ABSTRACT
Cardiorenal syndrome (CRS) is a complex disease in which the heart and kidneys are simultaneously affected, and subsequently, the malfunction of one organ promotes the deterioration of the other. Heart failure (HF) with preserved ejection fraction (HFpEF) is the most common form of HF. The pathophysiology of CRS is not well known and several mechanisms have been proposed. An elevation of central venous pressure seems to be one of the key points to consider, among others such as an increase in intraabdominal pressure. Several diagnostic tools have been identified to establish the diagnosis of CRS in patients with HFpEF. Currently, the availability of biomarkers of renal and cardiac injury, the use of pulmonary ultrasound, the monitoring of the size of the inferior vena cava and the study of the renal venous pattern offer a new dimension in accurately diagnosing and quantifying organ damage in CRS. Beyond the symptomatic treatment of congestion, until recently specific therapeutic tools for patients with CRS and HFpEF were not available. Interestingly, the development of new drugs such as the angiotensin/neprilysin inhibitors and sodium-glucose cotransporter-2 (SGLT-2) inhibitors offer new therapeutic strategies with potential benefits in reduction of cardiorenal adverse outcomes in this population. Randomized clinical trials that focus on patients with HFpEF are currently ongoing to delineate optimal new treatments that may be able to modify their prognosis. In addition, multidisciplinary teamwork (nephrologist, cardiologist and nurse) is expected to decrease the number of visits and the rate of hospitalizations, with a subsequent patient benefit.
Keywords: AKI, cardiorenal syndrome, chronic renal failure, diuretics, heart failure
INTRODUCTION
Cardiac and renal diseases are both common and often coexist in the same patient, interacting and sharing similarities in their pathophysiologic mechanisms. Cardiorenal syndromes (CRS) are broadly defined as disorders of the heart and kidneys whereby acute or chronic dysfunction in one organ may induce acute or chronic dysfunction of the other [1, 2]. Chronic kidney disease (CKD) has a high global prevalence estimated between 9 and 15% [3, 4] and currently, the sum of individuals with CKD, acute kidney injury (AKI) and those on renal replacement therapy (RRT) exceeds 850 million people worldwide [5, 6]. Among patients with CKD defined as glomerular filtration rate (GFR) <60 mL/min/1.73 m2, cardiovascular disease is the leading cause of death [7] and renal dysfunction is one of the most important risk factors predicting mortality and poor outcomes in heart failure (HF) patients, with more than double risk of death if CKD is present [8, 9]. HF is a major clinical and public health problem, the current worldwide prevalence is estimated at 64.34 million cases (8.52 per 1000 inhabitants, 29% of which are mild, 19% moderate and 51% severe HF) [10]. Estimating the prevalence of HF in the CKD population has been challenging [11].
Almost 30% of CKD Medicare patients have HF, compared with just 6% of Medicare patients without CKD [12, 13], and among dialysis patients the incidence of HF is 7% per year [14]. In other series, about 40%–50% of patients with HF have coexisting chronic renal dysfunction [15]. Figure 1 shows the estimated prevalence of CKD. Given the high burden of both HF and CKD, their complex interaction and challenging management, along with the prognostic implications regarding comorbidity and mortality, a comprehensive approach to CRS is mandatory, especially in the HFpEF population where HF diagnosis has been more evasive for years. Here, we propose a comprehensive review of the pathophysiology, diagnostic workup and management of CRS in HFpEF (Fig. 2).
FIGURE 1:
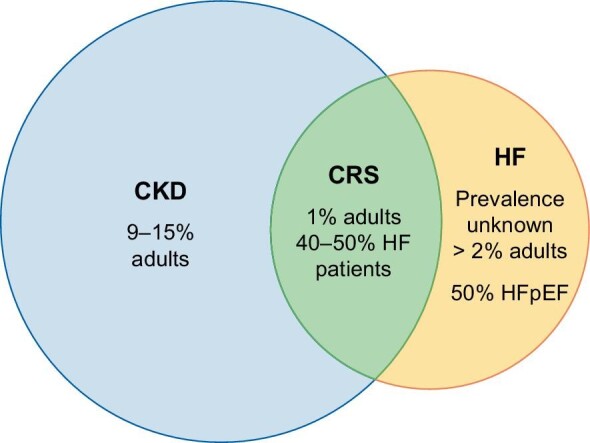
Estimated prevalence of CKD. CKD, chronic kidney disease; HF, heart failure; CRS, cardiorenal syndrome; HFpEF, heart failure with preserved ejection fraction.
FIGURE 2:
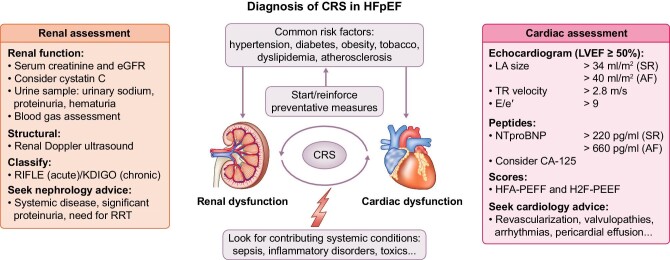
Suggested algorithm for CRS diagnosis. HfpEF, heart failure with preserved ejection fraction; CRS, cardiorenal syndrome; eGFR, estimated glomerular filtration rate; LA, left atria; TR, tricuspid regurgitation; SR, sinus rhythm; AF, atrial fibrillation; NTproBNP, N-terminal prohormone of brain natriuretic peptide; CA-125, cancer antigen 125.
DEFINITION OF CRS
CRS is a complex disease in which the heart and kidneys are affected simultaneously. There is an imbalance between the two, in which the malfunction of one organ promotes, consequently, the deterioration of the other. Depending on this, as well as the onset, acute or chronic, there is a classification that differentiates five types of CRS. The origin of the CRS can be the heart, the kidneys or even both, and its onset can be acute or chronic, based on which a classification has been established [16]. Thus, type 1 refers to the AKI caused by acute cardiac disease; type 2 to CKD caused by chronic heart disease; type 3 to the heart dysfunction caused by the acute worsening of kidney function; type 4 to cardiac disease determined by CKD; and finally, type 5 is characterized by a simultaneous injury of the heart and kidneys caused by systemic diseases [1].
HEART FAILURE WITH PRESERVED LVEF
The diagnosis of HFpEF is challenging. These patients generally do not have a dilated left ventricle (LV), but they often show a thickened LV or increased myocardial mass and/or increased left atrial size as a sign of augmented filling pressures. Most have additional ‘evidence’ of impaired LV filling or suction capacity, also referred to as diastolic dysfunction, which is generally accepted as the likely cause of HF in these patients [17].
Two score-based algorithms (H2FPEF and HFA-PEFF) have been developed for HFpEF diagnosis [18, 19]. The first relies on clinical characteristics and echocardiogram findings and the latest uses biomarkers, functional and morphological parameters from an echocardiogram to estimate HF probability. Both have their limitations and perform differently depending on the scenario. For example, in H2FPEF score, obesity and older age, both of them highly prevalent in CKD, lead to a high estimated probability of HF and in HFA-PEFF, natriuretic peptides (NP) might be elevated in CKD regardless of HF status. Recently, the latest HF Guidelines [17] define the HFpEF as those patients with normal LVEF, typically considered as ≥50%, symptoms and signs of HF and objective evidence of cardiac structural and/or functional abnormalities consistent with the presence of LV diastolic dysfunction/raised LV filling pressures, including raised NP. As a result of increasing awareness and clarification of diagnostic criteria [20], there has been an increase in the rate of diagnosed patients and nowadays HFpEF is the most common form of heart failure, comprising more than 50% of all patients [21], and the prevalence is still increasing over recent years [22]. HFpEF is associated with comorbidities including older age, obesity, CKD, atrial fibrillation and hypertension. These conditions, especially in patients with CKD, may exacerbate sodium and fluid retention leading to congestion [23].
In HFpEF, there is a disproportionate increase in intracavitary diastolic pressure for any rise in volume. This leads to a retrograde increase in left atrial and pulmonary venous pressure, which causes symptoms and signs of pulmonary venous congestion. Postcapillary pulmonary hypertension resulting from increased pulmonary venous pressure may precipitate right heart failure. Left ventricular stroke volume and cardiac output may also decline because of decreased end-diastolic volume (preload dependent) [24].
PATHOPHYSIOLOGY OF CRS IN THE PATIENT WITH HFpEF
Intraabdominal and central venous pressure elevation
HF, and therefore also patients with HFpEF, is characterized by an elevation in central venous pressure (CVP), which attenuates the gradient through the glomerular capillary network, leading to decreased renal perfusion and lower glomerular filtration. An increased CVP also produces a cascade of elevated intraabdominal pressure, pulmonary hypertension and endothelial dysfunction. Rising renal venous pressure limits urine formation and renal blood flow more than a reduction in arterial pressure [25].
The concept that venous congestion, not arterial blood flow, is an important mediator of cardiorenal failure is supported by the findings of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial, in which only baseline right atrial pressure, not arterial blood flow, correlated with baseline serum creatinine [26]. In accordance with this, Mullens et al. [27] also demonstrated that in patients with advanced HF admitted for HF decompensation, worsening renal function is commonly observed despite haemodynamic improvements with intensive medical therapy.
Renin-angiotensin-aldosterone axis and renal dysfunction
Classic studies in patients with decompensated HF showed relative preservation of the GFR despite having reductions in renal blood flow [28]. This could be, in part, explained by the reduction of renal blood flow, an elevation of renin levels, which leads to vasoconstriction of the efferent arteriole, conditioning an increase in the filtration fraction derived from high intraglomerular pressure. Due to the fact that in decompensated HF these elevated renal venous pressures are perpetuated, there is an activation of the renin-angiotensin-aldosterone system (RAAS) and neurohormonal system, which causes vasoconstriction of the afferent arteriole to ensure renal blood flow that ultimately produces a reduction in the GFR. In addition, there is an increased reabsorption of sodium and water in the proximal tubules to maintain effective plasma volumes, eventually resulting in oliguria and worsening of congestion [29]. This is the same mechanism that results in the worsening of kidney function when RAAS inhibitor drugs are started in these patients.
Chronic RAAS activation in HF may contribute to cell hypertrophy, inflammation, apoptosis, fibrosis and oxidative stress in both the heart and kidneys. In addition, it impairs mitochondrial function and increases mitochondrial-derived oxidative stress, which in turn can lead to kidney damage and sodium and water retention, contributing to worsening congestion [30].
Other mechanisms
Comorbidities in HFpEF such as obesity, hypertension and diabetes mellitus, among others, produce a systemic pro-inflammatory state, which causes coronary microvascular endothelial inflammation, and it is known that endothelial dysfunction is the primary pathophysiologic abnormality in HFpEF. Furthermore, coronary microvascular dysfunction has been proposed to be a novel mechanism underlying the pathogenesis of HFpEF [31]. Pulmonary hypertension is widely prevalent in patients with HFpEF [32], and those with right heart failure have a poor prognosis. Additionally, the neurohormonal and sympathetic activation in these patients promotes CKD [33].
Chronotropic incompetence has also emerged as a crucial pathophysiological mechanism in HFpEF and might be related to autonomic dysfunction with decreased baroreflex sensitivity and increased sympathetic stimulation [34]. A recent Spanish trial suggests that deprescribing beta-blockers in patients with HFpEF and chronotropic incompetence seems a plausible intervention to improve functional capacity [35].
DIAGNOSIS OF CRS FROM BIOMARKERS TO CLINICAL IMAGE TOOLS
Diagnosis of HF requires the presence of signs and symptoms, along with evidence of a structural or functional cardiac abnormality [17] and in CRS, this requirement extends to the heart and kidneys. As signs and symptoms of volume overload and increased filling pressures are commonly found in both of them, to date several diagnostic tools can help to establish the diagnosis, including biomarkers, non-invasive imaging modalities or invasive haemodynamic monitoring, which are summarized in the following sections (Fig. 2).
Biomarkers in CRS
The use of biomarkers has expanded during the last decades in HF and CKD, being used as diagnostic, prognostic and therapy response tools. This field has been rapidly expanding and many biomarkers have been proposed such as B-type natriuretic peptide (BNP), Troponin I and T(cTnI, cTnT) and Carbohydrate antigen 125 (CA125) [1, 36].
BNP and its inactive cleavage protein N-terminal pro-B-type natriuretic peptide (NTproBNT) are well-established markers of myocardial stretch widely used in HF management, with diuretic, natriuretic, vasodilation and other cardioprotective properties. However, in the presence of CKD, NP remain elevated, not simply because of a diminished clearance but as a counter-regulatory response from the heart to the kidneys [37]. In patients with CKD, adjusted BNP and NTproBNT cut-points are not available for HF diagnosis and for this reason its use in the setting of advanced CKD is controversial, especially in patients on dialysis. In addition, NP are less sensible in HFpEF compared with HFrEF and more strict cut-offs have been proposed, which limits its usefulness even more in the scenario of altered kidney function [19]. Nevertheless, high NP should not be ignored in the setting of renal dysfunction, as clearly elevated levels point toward cardiac disease. Future work is needed to establish and accurately adjust the cut-off of these markers in patients with advanced CKD.
cTnT and cTnI are specific biomarkers of myocardial injury and infarction and correlate with ventricular remodelling after HF and increase as HF progresses and mortality rises. Patients with CKD have elevated cTnT and cTnI levels because of the reduced excretion from the kidney [38], however, in CKD, cardiac troponins can predict cardiovascular and all-cause mortality rates in patients with mild-to-moderate CKD or end-stage renal disease.
CA125, a glycoprotein synthesized by epithelial serosa cells, has gained a lot of attention in recent years. Being widely available for ovarian cancer monitoring, it has emerged as a potential surrogate marker of fluid retention and inflammatory activity in HF. Even more, in Carbohydrate Antigen-125-Guided Therapy in Acute Heart Failure [39] it was successfully used as a diagnostic, prognostic and monitoring biomarker demonstrating a target value of <35 U/mL to reduce adverse events. Interestingly, CA125 appears not significantly influenced by gender, LVEF or renal function, which is particularly appealing in CRS [40].
Inflammatory markers such as C-reactive protein have been shown to predict prognosis both in cardiac and renal dysfunction independently from traditional cardiovascular risk factors [41, 42], and although it is linked to prognosis in HFpEF, this association might be comorbidity driven [43]. The endothelium also plays an important role in CRS, and given the close link between microvascular disease in HF and CKD, endothelial peptides as a vascular endothelial growth factor, platelet-derived growth factor and soluble vascular endothelial growth factor receptors-1 are elevated in HF and CKD [44].
Soluble suppression of tumorigenicity 2 (sST2) is the circulating form of the cellular receptor ST2L, expressed by cardiomyocytes and vascular endothelial cells together with its ligand interleukin-33 (IL-33) after cardiovascular injury. It is a biomarker of cardiomyocyte stress and fibrosis, which provides incremental value to NP for risk stratification of patients across a wide spectrum of cardiovascular diseases [45, 46]. Apparently, sST2 is able to predict cardiovascular events being less affected by GFR and age and with a good performance in HFpEF, correlating with a cardiac remodelling in CKD patients. Added to NP, it has been shown to better stratify the risk of CV events and/or death in CKD patients. The good performance of sST2 independently of GFR seems an attractive feature [47].
Galectin-3 is a protein that in humans is encoded by the LGALS3 gene that has been intensively studied in HF, as might reflect inflammation and cardiac fibrosis, but seems to be too influenced by renal dysfunction, which reduces its performance in CRS [48, 49].
Focussing on kidneys, as GFR estimating equations based on creatinine have various limitations, with a nonlinear correlation, difficulties in detecting slight alterations in GFR and influence of several confounding non-renal factors (age, race, sex, muscle mass and medication), new biomarkers are needed for the early detection of worsening renal function.
Cystatin C, an inhibitor of cysteine protease produced by nuclear cells, is completely filtered and reabsorbed by the kidneys. It is not affected by age, sex, race or muscle volume, and can serve as a better biomarker of AKI than creatinine [50]. Also, Cystatin C is related to not only HF progression but also cardiovascular events and deaths, independently of renal function, in patients with HF [51, 52, 53]. The combination of Cystatin with NP seems promising as a better stratification and prognosis tool [54, 55].
Neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), N-acetyl-beta-d-glucosaminidase (NAG) and IL-18, identify with high specificity ischaemic and nephrotoxic AKI and not prerenal azotaemia [1]. Nevertheless, the heterogeneous findings and their insufficient performance in isolation suggest that these last biomarkers do not represent a class of interchangeable tubular injury markers [56] and only one biomarker might just not be enough.
As every biomarker has its strengths and weakness, it seems that a combined approach might be reasonable, although the optimal combination needs still to be defined. In the future, it is crucial that a good biomarker or a combination of them would be capable of offering information in terms of the diagnosis, therapy and prognosis of CRS. Table 1 provides a summary of the principal mentioned biomarkers.
Table 1.
. Summary of the principal biomarkers’ pros and cons in heart failure with preserved ejection fraction and chronic kidney disease.
| Biomarker | Advantages | Disadvantages | Evidence |
|---|---|---|---|
| BNP and NTproBNP | • Stabilized diagnostic and prognostic value• Might aid guiding therapy• Widely available• Large evidence from clinical trials as a selection criteria for HF therapy | • Might be elevated in CKD regardless of HF status• Less sensible in HFpEF | +++ |
| CA125 | • Diagnostic and prognostic value• Might aid guiding therapy• Not influenced by GFR or LVEF• Widely available | • Cut-off not clearly stablished• HF studies generally based in NP | ++/+++ |
| Troponin I and T | • Predictive of cardiovascular events | • Might be elevated in CKD regardless of HF status• Not recommended for HF management | + |
| sST2 | • Incremental value to NP for risk stratification• Less affected from GFR and age than NP | • Not available in many centres• Remains in investigational field | + |
| Creatinine | • Reflects eGFR• Strong evidence for CKD progression• Prognostic value in HF• Widely available | • Not sensible for slight alterations in GFR• Influenced by several non-renal factors | +++ |
| Cystatin C | • Better biomarker of AKI than creatinine• Predictive of CV events and HF regardless of renal function | • Not available in many centres | ++ |
Evidence scale reflects a personal opinion from authors.
Pulmonary ultrasound
The use of pulmonary ultrasound to assess the presence of congestion in HF based on line B artefacts has expanded notoriously, given its fast learning curve, the possibility to perform it with any echo device and transducer, and its high sensitivity and specificity [57]. The number of B-lines has been found to be a good indicator of the presence of extravascular lung water, allows identification of HF patients with a worse prognosis and reflects dynamic changes in pulmonary water content after diuretic administration. It has been tested as a guidance tool in randomized trials demonstrating a reduction in the number of HF decompensations without triggering more worsening renal function events [58, 59], and CKD [60].
Inferior vena cava echo assessment
The diameter of the inferior vena cava (IVC) and its variation with respiration reflects the elasticity of this capacitance vessel. Intrathoracic pressure decreases during inspiration, thereby increasing venous return and causing the collapse of the IVC [61, 62]. In acutely decompensated HF, volume overload dilates the IVC and respirations produce only minimal changes in IVC diameter. An IVC >21 mm is a predictor of adverse outcomes and worsening renal function and is a valuable tool to assess rapidly and noninvasively the volaemia [63, 64].
Pulmonary artery pressure sensors and other device-driven analysis
Increased filling pressures gradually increase before overt clinical decompensation. In the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients trial [65], HF treatment guided by pulmonary artery pressured by CardioMEMS sensor was associated with a reduction in HF hospitalization compared with clinical management. A target diastolic blood pressure of 20 mmHg helped in the dosing of diuretic and vasodilators, avoiding both congestion and hypovolaemia leading to worsening renal function. Although it is not widely available, this could be a valuable tool to assess volume status in patients with HFpEF and frequent decompensations.
Implantable electronic devices are frequently used in HF. Some of them now include algorithms to estimate thoracic fluid content, which can help to predict HF decompensation early [66], but still remain in investigation. There is also a general agreement suggesting that bioimpedance vector analysis and bioreactance measures may contribute to a better definition of the patient's hydration status [1, 67] and can predict mortality in CKD [68], however its introduction in daily clinical practice is scarce and still remains in investigational fields.
Intrarenal haemodynamic evaluation by Doppler ultrasonography
Renal congestion constitutes an important mechanism of worsening renal function in patients with CRS. Intrarenal resistive index (RI) and intrarenal venous flow (IRVF) are parameters widely used for assessing renal function in different renal diseases [69]. Doppler ultrasonography as a non‐invasive method to evaluate IRVF in acute HF is a useful tool to evaluate renal congestion. Three patterns of IRVF have been described in the Doppler of interlobar veins: continuous, biphasic and discontinuous. Discontinuous IRVF patterns but no RI are associated with an increase in right atrium pressure and adverse clinical outcome defined as death from cardiovascular disease or unplanned hospitalization for HF [70]. In a study that evaluated the variations in IRVF signals after volume loading and after diuretics treatment in stable HF patients and control subjects, the continuous IRVF pattern was the most common pattern found in both groups. However, after fluid administration, most HF patients developed a biphasic pattern in renal Doppler ultrasound. After diuretic treatment, 70% of HF patients returned to the baseline situation (continuous flow pattern), indicating the recovery of the renal congestion [71].
Taken together, IRVF patterns assessed by renal Doppler ultrasound in intrarenal veins may have a role in identifying the renal haemodynamic disturbances in patients with HF. Biphasic and monophasic discontinuous IRVF may be associated with a phenotype of renal congestion in which an aggressive diuretic treatment would be useful to improve renal function. On the contrary, the presence of a continuous venous flow pattern indicates a normal renal venous pressure, indicating that diuretic needs are lower in these patients. Renal Doppler ultrasound may constitute a tool for monitoring the intrarenal haemodynamic changes in response to treatment [72].
MANAGEMENT OF CRS
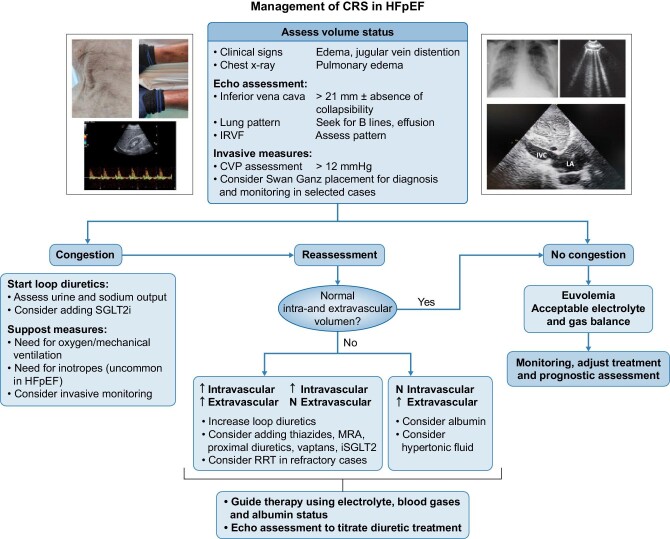
Several randomized clinical trials have been centred on the management of HF and CKD, however there is a gap in the knowledge in the management of the CRS. Two main strategies have been developed for its treatment (mainly focussed on CRS type 1): (i) strategies to relieve congestion: mainly based on diuretics and ultrafiltration; and (ii) strategies to improve cardiac output: neurohormonal modulation, vasodilator, inotropic therapy, RAAS inhibition in chronic CRS (angiotensin-converting inhibitors, angiotensin receptor blockers, mineralocorticoids antagonists, beta-blockers and the new antidiabetic drugs). A therapeutic algorithm for CRS is offered in Fig. 3.
FIGURE 3:
Suggested algorithm for CRS treatment. HFpEF, heart failure with preserved ejection fraction; CRS, cardiorenal syndrome; IRVF, intrarenal venous flow; CVP, central venous pressure; SGLTi, sodium-glucose co-transporter 2 inhibitors; MRA, mineralocorticoid receptor antagonists; RRT, renal replacement therapy; IVC, inferior vena cava; LA, left atria.
Currently, diuretics continue to be the drug of choice for the initial treatment of patients with type 1 CRS. However, they have not been demonstrated to improve hard cardiac endpoints in monotherapy. They are useful for decreasing fluid retention and peripheral oedema as a clinical sign of congestion. Aggressive treatment with diuretics in patients with acute decompensated HF has been demonstrated to improve renal function in type 1 and type II CRS. Patients with HF often develop diuretic resistance during the evolution of their disease, making congestion notoriously challenging to manage and giving them a worse prognosis in terms of survival. In patients with obvious criteria for resistance to diuretics, the only alternative to improve water and salt retention is ultrafiltration. Ultrafiltration can be achieved through two methods, using extracorporeal circulation during haemodialysis (HD), or peritoneal dialysis (PD). Extracorporeal ultrafiltration has been tested in several studies for years, however, it has not demonstrated to increase the survival in CRS patients. In early 2012, Bart et al. [73] demonstrated in a randomized clinical trial in hospitalized patients for acute decompensated HF, worsened renal function and persistent congestion, that the use of a stepped pharmacologic-therapy algorithm was superior to a strategy of ultrafiltration in terms of the preservation of renal function at 96 h, with a similar amount of weight loss with the two approaches. Surprisingly, ultrafiltration was associated with a higher rate of adverse events. In concordance with this study, a substudy of the CARRESS-HF (Cardiorenal Rescue Study in Acute Decompensated Heart Failure) that examined differential outcomes of high versus low fluid removal and preserved versus reduced ejection, demonstrated that in patients with HFpEF, ultrafiltration was associated with worsening renal function irrespective of fluid removal rate and higher initial fluid removal was associated with higher rates of adverse clinical outcomes, highlighting variable responses to decongestive therapy. For that reason, patients with HFpEF and resistance to diuretics and other pharmacological drugs have clinical benefits in terms of decreasing hospitalization rates with PD treatment [74]. PD is a home-based treatment that allows a gentle removal of the excess water and sodium with minimal haemodynamic changes, with a great capacity to be able to adapt the requirements according to the clinical situation and the lifestyle of the patients. Icodextrin is a glucose polymer with minimal peritoneal absorption that produces an osmotic gradient that allows significant sodium elimination during PD exchange.
The beneficial effects of the use of PD in patients with HF are related to adequate decongestion, which improves functional class, the rate of hospital admissions and a reduction in hospital days, which has a notable impact on a better quality of life [75]. Despite all these beneficial effects of PD, there are clinical contraindications such as inflammatory bowel disease, ostomy patients and large abdominal surgeries. In addition, there are sociocultural contraindications such as patients with housing problems or patients without family or social support.
To our knowledge, there has been little evidence for effective therapy in HFpEF that improves cardiorenal outcomes such as cardiovascular mortality or HF hospitalizations. In the Prospective Comparison of ARNI with ARB Global Outcomes in HF with Preserved Ejection Fraction randomized clinical trial (efficacy and safety of LCZ696 compared with valsartan, on morbidity and mortality in HFpEF patients), sacubitril-valsartan failed to reduce the rate of total hospitalizations for HF and death from cardiovascular causes among patients of HFpEF [76]. Last year, the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction (EMPEROR-Preserved) demonstrated the beneficial effect of empagliflozin in terms of reduction of the combined risk of cardiovascular death or hospitalization for HF in patients with HFpEF. The beneficial effects of empagliflozin appeared consistent in patients with or without diabetes. The total number of hospitalizations for HF was lower in the empagliflozin group than in the placebo group [407 with empagliflozin and 541 with placebo; hazard ratio (HR), 0.73; 95% confidence interval (CI), 0.61–0.88; P < .001] [77]. Taking these results together with the positive effects demonstrated by the sodium-glucose cotransporter 2 (SGLT2) inhibitors at the renal level in terms of decreasing the combined renal outcome, these drugs seem to be promising for treating patients with CRS [78]. Other large trials focussed on patients with HFpEF such as the Study to Evaluate the Efficacy and Safety of Finerenone on Morbidity & Mortality in Participants With Heart Failure and Left Ventricular Ejection Fraction (FINEARTS-HF, NCT04435626) and a study with Tirzepatide (LY3298176) in Participants With Heart Failure With Preserved Ejection Fraction and Obesity (SUMMIT, NCT 04847557) are currently underway. Hence this point will be extensively discussed in the next section, as patients with CRS need multidisciplinary care to minimize the number of health care visits and their management [79].
RATIONALE FOR A CARDIORENAL UNIT
Cardiovascular disease, especially HF, is a common thread between nephrologists and cardiologists. This relationship was for the first time described by Robert Bright in 1836 when he observed cardiac structural changes in patients with advanced kidney disease [29]. Since then, numerous advances have been made in summarizing the cardiorenal link in terms of haemodynamic phenotypes, pathophysiology, therapeutic options and clinical outcomes.
Mechanisms involved in the kidney–heart cross-talk are complex, involving different haemodynamic and non-haemodynamic pathways, which allows targeting different therapeutic strategies. In this regard, a multidisciplinary approach is fundamental. A strong ‘marriage’ of cardiologists and nephrologists enhances patient-tailored decisions with a wide vision based on the characteristics of each case. A close working relationship between nephrologists and cardiologists is the key to successfully managing symptoms and prolonging life where possible; treating with diuretics, angiotensin-converting enzyme inhibitors and mineralocorticoid receptor antagonists, while avoiding electrolyte abnormalities and AKI; and multidisciplinary team decisions for dialysis and device therapy, altogether, maybe the key for the CRS treatment [80]. It has been demonstrated that to achieve appropriate therapy and the best possible outcome, combined cardiology–nephrology clinics are needed [80]. In addition, these new multidisciplinary strategies are welcomed by patients, given that with a single visit they receive excellent, complete and coherent feedback regarding pharmacologic therapy, intravenous iron administration, congestion management, education and device therapies. The main problem is that it requires proper resources, including the presence of a nephrologist, cardiologist, nurse and an appropriate shared location [79]. However, as the multidisciplinary strategy might lead to a decreased number of visits and hospitalization rate, a huge economic benefit for the health system should also be expected. More studies promoted by cardiologist and nephrologist societies are needed to ascertain the evidence of cardiorenal unit benefits in this group of patients with CRS.
CONCLUSIONS
HF is a major clinical and public health problem and HFpEF is the most common form of HF. CKD has also a high global prevalence, and renal dysfunction is one of the most important risk factors predicting mortality and poor outcomes in HF patients. Therefore, understanding the interrelation between the heart and kidneys in the clinical scenario of CRS is essential. We currently have new tools that help us in the diagnosis, such as biomarkers and imaging techniques. Altogether this allows the early identification of these patients, with the aim of offering them the best treatment. The appearance of new drugs, such as SGLT2 inhibitors, represents a start for the prognostic improvement of CRS. The complexity of these patients requires multidisciplinary management and the implementation of cardiorenal units, which are expected to reduce the number of visits as well as the hospitalization rate, with a positive impact not only on the patient but also on the health system.
Contributor Information
Ana Belén Méndez, Cardiology Department, Vall d'Hebron University Hospital, Vall d'Hebron Research Institute (VHIR), Barcelona, Spain.
Maria Antonieta Azancot, Nephrology Department, Vall d'Hebron University Hospital, Universitat Autònoma de Barcelona, Nephrology and Kidney Transplant Research Group, Vall d'Hebron Research Institute (VHIR), Barcelona, Spain.
Aleix Olivella, Cardiology Department, Vall d'Hebron University Hospital, Vall d'Hebron Research Institute (VHIR), Barcelona, Spain.
María José Soler, Nephrology Department, Vall d'Hebron University Hospital, Universitat Autònoma de Barcelona, Nephrology and Kidney Transplant Research Group, Vall d'Hebron Research Institute (VHIR), Barcelona, Spain.
CONFLICT OF INTEREST STATEMENT
M.J.S. is the Editor-in-Chief Elect of CKJ.
REFERENCES
- 1. Ronco C, McCullough P, Anker SDet al. . Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J 2010; 31: 703–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Afsar B, Ortiz A, Covic Aet al. . Focus on renal congestion in heart failure. Clin Kidney J 2016; 9: 39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. González AO, de Francisco A, Gayoso Pet al. . Prevalence of chronic renal disease in Spain: Results of the EPIRCE study. Nefrologia 2010; 30: 78–86 [DOI] [PubMed] [Google Scholar]
- 4. Gorostidi M, Sánchez-Martínez M, Ruilope LMet al. . Prevalencia de enfermedad renal crónica en España: impacto de la acumulación de factores de riesgo cardiovascular. Nefrología 2018; 38: 606–615 [DOI] [PubMed] [Google Scholar]
- 5. Hill NR, Fatoba ST, Oke JLet al. . Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS One 2016; 11: e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jager KJ, Kovesdy C, Langham Ret al. . A single number for advocacy and communication—Worldwide more than 850 million individuals have kidney diseases. Kidney Int, 2019; 96: 1048–1050 [DOI] [PubMed] [Google Scholar]
- 7. Thompson S, James M, Wiebe Net al. . Cause of death in patients with reduced kidney function. J Am Soc Nephrol 2015; 26: 2504–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation 2010; 121: 2592–2600 [DOI] [PubMed] [Google Scholar]
- 9. Damman K, Valente MAE, Voors AAet al. . Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J 2014; 35: 455–469 [DOI] [PubMed] [Google Scholar]
- 10. Lippi G, Sanchis-Gomar F. Global epidemiology and future trends of heart failure. AME Med J 2020; 5: 1–6 [Google Scholar]
- 11. Park M, Hsu CY, Li Yet al. . Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol 2012; 23: 1725–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tuegel C, Bansal N. Heart failure in patients with kidney disease. Heart 2017; 103: 1848–1853 [DOI] [PubMed] [Google Scholar]
- 13. Saran R, Robinson B, Abbott KCet al. . US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2017; 69: A7–A8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Foley RN. Clinical epidemiology of cardiac disease in dialysis patients: left ventricular hypertrophy, ischemic heart disease, and cardiac failure. Semin Dial 2003; 16: 111–117 [DOI] [PubMed] [Google Scholar]
- 15. Hillege HL, Nitsch D, Pfeffer MAet al. . Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 2006; 113: 671–678 [DOI] [PubMed] [Google Scholar]
- 16. Lazzeri C, Valente S, Tarquini Ret al. . Cardiorenal syndrome caused by heart failure with preserved ejection fraction. Intern J Nephrol 2011; 24: 421–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McDonagh TA, Metra M, Adamo Met al. . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599–3726 [DOI] [PubMed] [Google Scholar]
- 18. Reddy YNV, Carter RE, Obokata Met al. . A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018; 138: 861–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pieske B, Tschöpe C, De Boer RAet al. . How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019; 40: 3297–3317 [DOI] [PubMed] [Google Scholar]
- 20. Task A, Members F, Mcdonagh TAet al. . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 36: 1–128 [DOI] [PubMed] [Google Scholar]
- 21. Kumar U, Wettersten N, Garimella PS. Cardiorenal syndrome: pathophysiology. Cardiol Clin 2019; 37: 251–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McDonald K. Diastolic heart failure in the elderly: underlying mechanisms and clinical relevance. Int J Cardiol 2008; 125: 197–202 [DOI] [PubMed] [Google Scholar]
- 23. Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2014; 11: 507–515 [DOI] [PubMed] [Google Scholar]
- 24. Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure—abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 2004; 350: 1953–1959 [DOI] [PubMed] [Google Scholar]
- 25. Van Aelst LNL, Arrigo M, Placido Ret al. . Acutely decompensated heart failure with preserved and reduced ejection fraction present with comparable haemodynamic congestion. Eur J Heart Fail 2018; 20: 738–747 [DOI] [PubMed] [Google Scholar]
- 26. Nohria A, Hasselblad V, Stebbins Aet al. . Cardiorenal Interactions. Insights from the ESCAPE trial. J Am Coll Cardiol 2008; 51: 1268–1274 [DOI] [PubMed] [Google Scholar]
- 27. Mullens W, Abrahams Z, Francis Get al. . Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. Eur J Heart Fail Suppl 2008; 53: 589–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Merrill AJ. Edema and decreased renal blood flow in patients with chronic congestive heart failure; evidence of forward failure as the primary cause of edema. J Clin Invest 1946; 25: 389–400 [PubMed] [Google Scholar]
- 29. Rangaswami J, Bhalla V, Blair JEAet al. . Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation 2019; 139: e840–e878 [DOI] [PubMed] [Google Scholar]
- 30. Giam B, Kaye DM, Rajapakse NW. Role of renal oxidative stress in the pathogenesis of the cardiorenal syndrome. Heart Lung Circ 2016; 25: 874–880 [DOI] [PubMed] [Google Scholar]
- 31. Shah SJ, Lam CSP, Svedlund Set al. . Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: pROMIS-HFpEF. Eur Heart J 2018; 39: 3439–3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Agrawal A, Naranjo M, Kanjanahattakij Net al. . Cardiorenal syndrome in heart failure with preserved ejection fraction—an under-recognized clinical entity. Heart Fail Rev 2019; 24: 421–437 [DOI] [PubMed] [Google Scholar]
- 33. Nickel NP, O'Leary JM, Brittain ELet al. . Kidney dysfunction in patients with pulmonary arterial hypertension. Pulmonary Circulation 2017; 7: 38–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Borlaug BA, Melenovsky V, Russell SDet al. . Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation 2006; 114: 2138–2147 [DOI] [PubMed] [Google Scholar]
- 35. Palau P, Seller J, Domínguez Eet al. . Beta-blockers withdrawal in patients with heart failure with preserved ejection fraction and chronotropic incompetence: effect on functional capacity rationale and study design of a prospective, randomized, controlled trial (The Preserve-HR trial). Clin Cardiol 2020; 43: 423–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fu S, Zhao S, Ye P, Luo L. Biomarkers in cardiorenal syndromes. Biomed Res Int 2018; 2018: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maisel A, Mueller C, Adams Ket al. . State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail 2008; 10: 824–839 [DOI] [PubMed] [Google Scholar]
- 38. Yang H, Liu J, Luo Het al. . Improving the diagnostic accuracy of acute myocardial infarction with the use of high-sensitive cardiac troponin T in different chronic kidney disease stages. Sci Rep 2017; 7: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Núñez J, Llàcer P, Bertomeu-González Vet al. . Carbohydrate Antigen-125–Guided Therapy in Acute Heart Failure: CHANCE-HF: A Randomized Study. JACC Heart Fail 2016; 4: 833–843 [DOI] [PubMed] [Google Scholar]
- 40. Llàcer P, Bayés-Genís A, Núñez J. Carbohydrate antigen 125 in heart failure. New era in the monitoring and control of treatment. Medicina Clínica 2019; 152: 266–273 [DOI] [PubMed] [Google Scholar]
- 41. Kang S, Fan L-Y, Chen Met al. . Relationship of high-sensitivity C-Reactive protein concentrations and systolic heart failure. Curr Vasc Pharmacol 2017; 15: 390–396 [DOI] [PubMed] [Google Scholar]
- 42. Li W, Xiong L, Fan Let al. . Association of baseline, longitudinal serum high-sensitive C-reactive protein and its change with mortality in peritoneal dialysis patients. BMC Nephrol 2017; 18: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. DuBrock HM, AbouEzzeddine OF, Redfield MM. High-sensitivity C-reactive protein in heart failure with preserved ejection fraction. PLoS One 2018; 13: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vorovich E, French B, Ky Bet al. . Biomarker predictors of cardiac hospitalization in chronic heart failure: a recurrent event analysis. J Card Fail 2014; 20: 569–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Manzano-Fernndez S, Mueller T, Pascual-Figal Det al. . Usefulness of soluble concentrations of interleukin family member ST2 as predictor of mortality in patients with acutely decompensated heart failure relative to left ventricular ejection fraction. Am J Cardiol 2011; 107: 259–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ky B, French B, McCloskey Ket al. . High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail 2011; 4: 180–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Plawecki M, Morena M, Kuster Net al. . sST2 as a new biomarker of chronic kidney disease-induced cardiac remodeling: impact on risk prediction. Mediators Inflamm 2018; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zamora E, Lupón J, De Antonio Met al. . Renal function largely influences Galectin-3 prognostic value in heart failure. Int J Cardiol 2014; 177: 171–177 [DOI] [PubMed] [Google Scholar]
- 49. Besler C, Lang D, Urban Det al. . Plasma and cardiac galectin-3 in patients with heart failure reflects both inflammation and fibrosis: implications for its use as a biomarker. Circ Heart Fail 2017; 10: 1–9 [DOI] [PubMed] [Google Scholar]
- 50. Roos JF, Doust J, Tett SEet al. . Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children—A meta-analysis. Clin Biochem 2007; 40: 383–391 [DOI] [PubMed] [Google Scholar]
- 51. Lassus JPE, Nieminen MS, Peuhkurinen Ket al. . Markers of renal function and acute kidney injury in acute heart failure: definitions and impact on outcomes of the cardiorenal syndrome. Eur Heart J 2010; 31: 2791–2798 [DOI] [PubMed] [Google Scholar]
- 52. Rafouli-Stergiou P, Parissis J, Farmakis Det al. . Prognostic value of in-hospital change in cystatin C in patients with acutely decompensated heart failure and renal dysfunction. Int J Cardiol 2015; 182: 74–76 [DOI] [PubMed] [Google Scholar]
- 53. Jang SY, Yang DH, Kim HJet al. . Prognostic value of cystatin C-derived estimated glomerular filtration rate in patients with acute heart failure. Cardiorenal Med 2020; 10: 232–242 [DOI] [PubMed] [Google Scholar]
- 54. Alehagen U, Dahlström U, Lindahl TL. Cystatin C and NT-proBNP, a powerful combination of biomarkers for predicting cardiovascular mortality in elderly patients with heart failure: results from a 10-year study in primary care. Eur J Heart Fail 2009; 11: 354–360 [DOI] [PubMed] [Google Scholar]
- 55. Truong QA, Szymonifka J, Januzzi JLet al. . Cardiorenal status using amino-terminal pro–brain natriuretic peptide and cystatin C on cardiac resynchronization therapy outcomes: from the BIOCRT Study. Heart Rhythm 2019; 16: 928–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Testani JM, Tang WHW. Biomarkers of acute kidney injury in chronic heart failure: what do the signals mean? JACC Heart Fail 2013; 1: 425–426 [DOI] [PubMed] [Google Scholar]
- 57. Price S, Platz E, Cullen Let al. . Expert consensus document: echocardiography and lung ultrasonography for the assessment and management of acute heart failure. Nat Rev Cardiol 2017; 14: 427–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Araiza-Garaygordobil D, Gopar-Nieto R, Martinez-Amezcua Pet al. . A randomized controlled trial of lung ultrasound-guided therapy in heart failure (CLUSTER-HF study). Am Heart J 2020; 227: 31–39 [DOI] [PubMed] [Google Scholar]
- 59. Rivas-Lasarte M, Álvarez-García J, Fernández-Martínez Jet al. . Lung ultrasound-guided treatment in ambulatory patients with heart failure: a randomized controlled clinical trial (LUS-HF study). Eur J Heart Fail 2019; 21: 1605–1613 [DOI] [PubMed] [Google Scholar]
- 60. Covic A, Siriopol D, Voroneanu L. Use of lung ultrasound for the assessment of volume status in CKD. Am J Kidney Dis 2018; 71: 412–422 [DOI] [PubMed] [Google Scholar]
- 61. Guiotto G, Masarone M, Paladino Fet al. . Inferior vena cava collapsibility to guide fluid removal in slow continuous ultrafiltration: a pilot study. Intensive Care Med 2010; 36: 692–696 [DOI] [PubMed] [Google Scholar]
- 62. Costanzo MR. The cardiorenal syndrome in heart failure. Heart Fail Clin 2020; 16: 81–97 [DOI] [PubMed] [Google Scholar]
- 63. Lee HF, Hsu LA, Chang CJet al. . Prognostic significance of dilated inferior vena cava in advanced decompensated heart failure. Int J Cardiovasc Imaging 2014; 30: 1289–1295 [DOI] [PubMed] [Google Scholar]
- 64. Blehar DJ, Dickman E, Gaspari R. Identification of congestive heart failure via respiratory variation of inferior vena cava diameter. Am J Emerg Med 2009; 27: 71–75 [DOI] [PubMed] [Google Scholar]
- 65. Abraham WT, Adamson PB, Bourge RCet al. . Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet North Am Ed 2011; 377: 658–666 [DOI] [PubMed] [Google Scholar]
- 66. Boehmer JP, Hariharan R, Devecchi FGet al. . A multisensor algorithm predicts heart failure events in patients with implanted devices: results from the MultiSENSE Study. JACC Heart Fail 2017; 5: 216–225 [DOI] [PubMed] [Google Scholar]
- 67. Hankinson SJ, Williams CH, Ton VKet al. . Should we overcome the resistance to bioelectrical impedance in heart failure? Expert Rev Med Devices 2020; 17: 785–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wizemann V, Wabel P, Chamney Pet al. . The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant 2009; 24: 1574–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Di Nicolò P, Granata A. Renal intraparenchymal resistive index: the ultrasonographic answer to many clinical questions. J Nephrol 2019; 32: 527–538 [DOI] [PubMed] [Google Scholar]
- 70. Iida N, Seo Y, Sai Set al. . Clinical implications of intrarenal hemodynamic evaluation by doppler ultrasonography in heart failure. JACC Heart Fail 2016; 4: 674–682 [DOI] [PubMed] [Google Scholar]
- 71. Nijst P, Martens P, Dupont Met al. . Intrarenal flow alterations during transition from euvolemia to intravascular volume expansion in heart failure patients. JACC Heart Fail 2017; 5: 672–681 [DOI] [PubMed] [Google Scholar]
- 72. de la Espriella-Juan R, Núñez E, Miñana Get al. . Intrarenal venous flow in cardiorenal syndrome: a shining light into the darkness. ESC Heart Failure 2018; 5: 1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bart BA, Goldsmith SR, Lee KLet al. . Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012; 367: 2296–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fudim M, Brooksbank J, Giczewska Aet al. . Ultrafiltration in acute heart failure: implications of ejection fraction and early response to treatment from CARRESS-HF. J Am Heart Assoc 2020; 9: e015752, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chionh CY, Clementi A, Poh CBet al. . The use of peritoneal dialysis in heart failure: a systematic review. Perit Dial Int 2020; 40: 527–539 [DOI] [PubMed] [Google Scholar]
- 76. Solomon SD, McMurray JJV, Anand ISet al. . Angiotensin-Neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019; 381: 1609–1620 [DOI] [PubMed] [Google Scholar]
- 77. Anker SD, Butler J, Filippatos Get al. . Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021; 385: 1451–1461 [DOI] [PubMed] [Google Scholar]
- 78. Fernandez-Fernandez B, Fernandez-Prado R, Górriz JLet al. . Canagliflozin and renal events in diabetes with established nephropathy clinical evaluation and study of diabetic nephropathy with atrasentan: what was learned about the treatment of diabetic kidney disease with canagliflozin and atrasentan? Clin Kidney J 2019; 12: 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. de la Espriella R, González M, Górriz JLet al. . Setting up a cardiorenal clinic. Consensus document of the Cardiorenal Working Groups of the Spanish Society of Cardiology and the Spanish Society of Nephrology. REC: CardioClinics 2021; 56: 284–295 [Google Scholar]
- 80. Banerjee D, Rosano G, Herzog CA. Management of heart failure patient with CKD. Clin J Am Soc Nephrol 2021; 16: 1131–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]