ABSTRACT
Background
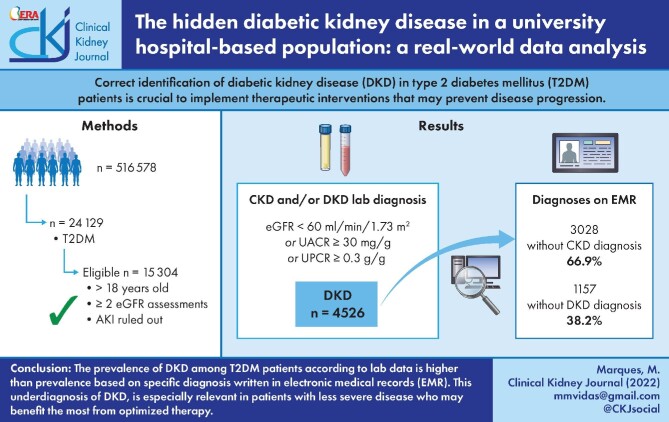
Correct identification of diabetic kidney disease (DKD) in type 2 diabetes mellitus (T2DM) patients is crucial to implement therapeutic interventions that may prevent disease progression.
Methods
We compared the real prevalence of DKD in T2DM patients according to actual serum and urine laboratory data with the presence of the diagnostic terms DKD and/or CKD on the electronic medical records (EMRs) using a natural language processing tool (SAVANA Manager). All patients ˃18 years of age and diagnosed with T2DM were selected. DKD was defined as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 or a urinary albumin:creatinine ratio (UACR) >30 mg/g or a urinary protein:creatinine ratio (UPCR) >0.3 g/g after excluding acute kidney injury.
Results
A total of 15 304 T2DM patients identified on EMRs were eligible to enter the study. A total of 4526 (29.6%) T2DM patients had DKD according to lab criteria. However, the terms CKD or DKD were only present in 33.1% and 7.5%, representing a hidden prevalence of CKD and DKD of 66.9% and 92.5%, respectively. Less severe kidney disease (lower UACR or UPCR, higher eGFR values), female sex and lack of insulin prescription were associated with the absence of DKD or CKD terms in the EMRs (P < .001)
Conclusions
The prevalence of DKD among T2DM patients defined by lab data is significantly higher than that reported on hospital EMRs. This could imply underdiagnosis of DKD, especially in patients with the least severe disease who may benefit the most from optimized therapy.
Keywords: chronic kidney disease, diabetic kidney disease, gender, real-world data, underdiagnosis
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Chronic kidney disease (CKD) is one of the most severe complications of diabetes mellitus (DM) and its severity strongly correlates with adverse outcomes. In fact, estimated glomerular filtration rate (eGFR) and urinary albumin excretion are synergistic and independent predictors of mortality in type 2 DM (T2DM) [1].
Strikingly, despite better control of glycaemia, arterial blood pressure and lipid profile or the appropriate use of renin–angiotensin–aldosterone system blockers, the overall incidence of diabetic kidney disease (DKD) has not significantly decreased [2]. DKD prevalence is widely variable between series, probably reflecting disparities in screening procedures in T2DM patients, inconsistent DKD diagnostic criteria [3], or healthcare level bias. DKD was originally described as a progressive disease that initiates with the detection of microalbuminuria and slowly progresses to macroalbuminuria and a decline in GFR. However, some type 1 and most T2DM patients do not follow this course, as revealed in the UK Prospective Diabetes Study (UKPDS): of the 28% of the cohort that developed an eGFR <60 mL/min/1.73 m2, only half had preceding pathological albuminuria [4]. Moreover, the excess risk for kidney disease progression in DM is not restricted to albuminuric patients [5].
Since the preventive and therapeutic approach for CKD in diabetic patients is common to the albuminuric and non-albuminuric phenotypes of diabetes-related CKD, most scientific societies advocate to include both presentations under the term DKD or the preferred ‘diabetes and CKD’ term [6].
Studies based on big data in comparison with classic observational epidemiological studies are a powerful tool to extract meaningful information from very large data sets and electronic medical records (EMRs). Real-world data is defined as the information that is gathered through observations of routine clinical practice from multiple sources that can be linked to provide meaningful patterns. Big data and real-world data are not synonyms, however, there are important aspects of real-world data that also apply to big data, as is the use of natural language processing (NLP) systems [7].
The aim of this study was to assess the prevalence of DKD and the accuracy of DKD diagnosis using real-world data analysis in a cross-sectional study of T2DM patients admitted to a university hospital. The real prevalence of DKD in T2DM patients is based on serum and urine laboratory data and was compared with the existence of diagnostic terms ‘DKD’ and/or ‘CKD’ on the EMRs using the SAVANA Manager tool.
MATERIALS AND METHODS
The Big Data in Diabetic Kidney Disease (BIGERD) study is a non-experimental, observational, cross-sectional study performed using free-text data captured in the EMRs of the hospitals from the health system in Madrid.
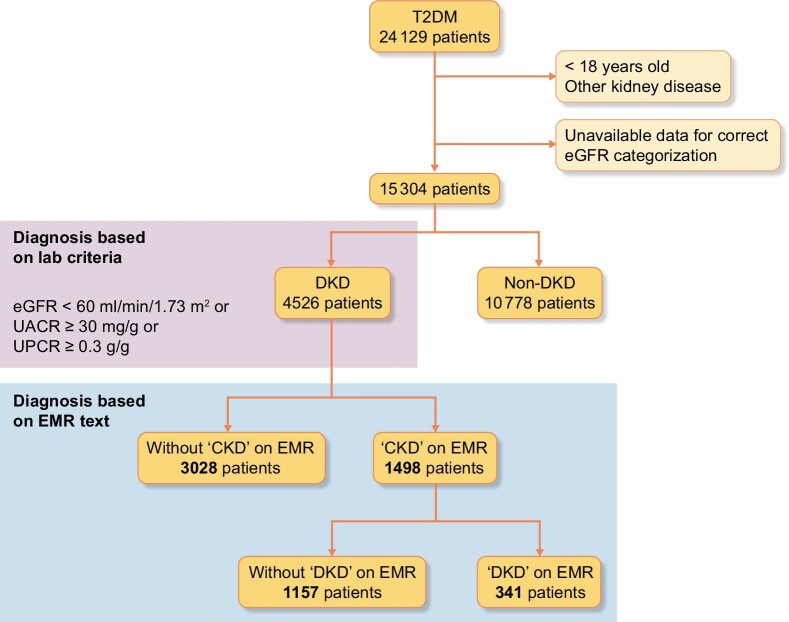
All T2DM patients ˃18 years of age admitted to any clinical department of the Hospital Universitario Puerta de Hierro Majadahonda (HUPH) from January 2009 to December 2018 and with enough lab data to be categorized were included. T2DM was defined as fasting glucose ≥126 mg/dL or ongoing pharmacological treatment for T2DM. Patients diagnosed with glomerular disease, adult polycystic kidney disease and acute or chronic interstitial disease were excluded. Figure 1 depicts the patient selection and categorization process.
FIGURE 1:
Patient flow chart.
The hidden prevalence of CKD was defined as the percentage of patients meeting the criteria for DKD according to lab data but missing a diagnosis of CKD or DKD on the EMRs.
Information extraction from the EMRs
The information from EMRs—T2DM, CKD and/or DKD diagnosis and all other diagnoses that were considered as exclusion criteria for entering the study—was extracted with NLP and artificial intelligence techniques using the SAVANA Manager clinical platform (through EHRead technology). SAVANA Manager is a powerful multilingual engine for the analysis of free-text clinical information. This tool can capture numerical values and clinical notes and transform them into accessible variables, thus allowing for reuse of the information captured in large-scale collections of clinical records (i.e. big data). Furthermore, EHRead technology combines modules for sentence segmentation, tokenization, text normalization, acronym disambiguation and negation detection that are considered in the validation process to be able to read typos, misspellings, acronyms and other particularities within the free-text narratives of patients’ EMRs. SAVANA methodological specifications have been included in the supplementary data.
Simultaneously, laboratory data were used to categorize the study group into CKD and/or DKD based on the eGFR and the presence of albuminuria or proteinuria. eGFR was calculated from serum creatinine (sCr) by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [8]. Patients with less than two sCr values as well as those fulfilling the Kidney Disease: Improving Global Outcomes (KDIGO) criteria for acute kidney injury (AKI) [9] were excluded (missing data were discarded in multicentre methods). The last available sCr value was selected to calculate the patient's eGFR in mL/min/1.73 m2. Urine albumin:creatinine ratio (UACR) or urine protein:creatinine ratio (UPCR) was only analysed in patients with two or more UACR or UPCR values. The highest value was used to categorize patients into non-albuminuric (UACR <30 mg/g), albuminuric (UACR ≥30 mg/g) or proteinuric (UPCR ≥0.3 g/g) CKD. DKD was defined according to KDIGO criteria as the presence of UACR ≥30 mg/g or UPCR ≥0.3 g/g or eGFR <60 mL/min/1.73 m2 in a DM patient (T2DM in this study) once other causes of CKD had been reasonably ruled out [10].
Next, DKD patients were classified according to the presence or absence of the diagnostic terms DKD and/or CKD on the EMRs. SAVANA Manager groups diabetic kidney disease, diabetic nephropathy and diabetic chronic kidney disease under the term DKD. CKD includes the terms end-stage renal disease, renal disease, chronic renal disease/chronic kidney disease and nephropathy. Patients with a DKD diagnosis but insufficient lab data to be correctly categorized were rare in the analysis and were excluded.
All EMRs were previously anonymized by the information technology service, so neither investigator has access to the patients’ identification. The study was approved by the ethics committee of the Hospital Universitario Puerta de Hierro Majadahonda (138/18) and they determined that informed consent was not necessary due to the number of patients and the characteristics of the study.
Demographic data (age and sex) and antidiabetic drug prescriptions were collected from the medical history. Quantitative variables are expressed as mean and standard deviation (SD) and categorical variables are presented as percentages. Differences between variables were evaluated using the Student's t-test or chi-squared test according to the nature of the variables; a post hoc test was done. For significant chi-squared results we conducted Tukey post hoc analyses to identify the discrepancy. P-values <.05 were considered statistically significant. All analyses were performed with Stata 14 (StataCorp, College Station, TX, USA).
RESULTS
Study population
Of 516 578 patients available for analysis in SAVANA Manager, a total of 24 129 patients were categorized as having T2DM [median age 72 years (interquartile range 61.5–81.2), 54.7% male].
After selecting just the adult population and excluding patients with diagnosed non-diabetic forms of CKD or with less than two eGFR assessments or those in whom AKI could not be ruled out (Figure 1), 15 304 T2DM patients were eligible for the study.
DKD, defined as an eGFR <60 mL/min/1.73 m2 or pathological UACR or UPCR, was diagnosed in 4526 (29.6%) patients; 3204 (20.9%) had an eGFR <60 mL/min/1.73 m2 (Table 1). Pathological UACR (>30 mg/g) or UPCR (>0.3 g/g) were present in 1556/2490 (62.5%) and 1044/3655 (28.6%) patients with available UACR or UPCR data, respectively (Table 1).
Table 1.
DKD categorization by laboratory data
| Clinical description from laboratory data | Values, n (%), 95% CI |
|---|---|
| T2DM eligible for analysis, n | 15 304 |
| DKD based on eGFR (CKD-EPI; mL/min 1.73 m2) | 3204 (20.9), 20.3–21.6 |
| DKD based on UACR or UPCR and eGFR >60 mL/min/1.73 m2 | 1322 (9.0), 8.5–9.4a |
| Total DKD by lab criteria | 4526 (29.6), 28.9–30.3 |
| Prevalence of UACR >30 mg/g or UPCR >0.3 g/g among patients with UACR or UPCR data | |
| UACR ≥30 mg/g | 1556 (62.5), 60.6–64.4b |
| UPCR ≥0.3 g/g | 1044 (28.6), 27.1–30.0 |
CI, confidence interval.
aPercentage expressed over total patients having an eGFR >60 mL/min/1.73 m2 and UACR (n = 2490) or UPCR data (n = 3655).
bPercentage expressed over total patients having UACR or UPCR (data n = 14 750).
The analysis of the 4526 patients identified as having DKD according to lab data revealed that a CKD diagnosis was found on EMRs in 1498 (33.1%) and a DKD diagnosis in 341 (7.5%) of DKD patients diagnosed by lab criteria. This means that 66.9% of lab-diagnosed DKD did not show a CKD diagnosis on the EMRs, and when evaluating the term DKD, this percentage was even higher, reaching 92.5% of T2DM patients diagnosed with DKD by lab criteria (Table 2).
Table 2.
Main characteristics of patients diagnosed with DKD by laboratory criteria who were correctly or incorrectly identified as CKD (upper table) or DKD (lower table) patients in the EMRs
| CKD by EMR | Matched | Unmatched | P-value |
|---|---|---|---|
| Patients, n (%) | 1498 (33.1) | 3028 (66.9) | |
| Age (years), n (%) | <.001 | ||
| >70 | 1177 (78.6) | 2121 (70.1) | |
| 60–69 | 216 (14.4) | 520 (17.2) | |
| 50–59 | 83 (5.5) | 269 (8.9) | |
| 40–49 | 21 (1.4) | 74 (2.4) | |
| 18–39 | 1 (0.1) | 44 (1.5) | |
| Male, n (%) | 894 (59.7) | 1488 (49.1) | <.001 |
| Insulin, n (%) | 693 (46.3) | 1050 (34.7) | <.001 |
| UACR (mg/g), (n)(%) | 504 | 826 | <.001 |
| <30 | 88 (17.5) | 213 (25.8) | |
| 30–300 | 200 (39.7) | 495 (59.9) | |
| >300 | 216 (42.9) | 118 (14.3) | |
| UPCR (g/g), (n) (%) | 775 | 1411 | <.001 |
| <1 | 443 (42.8) | 1106 (21.6) | |
| ≥1 | 332 (57.2) | 305 (78.4) | |
| eGFR (mL/min/1.73 m2), n (%) | 1470 | 2943 | <.001 |
| <30 | 461 (31.4) | 201 (6.8) | |
| 30–60 | 834 (56.7) | 1635 (55.6) | |
| >60 | 175 (11.9) | 1107 (37.6) | |
| eGFR <30 mL/min/1.73/ m2 + UPCR ≥1 g/g | 270/363 (74.4) | 93/363 (25.6) | |
| DKD by EMR | |||
| Patients, n (%) | 341 (7.5) | 4185 (92.5) | |
| Age (years), n (%) | <.001 | ||
| ≥70 | 214 (62.8) | 3084 (73.7) | |
| 60–69 | 84 (24.6) | 652 (15.6) | |
| 50–59 | 31 (9.1) | 321 (7.7) | |
| 40–49 | 11 (3.2) | 84 (2.0) | |
| 18–39 | 1 (0.3) | 44 (1.1) | |
| Male, n (%) | 236 (69.2) | 2146 (51.3) | <.001 |
| Insulin, n () | 218 (63.9) | 1525 (36.4) | <.001 |
| UACR (mg/g), (n) (%) | 178 | 1152 | <.001 |
| <30 | 17 (9.6) | 284 (24.7) | |
| 30–300 | 59 (33.2) | 636 (55.2) | |
| >300 | 102 (57.3) | 232 (20.1) | |
| UPCR (g/g), n (%) | 235 | 1951 | <.001 |
| <1 | 101 (43.0) | 1448 (74.2) | |
| ≥1 | 134 (57.0) | 503 (25.8) | |
| eGFR (mL/min/1.73 m2), n (%) | 335 | 4078 | <.001 |
| <30 | 151 (45.1) | 511 (12.5) | |
| 30–60 | 114 (34.0) | 2355 (57.8) | |
| >60 | 70 (20.9) | 1212 (29.7) | |
| eGFR <30 mL/min 1.73 m2 + UPCR ≥1 g/g | 109/363 (30.0) | 254/363 (70.0) |
Matched: CKD by EMR or DKD by EMR.was calculated with the CKD-EPI equation
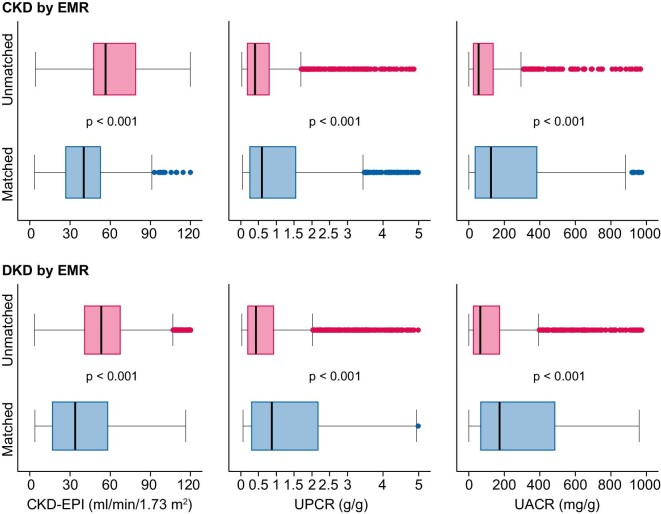
Patients having a DKD diagnosis as per biochemistry lab values while missing a diagnosis of CKD or DKD in the EMR had a higher eGFR and a lower UACR or UPCR than those not missing the diagnosis (Figure 2). Indeed, patients with milder CKD (higher eGFR, lower UACR or UPCR) more frequently missed the diagnoses of CKD or DKD in the EMRs than patients with eGFR <30 mL/min/m2, UACR >300 mg/g or UPCR >1.0 g/g (Table 2, Figure 2). Additionally, female patients or patients not on insulin more frequently missed a diagnosis of CKD or DKD. Finally, patients ≥70 years of age were more frequently misdiagnosed with DKD or CKD in the EMRs compared with younger patients (Table 2).
FIGURE 2:
Biochemistry laboratory values for T2DM patients diagnosed with DKD according to lab values. eGFR UACR and UPCR are shown. Upper panel: CKD results. Patients are categorized as unmatched when a laboratory diagnosis of DKD was not matched by a medical records diagnosis of CKD and matched when a laboratory diagnosis of DKD was matched by a medical records diagnosis of CKD. Lower panel: DKD results. Patients are categorized as unmatched when a laboratory diagnosis of DKD was not matched by a medical records diagnosis of DKD and matched when a laboratory diagnosis of DKD was matched by a medical records diagnosis of DKD. Higher values of eGFR and lower values of UACR or UPCR in patients diagnosed with DKD according to biochemistry lab values lacked a diagnosis for CKD or DKD in the medical records. Statistical comparisons for this set of data are presented in Table 2. Box plot represents the median and interquartile range.
DISCUSSION
To our knowledge, this is the first study that approaches the relevance of undiagnosed DKD in T2DM patients in a broad population under real-world data conditions and the results are striking and worth a detailed analysis of the potential consequences. The main finding is the underdiagnosis of CKD or DKD in the EMRs. Despite international consensus definitions, these data suggest that physicians may not be translating objective diagnostic laboratory criteria to an actual diagnosis. Thus a DKD diagnosis was present in the EMRs in only 7.5% of T2DM patients who fulfilled diagnostic lab criteria for DKD and the less accurate term CKD was present in only 33.1% of DKD patients diagnosed according to lab criteria. Hence, approximately two-thirds of CKD occurring in T2DM patients goes unnoticed and only 1 in 10 T2DM patients suffering from DKD is correctly identified in the EMRs, uncovering a critical issue in DKD management, namely correct identification of T2DM patients suffering from DKD. This may have consequences for patient management, from optimizing therapy for CKD/DKD to limiting the use of nephrotoxic agents to providing adequate preventive care for nephrotoxicity or dose adjustment of kidney excreted or metabolized molecules. It may additionally confound policymakers as to the true prevalence of DKD.
The DKD prevalence (29.6%) according to current KDIGO diagnostic criteria is in line with previous reports from the Spanish prospective series using conventional database analysis: 27.9% of T2DM patients develop end-stage renal disease (ESRD) and at least 35% have albuminuria, proteinuria or CKD [11], supporting the representativeness of the study population.
Correct identification of DKD in T2DM patients is key for optimal therapeutic decision-making aimed at preventing the development or progression of DKD to ESRD. Several effective strategies have been demonstrated to slow or even prevent DKD progression [12]. However, the progression of non-albuminuric DKD and the general prevalence of ESRD caused by DKD has remained stable over the last 20 years [2]. Recently the use of sodium-glucose cotransporter 2(SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists was reported to provide add-on renal protective effects in T2DM patients, even in advanced CKD stages and including non-albuminuric DKD [13].
Diagnosis of DKD is based on screening for albuminuria and low eGFR and most T2DM patients in the present study had several in-hospital laboratory assessments and most probably many others at their primary care laboratory. However, the correct interpretation of these results and integration into a diagnosis was missing in most EMRs from T2DM patients admitted to our centre. Several patient characteristics were identified as being associated with a lower prevalence of diagnoses of CKD or DKD reflected in EMRs. T2DM patients on insulin are usually in the later stages of T2DM natural history or display more comorbidities, therefore, they usually receive more medical and laboratory assessments. That could explain the observed more accurate diagnosis of CKD or DKD in EMRs of patients on insulin. Similarly, patients with more severe GFR impairment and significant albuminuria or proteinuria were, in general, better categorized than patients with milder forms of CKD. Yet, an eGFR <60 mL/min/1.73 m2 denotes an already important loss of renal function and albuminuria in the range of 30–300 mg/g is associated with worse renal and cardiovascular outcomes [14]. Moreover, early initiation of therapy aimed at preventing CKD progression has also been demonstrated to be effective in preventing cardiovascular events and premature death. Current American Diabetes Association and European Association for the Study of Diabetes guidelines recommend the preferential use of SGLT2 inhibitors on top of metformin when DKD is diagnosed [15] and improved renal and cardiovascular outcomes were observed when SGLT2 inhibitors were started in patients with an eGFR ˃30 mL/min/1.73 m2 and maintained until dialysis initiation [16, 17].
The analysis of risk factors for missing diagnoses yielded results that could be understood as widely extended default attitudes by physicians. Thus we hypothesized the underdiagnosis of CKD or DKD in the EMRs of female patients may reflect the lower serum creatinine values in females than in males for the same eGFR values as well as lower UACR values [18]. Similarly, physicians may be more reluctant to attribute CKD to T2DM in the elderly, in whom hypertensive nephropathy is a frequent diagnosis in Spain, especially if albuminuria is not prominent.
Big data is a powerful tool for providing insight from very large data sets and EMRs. We have shown that real-world data analysis can be a useful tool in detecting undiagnosed patients that can benefit from tailored therapeutic options [19]. As shown in this study, the combination of two tools (NLP technology and lab data processing algorithms) could easily evolve into automated diagnostic tools of DKD to be validated by physicians, facilitating the correct detection of kidney disease in T2DM and early intervention.
Several limitations should be acknowledged. We have not directly accessed the information from the primary care health system. However, it is unlikely that a diagnosis of CKD or DKD previously included in the general practice physician’s EMRs was skipped from discharge forms, since this comorbid diagnosis added value to hospital reimbursement. Additionally, the observed DKD prevalence was inline with published data from the same region, supporting that those results can be extrapolated to broader populations. A second limitation is that being a single-centre study, it may have reflected local practices not representative of other centres. However, we estimate that this is also unlikely, given the mobility of health care workers in our region.
In conclusion, a high rate of hidden DKD was observed among hospitalized T2DM patients, which was more frequent in patients with less severe DKD. Since effective therapies are now available to modify the renal outcomes of these patients, including antidiabetic drugs that should be preferentially used in patients with DKD, educational efforts should be directed towards increasing DKD diagnosis accuracy at every health system level. Whether this miscategorization is really interfering with optimal treatment and impairing renal or cardiovascular outcomes is beyond the scope of the present study but deserves further analysis. Additionally, this use of real-world data is a powerful and safe tool to identify and select groups of patients for clinical, epidemiological, or investigational purposes.
Supplementary Material
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the cooperation of Juan Manuel Martín Giner and Dr Arturo Ramos Martín-Vegué for their assistance in database and medical records technical aspects of this study and the SAVANA team for their help in data acquisition. The project was approved by the Ethics Committee of the Hospital Universitario Puerta de Hierro Majadahonda.
Contributor Information
María Marques, Nephrology Department, Hospital Universitario Puerta de Hierro, IDIPHISA, Madrid, Spain; Research Network REDInREN 016/009/009 Instituto Salud Carlos III, Madrid, Spain.
Paula López-Sánchez, Nephrology Department, Hospital Universitario Puerta de Hierro, IDIPHISA, Madrid, Spain.
Fernando Tornero, Nephrology Department, Hospital Universitario del Sureste, Madrid, Spain.
Pedro Gargantilla, Internal Medicine Department, Hospital El Escorial, Madrid, Spain.
Alba Maroto, Nephrology Department, Hospital Universitario Puerta de Hierro, IDIPHISA, Madrid, Spain.
Alberto Ortiz, Research Network REDInREN 016/009/009 Instituto Salud Carlos III, Madrid, Spain; Nephrology Department, Fundación Jiménez Díaz, Madrid, Spain.
José Portolés, Nephrology Department, Hospital Universitario Puerta de Hierro, IDIPHISA, Madrid, Spain; Research Network REDInREN 016/009/009 Instituto Salud Carlos III, Madrid, Spain.
FUNDING
The BIGERD project was co-founded by a competitive unrestricted grant from Madrid Nephrology Foundation (2019–2020) and Public Research Network REDinREN RETIC ISCIII 16/009/009 and Research Institute Puerta de Hierro-- Segovia Arana. This study has not been funded by SAVANA. Neither the funders or our institution had any role in the study design; collection, analysis and interpretation of data; writing the report or the decision to submit the report for publication. Neither the sponsor or our institution imposed any limits on the authors’ access to all the study's data.
CONFLICT OF INTEREST STATEMENT
A.O. is the Editor-in-Chief of CKJ. The other authors declare no conflicts of interest in the scope of this study. The results presented in this article have not been presented previously in whole or part.
DATA AVAILABILITY STATEMENT
Data will be available after a formal request to the corresponding author.
REFERENCES
- 1. Gimeno-Orna JA, Blasco-Lamarca Y, Campos-Gutierrez Bet al. Risk of mortality associated to chronic kidney disease in patients with type 2 diabetes mellitus: a 13-year follow-up. Nefrologia 2015; 35: 487–492 [DOI] [PubMed] [Google Scholar]
- 2. Afkarian M, Zelnick LR, Hall YNet al. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA 2016; 316: 602–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rodríguez Artalejo F. Epidemiología de la nefropatía diabética en españa (hechos y cifras). Rev Española Cardiol Suppl 2007; 7: 5A–8A [Google Scholar]
- 4. Retnakaran R, Cull CA, Thorne KIet al. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006; 55: 1832–1839 [DOI] [PubMed] [Google Scholar]
- 5. Zoccali C, Mallamaci F.. Nonproteinuric progressive diabetic kidney disease. Curr Opin Nephrol Hypertens 2019; 28: 227–232 [DOI] [PubMed] [Google Scholar]
- 6. Kidney Disease: Improving Global Outcomes Diabetes Work Group . KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int 2020; 98(4 Suppl): S1–S115 [DOI] [PubMed] [Google Scholar]
- 7. Klonoff DC. The new FDA real-world evidence program to support development of drugs and biologics. J Diabetes Sci Technol 2019; 14: 345–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levey AS, Stevens LA, Schmid CHet al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kidney Disease: Improving Global Outcomes Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury . Kidney Int Suppl 2012; 2: 1–138 [Google Scholar]
- 10. Kidney Disease: Improving Global Outcomes CKD Work Group . KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 11. Rodriguez-Poncelas A, Garre-Olmo J, Franch-Nadal Jet al. Prevalence of chronic kidney disease in patients with type 2 diabetes in Spain: PERCEDIME2 study. BMC Nephrol 2013; 14: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fox CS, Matsushita K, Woodward Met al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 2012; 380: 1662–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heerspink HJL, Kosiborod M, Inzucchi SEet al. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int 2018; 94: 26–39 [DOI] [PubMed] [Google Scholar]
- 14. KDOQI . KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis 2007; 49(2 Suppl 2): S12–S54 [DOI] [PubMed] [Google Scholar]
- 15. Buse JB, Wexler DJ, Tsapas Aet al. 2019 Update to: Management of Hyperglycaemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2020; 63: 221–228 [DOI] [PubMed] [Google Scholar]
- 16. Perkovic V, Jardine MJ, Neal Bet al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306 [DOI] [PubMed] [Google Scholar]
- 17. Cherney DZI, Odutayo A, Verma S. A big win for diabetic kidney disease: CREDENCE. Cell Metab 2019; 29: 1024–1027 [DOI] [PubMed] [Google Scholar]
- 18. Fernandez-Fernandez B, Mahillo I, Sanchez-Rodriguez Jet al. Gender, albuminuria and chronic kidney disease progression in treated diabetic kidney disease. J Clin Med 2020; 9: 1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arora P, Boyne D, Slater JJet al. Bayesian networks for risk prediction using real-world data: a tool for precision medicine. Value Health 2019; 22: 439–445 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be available after a formal request to the corresponding author.