric case–control design with a 4:1 ratio enables the inclusion of a large number of patients increasing statistical relevance. Regarding potential notification bias, the authors did not include reports when concomitant medications were lacking. Regarding duplication, a patient subjected to several reports (ICI-AKI and extrarenal irAEs) was tagged, allowing authors to remove potential duplicates.
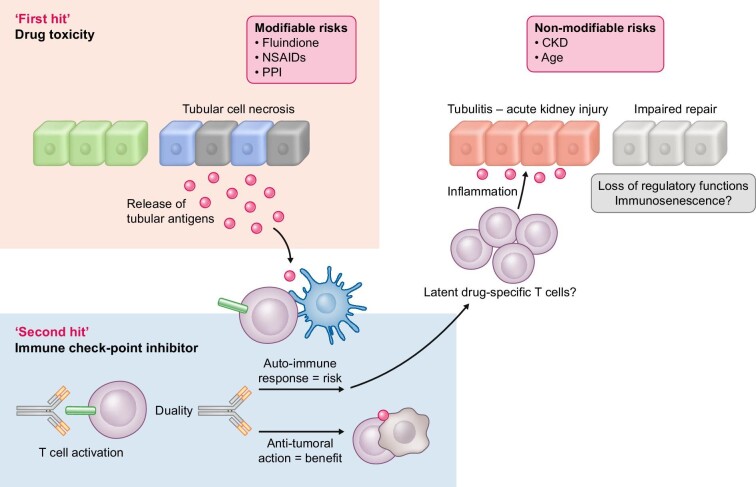
The authors confirm CKD, PPI use and the presence of simultaneous extrarenal irAEs as risk factors for the development of ICI-AKI. In the current study, extrarenal irAEs occurred simultaneously in almost 40% of ICI-AKI patients, a number consistent with existing data [4, 5]. However, for the first time fluindione, a vitamin K antagonist, was identified as an ICI-AKI-associated drug, with the highest OR. It has been established that fluindione can induce ATIN [6–8], but it had never been documented in this context, probably because it is neither available nor easily used in countries other than France. Finally, renal cancer was significantly more frequent in patients with ICI-AKI in the univariate analysis in line with a recently published report suggesting that genitourinary cancers were associated with higher probabilities of nephritis (OR versus gastro-intestinal cancer 1.99) [9]. Thus, the present study underscores non-modifiable (CKD, age) and modifiable (drug-related) risk factors for ICI-AKI. This work brings new arguments to communicate about the danger of co-medications in patients exposed to ICI. As depicted in Fig. 1, ICI may be a ‘second hit’ that precipitates AKI caused by another concomitant drug.
FIGURE 1:
ATIN-inducing drugs could be seen as ‘modifiable’ risk factors acting as a ‘first hit’ in ICI-AKI development, whereas age and CKD are ‘non-modifiable’ risk factors.
In-depth mechanisms involved in ICI-AKI: a long story to write
ATIN is the most common form of ICI-AKI. Until now, its exact pathophysiology is unknown. Whereas CTLA4 signalling occurs in the tumour-draining lymph nodes, PD1/PDL1 blockade occurs at the tissue level and in the tumour microenvironment, both exert immune effects in a systemic manner. Four non-mutually exclusive processes are supposed to contribute to ICI-related [10]:
(i) Re-activation of drug-specific T cells: The present study by Gerard et al. [3] reinforces the strong association between drug exposure and occurrence of ICI-AKI. It is possible that T cell primed by different drugs (e.g. fluindione, PPIs or NSAIDs) became dormant over the time and are reactivated following ICI introduction through loss of tolerance. However, the demonstration of PPI, NSAIDs and fluindione-specific T cells has not yet been done to the same extent as was previously proven for flucloxacillin.
(ii) Loss of tolerance versus self-antigens: Firstly, ICI could activate self-reactive T cell clones. This was previously described in a case report of a patient presenting with fulminant myocarditis in which the selective clonal T cell populations infiltrating the myocardium were identical to those in the tumour and skeletal muscle [11]. Recently, a very detailed study focussing on peripheral blood samples from melanoma patients treated with ICI analysed with deep resolution [combining single-cell RNA sequencing, single cell V(D)J and T cell receptor (TCR) sequencing] demonstrated that T cell clones characteristics associated with ICI-induced irAES. Pre-treatment activated CD4 memory T cell abundance and TCR diversity were associated with severe irAES development regardless of organ system involvement [12]. So, ICI could generate T cell clones with auto-reactivity. Secondly, these self-reactive T cell clones could theoretically activate self-reactive B cells leading to auto-antibody release, explaining the rare ICI-AKI related to glomerular diseases (lupus-like nephropathy) [13] or anti-thrombospondin type 1-domain containing 7A (THSD7A) membranous nephropathy [14].
(iii) Off-target effect: Regarding kidneys, antigenic overlap between normal tubular cells and tumour cells could involve PD-L1 itself. In fact, renal tubular cells express PD-L1, which protects them from T cell–mediated autoimmunity. PD-L1 is constitutively expressed on tubular cells and is dramatically up-regulated by inflammatory signalling and AKI. PD-L1 is frequently expressed in various renal diseases unrelated to ICI therapy and could be a prerequisite for susceptibility to developing AKI and deleterious immune-related AIN. The up-regulation of PD-L1 on renal tubular epithelial cells can lead to kidney damage by effector T lymphocytes infiltration resulting in ATIN. Recently, it was demonstrated that PD-L1 localizes to different compartments in ICI-AKI and ICI-naïve kidneys, suggesting that PD-L1 staining on kidney biopsies could be of great help to reinforce ICI accountability [15]. However, this explanation is not entirely valid for ICI-AKI related to other ICI than anti-PD-L1 (e.g. anti-CTLA4).
(iv) Pro-inflammatory cytokines: ICI also promote the migration and activation of effector T cells in renal tissue, the infiltration of other immune cells as B cells together with pro-inflammatory cytokines release. CXCL10, TNFalpha, interleukin (IL)-6 subsequently contribute to the generation of an inflammatory milieu, leading to renal damage. In murine models, IL-6 blockade was associated with improved tumour control and a higher density of CD4+/CD8+ effector T cells, with reduced Th17, macrophages and myeloid cells. In an experimental model of autoimmune encephalomyelitis with concomitant tumours, combined IL-6 blockade and ICI enhanced tumour rejection while simultaneously mitigating experimental autoimmune encephalitis (EAE) symptoms compared with immune checkpoint blockade alone. IL-6 blockade with ICI could de-couple autoimmunity from antitumour immunity [16]. Tocilizumab could also be proposed in patient’s refractory or cortico-dependant ATIN.
In brief, ICI disrupt peripheral immune tolerance between tubular cells, dormant auto-reactive T cells and tolerogenic dendritic cells, and promote the migration and activation of effector T cells in renal tissue. Additional, basic science data are needed to confirm the relative contribution of: (i) direct tubular toxicity of the drug leading to the release of tubular antigen that mimics tumour antigens and becomes the target of T cells, and (ii) T cells specific for a drug that are activated in kidney and orchestrate an immune response in renal tissue, which is amplified by ICI treatment.
Shortcomings and future directions in preventing ICI-AKI
The following shortcomings should be acknowledged. Firstly, queries for ICI encompassed only atezolizumab, avelumab, cemiplimab, durvalumab, ipilimumab, nivolumab, pembrolizumab and not newly developed ICI [e.g. anti lymphocyte activating 3 (LAG3), T cell immunoglobulin and mucin-domain containing protein 3 (TIM3), T cell immunoreceptor with immunoglobulin and ITIM domain (TIGIT), V-domain Immunoglobulin suppressor of T cell activation (VISTA)] that may be associated with other immune responses or clinical phenotypes. Secondly, data are lacking on the precise timing of sequential irAEs. Up to now, it is not known whether ICI-AKI occurring simultaneously with extrarenal irAEs is more or less severe than ICI-AKI occurring after irAEs. Thirdly, there is no evidence about the proportion of biopsy-proven ATIN among the ICI-AKI reports, which prevents the drawing of any definite conclusion about the pathophysiological mechanism. The distinction between acute tubular necrosis and ATIN is crucial because the first case does not need any ICI discontinuation or steroids, whereas the latter impacts the patient global management [17]. The better we understand the pathophysiology of ATIN, the more sensitive biomarkers we will be able to identify in addition to the clinical risk factors identified in the current study.
PPI danger beyond AKI
Several studies, including the current one, have now identified PPI as a risk factor for ICI-AKI development. The highest risk for ICI-AKI development may be the risk of ICI-AKI relapse after PPI rechallenge, which is clearly an iatrogenic event [18]. However, this has only been partially incorporated into current guidelines. Beyond renal risk, PPI seems to associate with worse prognosis as a recent meta-analysis suggested that concomitant PPI use is significantly associated with low clinical benefit in ICI treatment, revealing a significantly decreased performance status and overall survival in advanced cancer patients receiving ICIs who are also exposed to PPI [19]. Indeed, PPI through altering intestinal microbiota may affect the efficacy of ICI among cancer patients, given the fact that the intestinal microbiota plays an important role in shaping systemic immune responses [20].
CONCLUSION
There is a need for awareness concerning the potential risk factors of ICI-AKI in ICI prescribers. Guidelines should now include a clear warning about comedications like fluindione, NSAIDs and especially PPI regarding renal and potential systemic effects. A careful query about comedications should be taken in every patient before ICI initiation and general practitioners, as well as patients, should receive therapeutic education about PPI and other nephrotoxic drugs. The estimation of a patient's individual susceptibility to develop ICI-AKI may be facilitated by an approach, combining T-cell clone analysis, PD-L1+ urinary cells detection and other risk factors such as immunological age, microbiota content or renal senescence markers.
Contributor Information
Julie Belliere, Department of Nephrology and Organ Transplantation, Referral Centre for Rare Kidney Diseases, French National of Health and Medical Research, U1297 (Institute of Metabolic and Cardiovascular Diseases), University Paul Sabatier, University Hospital of Toulouse, Toulouse, France.
Ben Sprangers, Department of Microbiology, Immunology and Transplantation, Laboratory of Molecular Immunology, Rega Institute, KU Leuven, Leuven Belgium and Division of Nephrology, University Hospitals Leuven, Leuven, Belgium.
CONFLICT OF INTEREST STATEMENT
B.S. is member of the CKJ editorial board.
FUNDING
B.S. is a senior clinical investigators of The Research Foundation Flanders (F.W.O., 1 842 919 N) and received funding from the Foundation against Cancer (Stichting tegen Kanker, grant number C/2020/1380).
References
- 1. Perazella MA, Shirali AC. Immune checkpoint inhibitor nephrotoxicity: what do we know and what should we do? Kidney Int 2020; 97: 62–74 [DOI] [PubMed] [Google Scholar]
- 2. Wanchoo R, Karam S, Uppal NNet al. . Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol 2017; 45: 160–9 [DOI] [PubMed] [Google Scholar]
- 3. Gerard AO, Barbosa S, Parassol Net al. . Risk factors associated with immune checkpoint inhibitor-induced nephropathy: a case-control study. Clin Kidney J 2022; this issue [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cortazar FB, Kibbelaar ZA, Glezerman IGet al. . Clinical features and outcomes of immune checkpoint inhibitor-associated AKI: a multicenter study. J Am Soc Nephrol 2020; 31: 435–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gupta S, Short SAP, Sise MEet al. . Acute kidney injury in patients treated with immune checkpoint inhibitors. J Immunother Cancer 2021; 9: e003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belmouaz S, Desport E, Abou ARet al. . Acute immuno-allergic interstitial nephritis caused by fluindione. Clin Nephrol 2006; 66: 455–8 [DOI] [PubMed] [Google Scholar]
- 7. Crepin T, Bamoulid J, Courivaud Cet al. . Reversible fluindione-induced chronic interstitial nephritis. Case Rep Nephrol 2016; 2016: 9818195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cam G, Kwetcheu AT, Vigneau Cet al. . Acute and chronic nephropathy induced by fluindione must be addressed. Nephrol Dial Transplant 2012; 27: 1554–8 [DOI] [PubMed] [Google Scholar]
- 9. Abdel-Rahman O. Association between PD-L1 inhibitor, tumor site and adverse events of potential immune etiology within the USA FDA adverse event reporting system. Immunotherapy 2021; 13: 1407–17 [DOI] [PubMed] [Google Scholar]
- 10. Franzin R, Netti GS, Spadaccino Fet al. . The use of immune checkpoint inhibitors in oncology and the occurrence of AKI: where do we stand? Front Immunol 2020; 11: 574271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson DB, Balko JM, Compton MLet al. . Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016; 375: 1749–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lozano AX, Chaudhuri AA, Nene Aet al. . T cell characteristics associated with toxicity to immune checkpoint blockade in patients with melanoma. Nat Med 2022; 28: 353–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fadel F, El KK, Knebelmann B. Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med 2009; 361: 211–2 [DOI] [PubMed] [Google Scholar]
- 14. Chen M, Zhang L, Zhong Wet al. . Case report: tHSD7A-Positive membranous nephropathy caused by tislelizumab in a lung cancer patient. Front Immunol 2021; 12: 619147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hakroush S, Kopp SB, Tampe Det al. . Variable expression of programmed cell death protein 1-Ligand 1 in kidneys independent of immune checkpoint inhibition. Front Immunol 2020; 11: 624547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hailemichael Y, Johnson DH, Abdel-Wahab Net al. . Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell 2022; 40: 509–23.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eijgelsheim M, Sprangers B. Kidney biopsy should be performed to document the cause of checkpoint inhibitor-associated acute kidney injury: PRO. Kidney360 2020; 1: 158–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee MD, Seethapathy H, Strohbehn IAet al. . Rapid corticosteroid taper versus standard of care for immune checkpoint inhibitor induced nephritis: a single-center retrospective cohort study. J Immunother Cancer 2021; 9: e002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qin BD, Jiao XD, Zhou XCet al. . Effects of concomitant proton pump inhibitor use on immune checkpoint inhibitor efficacy among patients with advanced cancer. OncoImmunology 2021; 10: 1929727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giordan Q, Salleron J, Vallance Cet al. . Impact of antibiotics and proton pump inhibitors on efficacy and tolerance of anti-pd-1 immune checkpoint inhibitors. Front Immunol 2021; 12: 716317. [DOI] [PMC free article] [PubMed] [Google Scholar]