ABSTRACT
Background
Immune checkpoint inhibitors (ICIs) foster anti-cancer immune responses. Their efficacy comes at the cost of immune-related adverse events (IRAEs). The latter affects various organs, including kidneys, mostly as acute tubulointerstitial nephritis, the pathophysiology of which remains unclear. We conducted a multicentre case–control study to compare the characteristics of patients with renal IRAEs (ICI-AKI) with those of patients diagnosed with other IRAEs.
Methods
We queried the French pharmacovigilance database for all adverse events involving ICIs. Reports were classified as ICI-AKI or extrarenal IRAE. For each ICI-AKI report, four reports of extrarenal IRAEs were randomly included (control group, 4:1 ratio). Variables showing an association with a P < 0.05 were included as covariates in a multivariate analysis.
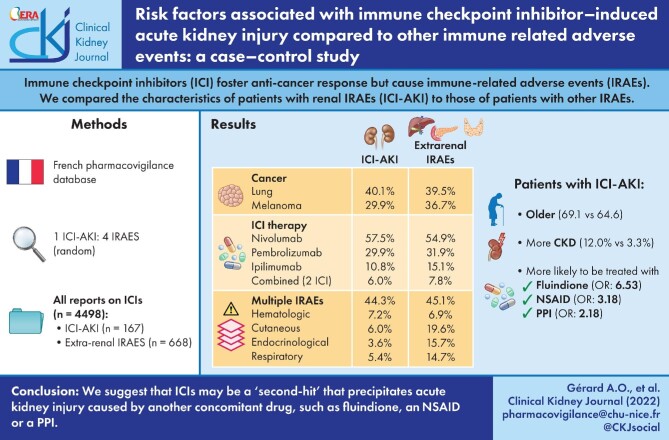
Results
Therefore, 167 ICI-AKI reports were compared with 668 extrarenal IRAEs. At least one concomitant extrarenal IRAE was mentioned in 44.3% of ICI-AKI reports. Patients with ICI-AKI were significantly older than patients with extrarenal IRAEs (69.1 versus 64.6 years; P = 0.0135), and chronic kidney disease was significantly more prevalent (12.0% versus 3.3%; P = 0.0125). Patients with ICI-AKI were significantly more likely to be treated with fluindione [adjusted odds ratio (OR) 6.53, 95% confidence interval (95% CI) 2.21–19.31; P = 0.0007], a non-steroidal anti-inflammatory drug (NSAID, OR 3.18, 95% CI 1.07–9.4; P = 0.0368) or a proton-pump inhibitor (PPI, OR 2.18, 95% CI 1.42–3.34; P = 0.0004).
Conclusion
This study is limited by a lack of data, preventing confirmation of numerous reports therefore not included in the analysis. We are unable to draw definite pathophysiological conclusions from our data. Nonetheless, we suggest that ICIs may be a ‘second-hit’ that precipitates acute kidney injury caused by another concomitant drug (fluindione, NSAID or PPI).
Keywords: acute kidney injury, allergy, immune checkpoint inhibitors, immunotherapy, nephrology, nephrotoxicity, pharmacovigilance
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Immune checkpoint inhibitors (ICIs) are a recent class of anti-cancer agents, intended to foster the physiological immune response against malignancies. Those ICIs target either cytotoxic T-lymphocyte-associated protein 4 (CTLA4, ipilimumab) or the programmed cell death protein 1 (PD1) pathway (nivolumab, pembrolizumab, cemiplimab, atezolizumab, avelumab and durvalumab) [1].
This class has profoundly changed the management of a broad spectrum of advanced malignancies, including melanoma and lung cancer [2], so that the indications of ICIs keep expanding at a fast pace. However, ICIs are also associated with a specific array of immune-related adverse events (IRAEs) affecting various organs such as the skin, the gastrointestinal tract and the endocrine organs [3].
Acute kidney injury (AKI) associated with ICIs has also been reported [4–8], with an estimated incidence between 2% and 30% in ICI-treated patients [9–11]. Even though glomerular diseases have been described [12], the most frequent cause of AKI is acute tubulointerstitial nephritis (ATIN) [8, 13–15]. The pathophysiology of those ICI-induced nephropathies (ICI-AKI) remains unclear, but may involve concomitant treatments [16–20]. Likewise, evidence is scarce regarding the optimal management of ICI-AKI [21].
To suggest potential mechanisms and specific risk factors for ICI-AKI, we conducted a multicentre case–control study, based on reports registered in the French Pharmacovigilance Database (PVD). We aimed to compare the characteristics of patients with ICI-induced nephropathy to the ones of patients diagnosed with other IRAEs.
MATERIALS AND METHODS
Database
The PVD gathers all adverse drug reactions (ADRs) reported spontaneously by healthcare professionals and patients. All reports are analysed by clinical pharmacologists of each regional pharmacovigilance centre. It is mandatory for every healthcare professional to notify all ADRs to the corresponding regional pharmacovigilance centre, particularly if serious and/or unexpected. The PVD respects the anonymity of both patients and notifiers.
The PVD has been approved by the National Data Protection Agency. All our data originate from this database. In accordance with European regulation, this observational study did not need the approval of an Institutional Review Board/Independent Ethics Committee [22]. This research was allowed by the Pharmacovigilance network, which was kept informed.
Query
The PVD was queried for all ADR reports registered from 1985 (creation of the PVD) up to 23 September 2020 and involving, as ‘active ingredients’, one or more of the following suspected drugs: atezolizumab, avelumab, cemiplimab, durvalumab, ipilimumab, nivolumab and pembrolizumab.
The queried reports were then divided into two groups depending on the type of ADR. All queried reports mentioning at least one of the following terms were classified in the group ‘ICI-AKI’: the ‘High Group Level Term’ (HLGT) ‘nephropathies’ or the HGLT ‘renal disorders’ or the HLT ‘renal function analyses’, according to MedDRA (version 22.1) [23]. All queried reports mentioning an effect belonging to any other MedDRA term (to the exclusion of the three abovementioned) were classified in the group ‘extrarenal IRAEs’.
For each report, whenever available, data were gathered about the patient [age, sex, body mass index (BMI) and comorbidities], the ICI (therapeutic indication, dosage, date of introduction and of last course of treatment), concomitant drugs and the main features of the ADR (description, notified MedDRA terms, time to onset and outcome). Preexisting chronic kidney disease (CKD), as well as ICI-AKI, were assessed according to the data included in the PVD report (KDIGO classification, estimated glomerular filtration rate, medical history or creatininemia). Notified IRAEs were classified according to MedDRA System Organ Class. We focused on concomitant drugs known for their propensity to induce ATIN: non-steroidal anti-inflammatory drugs (NSAIDs), calcium channel blockers, thiazide and loop diuretics, proton-pump inhibitors (PPIs), hypouricemic drugs, H2 antagonists and fluindione.
Screening of reports
All reports of ‘ICI-AKI’ were reviewed by nephrologists and clinical pharmacologists trained in pharmacovigilance to assess their relevance. To increase specificity, cases were not included if AKI could not be directly attributed to the ICI in view of the available data (i.e. prerenal AKI following ICI-induced diarrhea) or when reports were not assessable because of missing data (especially when data on concomitant drugs were not available).
A sample of reports from the ‘extrarenal IRAEs’ group was randomly included, following a 4:1 ratio of four ‘extrarenal IRAEs’ for one ‘ICI-AKI’. This 4:1 ratio was intended to increase statistical confidence. It is usually not worth going beyond a ratio of four or five controls to one case in case–control studies [24].
When a single patient is subject to several successive distinct reports, this is usually mentioned as a ‘related case’ in the PVD, and therefore linked to previous reports. This tool was used to seek for potential duplicates.
Analysis
Descriptive statistics were expressed as mean ± standard deviation or as median [interquartile range (IQR)] whenever appropriate. Percentages were calculated for qualitative data. The patients’ characteristics were compared with Pearson's Chi-Squared test (with Yates's correction) for categorical variables or the Fisher exact test when the expected number of observations was <5. Student's t-test was used for continuous variables. All variables showing an association with a P < 0.05 were included as covariates in a multivariate analysis, along with sex and BMI. Multivariate analysis was conducted in R [25]. Logistic regressions, with renal injury as an outcome, were run over 40 multiple imputed datasets, using the ‘mice’ package to impute missing values. Results of the multivariate analysis were expressed as adjusted odds ratios (OR) with their 95% confidence intervals (95% CI), and P < 0.05 was considered statistically significant.
RESULTS
Included reports
The query of all specified ADR reports (involving one or more of the ICI and reported between 1985 and 23 September 2020) yielded 4498 reports (Figure 1), 266 (5.9%) of which were classified as ICI-AKI and 4232 (94.1%) as extrarenal IRAEs. After screening this comprehensive list of reports, 99 reports of ICI-AKI were not included because of either insufficient data (especially regarding concomitant drugs) or because AKI could not be directly attributed to the ICI after review, thus 167 reports of ICI-AKI were included (3.7% of the 4498 reports). Among extrarenal IRAEs, 668 reports were sampled to comply with the 4:1 ratio. Among the sample of extrarenal IRAEs, no report was identified as a ‘related case’ linked to a report of ICI-AKI for the same patient and vice versa.
FIGURE 1:
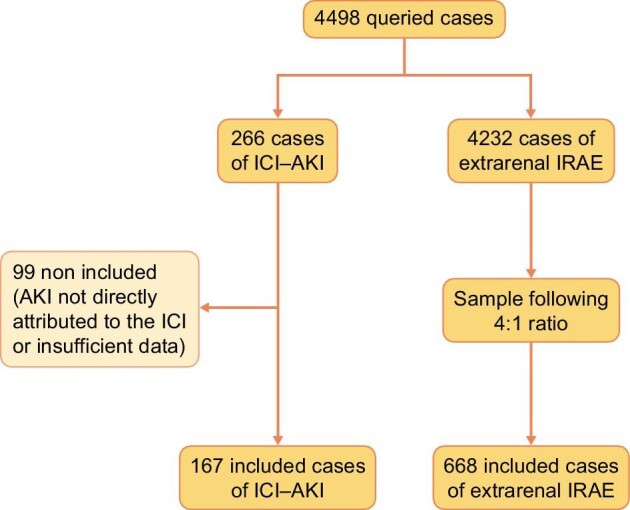
Flowchart. AKI: acute kidney injury; ICI-AKI: patients with renal IRAEs; IRAE: immune-related adverse events.
Patients’ baseline characteristics
Baseline characteristics of patients with ICI-AKI (N = 167) and controls with extrarenal IRAEs (N = 668) are detailed in Table 1. Most patients in both groups were male: 107 (64.1%, sex ratio: 1.8) and 410 (61.4%, sex ratio: 1.6), respectively. Patients with ICI-AKI were older (mean age: 69.1 years) than those in the control group (64.6 years). This difference was statistically significant in the univariate analysis (P < 0.0001) and confirmed in the multivariate analysis (OR 1.02, 95% CI 1.00–1.04; P = 0.0135, Table 2). Mean BMI was not significantly different between the two groups.
Table 1.
Patients’ characteristics at baseline
| Characteristics | ICI-AKI N = 167 (%) | Extrarenal IRAEs N = 668 (%) | P-value |
|---|---|---|---|
| Sex | 0.5807 | ||
| Male | 107 (64.1) | 410 (61.4) | |
| Female | 60 (35.9) | 258 (38.6) | |
| Age at notification, years | 69.1 ± 11.3 | 64.6 ± 13.1 | <0.0001 |
| BMI | 24.5 ± 4.3 | 24.7 ± 5.1 | 0.7121 |
| Comorbidities | |||
| Chronic kidney disease | 20 (12.0) | 22 (3.3) | <0.0001 |
| Hypertension | 58 (34.7) | 156 (23.4) | 0.0036 |
| Heart failure | 3 (1.8) | 4 (0.6) | 0.1472 |
| Coronaropathy | 8 (4.5) | 14 (2.1) | 0.0940 |
| Diabetes | 15 (9.0) | 58 (8.7) | 0.9999 |
| Peripheral vascular disease | 9 (5.4) | 18 (2.7) | 0.1295 |
| COPD | 17 (10.2) | 42 (6.3) | 0.1126 |
| Tobacco use | 22 (13.2) | 95 (14.2) | 0.8225 |
| Malignancy | |||
| Lung cancer | 67 (40.1) | 263 (39.4) | 0.9295 |
| Melanoma | 50 (29.9) | 245 (36.7) | 0.1239 |
| Renal cancer | 14 (8.4) | 27 (4.0) | 0.0338 |
| Urothelial cancer | 6 (3.6) | 9 (1.3) | 0.0934 |
| Hodgkin lymphoma | 2 (1.2) | 6 (0.9) | 0.6638 |
| Other | 28 (16.8) | 118 (17.7) | 0.8733 |
| Immunotherapya | |||
| Nivolumab | 96 (57.5) | 361 (54.9) | 0.4761 |
| Pembrolizumab | 50 (29.9) | 213 (31.9) | 0.6957 |
| Ipilimumab | 18 (10.8) | 101 (15.1) | 0.8527 |
| Atezolizumab | 7 (4.2) | 21 (3.1) | 0.6654 |
| Durvalumab | 4 (2.4) | 16 (2.4) | 1 |
| Avelumab | 1 (0.6) | 5 (0.7) | 0.7990 |
| Cemiplimab | 1 (0.6) | 3 (0.4) | 1 |
| Nivolumab ± ipilimumab | 8 (4.8) | 49 (7.3) | 0.3198 |
| Pembrolizumab ± ipilimumab | 2 (1.2) | 2 (0.3) | 0.1804 |
| Nivolumab ± pembrolizumab | 0 | 1 (0.1) | 1 |
| Concomitant drugs | |||
| NSAIDs | 6 (3.6) | 9 (1.3) | 0.1034 |
| Calcium channel blocker | 23 (13.8) | 39 (5.8) | 0.0009 |
| Thiazide diuretic | 14 (8.4) | 24 (3.6) | 0.0143 |
| Loop diuretic | 15 (9.0) | 24 (3.6) | 0.0060 |
| PPI | 52 (31.1) | 94 (14.1) | <0.0001 |
| Hypouricemic | 8 (4.8) | 11 (1.6) | 0.0318 |
| H2 antagonist | 1 (0.6) | 0 | 0.2000 |
| Fluindione | 11 (6.6) | 6 (0.9) | <0.0001 |
Mean ± standard deviation for continuous variables; COPD: chronic obstructive pulmonary disease; thiazide diuretic: includes hydrochlorothiazide and indapamide.
aPatients with combined immunotherapy are also counted in each single immunotherapy's category.
Bold values are p-values of significant results (P < 0.05).
Table 2.
Multivariate analysis
| Characteristics | Odds ratio (95% confidence interval) | P-value |
|---|---|---|
| Male | 1.05 (0.72–1.53) | 0.8073 |
| Age at notification, years | 1.02 (1.00–1.04) | 0.0135 |
| BMI | 0.75 (0.26–2.18) | 0.5951 |
| Chronic kidney disease | 2.50 (1.22–5.14) | 0.0125 |
| Hypertension | 1.02 (0.66–1.56) | 0.9451 |
| Renal cancer | 1.42 (0.67–3.00) | 0.3610 |
| Concomitant drugs | ||
| NSAIDs | 3.18 (1.07–9.4) | 0.0368 |
| Thiazide diuretic | 1.56 (0.77–3.19) | 0.2192 |
| Loop diuretic | 1.37 (0.65–2.89) | 0.4052 |
| PPI | 2.18 (1.42–3.34) | 0.0004 |
| Hypouricemic | 1.81 (0.66–4.99) | 0.2489 |
| Fluindione | 6.53 (2.21–19.31) | 0.0007 |
Thiazide diuretic includes hydrochlorothiazide and indapamide.
Bold values are p-values of significant results (P < 0.05).
CKD was significantly more prevalent in patients with ICI-AKI (12.0% versus 3.3%) in the univariate analysis (P < 0.0001). This result was confirmed in the multivariate analysis (OR 2.50, 95% CI 1.22–5.14; P = 0.0125). Hypertension was significantly more frequent in patients with ICI-AKI (34.7% versus 23.4%) in univariate analysis, but did not stand out in the multivariate analysis (P = 0.9451). Other comorbidities were not significantly associated with the occurrence of ICI-AKI.
Malignancies and ICIs
The most frequent malignancies at the origin of treatment were lung cancer (40.1% among ICI-AKI patients and 39.4% among patients with extrarenal IRAEs) and melanoma (29.9% and 36.7%, respectively) in both groups. Renal cancer was significantly more frequent in patients with ICI-AKI in the univariate analysis (8.4% versus 4.0%; P = 0.0338) but not in the multivariate analysis (P = 0.3610). Other malignancies were not significantly associated with the occurrence of ICI-AKI.
More than half of the patients were treated with nivolumab in each group (Table 1): 57.5% in ICI-AKI patients and 54.9% in patients with extrarenal-IRAEs. Among patients with ICI-AKI, 10 (6.0%) were treated with two concomitant ICIs. In patients with extrarenal IRAEs, 52 (7.8%) were treated with two concomitant ICIs. The relative share of the different ICIs was not significantly different between the two groups.
Immune-related adverse events
Time to onset for ICI-AKI could be assessed in 136 of the 167 reports, with a median of 83 days (IQR 31–168). Among 167 ICI-AKI reports, 74 (44.3%) mentioned at least one concomitant extrarenal IRAE, mostly haematologic (12, 7.2%), hepatobiliary (11, 6.6%) and/or cutaneous (10, 6.0%) (Table 3).
Table 3.
Repartition of reported IRAEs
| System organ class | ICI-AKI N = 167 (%) | Extrarenal IRAEs N = 668 (%) |
|---|---|---|
| >1 concomitant SOC reported | 74 (44.3) | 301 (45.1) |
| Blood and lymphatic system disorders | 12 (7.2) | 46 (6.9) |
| Cardiac disorders | 4 (2.4) | 44 (6.6) |
| Endocrine disorders | 6 (3.6) | 105 (15.7) |
| Eye disorders | 1 (0.6) | 29 (4.3) |
| Gastrointestinal disorders | 5 (3.0) | 97 (14.5) |
| Hepatobiliary disorders | 11 (6.6) | 80 (12.0) |
| Immune system disorders | 0 | 16 (2.4) |
| Infections and infestations | 2 (1.2) | 21 (3.1) |
| Metabolism and nutrition disorders | 9 (5.4) | 38 (5.7) |
| Musculoskeletal and connective tissue disorders | 3 (1.8) | 58 (8.7) |
| Nervous system disorders | 5 (3.0) | 46 (6.9) |
| Psychiatric disorders | 0 | 7 (1.0) |
| Renal and urinary disorders | 167 (100) | 0 |
| Respiratory, thoracic and mediastinal disorders | 9 (5.4) | 98 (14.7) |
| Skin and subcutaneous tissue disorders | 10 (6.0) | 131 (19.6) |
| Vascular disorders | 2 (1.2) | 16 (2.4) |
IRAEs are classified depending on their system organ class (SOC; MedDRA) and are not mutually exclusive as some patients experienced several concomitant IRAEs (>1 concomitant SOC reported).
In the extrarenal IRAEs group, 301 (45.1%) reports mentioned IRAEs belonging to at least two different organs. The most frequent (non-mutually exclusive) IRAEs were cutaneous (131, 19.6%), endocrine (105, 15.7%), respiratory (98, 14.7%) and/or gastrointestinal (97, 14.5%) (Table 3).
Concomitant drugs
In the multivariate analysis, patients with ICI-AKI were significantly more likely to be treated with fluindione (OR 6.53, 95% CI 2.21–19.31; P = 0.0007), an NSAID (OR 3.18, 95% CI 1.07–9.4; P = 0.0368) or a PPI (OR 2.18, 95% CI 1.42–3.34; P = 0.0004) (Table 2). Among patients with ICI-AKI, five (3.0%) were treated with both fluindione and a PPI, two (1.2%) were treated with both an NSAID and a PPI, and none was treated with both fluindione and a NSAID. In the univariate analysis, patients with ICI-AKI were significantly more likely to be cotreated by a thiazide diuretic, a loop diuretic or a hypouricemic agent (Table 1). However, this association did not remain significant in the multivariate analysis (Table 2).
DISCUSSION
In this multicentre pharmacovigilance case–control study of 167 patients with ICI-AKI, we shed some light on the specific features associated and possibly concurring to ICI-AKI, compared with reports of extrarenal IRAEs as controls.
Most patients (60–65%) were male in both groups, as found in previous studies [17, 26, 27]. This result may seem surprising, as females are generally more prone to autoimmune diseases than males. Nonetheless, it may merely reflect the epidemiology of cancer [28], as no significant sex-associated differences have been described for IRAEs [27].
We found an independent association between the occurrence of ICI-AKI and age or preexisting CKD. Lower baseline renal function has already been retrospectively reported as a risk factor for ICI-AKI [17], while the association between age and ICI-AKI is, to our knowledge, unprecedented [29]. The increased risk of ICI-AKI in elderly seems rational, since age may reflect increased renal frailty in older patients.
Renal cancer was more frequent in ICI-AKI patients in univariate analysis. However, this result did not stand out in the multivariate analysis. This result might have been subject to a confusion bias. Should it be confirmed by further studies, this finding could also be underpinned by the two-way association between CKD and renal cancer [30]. Renal cancer might also foster immune tolerance loss against kidney antigen, but we are unable to draw definite conclusions as it stands.
The median time to onset (around 2 months) dovetails with the available literature on ICI-AKI [9, 11, 15]. On another note, we confirm the high proportion of patients with concomitant IRAEs affecting more than one organ in nearly half of the reports (in both groups). The literature suggests that concomitant extrarenal IRAE may lower the likelihood of renal recovery. Conversely, concomitant treatment with a drug known to cause ATIN may improve renal prognosis [17].
Regarding concomitant drugs, we confirm an independent association between concomitant treatment with a PPI or an NSAID and the occurrence of ICI-AKI [17, 19, 31]. In addition, we report for the first time an independent association between long-term treatment with the vitamin-K antagonist fluindione and ICI-AKI. This finding seems relevant, given that fluindione bears a definite immuno-allergic risk (including ATIN) [32, 33].
There is growing evidence that ICIs may sometimes precipitate (rather than directly induce) ATIN caused by another concomitant nephritogenic treatment such as a PPI, an NSAID, an antibiotic or fluindione [14, 20]. This loss of tolerance to a concomitant treatment may be explained by the activation of latent drug-specific T cells. Other (not mutually exclusive) mechanistic hypotheses for ICI-AKI include recognition by the T-cell of an off-target kidney antigen after immune checkpoint blockade or the formation of auto-antibodies directed against kidney tissue [14, 16, 20].
This multicentre case–control study relies on a large cohort of patients, with a 4:1 ratio and a multivariate analysis to mitigate the impact of potential confounders. The control group included patients with extrarenal IRAEs only, thus helping to approach the specific risk factors for ICI-AKI rather than the risk factors for IRAEs broadly speaking. However, it is limited by classical flaws inherent to pharmacovigilance studies, such as lack of data and under-notification. We did not include reports when concomitant drugs were not available to try to minimize the notification bias, whereby physicians reporting ICI-AKI are more prone to list the concomitant medications. However, this approach led us to rule out a substantial number of reports from the analysis. The heterogeneity of data prevented us from relying on established classifications such as KDIGO [34] and from precisely tracking the timing of sequential IRAEs. Besides, this case–control study cannot pretend to draw definite conclusions regarding the causal relationship between the studied characteristics and the occurrence of ICI-AKI. Moreover, we may have lacked the power to identify a significant association between some concomitant treatments and ICI-AKI.
This case–control study highlights potential risk factors for ICI-AKI, with a special focus on the role of co-treatments known to cause immuno-allergic ATIN, such as PPIs, NSAIDs and fluindione. ICIs may be a ‘second hit’ that precipitates AKI caused by another concomitant drug. Further causal confirmation of this pathophysiological hypothesis is awaited. Meanwhile, clinicians should be aware to minimize exposure to co-treatments at risk in patients treated with ICIs.
ACKNOWLEDGEMENTS
The authors are grateful for the proofreading of this manuscript by Elise Van Obberghen (MD), a native English speaker. She received no compensation for this work. The views expressed in this article are the authors’ personal views, and may not be understood or quoted as being made on behalf of or reflect the position of the ANSM, the EMA or one of their committees or working parties.
Contributor Information
Alexandre O Gérard, Department of Nephrology-Dialysis-Transplantation, University Hospital Centre of Nice, Nice, France; Department of Pharmacology and Pharmacovigilance Centre of Nice, University Hospital Centre of Nice, Nice, France.
Susana Barbosa, Institute of Molecular and Cellular Pharmacology (IPMC), UMR 7275, CNRS, University Côte d'Azur, Valbonne, France.
Nadège Parassol, Department of Pharmacology and Pharmacovigilance Centre of Nice, University Hospital Centre of Nice, Nice, France.
Marine Andreani, Department of Nephrology-Dialysis-Transplantation, University Hospital Centre of Nice, Nice, France.
Diane Merino, Department of Pharmacology and Pharmacovigilance Centre of Nice, University Hospital Centre of Nice, Nice, France.
Marion Cremoni, Department of Nephrology-Dialysis-Transplantation, University Hospital Centre of Nice, Nice, France.
Audrey Laurain, Department of Nephrology-Dialysis-Transplantation, University Hospital Centre of Nice, Nice, France.
Sylvine Pinel, Pharmacovigilance Center of Paris–Fernand Widal, Assistance Publique–Hôpitaux de Paris, Paris, France.
Delphine Bourneau-Martin, Pharmacovigilance Center of Angers, University Hospital Centre of Angers, Angers, France.
Fanny Rocher, Department of Pharmacology and Pharmacovigilance Centre of Nice, University Hospital Centre of Nice, Nice, France.
Vincent L M Esnault, Department of Nephrology-Dialysis-Transplantation, University Hospital Centre of Nice, Nice, France.
Delphine Borchiellini, Department of Medical Oncology, Centre Antoine Lacassagne, University Côte d'Azur, Nice, France.
Antoine Sicard, Department of Nephrology-Dialysis-Transplantation, University Hospital Centre of Nice, Nice, France; Laboratory of Molecular Physio Medicine (LP2M), UMR 7370, CNRS, University Côte d'Azur, Nice, France; Clinical Research Unit of University Côte d'Azur (UR2CA), University Côte d'Azur, Nice, France.
Milou-Daniel Drici, Department of Pharmacology and Pharmacovigilance Centre of Nice, University Hospital Centre of Nice, Nice, France.
FUNDING
The authors do declare that there is no source of funding for this study.
AUTHORS’ CONTRIBUTIONS
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by A.O.G., S.B. and N.P. The first draft of the manuscript was written by A.O.G., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors do not declare any conflict of interest relevant to the content of this article. The results presented in this paper have not been published previously in whole or in part.
DATA AVAILABILITY STATEMENT
The French Pharmacovigilance Database (PVD) has been approved by the National Data Protection Agency. All our data originate from this database. In accordance with European regulation, this retrospective observational study of anonymous reports did not need the approval of an Institutional Review Board/Independent Ethics Committee. This research was allowed by the Pharmacovigilance network, which was kept informed.
REFERENCES
- 1. Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med 2016; 375: 1767–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim JY, Lee KH, Eisenhut Met al. Efficacy of cancer immunotherapy: an umbrella review of meta-analyses of randomized controlled trials. Cancers 2019; 11: 1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baraibar I, Melero I, Ponz-Sarvise Met al. Safety and tolerability of immune checkpoint inhibitors (PD-1 and PD-L1) in cancer. Drug Saf 2019; 42: 281–294 [DOI] [PubMed] [Google Scholar]
- 4. Braet P, Sartò GVR, Pirovano Met al. Treatment of acute kidney injury in cancer patients. Clin Kidney J 2022; 15: 873–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Izzedine H, Gueutin V, Gharbi Cet al. Kidney injuries related to ipilimumab. Invest New Drugs 2014; 32: 769–773 [DOI] [PubMed] [Google Scholar]
- 6. Izzedine H, Mateus C, Boutros Cet al. Renal effects of immune checkpoint inhibitors. Nephrol Dial Transplant 2017; 32: 936–942 [DOI] [PubMed] [Google Scholar]
- 7. Izzedine H, Mathian A, Champiat Set al. Renal toxicities associated with pembrolizumab. Clin Kidney J 2019; 12: 81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gérard AO, Andreani M, Fresse Aet al. Immune checkpoint inhibitors-induced nephropathy: a French national survey. Cancer Immunol Immunother CII 2021; 70: 3357–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cortazar FB, Marrone KA, Troxell MLet al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016; 90: 638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manohar S, Kompotiatis P, Thongprayoon Cet al. Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: meta-analysis. Nephrol Dial Transplant 2019; 34: 108–117 [DOI] [PubMed] [Google Scholar]
- 11. Wanchoo R, Karam S, Uppal NNet al. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol 2017; 45: 160–169 [DOI] [PubMed] [Google Scholar]
- 12. Kim Y. Relapse of membranous nephropathy with cancer immunotherapy. Clin Kidney J 2021; 14: 418–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oleas D, Bolufer M, Agraz Iet al. Acute interstitial nephritis associated with immune checkpoint inhibitors: a single-centre experience. Clin Kidney J 2021; 14: 1364–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gupta S, Cortazar FB, Riella LV, Leaf DE. Immune checkpoint inhibitor nephrotoxicity: update 2020. Kidney 360 2020; 1: 130–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hultin S, Nahar K, Menzies AMet al. Histological diagnosis of immune checkpoint inhibitor induced acute renal injury in patients with metastatic melanoma: a retrospective case series report. BMC Nephrol 2020; 21: 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Draibe JB, García-Carro C, Martinez-Valenzuela Let al. Acute tubulointerstitial nephritis induced by checkpoint inhibitors versus classical acute tubulointerstitial nephritis: are they the same disease? Clin Kidney J 2021; 14: 884–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cortazar FB, Kibbelaar ZA, Glezerman IGet al. Clinical features and outcomes of immune checkpoint inhibitor–associated AKI: A multicenter study. J Am Soc Nephrol 2020; 31: 435–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murakami N, Borges TJ, Yamashita Met al. Severe acute interstitial nephritis after combination immune-checkpoint inhibitor therapy for metastatic melanoma. Clin Kidney J 2016; 9: 411–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perazella MA, Shirali AC. Immune checkpoint inhibitor nephrotoxicity: what do we know and what should we do? Kidney Int 2020; 97: 62–74 [DOI] [PubMed] [Google Scholar]
- 20. Perazella MA, Shirali AC. Nephrotoxicity of cancer immunotherapies: past, present and future. J Am Soc Nephrol 2018; 29: 2039–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perazella MA, Sprangers B. Checkpoint inhibitor therapy-associated acute kidney injury: time to move on to evidence-based recommendations. Clin Kidney J 2021; 14: 1301–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the Member States relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. Med Etika Bioet 2002; 9: 12–19 [PubMed] [Google Scholar]
- 23. Welcome to MedDRA | MedDRA. https://www.meddra.org/. (14 November 2020, last date accessed)
- 24. (2020) Chapter 8. Case-control and cross sectional studies | The BMJ. In: BMJ BMJ Lead. Gen. Med. J. Res. Educ. Comment. https://www.bmj.com/about-bmj/resources-readers/publications/epidemiology-uninitiated/8-case-control-and-cross-sectional (8 March 2022, date last accessed) [Google Scholar]
- 25. R Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing, 2020 [Google Scholar]
- 26. Gupta S, Short SAP, Sise MEet al. Acute kidney injury in patients treated with immune checkpoint inhibitors. J Immunother Cancer 2021; 9: e003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jing Y, Zhang Y, Wang Jet al. Association between sex and immune-related adverse events during immune checkpoint inhibitor therapy. JNCI J Natl Cancer Inst 2021; 113: 1396–1404 [DOI] [PubMed] [Google Scholar]
- 28. Zhu Y, Shao X, Wang Xet al. Sex disparities in cancer. Cancer Lett 2019; 466: 35–38 [DOI] [PubMed] [Google Scholar]
- 29. Huang X, Tian T, Zhang Yet al. Age-associated changes in adverse events arising from anti-PD-(L)1 therapy. Front Oncol 2021; 11: 1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Russo P. End stage and chronic kidney disease: associations with renal cancer. Front Oncol 2012; 2: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shirali AC, Perazella MA, Gettinger S. Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis 2016; 68: 287–291 [DOI] [PubMed] [Google Scholar]
- 32. Leven C, Hudier L, Picard Set al. [Prospective study of drug-induced interstitial nephritis in eleven French nephrology units]. Presse Medicale Paris Fr 2014; 43: e369–e376 [DOI] [PubMed] [Google Scholar]
- 33. Reynaud F, Giraud P, Cisterne J-Met al. [Acute immuno-allergic interstitial nephritis after treatment with fluindione. Seven cases]. Nephrol Ther 2009; 5: 292–298 [DOI] [PubMed] [Google Scholar]
- 34. Levey AS, Eckardt K-U, Tsukamoto Yet al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005; 67: 2089–2100 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The French Pharmacovigilance Database (PVD) has been approved by the National Data Protection Agency. All our data originate from this database. In accordance with European regulation, this retrospective observational study of anonymous reports did not need the approval of an Institutional Review Board/Independent Ethics Committee. This research was allowed by the Pharmacovigilance network, which was kept informed.