ABSTRACT
Background
There are limited renal replacement therapy (RRT) prediction models with good performance in the general population. We developed a model that includes lifestyle factors to improve predictive ability for RRT in the population at large.
Methods
We used data collected between 1996 and 2017 from a medical screening in a cohort comprising 442 714 participants aged 20 years or over. After a median follow-up of 13 years, we identified 2212 individuals with end-stage renal disease (RRT, n: 2091; kidney transplantation, n: 121). We built three models for comparison: model 1: basic model, Kidney Failure Risk Equation with four variables (age, sex, estimated glomerular filtration rate and proteinuria); model 2: basic model + medical history + lifestyle risk factors; and model 3: model 2 + all significant clinical variables. We used the Cox proportional hazards model to construct a points-based model and applied the C statistic.
Results
Adding lifestyle factors to the basic model, the C statistic improved in model 2 from 0.91 to 0.94 (95% confidence interval: 0.94, 0.95). Model 3 showed even better C statistic value i.e., 0.95 (0.95, 0.96). With a cut-off score of 33, model 3 identified 3% of individuals with RRT risk in 10 years. This model detected over half of individuals progressing to RRT, which was higher than the sensitivity of cohort participants with stage 3 or higher chronic kidney disease (0.53 versus 0.48).
Conclusions
Our prediction model including medical history and lifestyle factors improved the predictive ability for end-stage renal disease in the general population in addition to chronic kidney disease population.
Keywords: cohort, end-stage renal disease, lifestyle risk factors, prediction model, renal replacement therapy
Graphical Abstract
Graphical Abstract.
INTRODUCTION
End-stage renal disease (ESRD) is a rising global health burden. Kidney disease, including chronic kidney disease (CKD), acute kidney injury and renal replacement therapy (RRT), is a hidden epidemic affecting more than 850 million people worldwide [1]. In 2010, over 2.5 million people underwent RRT, and this number is projected to increase to 5.4 million by 2030 [2, 3]. Key to reducing ESRD incidence is identifying individuals with CKD and determining those at high risk of the disease. Many ESRD and RRT prediction models have been developed, including the Kidney Failure Risk Equation (KFRE) model with four variables [4], the Kaiser Permanente Northwest (KPNW) score [5], Marks formula [6] and the Landray model [7] for individuals with stages 3 to 5 CKD. The major components of these models are age, sex, estimated glomerular filtration rate (eGFR) and urinary albumin–creatinine ratio.
Previous RRT prediction models have been validated in populations with CKD [4, 8] but not in the general population [9] or individuals without advanced CKD. Only a few RRT prediction models include lifestyle risk factors [9, 10] despite their substantial association with the risk of CKD and RRT [11–13]. Lifestyle factors such as being overweight or obese are potentially associated with RRT [14, 15]. Further, smoking is an important factor in the progression of kidney disease [16, 17]. Conversely, there is a positive association between physical activity and health outcomes in individuals with CKD and recipients of kidney transplants [18]. We included medical history and lifestyle factors in our model and applied it in a large cohort with high RRT incidence for higher accuracy and better predictability in both CKD and non-CKD individuals. We compared the ability of our model to predict progression to RRT with that of the KFRE model.
MATERIALS AND METHODS
Study design and participants
We conducted our analysis in a cohort comprising 543 667 participants aged 20 years or more who underwent a series of standardized medical screenings in Taiwan, which has the world's highest incidence of RRT [19]. Between 1996 and 2017, the 21-year study period yielded 5.67 million person-years of follow-up for RRT. We excluded 6.54% of participants for the following reasons: missing kidney function results, including missing data of eGFR (0.6%) and/or proteinuria (4.6%), history of kidney disease (1.3%), and undergoing dialysis before the screening (0.04%). In addition, some participants in early stages of the screenings were not accessed by questionnaires and thus data were missing for some factors: education level (7% missing), smoking (8.8% missing), physical activity (5% missing) and history of diabetes or hypertension (2% missing). Complete data were available for 42 714 individuals, including serum creatinine, eGFR and urine protein (Supplementary data, Figure S1). Ethical reviews were approved by the China Medical University. Data were de-identified and analyzed at the Data Science Center of the Ministry of Health and Welfare, Taiwan.
Measurement of clinical, lifestyle factors and clinical variables
The participants underwent a physical examination that included blood and urine tests and completed a questionnaire that requested self-reported demographic and medical history data, including self-reported history of diabetes or hypertension and use of medication used to treat these conditions. Resting heart rate was measured by electrocardiography in a lying position, and blood pressure was measured in a sitting position. Urine protein and glucose dipstick results were reported as trace, 1+ and 2+ or higher. We defined CKD stages by proteinuria and eGFR using the Chronic Kidney Disease Epidemiology Collaboration equation [20]. Blood markers, including blood glucose, cholesterol, triglycerides, blood urea nitrogen, serum albumin, white blood cell count and hemoglobin, were analyzed by a Hitachi 7150 autoanalyzer. Details are described elsewhere [21–23].
Follow-up strategy and outcome ascertainment
We calculated RRT incidence by linking the identification numbers of individual cohort members with the nationwide Registry of Patients with Catastrophic Illness through the end of 2017. We defined RRT cases as individuals undergoing long-term dialysis and/or having a kidney transplant [24].
Statistical analysis
We used the multivariable Cox proportional hazards model to establish a parsimonious model that includes lifestyle and clinical variables for predicting the incidence of RRT during follow-up. We selected 21 variables significantly (P < 0.05) associated with increased risk of RRT in multivariable models from a forward-selection stepwise Cox proportional hazards regression analysis of 31 variables taken during the first check-up after entering the cohort (Supplementary data, Table S1). The initial 31 candidate variables were associated with kidney disease and RRT based on health screenings and the literature [9, 25, 26].
We built three models for comparison:
(Model 1) Basic model (KFRE four variables): age, sex, eGFR and proteinuria;
(Model 2) Basic model + medical history: lifestyle risk factors: educational level, smoking status, physical activity, body mass index (BMI), resting heart rate, use of hypertension medication and diabetes medication; and
(Model 3) Full model: model 2 + urine glucose, systolic blood pressure, diastolic blood pressure, blood glucose, triglyceride, blood urea nitrogen (BUN), serum albumin, white blood cell count, hemoglobin and urine specific gravity.
We created scores based on the weighted sum of the identified predictors in each model with the weights based on coefficients from the multivariate Cox regression model from Sullivan et al. [25, 27]. We used the following formula to model individual risk:
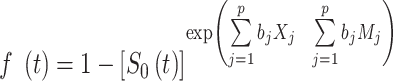 |
where  is the incidence of RRT in t years;
is the incidence of RRT in t years;  is the survival function of RRT in the study population;
is the survival function of RRT in the study population; 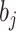 is the regression coefficient of variable
is the regression coefficient of variable 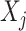 ;
; 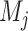 is the average value of variable
is the average value of variable 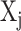 ; and p is the number of predictors [25, 27].
; and p is the number of predictors [25, 27].
We randomly and equally split the cohort dataset into training and validation datasets for internal validation. We evaluated the adequacy of each fitted model by calculating Harrell's concordance index for the total cohort, training set and validation set [28]. We assessed the predictive accuracy of the three models for participants with CKD as defined by KDIGO guidelines [21, 29] with receiver operating characteristic (ROC) curve analysis. We assessed the discriminatory ability of the predictive models using the time-dependent receiver operator characteristic curves in the validation set for a 10-year horizon. Further, we created calibration plots of participants’ RRT risk scores and plotted the predicted and observed incidence in each group. We also compared the slopes of the calibration plots of the three models. We compared the predictive effectiveness of various algorithms for individuals with different CKD stages (all stages, stage 3 and stage 4), levels of eGFR and diabetes or hypertension status using prevalence, sensitivity, specificity, positive predictive value and negative predictive value.
We developed a cross-table showing RRT cases found in our model compared with RRT cases determined by the standard definition of CKD [29]. We selected the cut-point as the point that maximizes the Youden function, which is the difference between the true positive rate and false positive rate over all possible values [30]. The KFRE prediction model is the hospital nephrology referral standard, and the 5-year dialysis risk should exceed 3% [31]. In our study, we used 10-year RRT risk exceeding 3% as the RRT reference standard for the general population.
We also used the Fine–Gray competing risk model as a sensitivity analysis to account for competing total mortality among participants progressing to RRT. This prediction model study follows the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) reporting guideline [32]. All statistical tests were two-sided, and P-values <0.05 were considered statistically significant. We used SAS 9.4 (SAS Institute Inc., Cary, NC) for all statistical analyses.
RESULTS
We recorded 2212 outcome cases, including 2091 RRT and 121 kidney transplantation cases with a median follow-up of 13 years. Table 1 shows the RRT risk predictors that were statistically significant in stepwise Cox proportional hazards regression selection (P-value < 0.05) (Supplementary data, Table S2). Supplementary data, Table S3 shows competing total mortality risk using the Fine–Gray model [33], which exhibits a similar direction for those subdistribution hazard ratios as the hazard ratios for the Cox proportional hazards model.
Table 1.
Characteristics of study participants by renal replacement therapy (RRT) status and identified RRT predictors in the total cohort
| Total | Non-RRT | RRT | |||
|---|---|---|---|---|---|
| N | (%) | (%) | (%) | ||
| Model 1: Basic model Kidney Failure Risk Equations (KFRE) four variables | |||||
| Age (years) | 20–29 | 108 611 | (24.5) | (24.6) | (4.0) |
| 30–39 | 148 710 | (33.6) | (33.7) | (9.8) | |
| 40–49 | 76 673 | (17.3) | (17.3) | (13.2) | |
| 50–59 | 60 497 | (13.7) | (13.6) | (31.0) | |
| 60–69 | 35 676 | (8.1) | (8.0) | (29.5) | |
| 70–79 | 11 109 | (2.5) | (2.5) | (11.1) | |
| ≥80 | 1438 | (0.3) | (0.3) | (1.4) | |
| Sex | Men | 224 315 | (50.7) | (50.7) | (52.6) |
| Women | 218 399 | (49.3) | (49.3) | (47.4) | |
| Proteinuria | Negative | 415 324 | (93.8) | (94.1) | (43.6) |
| Trace | 21 692 | (4.9) | (4.8) | (17.5) | |
| 1+ | 3548 | (0.8) | (0.7) | (15.4) | |
| 2+ | 1350 | (0.3) | (0.2) | (11.3) | |
| ≥3+ | 800 | (0.2) | (0.1) | (12.2) | |
| eGFR (mL/min/1.73 m²) | ≥ 90 | 211 735 | (47.8) | (48.0) | (16.3) |
| 60-89 | 213 736 | (48.3) | (48.3) | (35.9) | |
| 45-59 | 14 357 | (3.2) | (3.2) | (20.3) | |
| 30-44 | 2290 | (0.5) | (0.4) | (14.8) | |
| <30 | 596 | (0.1) | (0.1) | (12.6) | |
| Model 2: Basic model + medical history + lifestyle predictors | |||||
| Education | Middle school or lower | 93 687 | (21.2) | (20.9) | (65.9) |
| High school | 93 825 | (21.2) | (21.2) | (16.6) | |
| Junior college | 90 357 | (20.4) | (20.5) | (8.9) | |
| College or higher | 164 845 | (37.2) | (37.4) | (8.5) | |
| Smoking status | Non-smoker | 313 219 | (70.7) | (70.8) | (64.6) |
| Ex-smoker | 28 307 | (6.4) | (6.4) | (10.1) | |
| Current smoker | 101 188 | (22.9) | (22.8) | (25.4) | |
| Hypertension medication | No | 406 727 | (91.9) | (92.0) | (59.0) |
| Yes | 35 987 | (8.1) | (8.0) | (41.0) | |
| Diabetes medication | No | 430 016 | (97.1) | (97.3) | (61.0) |
| Yes | 12 698 | (2.9) | (2.7) | (39.0) | |
| Physical activity | Inactive | 218 364 | (49.3) | (49.3) | (50.7) |
| Low | 116 018 | (26.2) | (26.2) | (19.3) | |
| Medium | 67 217 | (15.2) | (15.2) | (16.7) | |
| High | 25 724 | (5.8) | (5.8) | (9.4) | |
| Very high | 15 391 | (3.5) | (3.5) | (3.8) | |
| BMI (kg/m2) | <18.5 | 37 244 | (8.4) | (8.4) | (2.6) |
| 18.5–24.9 | 282 907 | (63.9) | (64.0) | (50.7) | |
| 25–29.9 | 103 499 | (23.4) | (23.3) | (37.0) | |
| ≥30 | 19 064 | (4.3) | (4.3) | (9.6) | |
| Resting heart rate (beats/min) | 40–59 | 46 310 | (10.5) | (10.5) | (6.6) |
| 60–69 | 145 021 | (32.8) | (32.8) | (20.3) | |
| 70–79 | 154 261 | (34.8) | (34.9) | (30.2) | |
| 80–89 | 70 845 | (16.0) | (16.0) | (24.3) | |
| 90–99 | 20 058 | (4.5) | (4.5) | (13.6) | |
| ≥100 | 6219 | (1.4) | (1.4) | (5.0) | |
| Model 3: Full model | |||||
| Urine glucose | Negative | 435 192 | (98.3) | (98.5) | (67.8) |
| Trace | 1577 | (0.4) | (0.3) | (5.0) | |
| 1+ | 1377 | (0.3) | (0.3) | (5.5) | |
| ≥2+ | 4568 | (1.0) | (0.9) | (21.8) | |
| Systolic blood pressure (mmHg) | <110 | 142 226 | (32.1) | (32.2) | (9.3) |
| 110–139 | 242 865 | (54.9) | (54.9) | (39.6) | |
| 140–159 | 42 312 | (9.6) | (9.5) | (25.2) | |
| ≥160 | 15 311 | (3.5) | (3.3) | (25.9) | |
| Diastolic blood pressure (mmHg) | <70 | 192 636 | (43.5) | (43.6) | (20.3) |
| 70–89 | 213 969 | (48.3) | (48.3) | (52.4) | |
| 90–109 | 33 425 | (7.6) | (7.5) | (22.9) | |
| ≥110 | 2684 | (0.6) | (0.6) | (4.4) | |
| Blood glucose (mg/dL) | <100 | 294 968 | (66.6) | (66.8) | (32.9) |
| 100–109 | 100 285 | (22.7) | (22.7) | (14.1) | |
| 110–125 | 29 036 | (6.6) | (6.5) | (10.4) | |
| 126–139 | 5734 | (1.3) | (1.3) | (5.3) | |
| ≥140 | 12 691 | (2.9) | (2.7) | (37.2) | |
| Triglyceride (mg/dL) | <65 | 113 038 | (25.5) | (25.6) | (4.2) |
| 65–90 | 107 057 | (24.2) | (24.2) | (11.8) | |
| 91–137 | 112 847 | (25.5) | (25.5) | (24.4) | |
| ≥138 | 109 772 | (24.8) | (24.6) | (59.5) | |
| BUN (mg/dL) | <11 | 113 614 | (25.7) | (25.8) | (5.9) |
| 11–13 | 110 305 | (24.9) | (25.0) | (8.5) | |
| 13–16 | 108 265 | (24.5) | (24.5) | (15.6) | |
| ≥16 | 110 530 | (25.0) | (24.7) | (69.9) | |
| Albumin (g/dL) | <4.4 | 101 179 | (22.9) | (22.7) | (45.3) |
| 4.4–4.51 | 128 525 | (29.0) | (29.1) | (24.5) | |
| 4.52–4.70 | 121 833 | (27.5) | (27.6) | (17.2) | |
| ≥4.71 | 91 177 | (20.6) | (20.6) | (13.0) | |
| White blood cells (103/uL) | <5.2 | 108 891 | (24.6) | (24.7) | (12.1) |
| 5.2–6.09 | 118 464 | (26.8) | (26.8) | (18.5) | |
| 6.1–7.19 | 107 549 | (24.3) | (24.3) | (26.6) | |
| ≥7.2 | 107 810 | (24.4) | (24.3) | (42.7) | |
| Hemoglobin (g/dL) | <13.3 | 113 672 | (25.7) | (25.6) | (38.8) |
| 13.3–14.2 | 105 806 | (23.9) | (23.9) | (19.6) | |
| 14.3–15.4 | 113 645 | (25.7) | (25.7) | (23.0) | |
| ≥15.5 | 109 591 | (24.8) | (24.8) | (18.6) | |
| Urine specific gravity | <1.015 | 66 434 | (15.0) | (15.0) | (14.8) |
| 1.015–1.019 | 135 304 | (30.6) | (30.6) | (32.6) | |
| 1.020–1.021 | 154 154 | (34.8) | (34.8) | (37.1) | |
| ≥1.021 | 86 822 | (19.6) | (19.6) | (15.5) | |
These variables were significantly associated with increased risk of RRT in stepwise multivariable models (P < 0.05) in the total cohort;
BMI, body mass index; BUN, blood urea nitrogen; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; N, number of participants; RRT, renal replacement therapy.
Risk score assignments and predictive performance of RRT
We assigned a risk score as integer points for each risk level (Supplementary data, Table S4). For example, in model 3, the reference group for eGFR was 90 mL/min/1.73 m2 or above, and the score for the 45–59 group was 5; the score for the 30–44 group was 10; and the score for the <30 group was 15. The reference group for proteinuria was negative. Trace proteinuria was scored 5; 1+ proteinuria was scored 10; 2+ proteinuria was scored 12; and 3+ proteinuria was scored 13.
Goodness of fit of the risk prediction models
To assess the goodness of fit of the models in terms of discriminatory accuracy (Table 2), we calculated Harrell's concordance index for all three models in the full, training and validation datasets. Compared with model 1 (basic model: KFRE) with index values of 0.911, 0.904 and 0.918 for the full, training and validation datasets, respectively, a statistically significant increase in the index was noted in model 2 (basic model + health history + lifestyle risk factors) with index values of 0.940, 0.935 and 0.945. The predictive performance improved further in model 3, which included all 21 predictive variables, with index values of 0.951, 0.947 and 0.955. The index values for the validation and training datasets were similar. The time-dependent receiver operator characteristic curves of the three prediction models for 10-year RRT risk were 0.909, 0.925 and 0.926, respectively, in the validation set (Fig. 1).
Table 2.
Harrell's C index of the full, training and validation datasets by model
| Harrell's C | |||
|---|---|---|---|
| Statistic | (95% CI) | ||
| Model 1 (Basic model, KFRE four variables) | Full data | 0.911 | (0.904, 0.918) |
| Training set | 0.912 | (0.902, 0.922) | |
| Validation set | 0.908 | (0.898, 0.918) | |
| Model 2 | Full data | 0.940 | (0.935, 0.945) |
| Training set | 0.939 | (0.930, 0.947) | |
| Validation set | 0.947 | (0.939, 0.955) | |
| Model 3 | Full data | 0.951 | (0.947, 0.955) |
| Training set | 0.952 | (0.946, 0.958) | |
| Validation set | 0.951 | (0.944, 0.959) | |
| Among CKD participants | |||
| Model 1 | Full data | 0.877 | (0.867, 0.887) |
| Training set | 0.877 | (0.863, 0.891) | |
| Validation set | 0.879 | (0.865, 0.892) | |
| Model 2 | Full data | 0.907 | (0.898, 0.915) |
| Training set | 0.910 | (0.899, 0.921) | |
| Validation set | 0.905 | (0.893, 0.917) | |
| Model 3 | Full data | 0.923 | (0.916, 0.930) |
| Training set | 0.924 | (0.914, 0.933) | |
| Validation set | 0.924 | (0.914, 0.934) | |
| Among non-CKD participants | |||
| Model 2 | Full data | 0.835 | (0.815, 0.855) |
| Training set | 0.828 | (0.799, 0.857) | |
| Validation set | 0.846 | (0.821, 0.871) | |
| Model 3 | Full data | 0.873 | (0.859, 0.887) |
| Training set | 0.867 | (0.842, 0.892) | |
| Validation set | 0.882 | (0.858, 0.905) | |
We defined CKD stages by proteinuria and eGFR, which we determined with the Chronic Kidney Disease Epidemiology Collaboration equation.
CI: confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
Model 1: basic model, Kidney Failure Risk Equations model (KFRE) four variables: age, sex, eGFR and proteinuria.
Model 2: basic model + health history + lifestyle risk factors: model 1 + educational level, smoking status, physical activity, BMI, resting heart rate, hypertension medication and diabetes medication.
Model 3: full model: model 2 + systolic blood pressure, diastolic blood pressure, blood glucose, triglyceride, BUN, serum albumin, white blood cell, hemoglobin, urine glucose and urine specific gravity.
FIGURE 1:
Discriminatory accuracy of the models in 10 years in the validation set. Model 1: basic model, Kidney Failure Risk Equations model (KFRE) four variables: age, sex, eGFR and proteinuria. Model 2: basic model + health history + lifestyle risk factors: model 1 + educational level, smoking status, physical activity, BMI, resting heart rate, hypertension medication and diabetes medication. Model 3: full model: model 2 + systolic blood pressure, diastolic blood pressure, blood glucose, triglyceride, BUN, serum albumin, white blood cell, hemoglobin, urine glucose and urine specific gravity. BMI, body mass index; BUN, blood urea nitrogen; eGFR: estimated glomerular filtration rate.
Figure 2 shows the calibration plots for RRT incidence per 1000 person-years for 10-year RRT risk for the three models. The fitted calibration regression formulas are y = 1.4261x − 11.016 in model 1, y = 1.4018x − 12.877 in model 2 and y = 1.213x + 0.3346 in model 3, which suggests that the slope for model 3 is closest to 1. We also calculated Harrell's concordance index for all three models in participants with CKD. The C statistic was slightly lower compared with the total cohort for model 1 [0.877, 95% confidence interval (CI): 0.867, 0.887], model 2 (0.907, 95% CI: 0.898, 0.915) and model 3 (0.923, 95% CI: 0.916, 0.930). For participants without CKD, the C statistic for model 2 and model 3 was 0.835 (95% CI: 0.815, 0.855) and 0.873 (95% CI: 0.859, 0.887), respectively.
FIGURE 2:
Calibration plot of the risk prediction models in the validation set. The calibration plots were created with participants’ RRT risk scores of predicted probability and plotting the predicted and observed incidence in each group.
Selected subgroups for detecting participants progressing to RRT
Table 3 shows the predictive effectiveness for selected subgroups, including different stages of CKD. Statistics for sensitivity, specificity, positive predictive value and negative predictive indicate that adding health history and lifestyle factors to the basic model (KFRE four variables) improved its predictive ability for participants with different stages of CKD. Model 3, with a cut-off point of 22 or above, had sensitivity of 0.85 and specificity of 0.90 and identified 10.8% of cohort participants (47 624). This figure is similar to the actual number of participants with CKD in the cohort (9.3%; 41 085), but sensitivity for predicting RRT was higher (0.85 versus 0.70). Model 3, with a cut-off point of 33 or above, identified 3% RRT risk in 10 years and more than half (sensitivity: 52.7%) of all participants progressing to RRT. This is higher than the sensitivity for stages 3 and 4 CKD. The threshold for model 3 (score ≥33) was similar to trace or higher proteinuria with a sensitivity of 56% in the cohort but a higher positive predictive value (0.18 versus 0.05). We compared our prediction model for various stages of CKD based on the number of RRT cases observed during the study period (Supplementary data, Table S5). For stage 3 and higher CKD, defined in our prediction model with a cut-off point of 22 or above, the model identified 37% of all RRT cases. In participants without CKD and those with stages 1 or 2 CKD, a score of 22 or above identified 14.4%, 2.4% and 5.7%, respectively, of those progressing to RRT. In comparison, in participants with stage 3 and higher CKD but a score lower than 22, 0.5% progressed to RRT.
Table 3.
Predictive effectiveness of selected subgroups for prediction of participants in the total cohort progressing to RRT
| Selected subgroups | No. of participants | Proportion (%) | No. of RRT | RRT incidence per 1000 | Sensitivity | (95% CI) | Specificity | (95% CI) | PPV | (95% CI) | NPV | (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total CKD | 41 085 | 9.3 | 1547 | 2.8 | 0.70 | (0.68, 0.72) | 0.91 | (0.91, 0.91) | 0.04 | (0.04, 0.04) | 0.998 | (0.998, 0.998) |
| CKD stage 3 or higher | 17 243 | 3.9 | 1056 | 4.9 | 0.48 | (0.46, 0.50) | 0.96 | (0.96, 0.96) | 0.06 | (0.06, 0.07) | 0.997 | (0.997, 0.997) |
| CKD stage 4 or higher | 596 | 0.1 | 278 | 67.7 | 0.13 | (0.11, 0.14) | 1.00 | (1.00, 1.00) | 0.47 | (0.43, 0.51) | 0.996 | (0.995, 0.996) |
| eGFR <90 | 230 979 | 52.2 | 1851 | 0.6 | 0.84 | (0.82, 0.85) | 0.48 | (0.48, 0.48) | 0.01 | (0.01, 0.01) | 0.998 | (0.998, 0.998) |
| eGFR <60 | 17 243 | 3.9 | 1056 | 4.9 | 0.48 | (0.46, 0.50) | 0.96 | (0.96, 0.96) | 0.06 | (0.06, 0.07) | 0.997 | (0.997, 0.997) |
| eGFR <45 | 2886 | 0.7 | 606 | 21.6 | 0.27 | (0.26, 0.29) | 1.00 | (1.00, 1.00) | 0.21 | (0.20, 0.23) | 0.996 | (0.996, 0.997) |
| Trace proteinuria or above | 27 390 | 6.2 | 1247 | 3.3 | 0.56 | (0.54, 0.59) | 0.94 | (0.94, 0.94) | 0.05 | (0.04, 0.05) | 0.998 | (0.998, 0.998) |
| 1+ or above | 5698 | 1.3 | 859 | 13.8 | 0.39 | (0.37, 0.41) | 0.99 | (0.99, 0.99) | 0.15 | (0.14, 0.16) | 0.997 | (0.997, 0.997) |
| Screened diabetes | 18 425 | 4.2 | 941 | 4.3 | 0.43 | (0.41, 0.45) | 0.96 | (0.96, 0.96) | 0.05 | (0.05, 0.05) | 0.997 | (0.997, 0.997) |
| Screened hypertension | 57 623 | 13.0 | 1131 | 1.5 | 0.51 | (0.49, 0.53) | 0.87 | (0.87, 0.87) | 0.02 | (0.02, 0.02) | 0.997 | (0.997, 0.997) |
| This model: score 22 or above (optimal cut-off point) | 47 624 | 10.8 | 1879 | 3.0 | 0.85 | (0.83, 0.86) | 0.90 | (0.90, 0.90) | 0.04 | (0.04, 0.04) | 0.999 | (0.999, 0.999) |
| This model: score 33 or above (3% in 10 years) | 6578 | 1.5 | 1165 | 16.7 | 0.53 | (0.51, 0.55) | 0.99 | (0.99, 0.99) | 0.18 | (0.17, 0.19) | 0.998 | (0.997, 0.998) |
The optimal cut-off point for the model was selected as the point maximizing the Youden function, which is the difference between the true positive rate and false positive rate over all possible cut-off point values. CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; NPV, negative predictive value; PPV, positive predictive value; RRT, renal replacement therapy.
Application of risk scores and prediction power of lifestyle predictors
Table 4 shows the results of applying the models to predict RRT in 10 years for hypothetical risk profiles that are representative of individuals in the general population with varied risk profiles. For example, without considering other risk factors, this model predicts a 55-year-old male with abnormal eGFR (50 mL/min/1.73 m²) and trace proteinuria (Example 1) would have a 0.05% (0.04%, 0.07%) risk of RRT in 10 years. The same participant, with a history of hypertension and diabetes and other lifestyle risks (Example 2), would have a 0.48% risk of RRT in 10 years. If a participant's kidney function progressed, with eGFR of 40 mL/min/1.73 m² and 1+ proteinuria, their risk of RRT in 10 years would increase to 3.36% (Example 3). If an individual with progressed kidney function modified their lifestyle risks and was a nonsmoker, had a normal BMI, engaged in a high amount of physical activity and had a low resting heart rate, combined with no history of hypertension or diabetes, their risk of RRT would be substantially attenuated to 0.39% in 10 years (Example 4). This risk is lower than Example 2 (better kidney function but unhealthy lifestyle factors). If a participant's unhealthy lifestyle persisted and their kidney function became progressively worse, with eGFR <30 mL/min/1.73 m² and 2+ proteinuria, their risk of RRT in 10 years would increase to 14.21%.
Table 4.
Application of the prediction models for different risk profiles
| Risk factors | Example 1 | Score | Example 2 | Score | Example 3 | Score | Example 4 | Score | Example 5 | Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 55 | 3 | 55 | 3 | 55 | 3 | 55 | 3 | 55 | 3 |
| Sex | Men | 1 | Men | 1 | Men | 1 | Men | 1 | Man | 1 |
| eGFR | 50 | 5 | 50 | 5 | 40 | 10 | 40 | 10 | <30 | 15 |
| Proteinuria | Trace | 5 | Trace | 5 | 1+ | 10 | 1+ | 10 | 2+ | 12 |
| Education | College or higher | 0 | Middle school or lower | 2 | Middle school or lower | 2 | College or higher | 0 | Middle school or lower | 2 |
| Smoking status | No | 0 | Yes | 1 | Yes | 1 | No | 0 | Yes | 1 |
| Hypertension medication | None | 0 | Yes | 1 | Yes | 1 | None | 0 | Yes | 1 |
| Diabetes medication | None | 0 | Yes | 4 | Yes | 4 | None | 0 | Yes | 4 |
| BMI (kg/m2) | 20 | 0 | 30 | 1 | 30 | 1 | 20 | 0 | 30 | 1 |
| Resting heart rate (beats/min) | 65 | 0 | 90 | 1 | 90 | 1 | 65 | 0 | 90 | 1 |
| Physical activity | Very high | 0 | Inactive | 1 | Inactive | 1 | Very high | 0 | Inactive | 1 |
| Score total | 14 | 25 | 35 | 24 | 42 | |||||
| Predicted probability of RRT in 10 years (95% CI), % | 0.05% | 0.48% | 3.63% | 0.39% | 14.21% | |||||
| (0.04%, 0.07%) | (0.36%, 0.62%) | (2.70%, 4.63%) | (0.29%, 0.51%) | (10.71%, 17.85%) |
CI, confidence interval; BMI, body mass index; eGFR, estimated glomerular filtration rate; RRT, replacement therapy.
DISCUSSION
In this study, we describe RRT prediction models developed based on data routinely collected during medical screening visits. Including lifestyle and medical history predictors in the model significantly improved the predictive performance for RRT in the general population, with C statistics ranging from 0.91 to 0.94. Model 3, which included 10 clinical variables, had an improved C statistic of 0.95. Model 3, with an optimal cut-off point of 22 or above, had sensitivity of 0.85 and specificity of 0.90. Using this cut-off point, the model accounted for 10.8% of the cohort, which is similar to the actual percentage of all participants with CKD (9.3%), but had higher sensitivity (0.85 versus 0.70). With a cut-off point of 33 or above, model 3 identified 3% RRT risk in 10 years and detected more than half of all individuals progressing to RRT, with a higher sensitivity than prediction in cohort participants with stage 3 or higher CKD (0.53 versus 0.48).
When defining high risk of RRT with a score of 33 or above, our model identified more than half of participants who progressed to RRT, which equates to an incidence of 16.7 per 1000 person-years. This prediction accuracy is higher than the risk for stage 3 and higher CKD (4.9 per 1000 person-years), which is equivalent to proteinuria of 1+ or above (incidence of 13.8 per 1000 person-years). A cross-table (Supplementary data, Table S5) shows that our prediction model can identify more individuals with RRT than individuals with stage 3 and higher CKD. We also found that our model identified some participants with high risk of RRT but without CKD. These participants were relatively younger, had a higher prevalence of diabetes (82% versus 43%) and engaged in poor lifestyle habits (i.e., smoking, lack of physical activity and high resting heart rate) compared with those with CKD (Supplementary data, Table S6). We also examined individuals who were not identified by our prediction model but had RRT. We found similar characteristics as in the general population as these individuals did not have CKD but did have slightly higher rates of diabetes and hypertension.
Existing RRT prediction models are mainly based on data from individuals with stages 3 to 5 CKD. The predictive power of these models is high with C-index values of 0.91 or above [4–6, 34]. The models show high accuracy for ethnic groups, including Asians [8, 35, 36], but there are limited RRT prediction models for the general population. Further, most RRT prediction models do not include lifestyle or other modifiable factors [9, 10] and even though most nephrologists consider lifestyle ie., physical activity counseling as within their scope of practice and beneficial, due to competing priorities, do not regularly counsel patients [37]. Hallan et al. using data with only 124 RRT cases found that including additional variables such as smoking status, physical activity, waist circumference, diabetes status, antihypertensive treatment and high-density lipoprotein cholesterol in addition to eGFR and albumin–creatinine ratio in a renal risk model did not substantially improve risk prediction and they can thus be omitted [38]. However, in our large cohort with high RRT incidence, including medical and lifestyle variables improved the prediction accuracy of the models.
While the most commonly used KFRE model includes only age, sex, eGFR and proteinuria [4, 39], our model includes seven additional predictors taken from health history data, including smoking status, BMI, physical activity level and resting heart rate. Smoking status and BMI are important factors for the progression of reduced kidney function [14, 16, 17, 40], and the benefits of physical activity for preventing CKD have been reported [41]. Further, heart rate is a potential surrogate of cardiovascular fitness [42], and studies have found that people with a rapid heart rate have a higher risk of kidney dialysis [43, 44]. Individuals with a resting heart rate above 90 had double the risk of dialysis [43]. Our model also includes traditional indicators, namely systolic and diastolic blood pressure, blood sugar, triglycerides, urine glucose, urine specific gravity, proteinuria, white blood cell count and hemoglobin. Biologically relevant and statistically significant risk factors have been previously reported [9, 39, 45], and some of the clinical factors found in this study are similar to those in previous studies, including renal function indicators, namely BUN and albumin [9].
Other models that include lifestyle and other variables have exhibited good performance for CKD and RRT prediction. For instance, Grams et al. developed a kidney-failure risk model in seven large general-population cohorts with a total of 4 933 314 participants and 3900 predicted long-term dialysis or kidney transplant cases [46]. The Grams model developed with living kidney donors included age, sex, eGFR, albumin–creatinine ratio, systolic blood pressure, use of antihypertensive drugs, diabetes mellitus status, BMI and smoking status. The C-index values for the seven cohorts ranged from 0.675 to 0.889. For comparison, our model 2 for participants without CKD, which includes all of these variables, has a comparable C-index of 0.835. In addition, the Nord-Trondelag Health Study (HUNT 2) model was developed using data from 65 589 participants, with 124 RRT cases and an average follow-up of 10.3 years [38]. The HUNT2 model included age, sex, physical activity, diabetes, systolic blood pressure, antihypertensive treatment, HDL other than eGFR, and albumin–creatinine ratio and had a C-index of 0.858, which is comparable to our model 2 (0.940).
Consider a patient with eGFR of 40 mL/min/1.73 m², 1+ proteinuria, a history of hypertension and diabetes, and lifestyle risks. Their risk of RRT in 10 years would be 3.36% (Example 3). By this classification (score ≥33), half of the participants with this score would progress to RRT at the end of the follow-up period. This cutoff point is more accurate than eGFR <60 mL/min/1.73 m² or 1+ proteinuria for RRT risk, and our model is thus capable of identifying more high-risk individuals. Hypothetically, RRT risk for individuals without a history of hypertension or diabetes and lifestyle risks such as smoking, BMI, physical activity, and resting heart rate would substantially lower the predicted risk of RRT to 0.39%, even with kidney function progressed to 40 mL/min/1.73 m² (see Example 4 in Table 4). While the results from the prediction model should not be interpreted as indicating causal effects by the included variables, they may encourage patients to prioritize changing modifiable risk factors.
Our study has several strengths. First, we used longitudinal data from a cohort with half a million participants with a sufficient number of RRT cases for developing a prediction model. Second, data were available on many predictive variables from a standardized screening cohort, ensuring high accuracy of the model. Third, the availability of data on lifestyle factors such as smoking, physical activity and BMI from a large cohort allowed us to improve performance of the developed model compared with standard KFRE predictors for RRT.
Despite its strengths, the study has some limitations. First, we used dipstick proteinuria instead of albumin–creatinine ratio. Albumin–creatinine ratio may represent kidney function more accurately because of its sensitivity and quantification of levels. Among participants, there was substantial variability of eGFR, from healthy individuals to individuals with various stages of CKD. This variability may make it easier to discriminate between individuals with high RRT risk (e.g., elderly individuals with low eGFR) and those with lower risk. However, it may overestimate the predictive performance of the model compared with models developed in a more homogeneous population comprising individuals with advanced CKD, in which it is difficult to discriminate between low- and high-risk participants. However, using a large and heterogeneous population could be an advantage as a model developed in a population with both younger individuals and those with normal kidney function may help to predict CKD in individuals who would not have been identified by a model using eGFR and age as major predictors. Second, the KFRE model with eight variables may have higher accuracy for RRT prediction. However, some variables such as urine bicarbonate were not collected during screenings, and serum phosphate and serum calcium were available for only some participants. Third, we developed our prediction model in a cohort with the ability to pay for an out-of-pocket medical screening, indicating that these individuals may have higher socioeconomic status than the general population. Fourth, the prediction models were internally cross-validated but not externally validated. Fifth, some self-reported lifestyle risks may be underestimated due to reporting bias. Sixth, some participants may have undergone a preemptive renal graft. In the study population, 5% of ESRD cases underwent kidney transplant (Supplementary data, Table S7). However, the rate of kidney transplant is very low in Taiwan, so the rate of preemptive renal grafts should be even lower in the cohort. Seventh, the ascertainment of RRT was from the nationwide Registry for Catastrophic Illness, and RRT cases who did not apply to the registry for exemption from co-payments may not have been included in follow-up. We believe that the number of individuals who were lost to follow-up or declined treatment is small as the incidence of RRT in our cohort is similar to the national incidence. Finally, because this study is in an observational cohort, the predictions and observations do not imply causal relationships.
In conclusion, we developed an RRT prediction model based on data routinely collected during medical screening visits. Including lifestyle and medical history predictors in the model significantly improved the predictive performance for RRT with C statistics ranging from 0.91 to 0.94. Model 3, which included 10 variables, further improved the C statistic to 0.95. Increasing number of people attending routine medical examinations contribute to availability of Big Data and consequently, better prediction models for larger population sizes involving both CKD and non-CKD individuals.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to the Health and Welfare Data Science Center and National Health Research Institutes for providing administrative and technical support. This study was supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW109-TDU-B-212-114004). The funding source had no role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit the paper for publication.
Contributor Information
Min-Kuang Tsai, Institute of Epidemiology and Preventive Medicine, College of Public Health, National Taiwan University, Taipei, Taiwan.
Wayne Gao, College of Public Health, Taipei Medical University, Taipei, Taiwan.
Kuo-Liong Chien, Institute of Epidemiology and Preventive Medicine, College of Public Health, National Taiwan University, Taipei, Taiwan.
Chih-Cheng Hsu, Institute of Population Health Sciences, National Health Research Institutes, Miaoli, Taiwan.
Chi-Pang Wen, Institute of Population Health Sciences, National Health Research Institutes, Miaoli, Taiwan; China Medical University Hospital, Taichung, Taiwan.
AUTHORS’ CONTRIBUTIONS
K.-L.C. and M.-K.T. were responsible for the study concept and design. M.-K.T. analyzed the data and drafted the first manuscript. M.-K.T., W.G., K.-L.C., C.-C.H. and C.-P.W. critically reviewed the manuscript for important intellectual content. All authors gave final approval of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no relevant financial interests.
DATA AVAILABILITY STATEMENT
All or part of the data used in this research were authorized by and received from MJ Health Research Foundation (Authorization Code: MJHRFB2014001C). Any interpretation or conclusions described in this paper do not represent the views of MJ Health Research Foundation. The MJ Health Survey Database and MJ BioData are available from the MJ Health Research Foundation website (http://www.mjhrf.org).
REFERENCES
- 1. Jager KJ, Kovesdy C, Langham Ret al. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int 2019; 96: 1048–1050 [DOI] [PubMed] [Google Scholar]
- 2. Cockwell P, Fisher L-A. The global burden of chronic kidney disease. Lancet 2020; 395: 662–664 [DOI] [PubMed] [Google Scholar]
- 3. GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020; 395: 709–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tangri N, Stevens LA, Griffith Jet al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA 2011; 305: 1553–1559 [DOI] [PubMed] [Google Scholar]
- 5. Schroeder EB, Yang X, Thorp MLet al. Predicting 5-year risk of RRT in Stage 3 or 4 CKD: development and external validation. Clin J Am Soc Nephrol 2017; 12: 87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marks A, Fluck N, Prescott GJet al. Looking to the future: predicting renal replacement outcomes in a large community cohort with chronic kidney disease. Nephrol Dial Transplant 2015; 30: 1507–1517 [DOI] [PubMed] [Google Scholar]
- 7. Landray MJ, Emberson JR, Blackwell Let al. Prediction of ESRD and death among people with CKD: the Chronic Renal Impairment in Birmingham (CRIB) prospective cohort study. Am J Kidney Dis 2010; 56: 1082–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tangri N, Grams ME, Levey ASet al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA 2016; 315: 164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramspek CL, de Jong Y, Dekker FWet al. Towards the best kidney failure prediction tool: a systematic review and selection aid. Nephrol Dial Transplant 2020; 35: 1527–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nishimoto M, Tagawa M, Matsui Met al. A prediction model with lifestyle in addition to previously known risk factors improves its predictive ability for cardiovascular death. Sci Rep 2019; 9: 12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dunkler D, Kohl M, Heinze Get al. Modifiable lifestyle and social factors affect chronic kidney disease in high-risk individuals with type 2 diabetes mellitus. Kidney Int 2015; 87: 784–791 [DOI] [PubMed] [Google Scholar]
- 12. Wakasugi M, Kazama JJ, Yamamoto Set al. A combination of healthy lifestyle factors is associated with a decreased incidence of chronic kidney disease: a population-based cohort study. Hypertens Res 2013; 36: 328–333 [DOI] [PubMed] [Google Scholar]
- 13. Kazancioglu R. Risk factors for chronic kidney disease: an update. Kidney Int Suppl (2011) 2013; 3: 368–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hsu CY, McCulloch CE, Iribarren Cet al. Body mass index and risk for end-stage renal disease. Ann Intern Med 2006; 144: 21–28 [DOI] [PubMed] [Google Scholar]
- 15. Vivante A, Golan E, Tzur Det al. Body mass index in 1.2 million adolescents and risk for end-stage renal disease. Arch Intern Med 2012; 172: 1644–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hallan SI, Orth SR. Smoking is a risk factor in the progression to kidney failure. Kidney Int 2011; 80: 516–523 [DOI] [PubMed] [Google Scholar]
- 17. Orth SR, Hallan SI. Smoking: a risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients–absence of evidence or evidence of absence? Clin J Am Soc Nephrol 2008; 3: 226–236 [DOI] [PubMed] [Google Scholar]
- 18. Bakker EA, Zoccali C, Dekker FWet al. Assessing physical activity and function in patients with chronic kidney disease: a narrative review. Clin Kidney J 2021; 14: 768–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. United States Renal Data System . 2018 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. https://www.usrds.org/annual-data-report/previous-adrs/ (12 May 2022, date last accessed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsushita K, Mahmoodi BK, Woodward Met al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 2012; 307: 1941–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wen CP, Cheng TYD, Tsai MKet al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet 2008; 371: 2173–2182 [DOI] [PubMed] [Google Scholar]
- 22. Wen CP, Wai JPM, Tsai MKet al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet 2011; 378: 1244–1253 [DOI] [PubMed] [Google Scholar]
- 23. Wu X, Tsai SP, Tsao CKet al. Cohort Profile: The Taiwan MJ Cohort: half a million Chinese with repeated health surveillance data. Int J Epidemiol 2017; 46: 1744–1744g [DOI] [PubMed] [Google Scholar]
- 24. Wu HH, Kuo CF, Li IJet al. Family aggregation and heritability of ESRD in Taiwan: a population-based study. Am J Kidney Dis 2017; 70: 619–626 [DOI] [PubMed] [Google Scholar]
- 25. Chien KL, Lin HJ, Lee BCet al. A prediction model for the risk of incident chronic kidney disease. Am J Med 2010; 123: 836–846.e2 [DOI] [PubMed] [Google Scholar]
- 26. Kelly JT, Su G, Zhang Let al. Modifiable lifestyle factors for primary prevention of CKD: a systematic review and meta-analysis. J Am Soc Nephrol 2021; 32: 239–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sullivan LM, Massaro JM, D'Agostino RB Sr.. Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med 2004; 23: 1631–1660 [DOI] [PubMed] [Google Scholar]
- 28. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996; 15: 361–387 [DOI] [PubMed] [Google Scholar]
- 29. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2012; Suppl. 2013: 1–150 [Google Scholar]
- 30. Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J 2005; 47: 458–472 [DOI] [PubMed] [Google Scholar]
- 31. Hingwala J, Wojciechowski P, Hiebert Bet al. Risk-based triage for nephrology referrals using the kidney failure risk equation. Can J Kidney Health Dis 2017; 4: 2054358117722782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moons KG, Altman DG, Reitsma JBet al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015; 162: W1–73 [DOI] [PubMed] [Google Scholar]
- 33. Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med 2017; 36: 4391–4400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tangri N, Inker LA, Hiebert Bet al. A dynamic predictive model for progression of CKD. Am J Kidney Dis 2017; 69: 514–520 [DOI] [PubMed] [Google Scholar]
- 35. Kang MW, Tangri N, Kim YCet al. An independent validation of the kidney failure risk equation in an Asian population. Sci Rep 2020; 10: 12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lim CC, Chee ML, Cheng CYet al. Simplified end stage renal failure risk prediction model for the low-risk general population with chronic kidney disease. PLoS One 2019; 14: e0212590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taryana AA, Krishnasamy R, Bohm Cet al. Physical activity for people with chronic kidney disease: an international survey of nephrologist practice patterns and research priorities. BMJ Open 2019; 9: e032322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hallan SI, Ritz E, Lydersen Set al. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol 2009; 20: 1069–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tangri N, Kitsios GD, Inker LAet al. Risk prediction models for patients with chronic kidney disease: a systematic review. Ann Intern Med 2013; 158: 596–603 [DOI] [PubMed] [Google Scholar]
- 40. Thorp ML. Body mass index and risk for end-stage renal disease. Ann Intern Med 2006; 144: 700–701; author reply 701–702 [DOI] [PubMed] [Google Scholar]
- 41. Stump CS. Physical activity in the prevention of chronic kidney disease. Cardiorenal Med 2011; 1: 164–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jensen MT, Suadicani P, Hein HOet al. Elevated resting heart rate, physical fitness and all-cause mortality: a 16-year follow-up in the Copenhagen Male Study. Heart 2013; 99: 882–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brotman DJ, Bash LD, Qayyum Ret al. Heart rate variability predicts ESRD and CKD-related hospitalization. J Am Soc Nephrol 2010; 21: 1560–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bohm M, Schumacher H, Schmieder REet al. Resting heart rate is associated with renal disease outcomes in patients with vascular disease: results of the ONTARGET and TRANSCEND studies. J Intern Med 2015; 278: 38–49 [DOI] [PubMed] [Google Scholar]
- 45. Echouffo-Tcheugui JB, Kengne AP. Risk models to predict chronic kidney disease and its progression: a systematic review. PLoS Med 2012; 9: e1001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grams ME, Sang Y, Levey ASet al. Kidney-failure risk projection for the living kidney-donor candidate. N Engl J Med 2016; 374: 411–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All or part of the data used in this research were authorized by and received from MJ Health Research Foundation (Authorization Code: MJHRFB2014001C). Any interpretation or conclusions described in this paper do not represent the views of MJ Health Research Foundation. The MJ Health Survey Database and MJ BioData are available from the MJ Health Research Foundation website (http://www.mjhrf.org).





