ABSTRACT
Background
Previous studies suggest that haemodiafiltration reduces mortality compared with haemodialysis in patients with end-stage kidney disease (ESKD), but the controversy surrounding its benefits remains and it is unclear to what extent individual patients benefit from haemodiafiltration. This study is aimed to develop and validate a treatment effect prediction model to determine which patients would benefit most from haemodiafiltration compared with haemodialysis in terms of all-cause mortality.
Methods
Individual participant data from four randomized controlled trials comparing haemodiafiltration with haemodialysis on mortality were used to derive a Royston-Parmar model for the prediction of absolute treatment effect of haemodiafiltration based on pre-specified patient and disease characteristics. Validation of the model was performed using internal-external cross validation.
Results
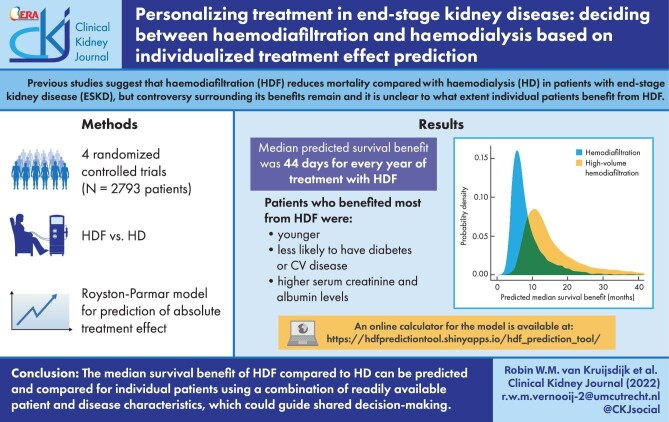
The median predicted survival benefit was 44 (Q1–Q3: 44–46) days for every year of treatment with haemodiafiltration compared with haemodialysis. The median survival benefit with haemodiafiltration ranged from 2 to 48 months. Patients who benefitted most from haemodiafiltration were younger, less likely to have diabetes or a cardiovascular history and had higher serum creatinine and albumin levels. Internal–external cross validation showed adequate discrimination and calibration.
Conclusion
Although overall mortality is reduced by haemodiafiltration compared with haemodialysis in ESKD patients, the absolute survival benefit can vary greatly between individuals. Our results indicate that the effects of haemodiafiltration on survival can be predicted using a combination of readily available patient and disease characteristics, which could guide shared decision-making.
Keywords: haemodiafiltration, haemodialysis, treatment effect heterogeneity, treatment effect prediction
Graphical Abstract
Graphical Abstract.
INTRODUCTION
The prevalence of end-stage kidney disease (ESKD) and the use of renal replacement therapy (RRT) are expected to increase over the next decades [1], driven by an ageing population and an increasing prevalence of diabetes and hypertension [2]. Despite improvements in the survival of patients with ESKD over past decades, morbidity and mortality in patients receiving haemodialysis remain high [3, 4]. Hence, there is an urgent medical need to improve the prognosis for patients with ESKD. Online haemodiafiltration, a technique combining convection and diffusion, has been shown to more effectively remove middle-molecular weight solutes than haemodialysis [5]. Moreover, an individual patient data (IPD) analysis of four randomized controlled trials (RCTs) indicated that haemodiafiltration reduces the mortality risk compared with haemodialysis, especially for patients receiving a higher convection volume [6]. However, controversy surrounding the benefits of haemodiafiltration remains and has impeded broad acceptance of this technique as routine therapy.
Previous studies on haemodiafiltration were designed to estimate group-level average treatment effects, but it is conceivable that not all patients benefit equally from haemodiafiltration. For example, absolute treatment effects may vary between patients due to differences in their prognosis and disease severity before treatment. Individualized treatment effect prediction provides a comprehensive approach to assess the presence of heterogeneity in treatment effect and to estimate an individual patient-specific expected absolute treatment effect, which is critical for personalized medicine [7–10]. In addition, such clinically interpretable estimates could help in decisions on whether to implement haemodiafiltration as routine therapy. In this study, we developed and validated a model to predict the median survival with haemodialysis and haemodiafiltration treatment for individual patients with ESKD to determine which patients will experience the greatest survival benefit from haemodiafiltration.
MATERIALS AND METHODS
Study design and population
Data from the HDF Pooling Project [6], including four RCTs that compared haemodialysis with haemodiafiltration treatment in terms of mortality, were used. The Convective Transport Study (CONTRAST) study, including 714 patients, was undertaken in the Netherlands, Canada and Norway [11]. The On-Line Haemodiafiltration Survival Study (ESHOL), including 906 patients, was conducted in Spain [12]. The French HDF study included 391 patients [13] and the Turkish HDF study 782 patients [14]. The primary outcome in all four trials was all-cause mortality. A detailed description of the study population and outcomes used for the present study is provided in Supplementary data, Appendix 1. Detailed descriptions of the four included RCTs are provided elsewhere [11–14].
Model development
A complete description of the methods used for developing and validating the model is provided in Supplementary data, Appendix 2–8. In short, using data from the HDF Pooling Project, a flexible parametric Royston-Parmar model [15] were developed to predict the absolute treatment effect, defined as the difference between the median survival time with haemodiafiltration and haemodialysis treatment. The model predictors were selected based on available literature (Supplementary data, Appendix 2) and comprised age, sex, body mass index (BMI) after dialysis, diabetes mellitus, history of cardiovascular disease, serum creatinine before dialysis, serum albumin and C-reactive protein (CRP). This model was used to predict the median survival in months with haemodiafiltration and haemodialysis treatment for all individual patients included in the HDF-Pooling project.
Baseline covariate data were missing in ≤4% of patients, except for CRP, which was missing for 20.3%. These missing data were multiplied and imputed 20 times, separately for the four studies (Supplementary data, Appendix 3) [16]. We accounted for competing risk by kidney transplantation (n = 355) and potential informative censoring due to missing outcome data after discontinuation of the randomized treatment (n = 57) by multiple imputations of censored failure times (Supplementary data, Appendix 4) [17]. Since log transformations of age and CRP consistently improved the model fit, these predictors were log-transformed in the final model (Supplementary data, Appendix 5).
To evaluate whether the relative treatment effect varies across individuals (i.e. treatment effect heterogeneity on the relative scale), we fitted a Cox proportional hazards model for each study and quantified the statistical interaction between treatment and the linear predictor of risk [10]. These interaction terms were then pooled using a random effects meta-analysis. If a significant risk-by-treatment interaction was found, the presence of treatment effect heterogeneity was further evaluated by including all possible treatment-covariate interactions in the model and selecting these with least the absolute shrinkage and selection operator (LASSO) regression. A detailed description of the evaluation of relative treatment effect heterogeneity is provided in Supplementary data, Appendix 6.
To enable the prediction of the absolute treatment effect, we fitted a Royston-Parmar model with a proportional odds scale and with two internal knots (Supplementary data, Appendix 7). Previous studies reported that higher convection volumes are associated with a more pronounced survival benefit for haemodiafiltration compared with haemodialysis treatment [6, 18]. To assess the effects of high-volume haemodiafiltration [i.e. body-surface area (BSA)-adjusted convection volume of ≥23 L/1.73m2 per session) we used the same steps as described above in the data excluding patients who were treated with haemodiafiltration with a BSA-adjusted convection volume of <23 L (n = 959) to obtain an effect estimate for high-volume haemodiafiltration. This effect estimate was subsequently used in the main model to predict the median survival with high-volume haemodiafiltration.
Model validation
Discrimination and calibration performance were evaluated using internal–external cross validation [19]. This approach combines model development with external validation and provides a method to detect heterogeneity in study populations, which may warrant model adjustment such as recalibration. For this purpose, a model was developed using three of the datasets and was then validated in the remaining fourth dataset, repeating for each permutation of the four studies. The prognostic performance of the model in terms of discrimination was evaluated using the C-index [20] (Supplementary data, Appendix 8a), and calibration was evaluated using calibration plots [21] (Supplementary data, Appendix 8b). Furthermore, we performed decision curve analyses to evaluate whether using the model to guide treatment decisions would improve clinical outcomes. This method is based on the calculation of net benefit, which is a decision-analytic measure that places benefits and harms on the same scale [22]. We used this technique to compare the following treatment strategies: (i) treat all patients with haemodiafiltration; (ii) treat all patients with haemodialysis; and (iii) prediction-based treatment. As the threshold for treatment is subjective, we calculated the net benefit for threshold ranges in terms of median survival benefit. A detailed description of the net benefit calculations is provided in Supplementary data, Appendix 8c. Performance measures were assessed at 30 months, i.e. the median follow-up time in the pooled data.
The analysis was guided by and reported in accordance with the Predictive Approaches to Treatment Heterogeneity (PATH) and Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD) statements [10, 23]. All statistical analyses were performed in R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
All 2793 patients that participated in the four trials were included in the analyses. The mean age was 64 (SD: 15) years, 63% were male, and the median dialysis vintage was 33 (Q1–Q3: 14–64) months (Table 1). Approximately one-third of the patients (n = 989) had a history of cardiovascular disease and 30% (n = 814) had diabetes. There were no major differences in baseline characteristics between study arms. During the median follow-up of 30 months, 769 (28%) died and 355 (13%) patients received a renal transplant (Supplementary data, Appendix Table A1). Random effects meta-analysis in the pooled data provided a hazard ratio (HR) for all-cause mortality of 0.79 [95% CI (confidence interval): 0.68–0.91] for haemodiafiltration compared with haemodialysis.
Table 1.
Baseline characteristics of study participants
| Total | CONTRAST | ESHOL | French study | Turkish study | |
|---|---|---|---|---|---|
| N (% haemodiafiltration) | 2793 (50) | 714 (50) | 906 (50) | 391 (50) | 782 (50) |
| Age, years | 64 (15) | 64 (14) | 65 (14) | 76 (6) | 57 (14) |
| Male sex | 63 | 62 | 67 | 61 | 59 |
| History of cardiovascular disease | 37 | 44 | 33 | 50 | 26 |
| Diabetes mellitus | 30 | 25 | 25 | 39 | 35 |
| Dialysis vintage, months | 33 (14–64) | 24 (12–48) | 28 (12–59) | 38 (17–71) | 50 (24–83) |
| Body mass index, kg/m2, post-dialysis | 25.2 (4.7) | 25.4 (4.8) | 24.9 (4.5) | 26.3 (4.9) | 24.9 (4.8) |
| Blood flow, mL/min | 337 (66) | 301 (40) | 386 (63) | 336 (42) | 294 (45) |
| Serum creatinine, mg/dL, pre-dialysis | 8.4 (2.5) | 9.7 (2.9) | 8.0 (2.4) | 7.6 (1.8) | 8.1 (2.2) |
| Haemoglobin, g/dL | 11.7 (1.4) | 11.8 (1.3) | 12.0 (1.4) | 11.6 (1.3) | 11.4 (1.5) |
| Serum albumin, g/dL | 4.0 (0.4) | 4.0 (0.4) | 4.1 (0.4) | 3.9 (0.4) | 3.8 (0.4) |
| C-reactive protein, mg/L | 3.5 (0.9–8.6) | 3.9 (1.4–10.4) | 6.3 (4.9–13.0) | 5.0 (1.9–12.6) | 0.9 (0.4–1.9) |
Values are expressed as % for categorical variables, and mean (SD) or median (interquartile range) for continuous variables.
Model derivation
Model coefficients accompanied by hazard ratios with corresponding 95% CIs, are reported in Supplementary data, Appendix Fig. A1. Results indicated heterogeneity in the relative treatment effect in the ESHOL study, where a significant treatment interaction was found for baseline risk (P = .002; Supplementary data, Appendix Fig. A2), and for log-transformed CRP (P = .007). These findings suggest that the relative effect on mortality of haemodiafiltration versus haemodialysis treatment increases as the patient's mortality risk increases (or as their CRP values increase). However, when data from all trials were pooled, evidence of heterogeneity in treatment effect on a relative scale was no longer significant (Pfor baseline risk-by-treatment interaction = .36 and Pfor CRP-by-treatment interaction = .63). Therefore, we assumed that the relative effect of haemodiafiltration versus haemodialysis is constant and did not include any treatment–covariate interactions in the final model.
Table 2 shows the ORs with 95% CI for the predictors in the final model. The full model parameters and computational formula for predicting absolute risk reduction for treatment with haemodiafiltration versus haemodialysis, as well as study-specific values for recalibration of the intercept are provided in Supplementary data, Appendix 10.
Table 2.
Relative effects of model predictors on all-cause mortality
| Predictor | Odds ratio (95% confidence interval) |
|---|---|
| Haemodiafiltration versus haemodialysis | 0.78 (0.65–0.93) |
| Male sex | 1.38 (1.13–1.68) |
| Natural logarithm of age, years | 8.71 (4.87–15.60) |
| Body mass index, kg/m2, post-dialysis | 0.97 (0.95–0.99) |
| Diabetes mellitus | 1.50 (1.23–1.83) |
| History of cardiovascular disease | 1.61 (1.34–1.94) |
| Serum creatinine, mg/dL, pre-dialysis | 0.94 (0.90–0.99) |
| Serum albumin, g/dL | 0.52 (0.40–0.67) |
| Natural logarithm of C-reactive protein, mg/L | 1.23 (1.15–1.31) |
Odds ratios with 95% confidence intervals of the predictors in the final Royston-Parmar proportional odds model for all-cause mortality. The model was fitted in all 20 imputation sets of the pooled data and results were pooled using Rubin's rules. The full model parameters and computational formula for predicting absolute risk reduction for haemodiafiltration versus haemodialysis are provided in Supplementary data, Appendix 10.
Treatment effect prediction
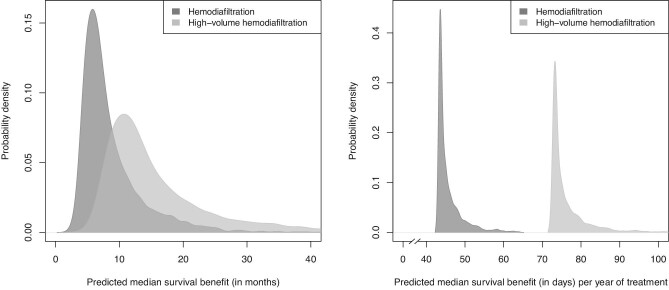
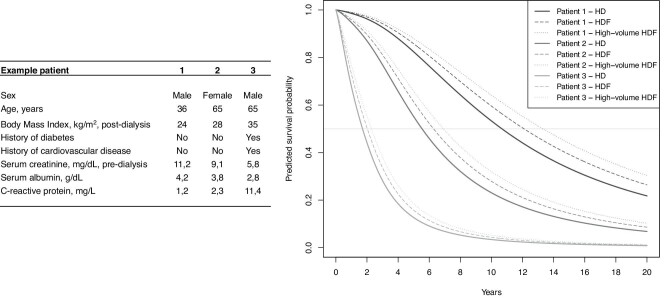
The median predicted survival benefit with haemodiafiltration versus haemodialysis treatment was 7.1 (Q1–Q3: 5.5–10.1) months (Fig. 1, left panel). For every year of treatment with haemodiafiltration the median predicted survival benefit was 44 (Q1–Q3: 44–46) days (Fig. 1, right panel). The characteristics of the patients in different strata of predicted survival benefits are shown in Table 3. Patients with a higher predicted survival benefit with haemodiafiltration treatment versus haemodialysis were younger, less likely to have diabetes or cardiovascular history and had higher serum creatinine and serum albumin levels. Supplementary data, Appendix Fig. A4 demonstrates the effects of the individual continuous predictors and the total linear predictor of risk on median survival benefit predictions with haemodiafiltration treatment versus haemodialysis. Examples of the predicted survival benefit with haemodiafiltration compared with haemodialysis for three patients are shown in Fig. 2.
FIGURE 1:
Probability densities for the predicted survival benefit (in months) with haemodiafiltration and for haemodiafiltration with a body surface area-adjusted convection volume of ≥23 L/1.73 m2 (i.e. high-volume haemodiafiltration) compared to hemodialysis (left panel), and for the predicted survival benefit (in days) per year of treatment with haemodiafiltration and high-volume haemodiafiltration compared with haemodialysis (right panel), in the pooled data.
Table 3.
Strata of predicted median survival benefit of online haemodiafiltration versus haemodialysis
| <6 months | ≥6 and <12 months | ≥12 and <24 months | ≥24 months | |
|---|---|---|---|---|
| N | 536 | 1301 | 630 | 265 |
| Age, years | 77 (7) | 69 (9) | 55 (10) | 39 (12) |
| Male sex | 79 | 64 | 49 | 54 |
| Body mass index, kg/m2, post-dialysis | 24.1 (3.8) | 25.6 (4.5) | 25.9 (5.4) | 24.0 (4.9) |
| Diabetes mellitus | 46 | 34 | 16 | 7 |
| History of cardiovascular disease | 73 | 38 | 12 | 6 |
| Serum creatinine, mg/dL, predialysis | 7.0 (5.6–8.4) | 7.9 (6.4–9.5) | 9.3 (7.6–10.8) | 10.0 (8.3–12.0) |
| Serum albumin, g/dL | 3.7 (0.4) | 4.0 (0.4) | 4.1 (0.4) | 4.2 (0.4) |
| C-reactive protein, mg/L | 10.5 (5.0–20.9) | 4.0 (1.3–8.3) | 1.4 (0.5–4.9) | 0.5 (0.2–1.4) |
Values are expressed as % for categorical variables, and mean (SD) or median (interquartile range) for continuous variables.
FIGURE 2:
Survival curves for three example patients on haemodialysis (HD) and haemodiafiltration (HDF) as predicted with the final model.
Model validation
Four-fold external validation using internal–external cross-validation showed that the C-index at 30 months ranged from 0.63 in the French study to 0.73 in the CONTRAST study. The pooled (using random effects meta-analysis) C-index at 30 months was 0.69 (95% CI: 0.61–0.77; Supplementary data, Appendix Fig. A5). Calibration at 30 months was adequate in the CONTRAST and ESHOL studies, but there was some overestimation of mortality in patients with the highest predicted risk in the Turkish and French studies (Supplementary data, Appendix Fig. A6). Supplementary data, Appendix 8c provides a detailed description of the decision curve analyses performed in the internal–external cross-validation framework. Compared with treating all patients with haemodiafiltration or treating all with haemodialysis, prediction-based treatment allocation resulted in the most favourable outcome across the range of evaluated treatment thresholds (Supplementary data, Appendix Fig. A7).
High-volume haemodiafiltration
After excluding patients who were treated with haemodiafiltration with a BSA-adjusted convection volume of <23 L per session the predicted median survival benefit with high-volume haemodiafiltration treatment versus haemodialysis was 13.1 (Q1–Q3: 10.1–18.7) months (Fig. 1, left panel). For every year of treatment with high-volume haemodiafiltration the median predicted survival benefit was 74 (Q1–Q3: 73–77) days (Fig. 1, right panel). This model showed similar performance in terms of discrimination, calibration, and net benefit compared with the other models (Supplementary data, Appendix Fig. A8–11).
DISCUSSION
In this study, we have developed a model for individualized prediction of the treatment effect with haemodiafiltration versus haemodialysis based on the data of four RCTs. Our results demonstrate that the treatment effect with haemodiafiltration versus haemodialysis can be predicted using a combination of patient and disease characteristics that are readily available in clinical practice. Although overall mortality is reduced by haemodiafiltration compared with haemodialysis in ESKD patients, the survival benefit greatly varied between individuals. For example, the patients who experienced the greatest survival benefit with haemodiafiltration were younger, less likely to have diabetes or cardiovascular history and had higher serum creatinine and serum albumin levels, suggesting better nutritional and physical status [24]. Internal–external cross-validation of the model showed adequate calibration and discrimination and decision curve analyses indicated that using the model to guide treatment decisions could improve clinical outcomes.
In contrast to previous prediction models for mortality in ESKD patients [25], our model predicts the median survival benefit for haemodiafiltration treatment compared with haemodialysis for individual patients. This approach facilitates weighing the expected benefits for individual patients against the potential harms and costs of a treatment. Although the predicted gain in median survival varied substantially between individual patients, we found no evidence that the relative treatment effect is affected by individual patient characteristics. Consequently, all patients benefit from haemodiafiltration treatment in terms of survival compared with haemodialysis. As haemodiafiltration is considered to be safe and cost-effective compared with haemodialysis [26, 27], it seems reasonable to have haemodiafiltration as standard-of-care. Yet, the use of haemodiafiltration varies substantially across the world, possibly because of differences in the acceptance of the idea that haemodiafiltration is superior to haemodialysis and required investments in machinery and training of personnel to start up haemodiafiltration programme [26]. Our model could help to ease these barriers by quantifying the individual patient's prognosis from haemodiafiltration treatment compared with haemodialysis. This also potentially improves the individual patient's understanding of the benefits of haemodiafiltration treatment compared with haemodialysis and facilitates shared decision-making.
The important heterogeneity in the absolute effect of treatment with haemodiafiltration versus haemodialysis that was found can be explained by the differences in predicted survival among ESKD patients because those patients who live the longest have the longest time to benefit from haemodiafiltration. Thus, younger patients benefited most from haemodiafiltration, whereas those with cardiovascular risk factors, such as higher BMI, diabetes and history of cardiovascular disease, which increase the mortality risk, had a lower predicted survival benefit with haemodiafiltration treatment versus haemodialysis.
Among the four studies included in the HDF-Pooling dataset, the convection volume, patient characteristics and practice practices varied considerably [6]. Possibly these differences explain the weaker discrimination found in the French study and the poorer calibration in the French and Turkish studies. To address this, we provided study-specific recalibration values, which can be used to tailor the baseline hazard function to specific patient populations.
The effects of high-volume haemodiafiltration treatment compared with haemodialysis on survival and cardiovascular diseases, but also on patient-reported outcomes measures and cost-effectiveness, are currently being evaluated in two large RCTs [28, 29]. Once these studies have been completed and the HDF-Pooling project database has been updated, including the new studies, our current results will warrant an update. Meanwhile, simultaneous evaluation of absolute treatment effects on all relevant characteristics at the level of an individual patient, such as utilized in this study, could provide a sensible approach to determine the value of haemodiafiltration in clinical practice. In line with previous studies [30, 31], our results suggest that those patients treated with the highest convection volume experienced the most survival benefit compared with haemodialysis. Given that the haemodiafiltration treatment arm in the two ongoing trials concerns high-volume haemodiafiltration, we expect that a definite answer on the effects of high-volume convection volume will be provided after finalizing these two trials [28, 29].
Some limitations of this study warrant caution in interpreting the present results. Although data from RCTs have the advantage of an unbiased treatment effect estimate, the study population included in RCTs are subject to eligibility criteria, which might include, but are not limited to, younger age, more males and fewer comorbidities. Hence, use of the presented model should be restricted to those patients who meet the inclusion criteria of the four included trials. In our analyses, we were only able to consider the patient- or disease-characteristics that are routinely available in clinical practice, i.e. were measured in the included RCTs. New biomarkers might further improve the predictions of response to haemodiafiltration if added to the model. The application of our prediction model warrants further evaluation in a real-world setting.
In conclusion, the effects of treatment with haemodiafiltration versus haemodialysis in patients with ESKD in terms of survival can be predicted by a prediction model that includes routinely available patient and disease characteristics. Although all patients were predicted to benefit from haemodiafiltration treatment compared with haemodialysis, there was considerable heterogeneity in the survival benefit by haemodiafiltration treatment in ESKD patients. Those patients who benefit most from treatment with haemodiafiltration were younger, less likely to have diabetes or cardiovascular disease history, and had higher serum creatinine and serum albumin levels. Treatment effect predictions can be used to determine which patients are likely to experience the most benefit from haemodiafiltration treatment and to guide shared decision-making.
ONLINE CALCULATOR
The model is available on https://hdfpredictiontool.shinyapps.io/hdf_prediction_tool/
Supplementary Material
ACKNOWLEDGEMENTS
The HDF-Pooling project was designed, conducted and analyzed independently of the financial contributors of the individual studies as listed below. Study data were collected and retained by the investigators and were not available for the financial contributors of the individual studies.
APPENDIX
List of the HDF pooling project investigators
Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht the Netherlands: Michiel L. Bots; Department of Nephrology and Hypertension, University Medical Center Utrecht, Utrecht, the Netherlands: Peter J. Blankestijn; Center of Excellence Medical, Fresenius Medical Care GmbH, Bad Homburg, Germany and University of Montpellier, Research and Training Unit Medicine, Montpellier, France: Bernard Canaud; Royal Free Hospital, University College London Medical School, London, UK: Andrew Davenport; Department of Nephrology and Amsterdam Cardiovascular Sciences, Amsterdam University Medical Centers, VU University, Amsterdam, the Netherlands: Muriel P.C. Grooteman and Menso J. Nubé; The George Institute for Global Health, University of Oxford, Oxford, UK: Sanne A.E. Peters; PhyMedExp, National Institute of Health and Medical Research (INSERM), French National Centre for Scientific Research (CNRS), University of Montpellier, Department of Biochemistry and Endocrinology, Central University Hospital (CHU) Montpellier, Montpellier, France: Marion Morena; Department of Nephrology, Hospital Clinic, Barcelona, Spain: Francisco Maduell and Ferran Torres; Division of Nephrology, Ege University School of Medicine, Izmir, Turkey: Ercan Ok and Gulay Asci; Department of Nephrology, Alessandro Manzoni Hospital, Lecco, Italy: Francesco Locatelli.
Contributor Information
Rob C M van Kruijsdijk, Department of Nephrology, Radboud University Medical Center, Nijmegen, The Netherlands; Department of Nephrology and Hypertension, University Medical Center Utrecht, Utrecht, The Netherlands.
Robin W M Vernooij, Department of Nephrology and Hypertension, University Medical Center Utrecht, Utrecht, The Netherlands; Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, The Netherlands.
Michiel L Bots, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, The Netherlands.
Sanne A E Peters, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, The Netherlands; The George Institute for Global Health, Imperial College London, London, UK.
Jannick A N Dorresteijn, Department of Vascular Medicine, University Medical Center Utrecht, Utrecht, The Netherlands.
Frank L J Visseren, Department of Vascular Medicine, University Medical Center Utrecht, Utrecht, The Netherlands.
Peter J Blankestijn, Department of Nephrology and Hypertension, University Medical Center Utrecht, Utrecht, The Netherlands.
Thomas P A Debray, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, The Netherlands.
The HDF Pooling Project investigators:
Michiel L Bots, Peter J Blankestijn, Bernard Canaud, Andrew Davenport, Muriel P C Grooteman, Menso J Nubé, Sanne A E Peters, Marion Morena, Francisco Maduell, Ferran Torres, Ercan Ok, Gulay Asci, and Francesco Locatelli
Funding
The authors of the four studies were financially supported by the EuDial working group. EuDial is an official working group of the European Renal Association (ERA, https://www.era-online.org/en/eudial/). No industry funding was received for any part of or activity related to the present analysis. The Turkish HDF study was supported by European Nephrology and Dialysis Institute with an unrestricted grant. The study was performed in Fresenius Medical Care haemodialysis clinics in Turkey. ESHOL was supported by The Catalan Society of Nephrology and by grants from Fresenius Medical Care and Gambro through the Catalan Society of Nephrology. The CONTRAST study was supported by a grant from the Dutch Kidney Foundation (Nierstichting Nederland Grant C02.2019), and unrestricted grants from Fresenius Medical Care, Netherlands, and Gambro Lundia AB, Sweden. Additional support was received from the Dr E.E. Twiss Fund, Roche Netherlands, the International Society of Nephrology/Baxter Extramural Grant Program, and the Netherlands Organization for Health Research and Development (ZONMw Grant 170882802). The French HDF study was supported by a national grant from the Health Ministry (Programme Hospitalier de Recherche Clinique, PHRC) as a means to improve the care and outcome of elderly chronic disease patients. RWMV, PJB, and MLB are funded by the CONVINCE study (European Union's Horizon 2020 research and innovation programme under grant agreement No 754803).
AUTHORS’ CONTRIBUTIONS
R.C.M.v.K. designed and carried out the data analyses, interpreted the results, and drafted and revised the manuscript; R.W.M.V. performed the literature search, interpreted the results, and drafted and revised the manuscript; M.L.B. and P.J.B. collected the data, interpreted the results and revised the manuscript; S.A.E.P. collected the data, interpreted the results and revised the manuscript; J.A.N.D. and F.L.J.V interpreted the results and revised the manuscript; T.P.A.D. designed the data analyses, interpreted the results, and revised the manuscript; the HDF-Pooling Project investigators collected the data, interpreted the results, and revised the manuscript.
CONFLICT OF INTEREST STATEMENT
P.J.B. reports grants from Gambro/Baxter, grants from Fresenius, grants from ZonMw (Dutch Government), grants from EDTA, during the conduct of the study; grants from Medtronic, grants from Recor, grants from Ablative Solutions and grants from European Commission, outside the submitted work. T.P.A.D. acknowledges financial support from the Netherlands Organization for Scientific Research (VENI grant 91 617 050), outside the submitted work. The other authors have no disclosures.
REFERENCES
- 1. Liyanage T, Ninomiya T, Jha Vet al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet North Am Ed 2015; 385: 1975–1982 [DOI] [PubMed] [Google Scholar]
- 2. Lozano R, Naghavi M, Foreman Ket al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet North Am Ed 2012; 380: 2095–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. United States Renal Data System . USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis, 2015; 66: S1–S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kramer A, Boenink R, Noordzij Met al. The ERA-EDTA Registry Annual Report 2017: a summary. Clin Kidney J 2020; 13: 693–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tattersall JE, Ward RA.. Online haemodiafiltration: definition, dose quantification and safety revisited. Nephrol Dial Transplant 2013; 28: 542–550 [DOI] [PubMed] [Google Scholar]
- 6. Peters SAE, Bots ML, Canaud BMMet al. Haemodiafiltration and mortality in end-stage kidney disease patients: a pooled individual participant data analysis from four randomized controlled trials. Nephrol Dial Transplant 2016; 31: 978–984 [DOI] [PubMed] [Google Scholar]
- 7. van Kruijsdijk RCM, Visseren FLJ, Boni Let al. Pemetrexed plus carboplatin versus pemetrexed in pretreated patients with advanced non-squamous non-small cell lung cancer: treating the right patients based on individualized treatment effect prediction. Ann Oncol 2016; 27: 1280–1286 [DOI] [PubMed] [Google Scholar]
- 8. Dorresteijn JAN, Visseren FLJ, Ridker PMet al. Estimating treatment effects for individual patients based on the results of randomised clinical trials. BMJ 2011; 343: d5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dorresteijn JAN, Kaasenbrood L, Cook NRet al. How to translate clinical trial results into gain in healthy life expectancy for individual patients. BMJ 2016; i1548. [DOI] [PubMed] [Google Scholar]
- 10. Kent DM, Paulus JK, van Klaveren Det al. The Predictive Approaches to Treatment effect Heterogeneity (PATH) statement. Ann Intern Med 2020; 172: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grooteman MPC, van den Dorpel MA, Bots MLet al. Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J Am Soc Nephrol 2012; 23: 1087–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maduell F, Moreso F, Pons Met al. High-Efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol 2013; 24: 487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morena M, Jaussent A, Chalabi Let al. Treatment tolerance and patient-reported outcomes favor online hemodiafiltration compared to high-flux hemodialysis in the elderly. Kidney Int 2017; 91: 1495–1509 [DOI] [PubMed] [Google Scholar]
- 14. Ok E, Asci G, Toz Het al. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: results from the Turkish OL-HDF study. Nephrol Dial Transplant 2013; 28: 192–202 [DOI] [PubMed] [Google Scholar]
- 15. Royston P, Parmar MKB. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med 2002; 21: 2175–2197 [DOI] [PubMed] [Google Scholar]
- 16. Bartlett JW, Seaman SR, White IRet al. Multiple imputation of covariates by fully conditional specification: accommodating the substantive model. Stat Methods Med Res 2015; 24: 462–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jackson D, White IR, Seaman Set al. Relaxing the independent censoring assumption in the Cox proportional hazards model using multiple imputation. Stat Med 2014; 33: 4681–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Canaud B, Bragg-Gresham JL, Marshall MRet al. Mortality risk for patients receiving hemodiafiltration versus hemodialysis: European results from the DOPPS. Kidney Int 2006; 69: 2087–2093 [DOI] [PubMed] [Google Scholar]
- 19. Debray TPA, Moons KGM, Ahmed Iet al. A framework for developing, implementing, and evaluating clinical prediction models in an individual participant data meta-analysis. Stat Med 2013; 32: 3158–3180 [DOI] [PubMed] [Google Scholar]
- 20. Gerds T A, Kattan MW, Schumacher Met al. Estimating a time-dependent concordance index for survival prediction models with covariate dependent censoring. Stat Med 2013; 32: 2173–2184 [DOI] [PubMed] [Google Scholar]
- 21. Steyerberg EW. Clinical Prediction Models. Springer, 2009 [Google Scholar]
- 22. Vickers AJ, Cronin AM, Elkin EBet al. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inf Decis Making 2008; 8: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Collins GS, Reitsma JB, Altman DGet al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 2015; 350: g7594. [DOI] [PubMed] [Google Scholar]
- 24. Canaud B, Ye X, Usvyat Let al. Clinical and predictive value of simplified creatinine index used as muscle mass surrogate in end-stage kidney disease haemodialysis patients—results from the international MONitoring Dialysis Outcome initiative. Nephrol Dialy Transplant 2020; 35: 2161–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramspek CL, Voskamp PW, van Ittersum FJet al. Prediction models for the mortality risk in chronic dialysis patients: a systematic review and independent external validation study. Clin Epidemiol 2017; 9: 451–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blankestijn PJ, Grooteman M, Nube M. Are there any downsides, barriers, or challenges in delivering hemodiafiltration in everyday clinical practice? Contrib Nephrol 2016; 189: 30–35 [DOI] [PubMed] [Google Scholar]
- 27. Ramponi F, Ronco C, Mason Get al. Cost-effectiveness analysis of online hemodiafiltration versus high-flux hemodialysis. Clinicoecon Outcomes Res 2016; 8: 531–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blankestijn PJ, Fischer KI, Barth Cet al. Benefits and harms of high-dose haemodiafiltration versus high-flux haemodialysis: the comparison of high-dose haemodiafiltration with high-flux haemodialysis (CONVINCE) trial protocol. BMJ Open 2020; 10: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. H4RT protocol . Bristol University, available at https://www.bristol.ac.uk/population-health-sciences/projects/h4rt-trial/ (1 February 2022, date last accessed) [Google Scholar]
- 30. Davenport A, Peters SAE, Bots MLet al. Higher convection volume exchange with online hemodiafiltration is associated with survival advantage for dialysis patients: the effect of adjustment for body size. Kidney Int 2016; 89: 193–199 [DOI] [PubMed] [Google Scholar]
- 31. Imamović G, Hrvačević R, Kapun Set al. Survival of incident patients on high-volume online hemodiafiltration compared to low-volume online hemodiafiltration and high-flux hemodialysis. Int Urol Nephrol 2014; 46: 1191–1200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.