ABSTRACT
Background
Hyperkalaemia is frequent in haemodialysis (HD) patients and associated with increased cardiovascular mortality. Despite routine clinical use, evidence regarding the efficacy of potassium (K+) binders in HD is scant. We wished to compare the efficacy of patiromer (PAT) and sodium polystyrene sulfonate (SPS) on K+ levels in this setting.
Methods
We screened patients in three HD centres with pre-HD K+ value between 5.0 and 6.4 mmol/L, after an initial 2-week washout period for those previously on K+ binders. We included patients in an unblinded two-arm crossover trial comparing SPS 15 g before each meal on non-dialysis days with PAT 16.8 g once daily on non-dialysis days with randomized attribution order and a 2-week intermediate washout period. The primary outcome was the mean weekly K+ value.
Results
We included 51 patients and analysed 48 with mean age of 66.4 ± 19.4 years, 72.9% men and 43.4% diabetics. Mean weekly K+ values were 5.00 ± 0.54 mmol/L, 4.55 ± 0.75 mmol/L and 5.17 ± 0.64 mmol/L under PAT (P = .003), SPS (P < .001) and washout, respectively. In direct comparison, K+ values and prevalence of hyperkalaemia were lower under SPS as compared with PAT (P < .001). While the incidence of gastrointestinal side effects was similar between treatments, SPS showed lower subjective tolerability score (6.0 ± 2.4 and 6.9 ± 1.9) and compliance (10.8 ± 20.4% and 2.4 ± 7.3% missed doses) as compared with PAT (P < .001 for both).
Conclusion
Both PAT and SPS are effective in decreasing K+ levels in chronic HD patients. However, at the tested doses, SPS was significantly more effective in doing so as compared with PAT, despite lower tolerability and compliance. Larger randomized controlled trials should be conducted in order to confirm our findings and determine whether they would impact clinical outcomes.
Keywords: haemodialysis, hyperkalaemia, patiromer, potassium binder, sodium polystyrene sulfonate
Graphical Abstract
Graphical Abstract.
INTRODUCTION
As kidneys are responsible for excreting 90% of dietary potassium (K+) intake, chronic haemodialysis (HD) patients rely mainly on dialytic removal to avoid complications associated with hyperkalaemia. Nevertheless, hyperkalaemia (defined as K+ ≥5.1 mmol/L) is frequent in this setting, affecting ˃25% of patients, despite the prescription of K+ binders in the majority of them [1]. Several studies have reported an association between hyperkalaemia and an increased risk of cardiovascular morbidity and mortality in HD patients [2, 3]. Moreover, this increased event rate is not evenly distributed throughout the week but rather clustered following the long inter-dialytic period [4]. Altogether, the evidence suggests that hyperkalaemia, as well as the associated sudden drop in K+ values during the first HD session of the week, are causally associated with adverse cardiovascular outcomes in this population.
In 1958, sodium polystyrene sulfonate (SPS) obtained Food and Drug Administration (FDA) approval for the treatment of hyperkalaemia in chronic kidney disease (CKD) [5]. Despite a relative lack of direct evidence for its efficacy, SPS has gained widespread use in chronic HD patients. A first randomized controlled trial (RCT) was eventually published in 2015 and showed the superiority of SPS compared with placebo in reducing serum K+ levels in 31 non-HD CKD patients over a 7-day follow-up [6]. However, to this day, no direct evidence of SPS efficacy in HD patients exists. Owing to concerns regarding tolerability and gastrointestinal (GI) side effects of SPS, patiromer (PAT) has recently been FDA-approved as a sodium-free, calcium-based K+ binder. PAT has been shown to reduce serum K+ levels in CKD patients with and without heart failure in large RCTs [7–9]. However, evidence remains scant in HD, with only one non-controlled study showing direct evidence of serum K+ reduction with PAT in six HD patients [10].
As K+ handling is deeply influenced by the dialytic procedure itself and HD patients are expected to benefit the most from satisfactory K+ control, evidence regarding the clinical use of K+ binders in this specific population is needed. In this regard, we conducted this study to evaluate the absolute and relative efficacy of SPS and PAT on serum K+ control as well as their tolerability in chronic HD patients.
MATERIALS AND METHODS
The data underlying this article will be shared on reasonable request to the corresponding author. This study was prospectively registered in the Swiss National Clinical Trials Portal (kofam.ch) under SNCTP000003912 as well as in the Business Administration System for Ethics Committees (swissethics.ch/en/basec) under BASEC2019-01656. Full study protocol is available as Supplementary data, document 1. CONSORT 2010 checklist is available as Supplementary data, document 2.
Participants
Patients were screened amongst chronic HD patients in three HD units attached to different hospitals in Switzerland in September 2020: Geneva University Hospitals (tertiary centre), Hôpital de la Providence (secondary centre) and Hôpital de la Tour (secondary centre). Patients were enrolled and assigned to interventions by respective local heads of HD units (F.S., T.E. and P.S.). Inclusion criteria were: (i) CKD patients on maintenance HD without significant recirculation (<20%) of vascular access, (ii) no change in medications potentially influencing K+ levels (renin-angiotensin-aldosterone blockers, loop or thiazide diuretics, beta-blockers, insulin, laxative, non-steroidal anti-inflammatory drugs) in the prior 2 weeks and (iii) pre-specified K+ levels (see below). Exclusion criteria were: (i) age <18 years, (ii) unable to provide informed consent, (iii) chronic GI disease, (iv) severe hyperkalaemia (>6.4 mmol/L) and (v) pregnant or breastfeeding women. In order to fulfil inclusion criteria, patients without K+ binders had to have K+ values between 5.0 and 6.5 mmol/L whereas patients under K+ binders had to have K+ values between 4.5 and 6.0 mmol/L. Patients under K+ binders were then submitted to a 2-week initial washout period where K+ binders were stopped. Finally, patients were included in the present study (at the end of the 2-week washout period for those initially under K+ binders) if they had K+ values between 5.0 and 6.4 mmol/L.
Study design, intervention and variables
Once included, patients took part in an unblinded two-arm crossover trial with an intermediate washout period. Patients were randomly allocated to one of two treatment arms. Randomization was generated using an online system (www.sealedenvelope.com) with a block size of four and no stratification [11]. In the first treatment arm, patients were prescribed PAT during 4 weeks, followed by a 2-week washout period without medication and finally SPS during 4 weeks. In the second arm, treatment attribution was the opposite and patients were prescribed SPS, followed by a washout period and finally PAT for equivalent durations. SPS was prescribed as 15 g before each meal (depending on the number of meals) on non-dialysis days. PAT was prescribed as 16.8 g once daily at 11 a.m. on non-dialysis days. In the absence of prior head-to-head comparison, respective PAT and SPS doses were empirically chosen as roughly equivalent based on prior study results [6–10, 12]. More specifically, a similar K+ reduction could be expected from those regimens when anticipating lower compliance under SPS. Moreover, selected doses also could be considered as relatively equivalent according to national recommendations on drug dosing (www.compendium.ch). No dose modification (increase or decrease) was allowed during the study period. K+ values were measured at the start of each HD session. Mean weekly K+ values were calculated. For each medication, the prevalence of hyperkalaemia (K+ ≥5.1 or ≥5.5 mmol/L) was calculated as: the number of study weeks with mean weekly K+ value ≥5.1 or 5.5 mmol/L divided by total number of study weeks on considered medication. Calcium, phosphate and magnesium values were measured on the first HD session of each week. Tolerability to SPS and PAT was assessed using a visual analogue scale ranging from 0 to 10. GI side effects were defined as any subjective noticeable change in bowel habits, abdominal discomfort and/or nausea/vomiting. Compliance with SPS and PAT was measured as a percentage of missed weekly doses and the proportion of patients missing ˃10% of weekly doses. The primary outcome was a mean weekly K+ value. Secondary outcomes were other electrolytes values, tolerability, side effects and compliance. Diabetes was defined based on the presence of related medications. Residual diuresis was defined as >200 mL of urine on the most recent 24 h urine collection. All patients were dialysed using haemodiafiltration (HDF) with post-dilution reinjection and high-flux polysulphone dialysers using Braun Dialog (Braun, Melsungen, Germany) or Fresenius 5008 (Fresenius AG, Bad Homberg, Germany) dialysis machines. Dialysate K+ concentration was 3 mmol/L. Dialysis prescriptions were not modified throughout the study.
Statistical analysis
Using a superiority framework, a sample size of 46 patients was estimated to reach 80% statistical power with 5% bilateral alpha-error, 0.5 mmol/L standard deviations (SD) and 0.3 mmol/L minimal detectable difference in means between SPS and PAT. All analyses were conducted as intention-to-treat. In the descriptive analysis, continuous variables were expressed as mean ± SD and categorical variables in number and relative frequencies. Continuous and categorical variables were compared between study arms using t-test and Chi-squared, respectively. In main analyses, linear regression models were used with mean weekly K+ value as the outcome and treatment (PAT or SPS) as the main predictor. In addition to unadjusted analysis, multivariate models were constructed with the inclusion of age, ethnicity, diabetes, residual diuresis and medication [renin-angiotensin-aldosterone system (RAAS) inhibitor, loop diuretics and beta-blocker] as covariates. As the effect of K+ binders on K+ values could potentially change over time, interaction effect between treatment and study week was tested by comparing models with interaction term (treatment × study week) to models without (treatment + study week). Interaction was considered significant if P-value for likelihood ratio test (LRT) comparing both models was <.05. In secondary analyses, variables representing compliance, tolerability and GI side effects as well as various electrolytes were considered as the outcome and treatment as the main predictor in similar models. As patients could be compared with themselves in this crossover trial, a multi-level effect was implemented in every regression model with a random effect on the intercept. P-values <.05 were considered statistically significant.
Ethics
This study was approved by the local ethics committee ‘Commission cantonale d’éthique de la recherche’ (CCER), Geneva, Switzerland and performed according to the declaration of Helsinki. All patients included in this trial provided written informed consent.
RESULTS
Baseline characteristics
In total, 51 patients were included in the present study (Supplementary data, Fig. S1). Of those, two resigned their consent during the first week and one was excluded before entering the study owing to severe sepsis. Thus, 48 patients were included in the present analysis. Of those, 25 were randomized to PAT-SPS sequence and 23 to SPS-PAT sequence. All these patients completed the study except one patient who was transplanted during week nine of the study. The mean age of participants was 66.4 ± 19.4 years with 72.9% men. Caucasians represented 81.2% of patients and the mean body mass index (BMI) was 24.9 ± 4.8 kg/m2. There were 3 (6.5%) smokers and 20 (43.4%) diabetics. Residual diuresis >200 mL/24 h was noted in 23 (47.9%) patients. Use of RAAS inhibitors, loop diuretics and beta-blockers were reported in 25 (52.0%), 16 (33.3%) and 23 (47.9%) patients, respectively. The mean number of HD weekly sessions per patient was 2.8 ± 0.4, with minimum and maximum numbers of one and four, respectively. On week one, the mean pre-HD K+ value on the first day was 5.23 ± 0.83 mmol/L. K+ values were not different between patients assigned to PAT and those assigned to SPS (P = .164). On week seven, the mean pre-HD K+ value on the first day was 5.37 ± 0.66 mmol/L. K+ values were not different between patients assigned to PAT and those assigned to SPS (P = .769). Baseline characteristics of included patients according to treatment randomization order are given in Table 1.
Table 1.
Baseline characteristics according to treatment randomization order
| Sequence PAT-SPS (N = 25) | Sequence SPS-PAT (N = 23) | P-value | |
|---|---|---|---|
| Age (years) | 69.4 ± 16.5 | 62.8 ± 22.2 | .252 |
| Gender (men), n (%) | 18 (72.0) | 17 (73.9) | .882 |
| Ethnicity (Caucasian), n (%) | 22 (88.0) | 17 (73.9) | .575 |
| BMI (kg/m2) | 24.9 ± 4.0 | 24.9 ± 5.7 | .981 |
| Diabetes, n (%) | 10 (41.6) | 10 (45.4) | .796 |
| Smoker, n (%) | 2 (8.3) | 1 (4.5) | .603 |
| Residual diuresis, n (%) | 14 (56.0) | 9 (39.1) | .243 |
| RAAS inhibitor, n (%) | 13 (52.0) | 12 (52.1) | .990 |
| Loop diuretic, n (%) | 9 (36.0) | 7 (30.4) | .683 |
| Beta-blocker, n (%) | 12 (48.0) | 11 (47.8) | .990 |
| K+ value on first session of week 1 | 5.39 ± 0.70 | 5.04 ± 0.95 | .164 |
| K+ value on first session of week 7 | 5.40 ± 0.60 | 5.34 ± 0.72 | .769 |
| Calcium (mmol/L) | 2.30 ± 0.19 | 2.24 ± 0.19 | .353 |
| Phosphate (mmol/L) | 1.73 ± 0.48 | 1.67 ± 0.51 | .686 |
| Magnesium (mmol/L) | 0.86 ± 0.17 | 0.90 ± 0.15 | .449 |
PAT, patiromer; SPS, sodium polystyrene sulfonate; BMI, body mass index; RAAS, renin-angiotensin-aldosterone system.
Effect on potassium values
The mean weekly K+ value throughout the study was 4.85 ± 0.70 mmol/L. The mean weekly K+ values under PAT, SPS and washout were 5.00 ± 0.54 mmol/L, 4.55 ± 0.75 mmol/L and 5.17 ± 0.64 mmol/L, respectively (Fig. 1). As compared with the washout period, the mean weekly K+ values were lower under PAT (P = .003) as well as under SPS (P < .001). Multivariate adjustment for age, ethnicity, residual diuresis, diabetes and concomitant medications (RAAS inhibitor, loop diuretic and beta-blocker) did not modify those results (P = .003 for PAT and P < .001 for SPS). No other considered covariate was significantly associated with mean weekly K+ values. When directly comparing values under PAT and SPS, mean weekly K+ values throughout the study were lower under SPS (P < .001). Multivariate adjustment for the above-mentioned variables did not modify this result (P < .001). No other considered covariate was significantly associated with mean weekly K+ values.
FIGURE 1:

Mean weekly potassium values according to treatment. PAT, patiromer; SPS, sodium polystyrene sulfonate.
Prevalence of hyperkalaemia as defined as K+ ≥5.1 mmol/L under PAT, SPS and washout was 44.1%, 22.9% and 54.2%, respectively (P < .001). When directly comparing values under PAT and SPS, prevalence of hyperkalaemia ≥5.1 mmol/L was lower under SPS (P < .001). Prevalence of hyperkalaemia as defined as K+ ≥5.5 mmol/L under PAT, SPS and washout was 17.0%, 12.0% and 34.0%, respectively (P < .001). When directly comparing values under PAT and SPS, prevalence of hyperkalaemia ≥5.5 mmol was not significantly different (P = .115).
When considering only last week of each study phase (weeks 4, 6 and 10), the mean weekly K+ values were 4.98 ± 0.49 mmol/L, 4.61 ± 0.72 mmol/L and 5.37 ± 0.65 mmol/L under PAT, SPS and washout, respectively (P < .001). When directly comparing values under PAT and SPS, the mean weekly K+ values were lower under SPS (P < .001). Prevalence of hyperkalaemia as defined as K+ ≥5.1 mmol/L under PAT, SPS and washout was 43.1%, 26.6% and 61.7%, respectively (P = .003). When directly comparing values under PAT and SPS, prevalence of hyperkalaemia was lower under SPS (P = .003). Prevalence of hyperkalaemia as defined as K+ ≥5.5 mmol/L under PAT, SPS and washout was 9.0%, 13.3% and 44.6%, respectively (P < .001). When directly comparing values under PAT and SPS, prevalence of hyperkalaemia was similar (P = .502).
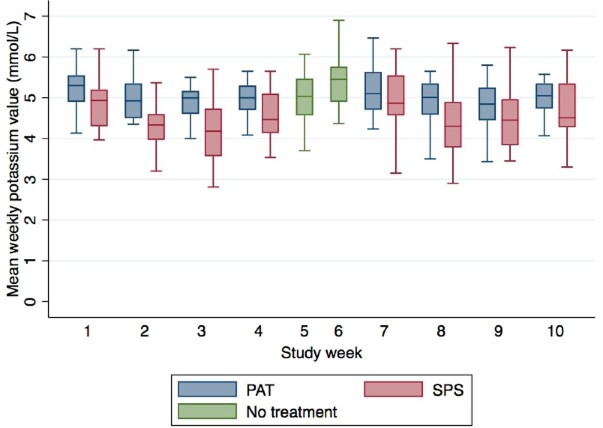
Under treatment, time (study week) had no statistically significant effect on mean weekly K+ values throughout the study (P = .267). Likewise, time (study week) did not significantly modify the effect of PAT or SPS on K values (P = 0.435). Figure 2 represents the effect of time (study week) on K+ values according to treatment.
FIGURE 2:
Mean weekly potassium values according to treatment and study week. PAT, patiromer; SPS, sodium polystyrene sulfonate.
Effect on other electrolytes
During the washout period, the mean phosphate value was 1.64 ± 0.47 mmol/L. As compared with the washout period, phosphate was lower under PAT, with a value of 1.56 ± 0.47 mmol/L (P = .044), but not under SPS, with a value of 1.67 ± 0.43 mmol/L (P = .676). During the washout period, mean calcium value was 2.26 ± 0.18 mmol/L. As compared with the washout period, calcium was lower under SPS with a value of 2.24 ± 0.19 mmol/L (P = .005) but not under PAT with a value of 2.27 ± 0.17 mmol/L (P = .291). During the washout period, mean magnesium value was 0.89 ± 0.13 mmol/L. As compared with the washout period, magnesium was lower under PAT with a value of 0.86 ± 0.14 mmol/L (P = .020) but not under SPS with a value of 0.87 ± 0.16 mmol/L (P = .167).
Safety, tolerability and compliance
Throughout the study, no patient had to be excluded based on pre-specified safety criteria. Moreover, no patient resigned owing to intolerance to either treatment. The percentage of missed medication doses was lower under PAT (2.4 ± 7.3%) as compared with SPS (10.8 ± 20.4%) (P < .001). Supplementary data, Fig. S2 represents the effect of time (study weeks) on the percentage of missed doses according to treatment. The proportion of patients missing 10% or more doses was lower under PAT (9.8%) as compared with SPS (28.8%) (P < .001). Tolerability score was higher under PAT (6.9 ± 1.9) as compared with SPS (6.0 ± 2.4) (P < .001). GI side effects were reported in 66 (25.4%) of the study weeks. The incidence of GI side effects was similar between treatment groups (P = .858). Pre-HD weight was 72.3 ± 16.2 kg and 71.8 ± 13.4 under SPS and PAT, respectively (P < .001). Post-HD weight was 70.5 ± 15.6 kg and 70.0 ± 12.9 kg under SPS and PAT, respectively (P < .001). Mean ultrafiltration was 1.79 ± 0.96 L per session, without significant difference between treatment groups (P = .881). A summary of PAT and SPS association with selected outcomes is presented in Table 2.
Table 2.
Summary of PAT and SPS associations with selected outcomes
| Washout | PAT | SPS | |
|---|---|---|---|
| K+ value (mmol/L) | 5.17 ± 0.64 | 5.00 ± 0.54a,b | 4.55 ± 0.75a,b |
| Calcium (mmol/L) | 2.26 ± 0.18 | 2.27 ± 0.17 | 2.24 ± 0.19a |
| Phosphate (mmol/L) | 1.64 ± 0.47 | 1.56 ± 0.47a | 1.67 ± 0.43 |
| Magnesium (mmol/L) | 0.89 ± 0.13 | 0.86 ± 0.14a | 0.87 ± 0.16 |
| Missed doses (%) | 2.4 ± 7.3b | 10.8 ± 20.4b | |
| Tolerability (0–10 scale) | 6.9 ± 1.9b | 6.0 ± 2.4b | |
| GI side effect (study weeks), n (%) | 35 (26.3)c | 31 (24.6)c |
P < .05 as compared with washout.
P < .001 between PAT and SPS.
P = NS between PAT and SPS.
PAT, patiromer; SPS, sodium polystyrene sulfonate; K+, potassium; GI, gastro intestinal.
DISCUSSION
In this crossover trial in three dialysis facilities, we confirmed high K+ values in chronic HD patients not taking K+ binders. Both PAT 16.8 g once daily on non-dialysis days and SPS 15 g at each meal on non-dialysis days were effective in decreasing K+ values. In addition, while subjective tolerability and compliance were lower under SPS, efficacy was superior as compared with PAT. Finally, the incidence of GI side effects was frequent but similar under both treatments.
We found a relatively higher prevalence of hyperkalaemia (based either on a 5.1 or a 5.5 mmol/L cut-off) in our sample population as compared with previous reports [1, 13]. This is, however, not surprising as this study was designed to include specific HD patients particularly prone to hyperkalaemia. Both PAT and SPS allowed a decrease in pre-HD K+ values. However, PAT offered a 0.17 mmol/L reduction only at the prescribed dose. As such, the prevalence of hyperkalaemia under PAT treatment was still 44% and 17% with 5.1 mmol/L and 5.5 mmol/L cut-offs, respectively, a result that might not be considered clinically acceptable in this population at high cardiovascular risk. A prior study on HD patients included six anuric hyperkalaemic HD patients who were prescribed PAT 12.6 g daily [10]. Authors reported a maximal K+ reduction of 0.6 ± 0.2 mmol/L as compared with pre-treatment week and the prevalence of hyperkalaemia ≥5.5 mmol/L decreased from 47.6% to 11.9% under treatment. Those higher performances could be explained by several reasons. First, this prior study was conducted in a highly controlled environment in a clinical research unit with controlled meals. Second, those patients presented significantly higher baseline K+ values as compared with our study and this has been consistently shown to translate into a larger absolute reduction in K+ values under treatment in large CKD studies [7]. Third, maximal differences in K+ values are reported by the authors, while we chose to compare time-averaged values over whole study periods in order to decrease intra-individual variability and increase statistical power in our study. Thus, our methodological approach would tend to yield more conservative results. Finally, PAT (as SPS) was prescribed on non-dialysis days only in our protocol according to standard practice in participating centres in an effort to reduce the global pill burden and maximize compliance. This could have potentially resulted in a slightly lower efficacy although it is theoretically unlikely as a significant reduction in K+ levels was observed as soon as 7 h after single dose of PAT 8.4 g [12]. Moreover, experimental studies showed complete faecal elimination of radio-labelled PAT in the first 24 h after a single dose ingestion, suggesting that any additive effect of PAT over time would result from progressively lower initial K+ values rather than from an incremental mechanistic effect in the GI tract [14].
On the other hand, SPS significantly outperformed PAT allowing a 0.62 mmol/L reduction in pre-HD K+ values and decreasing the prevalence of hyperkalaemia to 22 and 12% with 5.1 mmol/L and 5.5 mmol/L cut-offs, respectively. Direct comparison between the two drugs also showed consistent superiority of SPS on K+ control. Although no prior data exist on the efficacy of SPS in HD patients, 33 non-HD CKD patients with mild hyperkalaemia were included in a study comparing SPS 30 g once daily with a placebo for 7 days [6]. The reduction in K+ values was slightly higher compared with our study with a mean difference between arms of 1.0 mmol/L. However, the comments formulated above also apply here. Notably, the K+ value at the final follow-up only was considered in the main analysis, thus potentially magnifying differences between groups. Importantly, the highest efficacy of SPS was achieved despite lower subjective tolerability and observed prevalence as compared with PAT, with ˃10% of missed doses overall in the relatively controlled environment of this clinical trial. Lower SPS compliance was probably due to higher pill burden and lower tolerability as the incidence of GI side effects was similar between treatments affecting around one patient out of four. Although our study was not powered for safety outcomes, no serious adverse event was noted and no patient had to withdraw from protocol owing to side effects. While our study did not include formal cost-effectiveness analysis, the much higher cost of PAT as compared with SPS would certainly impact treatment decisions in routine clinical practice.
Finally, differences in electrolytes profile were noted between treatments. Although statistically lower calcium values under SPS and magnesium values under PAT were noted, the clinical significance of this finding is debatable as absolute differences were minute. Those results are, however, in agreement with previous reports. SPS non-specifically binds K+, as well as calcium in the GI tract and cases of symptomatic hypocalcaemia secondary to treatment with SPS, have been reported [15]. PAT also binds magnesium in addition to K+ in the GI tract as shown by studies reporting increased and decreased magnesium levels in the stools and urine, respectively [10, 16]. Of higher magnitude was the effect on phosphate, which was significantly lower under PAT treatment in our study. Prior reports also described an increase and a decrease in phosphate concentration in the stools and the urine, respectively [10, 16]. Theoretically, the reduction in serum phosphate under PAT might be secondary to GI binding of phosphate by calcium ions released from PAT, thus decreasing phosphate absorption. Treatment with phosphate binders was not constrained in our protocol and whether this phosphate- lowering effect of PAT might be of clinical relevance should be tested in dedicated studies. Finally, while ultrafiltration volume was similar, patients were slightly heavier under SPS as compared with PAT throughout the study. A potential explanation would be that SPS induced a mild weight gain owing to sodium intake that could not be compensated for by dialytic fluid removal.
Limitations must be considered when interpreting our findings. Dietary aspects were not controlled or measured in this study. However, as intra-individual variation of dietary K+ intake is rather low and as each participant serves as its own control in crossover trials, it seems unlikely that it could have significantly impacted our results. As stool and urine samples were not collected, differences in mechanistic aspects between treatments could not be inferred. Colonic necrosis is a rare but potentially fatal side effect attributed to SPS. Given the relatively limited sample size and short follow-up, analysis of such rare events was very unlikely. Patients and investigators were not blinded to the intervention given the fundamental galenic differences between SPS and PAT. In the absence of prior head-to-head comparison, selected fixed doses of respective medications were empirically chosen based on anticipated clinical effects. This was based on K+ reduction achieved in similar prior studies as well as relatively equivalent dosing according to national recommendations on respective drug dosing. Whether different drug dosing or dose titration could have modified our results is yet to be tested. Finally, our study was designed to describe serum K+ values, not clinical events. As such, whether observed differences in K+ reduction would have a significant clinical impact cannot be inferred from our results. An important strength of our study is the crossover design allowing time-varying variables. It allowed us not only to reach satisfactory statistical power but also to significantly decrease the impact of intra-individual fluctuations in K+ values over time. Accounting for potential confounding factors in study design and multivariate statistical analysis also comforts the robustness of our results. Finally, the multi-centre aspect tends to enhance generalizability of our findings.
CONCLUSION
In this multi-centre crossover trial comparing PAT with SPS in controlling pre-HD K+ values in chronic HD patients, we report efficacy of both treatments. Importantly, however, at the tested doses, the PAT effect was marginal only, while SPS showed superior efficacy despite lower subjective tolerability and compliance. Side effects were frequent under both treatments, with no serious adverse event reported. Finally, the electrolytes profile was influenced as PAT allowed a significant reduction in phosphate levels. Given the importance of K+ control on cardiovascular outcomes in HD patients, larger randomized controlled trial studies should be conducted in order to confirm our findings and determine whether they would impact clinical outcomes.
Supplementary Material
Contributor Information
David A Jaques, Division of Nephrology, Geneva University Hospitals, Geneva, Switzerland.
Fabien Stucker, Division of Nephrology, Hôpital de la Providence, Neuchâtel, Switzerland.
Thomas Ernandez, Division of Nephrology, Hôpital de la Tour, Geneva, Switzerland.
Cyrielle Alves, Division of Nephrology, Geneva University Hospitals, Geneva, Switzerland.
Pierre-Yves Martin, Division of Nephrology, Geneva University Hospitals, Geneva, Switzerland.
Sophie De Seigneux, Division of Nephrology, Geneva University Hospitals, Geneva, Switzerland.
Patrick Saudan, Division of Nephrology, Geneva University Hospitals, Geneva, Switzerland.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflict of interest. This article has not been published and is not being considered for publication elsewhere, in whole or in part, in any language.
FUNDING
This study was funded by an academic research and development grant from Geneva University Hospitals (PRD 14-2019-I).
REFERENCES
- 1. Rossignol P, Lamiral Z, Frimat Let al. Hyperkalaemia prevalence, recurrence and management in chronic haemodialysis: a prospective multicentre French regional registry 2-year survey. Nephrol Dial Transplant 2017; 32: 2112–2118 [DOI] [PubMed] [Google Scholar]
- 2. Kovesdy CP, Regidor DL, Mehrotra Ret al. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol 2007; 2: 999–1007 [DOI] [PubMed] [Google Scholar]
- 3. Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis 1990; 15: 458–482 [DOI] [PubMed] [Google Scholar]
- 4. Foley RN, Gilbertson DT, Murray Tet al. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med 2011; 365: 1099–1107 [DOI] [PubMed] [Google Scholar]
- 5. Scherr L, Ogden DA, Mead AWet al. Management of hyperkalemia with a cation-exchange resin. N Engl J Med 1961; 264: 115–119 [DOI] [PubMed] [Google Scholar]
- 6. Lepage L, Dufour AC, Doiron Jet al. Randomized clinical trial of sodium polystyrene sulfonate for the treatment of mild hyperkalemia in CKD. Clin J Am Soc Nephrol 2015; 10: 2136–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bakris GL, Pitt B, Weir MRet al. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease the AMETHYST-DN randomized clinical trial. JAMA 2015; 314: 151–161 [DOI] [PubMed] [Google Scholar]
- 8. Pitt B, Bakris GL, Bushinsky DAet al. Effect of patiromer on reducing serum potassium and preventing recurrent hyperkalaemia in patients with heart failure and chronic kidney disease on RAAS inhibitors. Eur J Heart Fail 2015; 17: 1057–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weir MR, Bakris GL, Bushinsky DAet al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 2015; 372: 211–221 [DOI] [PubMed] [Google Scholar]
- 10. Bushinsky DA, Rossignol P, Spiegel DMet al. Patiromer decreases serum potassium and phosphate levels in patients on hemodialysis. Am J Nephrol 2016; 44: 404–410 [DOI] [PubMed] [Google Scholar]
- 11. Sealed Envelope | Randomisation (randomization) and online databases for clinical trials. https://www.sealedenvelope.com/ (16 March 2022, date last accessed) [Google Scholar]
- 12. Bushinsky DA, Williams GH, Pitt Bet al. Patiromer induces rapid and sustained potassium lowering in patients with chronic kidney hyperkalemia. Kidney Int 2015; 88: 1427–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brunelli SM, Du Mond C, Oestreicher Net al. Serum potassium and short-term clinical outcomes among hemodialysis patients: impact of the long interdialytic interval. Am J Kidney Dis 2017; 70: 21–29 [DOI] [PubMed] [Google Scholar]
- 14. Li L, Harrison SD, Cope MJet al. Mechanism of action and pharmacology of patiromer, a nonabsorbed cross-linked polymer that lowers serum potassium concentration in patients with hyperkalemia. J Cardiovasc Pharmacol Ther 2016; 21: 456–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kakajiwala A, Barton KT, Rampolla Eet al. Acute hypocalcemia and metabolic alkalosis in children on cation-exchange resin therapy. Case Rep Nephrol 2017; 2017: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bushinsky DA, Spiegel DM, Gross Cet al. Effect of patiromer on urinary ion excretion in healthy adults. Clin J Am Soc Nephrol 2016; 11: 1769–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.