ABSTRACT
Chronic kidney disease (CKD) is defined as abnormalities of kidney structure or function, present for ˃3 months, with implications for health. The most used diagnostic criteria are a urinary albumin: creatinine ratio ≥30 mg/g or an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2. Either of these diagnostic thresholds is associated with adverse health outcomes. GFR decreases with age and the prevalence of CKD is highest in older adults; moreover, the presence of CKD is associated with an increased risk of all-cause and cardiovascular death related to accelerated ageing in all age ranges, and the absolute increase in risk is highest for those aged ˃75 years. Indeed, premature death is a more common outcome than CKD progression to kidney failure requiring kidney replacement therapy. The progressive ageing of the world population contributes to the projection that CKD will become the second most common cause of death before the end of the century in countries with long life expectancy. The current collection of selected studies on kidney disease and ageing published in Age&Ageing, NDT and CKJ provides an overview of key topics, including cognitive decline, sarcopaenia, wasting and cardiovascular and non-cardiovascular morbidity and mortality, the management of kidney failure and gender differences in CKD progression.
Keywords: ageing, chronic kidney disease, cognitive decline, haemodialysis, transplantation, sarcopaenia
INTRODUCTION
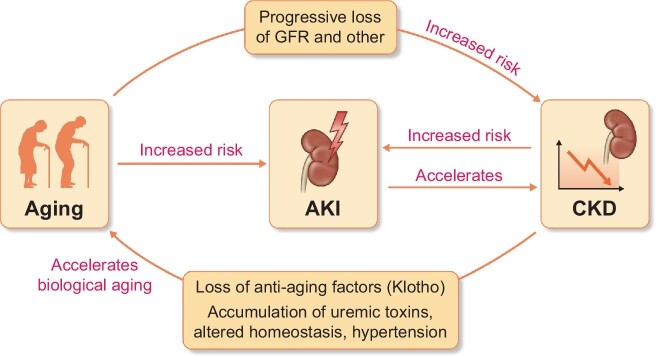
Chronic kidney disease (CKD) is one of the fastest-growing causes of morbidity and mortality, expected to become the fifth most common global cause of death by 2040 and the second most common before the end of the 21st century in countries with long life expectancy [1]. CKD has a bidirectional link with ageing (Fig. 1). The prevalence of CKD increases with age and CKD accelerates biological ageing through a variety of mechanisms that include the early loss of additional kidney functions not assessed in routine clinical care, such as the production of the anti-ageing protein Klotho, when CKD is already present [e.g. urinary albumin:creatinine ratio (UACR) ≥30 mg/g] but global kidney function [i.e. estimated glomerular filtration rate (eGFR)] is still normal, and later, the accumulation of uraemic toxins and altered homeostasis when GFR falls below 60 mL/min/1.73 m2 [2–4]. Indeed, GFR and UACR thresholds to define CKD were chosen because, when persisting for ˃3 months, they are associated with an increased risk of adverse outcomes including acute kidney injury (AKI), progression to kidney failure requiring kidney replacement therapy, and all-cause mortality. Despite the clear implications of a CKD diagnosis for health, and clear-cut diagnostic criteria, CKD remains an unrecognized condition, especially in older adults [5]. Moreover, recognition of CKD is important for adequate drug prescription. Thus, in older adults, community prescriptions for quinine, the most commonly prescribed medication in the UK and USA to treat leg cramps, were associated with an increased risk of AKI [6]. Furthermore, the risk of AKI increased with advancing CKD stages, which conferred the highest risk of AKI other than a prior history of AKI. The current collection of selected manuscripts on kidney disease and ageing published in Age&Ageing, NDT and CKJ provides an overview of key topics including cognitive decline, sarcopaenia, cardiovascular and non-cardiovascular morbidity and mortality, management of kidney failure, and gender differences in CKD progression, as older men with CKD G4 and G5 lost eGFR faster than women, whereas diabetes was a key determinant of loss of eGFR specifically in women [7].
FIGURE 1:
Interaction between ageing and kidney disease. Chronological ageing is associated to a progressive decrease of multiple kidney functions, including glomerular filtration. While the age-associated decrease in eGFR has been considered ‘physiological’ by some, when it reaches the threshold values to diagnose chronic kidney disease (CKD), it is associated with adverse outcomes, including increased mortality, and should no longer be viewed as ‘physiological’, in the same manner that the age-associated development of hypertension is not considered ‘physiological’. Both ageing and CKD are risk factors for acute kidney injury (AKI) and AKI may accelerate CKD progression. Through multiple mechanisms, CKD accelerates biological ageing.
COGNITIVE DECLINE
The cumulative effect of shared cardiovascular risk factors could both cause CKD and cognitive decline, the latter more probably in later life. Within the framework of the Whitehall II prospective population-based study which included 6050 older adults [8], kidney function increased the risk of incident dementia which was not attributable to stroke; moreover, in the same study, a decline of the eGFR was also associated with the incidence of dementia even in participants with eGFR ≥60 mL/min/1.73 m2 at baseline. These findings were not confirmed in a smaller study (n = 1127 older adults); in this case, no association was reported in older adults with mild to moderately low eGFR [9]. Further insight is provided by a prospective study of 250 CKD patients. Previous stroke, depression or anxiety, higher proteinuria and prescription of psychotropic medications were associated with an increased risk of cognitive impairment [10], emphasizing the need to add the albuminuria/proteinuria dimension on top of eGFR for the full evaluation of CKD and associated risks.
SARCOPAENIA, ASSESSMENT OF KIDNEY FUNCTION
Older adults with CKD will present with a higher risk of sarcopaenia and falls, which may be increased when both are present and there is interest in the contribution of physical activity to improve outcomes, including those involving cognition. Among 3223 older participants in National Health and Nutrition Examination Survey 2011–14 [11], CKD G4–G5 was associated with lower objective cognitive function only in those with low physical activity. This cross-sectional association may reflect a lower kidney function than indicated by eGFR in persons with low muscle mass which generates less creatinine. In this regard, eGFR has limitations and may be less reliable in older persons as loss of muscle mass lowers serum creatinine. In baseline data of the IMPROveFALL study, among 578 older participants, those with eGFR ≥90 mL/min had lower handgrip strength than those with lower eGFR [12]. They additionally had a smaller calf circumference. Overall, these data suggest that lower muscle mass may lead to overestimation of GFR (i.e. of kidney function), identifying an unmet need for biomarkers independent of muscle mass to estimate kidney function in older persons. Despite the potential interference of higher muscle mass with eGFR, in 1352 elderly men from the British Regional Heart Study, higher levels of objectively measured physical activity were associated with reduced odds ratios (ORs) for lower eGFR (<45 versus ≥45 mL/min/1.73 m2) after adjustment for covariates [13]. CKD is defined by an eGFR below 60 mL/min/1.73 m2 in adults, regardless of age, and the largest increase in absolute risk of death associated with an eGFR-based diagnosis of CKD (i.e. an eGFR below 60 mL/min/1.73 m2) was found in persons ≥75-year-old [14]. More recently, in the National Health Insurance Service (NHIS) database, the eGFR percentile showing minimum mortality was higher in the aged population: 28th percentile (84 mL/min/1.73 m2) for middle-aged males and 63rd percentile (86 mL/min/1.73 m2) for elderly males; 42nd percentile (103 mL/min/1.73 m2) for middle-aged females and 75th percentile (90 mL/min/1.73 m2) for elderly females [15]. These findings support the current eGFR thresholds to define CKD even in older people and argue against considering that a lower eGFR threshold in older people indicates a ‘physiological’ decrease in GFR.
Among older haemodialysis patients, physical performance was poor and declined faster than in the healthy population. In 714 prevalent haemodialysis patients, only a minority (15%) of patients maintained good physical condition; the remainder deteriorated or died [16]. Among older patients (≥75 years) only 4% remained in good physical condition. In this very high-risk population (75–95 years), a 12-week intradialytic lower limb exercise programme improved the 5-times sit-to-stand (STS-5) test and the Fried frailty scale [17]. Improvement persisted 12 weeks after completing the programme, suggesting that intermittent exercise programmes in resource-limited settings may also be beneficial.
CARDIOVASCULAR DISEASES
An increased risk of cardiovascular and non-cardiovascular death is also an integral part of the CKD definition. Loss of Klotho bioactivity is thought to drive the accelerated cardiovascular ageing phenotype of CKD. Additionally, among 2185 participants with CKD in the NEFRONA study, a combination of three Single nucleotide polymorphisms in the KL gene was associated with an increased risk for non-cardiovascular death, in line with a potential impact of Klotho deficiency on accelerated ageing beyond the cardiovascular system [18].
Regarding cardiovascular disease, there is still debate as to the optimal approach to improve outcomes in patients with CKD, especially those undergoing dialysis, since they are usually excluded from clinical trials. Moreover, CKD patients have an increased risk of multiple forms of cardiovascular disease. In CKD-REIN, both atheromatous (coronary artery disease, peripheral artery disease and stroke) and non-atheromatous (heart failure, cardiac arrhythmia and valvular heart disease) cardiovascular disease were highly prevalent and more frequent in older patients, peaking at 43% at 75–84 years and at 56% at ≥85 years, respectively [19]. Among 3033 adults with CKD Stages 3 and 4, older age (≥65 years) was not associated with the underuse of antithrombotic drugs (antiplatelet agents in coronary artery disease or oral anticoagulants in atrial fibrillation) but was associated with less use of renin-angiotensin system blockers, β-blockers and lipid-lowering therapy, especially in patients ˃75–85 years with coronary artery disease [20]. However, the multiple coexisting conditions of older CKD patients increase the risk of polypharmacy. In 1317 participants in the EQUAL cohort, polypharmacy (≥5 medications) was found in 91% and hyperpolypharmacy (≥10) in 43% [21], respectively. Cardiovascular medications were the most prescribed medications (mean 3.5 per person). However, there were international differences: hyperpolypharmacy was more frequent in Germany (almost 3-fold higher than in the UK), the Netherlands (almost 2-fold higher) and Italy (around 50% higher), and less common in Poland. The impact of practice variation on outcomes should be studied. The UK also has a less proactive approach in providing kidney replacement therapy than other Western European countries [22]. Interestingly, life expectancy at birth is similar in Germany and the UK, but higher in the Netherlands and Italy, and lower in Poland [23]. Unfortunately, key large European countries, such as Germany and Italy, do not contribute data to the ERA Registry.
The optimal target systolic blood pressure (SBP) in CKD is also disputed, with wildly different recommendations from major guidelines [24]. Among 158 713 participants with CKD3 and 6611 with CKD4 without diabetes or proteinuria, SBP 140–169 mmHg had little additional predictive value while <120 mmHg was associated with increased mortality [25], cautioning against recent Kidney Disease: Improving Global Outcomes (KDIGO) guidelines on SBP targets.
MANAGEMENT OF KIDNEY FUNCTION
Three main options are available for patients with kidney failure (i.e. eGFR <15 mL/min/1.73 m2): kidney transplantation from either a living or cadaveric donor, dialysis (haemodialysis or peritoneal dialysis) or conservative treatment [26]. The decision-making process for the modality of kidney replacement therapy (kidney transplantation or dialysis) in older adults merits specific consideration [27]. However, older patients may switch their choice of therapy over time, mainly between dialysis and conservative treatment [28]. A prediction tool of 2-year mortality was developed to provide older (˃70 years) advanced CKD patients with individualized prognosis estimates for both dialysis and conservative care, although it would need external validation in other CKD populations before widespread use to inform treatment decision-making [29]. In this regard, a smooth transition to dialysis may improve early outcomes after dialysis initiation among old and frail adults [30]. Kidney transplantation also presents age-related issues. The short- and medium-term graft and recipient survival were similar for kidneys from older donors after circulatory death and for older brain death donors, which is the conventional cadaveric kidney donor [31].
Contributor Information
Alberto Ortiz, Department of Nephrology and Hypertension, IIS-Fundacion Jimenez Diaz UAM, Madrid, Spain; RICORS2040, Madrid, Spain; Departamento de Medicina, Facultad de Medicina, Universidad Autónoma de Madrid, Madrid, Spain.
Francesco Mattace-Raso, Division of Geriatric Medicine, Erasmus MC University Medical Center, Rotterdam, The Netherlands.
María José Soler, Nephrology Department, Vall d'Hebron University Hospital, Universitat Autònoma de Barcelona, Nephrology and Kidney Transplant Research Group, Vall d'Hebron Research Institute (VHIR), Barcelona, Spain.
Denis Fouque, Department of Nephrology, Nutrition and Dialysis, Université de Lyon - Hospices Civils de Lyon, Lyon, France.
Funding
A.O. research is funded by Instituto de Salud Carlos III (ISCIII) RICORS program to RICORS2040 (RD21/0005/0001), FEDER funds. M.J.S. research is funded by Instituto de Salud Carlos III (ISCIII) RICORS program to RICORS2040 (RD21/0005/0016), FEDER funds and Marató TV3.
CONFLICT OF INTEREST STATEMENT
A.O. has received grants from Sanofi and consultancy or speaker fees or travel support from Advicciene, Astellas, Astrazeneca, Amicus, Amgen, Fresenius Medical Care, GSK, Bayer, Sanofi-Genzyme, Menarini, Mundipharma, Kyowa Kirin, Alexion, Freeline, Idorsia, Chiesi, Otsuka, Novo-Nordisk and Vifor Fresenius Medical Care Renal Pharma, and is Director of the Catedra Mundipharma-UAM of diabetic kidney disease and the Catedra Astrazeneca-UAM of chronic kidney disease and electrolytes. A.O. was the Editor-in-Chief for CKJ (up to 21 May 2022). M.J.S. has received grants from Boehringer and consultancy or speaker fees or travel support AstraZeneca, Novo-Nordsik, Esteve, Vifor, Bayer, Mundipharma, Fresenius Medical Care Renal Pharma, Boehringer, Ingelheim Lilly, Jansen, ICU Medical, Travere Therapeutics and Boehringer. M.J.S. is the new Editor-in-Chief for CKJ (starting from 21 May 2022). D.F. has received honoraria from Fresenius Kabi, Astellas, AstraZeneca, GSK, Vifor and BBraun. F.M.-R. reports no conflict of interest.
This Collection can be viewed at https://academic.oup.com/journals/pages/ageing-meets-kidney-disease.
REFERENCES
- 1. Ortiz A. RICORS2040: the need for collaborative research in chronic kidney disease. Clin Kidney J 2022; 15: 372–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perez-Gomez MV, Bartsch L-A, Castillo-Rodriguez Eet al. . Clarifying the concept of chronic kidney disease for non-nephrologists. Clin Kidney J 2019; 12: 258–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fernandez-Fernandez B, Izquierdo MC, Valino-Rivas Let al. . Albumin downregulates Klotho in tubular cells. Nephrol Dial Transplant 2018; 33: 1712–1722 [DOI] [PubMed] [Google Scholar]
- 4. Anders HJ, Peired AJ, Romagnani P. SGLT2 inhibition requires reconsideration of fundamental paradigms in chronic kidney disease, ‘diabetic nephropathy’, IgA nephropathy and podocytopathies with FSGS lesions. Nephrol Dial Transplant 2020; doi: 10.1093/ndt/gfaa329 [DOI] [PubMed] [Google Scholar]
- 5. Bosi A, Xu Y, Gasparini Aet al. . Use of nephrotoxic medications in adults with chronic kidney disease in Swedish and US routine care. Clin Kidney J 2022; 15: 442–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andrew DS Duncan, Simona Hapca, Nicosha De Souzaet al. . Quinine exposure and the risk of acute kidney injury: a population-based observational study of older people. Age Ageing 2020; 49: 1042–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chesnaye NC, Dekker FW, Evans Met al. . Renal function decline in older men and women with advanced chronic kidney disease—results from the EQUAL study. Nephrol Dial Transplant 2021; 36: 1656–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singh-Manoux A, Oumarou-Ibrahim A, Machado-Fragua MDet al. . Association between kidney function and incidence of dementia: 10-year follow-up of the Whitehall II cohort study. Age Ageing 2022; 51: 10.1093/ageing/afab259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grasing M, Kennedy K, Sarnak MJet al. . Mild to moderate decrease in eGFR and cognitive decline in older adults. Nephrol Dial Transplant 2021; gfab226, 10.1093/ndt/gfab226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tollitt J, Odudu A, Montaldi Det al. . Cognitive impairment in patients with moderate to severe chronic kidney disease: the Salford kidney cohort study. Clin Kidney J 2021; 14: 1639–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chu NM, Hong J, Harasemiw Oet al. . Chronic kidney disease, physical activity and cognitive function in older adults—results from the National Health and Nutrition Examination Survey (2011–2014). Nephrol Dial Transplant 2018; gfab338, 10.1093/ndt/gfab338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lisanne Tap, Nicole DA Boyé, Klaas A Hartholtet al. . Association of estimated glomerular filtration rate with muscle function in older persons who have fallen. Age Ageing 2018; 47: 269–274 [DOI] [PubMed] [Google Scholar]
- 13. Tessa J Parsons, Claudio Sartini, Sarah Ashet al. . Objectively measured physical activity and kidney function in older men; a cross-sectional population-based study. Age Ageing 2017; 46: 1010–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hallan S, Matsushita K, Sang Yet al. . Age and association of kidney measures with mortality and end-stage renal disease. JAMA 2012; 308: 2349–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim Y, Lee S, Lee Yet al. . The minimum-mortality estimated glomerular filtration rate percentile shifts upward in the aged population: a nationwide population-based study. Clin Kidney J 2021; 14: 1356–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ismay van Loon, Marije E. Hamaker, Franciscus TJ. Boereboomet al. . A closer look at the trajectory of physical functioning in chronic hemodialysis. Age Ageing 2017; 46: 594–599 [DOI] [PubMed] [Google Scholar]
- 17. Sanchez-Tocino ML, Gonzalez-Parra E, Serrano BMet al. . Evaluation of the impact of an intradialytic exercise program on sarcopenia in very elderly hemodialysis patients. Clin Kidney J; sfac046, 10.1093/ckj/sfac046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cambray S, Bermudez-Lopez M, Bozic Met al. . Association of a single nucleotide polymorphism combination pattern of the Klotho gene with non-cardiovascular death in patients with chronic kidney disease. Clin Kidney J 2020; 13: 1017–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Villain C, Metzger M, Combe Cet al. . Choosing end-stage kidney disease treatment with elderly patients: are data available? Nephrol Dial Transplant 2020; 35: 827–836 [DOI] [PubMed] [Google Scholar]
- 20. Villain C, Liabeuf S, Metzger Met al. . Impact of age on cardiovascular drug use in patients with chronic kidney disease. Clin Kidney J 2020; 13: 199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hayward S, Hole B, Denholm Ret al. . International prescribing patterns and polypharmacy in older people with advanced chronic kidney disease: results from the European Quality Study. Nephrol Dial Transplant 2021; 36: 503–511 [DOI] [PubMed] [Google Scholar]
- 22. Carriazo S, Ortiz A. The last pre-pandemic European Renal Association Registry Report: age at start of kidney replacement therapy in Europe. Clin Kidney J 2022; 15: 393–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Global age-sex-specific fertility, mortality, HeAlthy Life Expectancy (HALE), and population estimates in 204 countries and territories, 1950-2019: a comprehensive demographic analysis for the Global Burden of Disease Study. Lancet North Am Ed 2020; 396: 1160–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carriazo S, Sarafidis P, Ferro CJet al. . Blood pressure targets in CKD 2021: the never-ending guidelines debacle. Clin Kidney J 2022; 15: 845–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jane A H Masoli, Joao Delgado, Kirsty Bowmanet al. . Association of blood pressure with clinical outcomes in older adults with chronic kidney disease. Age Ageing 2019; 48: 380–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Villain C, Fouque D. Choosing end-stage kidney disease treatment with elderly patients: are data available? Nephrol Dial Transplant 2019; 34: 1432–1435 [DOI] [PubMed] [Google Scholar]
- 27. Schoot TS, Perry M, Hilbrands LBet al. . Kidney transplantation or dialysis in older adults—an interview study on the decision-making process. Age Ageing 2022; afac111, 10.1093/ageing/afac111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Voorend CGN, Verberne WR, van Oevelen Met al. . Changing the choice from dialysis to conservative care or vice versa in older patients with advanced chronic kidney disease. Nephrol Dial Transplant 2021; 36: 1958–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramspek CL, Verberne WR, van Buren Met al. . Predicting mortality risk on dialysis and conservative care: development and internal validation of a prediction tool for older patients with advanced chronic kidney disease. Clin Kidney J 2021; 14: 189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schaeffner E. Smoothing transition to dialysis to improve early outcomes after dialysis initiation among old and frail adults—a narrative review. Nephrol Dial Transplant 2021; gfab342, 10.1093/ndt/gfab342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buxeda A, Velis G, Arias-Cabrales Cet al. . Kidney transplantation outcomes from elderly donors after circulatory death: a comparison with elderly brain-dead donors. Clin Kidney J 2021; 14: 1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]