Abstract
Objectives
To provide a comprehensive overview of interventions that support shared decision-making (SDM) for treatment modality decisions in advanced kidney disease (AKD). To provide summarised information on their content, use and reported results. To provide an overview of interventions currently under development or investigation.
Design
The JBI methodology for scoping reviews was followed. This review conforms to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) checklist.
Data sources
MEDLINE, Embase, Web of Science, Cochrane Library, Emcare, PsycINFO, PROSPERO and Academic Search Premier for peer-reviewed literature. Other online databases (eg, clinicaltrials.gov, OpenGrey) for grey literature.
Eligibility for inclusion
Records in English with a study population of patients >18 years of age with an estimated glomerular filtration rate <30 mL/min/1.73 m2. Records had to be on the subject of SDM, or explicitly mention that the intervention reported on could be used to support SDM for treatment modality decisions in AKD.
Data extraction and synthesis
Two reviewers independently screened and selected records for data extraction. Interventions were categorised as prognostic tools (PTs), educational programmes (EPs), patient decision aids (PtDAs) or multicomponent initiatives (MIs). Interventions were subsequently categorised based on the decisions they were developed to support.
Results
One hundred forty-five interventions were identified in a total of 158 included records: 52 PTs, 51 EPs, 29 PtDAs and 13 MIs. Sixteen (n=16, 11%) were novel interventions currently under investigation. Forty-six (n=46, 35.7%) were reported to have been implemented in clinical practice. Sixty-seven (n=67, 51.9%) were evaluated for their effects on outcomes in the intended users.
Conclusion
There is no conclusive evidence on which intervention is the most efficacious in supporting SDM for treatment modality decisions in AKD. There is a lot of variation in selected outcomes, and the body of evidence is largely based on observational research. In addition, the effects of these interventions on SDM are under-reported.
Keywords: nephrology, internal medicine, end stage renal failure
Strengths and limitations of this study.
The search queries for this scoping review were conducted without time period restrictions and generated comprehensive results covering all possible interventions that support SDM for treatment modality decisions in AKD.
Two reviewers independently used predeveloped charting and data-extraction tables to screen the literature, select records for inclusion and extract the relevant data.
The interventions identified in the included records are presented based on the decisions they were developed to support, after which information is provided on their content, format, evidence and availability.
Included records were not formally assessed for quality; potential risks of bias in the reported outcomes remain undetermined.
Interventions and/or findings from records not written in English, or records inaccessible due to subscription limitations or internet protocol address geo-blocking, are not reported.
Introduction
Over 2 million patients with kidney failure currently rely on kidney replacement therapy (KRT) to stay alive.1 This number has been estimated to double by 2030,2 and many patients with advanced kidney disease (AKD) will have to make treatment modality decisions as their kidneys deteriorate over time.
Guidelines on the management of chronic kidney disease (CKD) emphasise the importance of timely kidney failure treatment modality education and decisional support as patients progress to the more advanced stages of kidney disease.3 4 Delays in the decision-making process can result in suboptimal dialysis initiation, which is associated with increased patient morbidity, mortality and healthcare costs.5
Shared decision-making (SDM) has been recognised as the preferred model to help patients with AKD understand their treatment options, and make informed decisions that align with their values and preferences.6 7 SDM requires that patients and clinicians proactively engage in a collaborative decision-making process.8–10 This process should be characterised by deliberation, during which patients become aware of their choice, understand all of their options and get to consider what matters most to them. A three-step framework has been developed to help guide this decision-making process with the following conversational steps in clinical practice: (1) team talk, (2) option talk and (3) decision talk.10 In addition, educational programmes (EPs) and decision support interventions such as patient decision aids (PtDAs) and prognostic tools (PTs) can be used to support deliberation and help patients and clinicians engage in SDM.10 Multiple efforts have been made to foster SDM across the international healthcare community,11 12 but there are still signs that patients experience a low degree of SDM,13 and efforts to incentivise SDM risk being limited to the promotion of PtDAs.14 15 A broader sense of awareness and knowledge of SDM is needed for it to become widely implemented,8 and stakeholders should share their experiences to speed up this process.16
We previously set out to write a scoping review on interventions that can support SDM for treatment modality decisions in AKD after we showcased a lack of a comprehensive overview of these interventions in the literature.17 We performed an additional preliminary search on the MEDLINE database prior to the conduct of this review and did not identify previous scoping reviews on the topic aligning to the same concept. We did identify a scoping review on the information available for clinicians counselling older patients with kidney failure,18 a systematic review on PtDAs developed to support SDM between dialysis and conservative care management (CCM) pathways,19 a scoping review on predialysis EPs,20 a systematic review on PTs developed to predict kidney failure21 and a Cochrane review on the effects of PtDAs in people facing treatment or screening decisions.22
We conducted this scoping review to provide clinicians, researchers and other stakeholders with one comprehensive, but digestible source of information on interventions that can support SDM for treatment modality decisions in AKD. An overview of interventions currently under development or investigation is also provided. We hope that this review will facilitate the future implementation of SDM in clinical practice, as well as stimulate development and research on new and effective interventions by exploring and defining knowledge gaps on the subject.
Methods
We followed the JBI methodology for scoping reviews23 and our scoping review protocol17 when we conducted this scoping review. Our objectives, research questions and methods are specified in our protocol (see online supplemental appendix 1). In addition to the protocol, we also used: (1) more detailed inclusion criteria during the screening and inclusion process and (2) the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) checklist24 (see online supplemental appendix 2) when we completed the review. No other changes were made in the methodology described in our protocol.
bmjopen-2021-055248supp001.pdf (158.3KB, pdf)
bmjopen-2021-055248supp002.pdf (4.8MB, pdf)
Objectives
In brief, our objectives were to provide:
a comprehensive overview of interventions that can support SDM for treatment modality decisions in AKD;
summarised information on their contents, use and reported results;
an overview of interventions currently under development or investigation.
Inclusion criteria
We searched the peer-reviewed and grey literature for records on interventions that support SDM for treatment modality decisions in AKD. We considered any intervention in standard care that can support deliberation and/or help patients and clinicians engage in SDM (eg, EPs, PtDAs, PTs) an SDM intervention. No time period restriction was used in an effort to be as comprehensive as possible. Records were eligible for inclusion if they were written in English, and if the study population consisted of patients >18 years of age with an estimated glomerular filtration rate <30 mL/min/1.73 m2. Records had to be on the subject of SDM or explicitly mention that the reported interventions could be used to support SDM for treatment modality decisions in AKD. Records that reported on interventions that could clearly be used to support SDM without explicitly mentioning it were also included.
Exclusion criteria
We excluded records if:
they only reported on interventions for advance care planning;
they only reported on interventions for the withdrawal of treatment.
Search methodology
We performed a three-step search strategy as explained in the JBI methodology for scoping reviews23 and in our review protocol17 (see online supplemental appendix 1). We searched MEDLINE, Embase, Web of Science, Cochrane Library, Emcare, PsycINFO, PROSPERO and Academic Search Premier for peer-reviewed literature. We searched OpenGrey, researchgate.net, clinicaltrials.gov, europepmc.org, Google Scholar and websites of the Kidney Disease Improving Global Outcomes Association, the Renal Physicians Association, the American Society of Nephrology, the Canadian Society of Nephrology, the National Institute of Health and Care Excellence, the European Renal Association—European Dialysis and Transplant Association, the Kidney Health Australia—Caring for Australians with Renal Impairment Association and the Ottawa Hospital Research Institute for grey literature.
A research librarian generated the search queries (see online supplemental appendix 3). The results were uploaded in RefWorks V.2.0.
bmjopen-2021-055248supp003.pdf (163.9KB, pdf)
Record selection and data extraction
We used previous publications, the International Patient Decision Aids Standards (IPDAS) minimum standards criteria25 and the Standards for UNiversal reporting of patient Decision Aid evaluation (SUNDAE) checklist26 to design charting and data-extraction tables used for record selection and data extraction. Two reviewers (NE and GNdG) independently performed the process of record selection and data extraction. Disagreements were resolved by discussion or consultation with the research team (PvdN, MvdD, WJB, AMS).
We initially screened the titles and abstracts of all identified records after which the charting table was used to register records selected for full-text analysis in Microsoft Excel V.16. We then performed full-text analysis of the selected records during which a final selection was made for data extraction. We also screened the references of this selection for additional records on the subject.
Selected records were categorised based on record type and on their scope and context as mentioned by the authors and developers. We categorised the interventions we identified in these records based on whether these interventions were PTs, EPs or PtDAs. Interventions were categorised as multicomponent initiatives (MIs) when two or more of these interventions were combined to support patients with AKD in treatment modality decisions, or implement SDM in clinical practice. We subsequently categorised the identified interventions based on the decisions they were developed to support.
Extracted data included: primary author, developer, date of publication, country of origin, type of record, study population/target demographic, study aims, study methods, sample size, study arms, intervention, format and context of the intervention, contents of the intervention, patient participation in development, comparator, study outcomes, reports on outcomes of SDM, use of International Consortium for Health Outcomes Measurement (ICHOM)27 or Standardised Outcomes in Nephrology (SONG)28 outcomes, main findings, implemented in clinical practice, recruitment status, date of completion and/or publication.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Results
Figure 1 illustrates a flow chart of the screening and inclusion process. We conducted the final search query in February 2021. We identified 1512 records and included a total of 158 records. Records were excluded because they were on another subject (n=1215, 80.3%), not available (n=127, 8.4%), not in English (n=57, 3.8%), duplicates (n=34, 2.2%), reviews (n=28, 1.9%) on the wrong population (n=27, 1.8%) or protocols for completed studies (n=24, 1.6%).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) flow chart of screening and inclusion process.
Figure 2 illustrates the included records stratified by type, scope and context. The majority of these records are observational (n=68, 43.0%) and experimental studies (n=39, 24.7%). A smaller proportion are study protocols (n=17, 10.8%), meeting abstracts (n=16, 10.1%), mixed-methods studies (n=12, 7.6%) and websites (n=6, 3.8%). Most records report on EPs (n=62, 39.2%), followed by PTs (n=42, 26.6%), PtDAs (n=37, 23.4%) and MIs (n=17, 10.8%).
Figure 2.
Included records stratified by record type, scope and context.
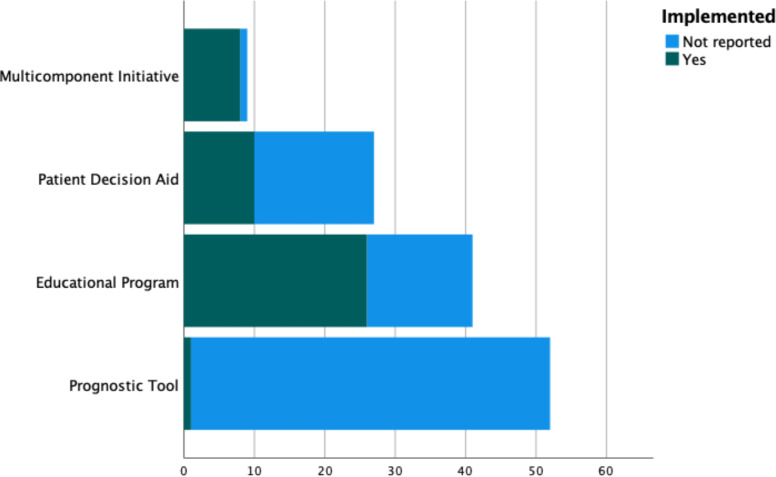
We identified 145 interventions in the included records. Figure 3 illustrates the distribution of these interventions. The majority of these records are PTs (n=52, 35.9%) and EPs (n=51, 35.2%), followed by PtDAs (n=29, 20.0%) and MIs (n=13, 8.9%). Some of these interventions were only identified in meeting abstracts (n=14, 9.7%). A minority were novel interventions that we identified in study protocols (n=16, 11.0%). Figures 4 and 5 illustrate the implementation and evaluation rates of the identified interventions. About one-third of the interventions (n=46, 35.7%) were reported to have been implemented in clinical practice. About half of the interventions (n=67, 51.9%) were evaluated for their effects on outcomes in the intended users. PTs were the interventions with the least information on implementation status and were the least evaluated interventions, followed by PtDAs, EPs and MIs. Interventions were generally evaluated on health-related outcomes and on knowledge, decisional quality, communication and patient activation. Patients that were exposed to the interventions generally had better outcomes than patients that were not exposed to the interventions.
Figure 3.
Distribution of the different types of interventions.
Figure 4.
Stacked bar count of the interventions stratified by implementation status. *Interventions currently under investigation (n=16, 11.0%) are not shown here.
Figure 5.
Stacked bar count of the interventions stratified by evaluation status. *Interventions currently under investigation (n=16, 11.0%) are not shown here.
Prognostic tools
We identified 52 PTs. All PTs were identified in peer-reviewed articles.29–59
Table 1 provides an overview of the identified PTs with their characteristics and performance metrics. Table S1 in online supplemental appendix 4 provides additional details on the identified PTs (eg, sources for publicly available PTs). Nineteen PTs predict the risk of progression to kidney failure (no. 1–19) and help patients and clinicians decide whether or not patients should start with preparations for kidney failure. One PT also predicts the risk of cardiovascular disease and death (no. 19). Twenty-eight PTs predict the risk of death after starting dialysis (no. 20–47) and help patients and clinicians decide whether or not patients should choose to start dialysis. Two PTs predicts the risk of death after starting CCM (no. 48, 49) and help patients and clinicians decide whether or not patients should choose to start CCM. One PT predicts and compares the risk of death after starting dialysis or transplantation (no. 50) and helps patients and clinicians decide between dialysis and transplantation options. One PT predicts the risk of deceased donor kidney graft failure (no. 51) and helps patients and clinicians decide whether or not patients should accept a deceased donor kidney transplantation offer. One PT predicts the risk of living donor kidney graft failure (no. 52) and helps patients and clinicians decide whether or not patients should accept a living donor kidney transplantation (LDKT) offer.
Table 1.
Overview of the identified PTs, their characteristics and performance metrics
| PT | Prediction* | Format | Population† | Validation | Discrimination‡ | Calibration§ | External validation | Implemented | Evaluated |
| Start preparation for kidney failure? | |||||||||
| No. 1: Johnson prognostic score | 5-year risk of kidney failure | Point-based scoring system | Patients with CKD stage 3–4 | Bootstrapping | C-statistic=0.89 | Calibration plot and Hosmer-Lemeshow test (p>0.99) | No | Not reported | No |
| No. 2: 4-variable kidney failure risk equation |
|
|
Patients with CKD stage 3–5 | External sample |
|
Calibration plot and Nam and D’Agostino statistic 3-year risk of kidney failure (32) | Yes | Not reported | No |
| No. 3: 8-variable kidney failure risk equation |
|
|
|
Calibration plot and Nam and D’Agostino statistic 3-year risk of kidney failure (19) | Yes | Not reported | No | ||
| No. 4: Drawz prognostic model | 1-year risk of kidney failure | Formula | Patients >65 years of age with an eGFR <30 mL/min/1.73 m2 |
|
|
Calibration plot | Yes | Not reported | No |
| No. 5: Marks prognostic model |
5-year risk of kidney failure | Formula | Patients with an eGFR <60 mL/min/1.73 m2 |
External sample | C-statistic=0.94 | Calibration plot and Hosmer-Lemeshow statistic (4.6) | Yes | Not reported | No |
| No. 6: Norouzi prognostic model |
|
Computer software package | Patients with an eGFR <60 mL/min/1.73 m2 |
Comparison of performance in training and testing datasets |
|
|
No | Not reported | No |
| No. 7: Tangri dynamic prognostic model | Time to kidney failure (dynamic) | Formula | Patients with CKD stage 3–5 |
|
C-statistic=0.91 | Calibration plot and Hosmer-Lemeshow statistic (<20) | No | Not reported | No |
| No. 8: Schroeder prognostic model | 5-year risk of kidney failure | Formula | Patients with CKD stage 3–4 |
|
C-statistic=0.95 | Calibration plot | No | Not reported | No |
| No. 9: 2-variable CKD-JAC clinical prediction model |
3-year risk of kidney failure | Formula | Patients with an eGFR <60 mL/min/1.73 m2 |
|
|
|
No | Not reported | No |
| No. 10: 3-variable CKD-JAC clinical prediction model |
3-year risk of kidney failure | Formula |
|
|
No | Not reported | No | ||
| No. 11: 4-variable CKD-JAC clinical prediction model |
3-year risk of kidney failure | Formula |
|
|
No | Not reported | No | ||
| No. 12: 5-variable CKD-JAC clinical prediction model |
3-year risk of kidney failure | Formula |
|
|
No | Not reported | No | ||
| No. 13: 6-variable CKD-JAC clinical prediction model |
3-year risk of kidney failure | Formula |
|
|
No | Not reported | No | ||
| No. 14: 7-variable CKD-JAC clinical prediction model |
3-year risk of kidney failure | Formula |
|
|
No | Not reported | No | ||
| No. 15: 8-variable CKD-JAC clinical prediction model |
3-year risk of kidney failure | Formula |
|
|
No | Not reported | No | ||
| No. 16: 9-variable CKD-JAC clinical prediction model |
3-year risk of kidney failure | Formula |
|
|
No | Not reported | No | ||
| No. 17: 10-variable CKD-JAC clinical prediction model |
3-year risk of kidney failure | Formula |
|
|
No | Not reported | No | ||
| No. 18: 11-variable CKD-JAC clinical prediction model |
3-year risk of kidney failure | Formula |
|
|
No | Not reported | No | ||
| No. 19: CKD-PC risk |
|
|
Patients with an eGFR <30 mL/min/1.73 m2 |
External sample | C-statistic 2-year risk of kidney failure=0.81 | Calibration plot 2-year risk of kidney failure | No | Not reported | No |
| Start with dialysis or not? | |||||||||
| No. 20: Foley prognostic score | Death within 6 months of dialysis initiation | Point-based scoring system | Patients on dialysis | No | No | No | Yes | Not reported | No |
| No. 21: Barret prognostic score | Death within 6 months of dialysis initiation | Point-based scoring system | Patients on dialysis (PD/HD) | No | No | No | No | Not reported | No |
| No. 22: Geddes multivariate prognostic model |
|
Formula | Patients on KRT | Split sample |
|
No | No | Not reported | No |
| No. 23: Geddes self-learning rule-based model |
|
Computer software package |
|
No | No | Not reported | No | ||
| No. 24: Mauri prognostic model | Death within 1 year of HD initiation | Formula | Patients on HD | Split sample | C-statistic=0.78 | Calibration plot and Hosmer-Lemeshow test (p=0.49) | No | Not reported | No |
| No. 25: 6-month REIN score |
Death within 6 months of dialysis initiation | Point-based scoring system | Patients >75 years of age on dialysis | Split sample | C-statistic=0.70 | Calibration plot and Hosmer-Lemeshow test (p=0.93) | Yes | Not reported | No |
| No. 26: 3-month REIN score |
Death within 3 months of dialysis initiation | Point-based scoring system | Patients >75 years of age on dialysis | Split sample | C-statistic=0.75 | No | Yes | Not reported | No |
| No. 27: Dusseux prognostic score | Death within 3 years of dialysis initiation | Point-based scoring system | Patients with an eGFR <60 mL/min/1.73 m2 | External sample | C-statistic=0.71 | Calibration plot and Hosmer-Lemeshow test (p=0.20) | No | Not reported | No |
| No. 28: Weiss prognostic score (age 65–79 years) |
|
Point-based scoring system | Patients >70 years of age on dialysis (PD/HD) | Bootstrapping |
|
|
No | Not reported | No |
| No. 29: Weiss prognostic score (age >80 years) |
|
Point-based scoring system |
|
|
No | Not reported | No | ||
| No. 30: 7-variable Thamer prognostic score |
|
Point-based scoring system | Patients >67 years of age on dialysis | Split sample |
|
No | Yes | Not reported | No |
| No. 31: 14-variable Thamer prognostic score |
|
Point-based scoring system |
|
|
Yes | Not reported | No | ||
| No. 32: Doi prognostic score |
Death within 1 year of HD initiation | Point-based scoring system | Patients on HD | Bootstrapping | C-statistic=0.83 | Calibration plot | No | Not reported | No |
| No. 33: Wick prognostic score | Death within 6 months of dialysis initiation | Point-based scoring system | Patients >65 years of age on dialysis (PD/HD) | Cross-validation | C-statistic=0.72 | Calibration plot and Hosmer-Lemeshow test (p=0.20) | Yes | Not reported | No |
| No. 34: Chen prognostic score | Death within 5 years of dialysis initiation | Point-based scoring system | Patients >70 years of age on dialysis (PD/HD) |
|
|
No | No | Not reported | No |
| No. 35: Haapio prognostic model | Death within 1 year of dialysis initiation | Formula | Patients on dialysis (PD/HD) | External sample | C-statistic=0.76 | Calibration plot and Hosmer-Lemeshow test (p=0.041) | No | Not reported | No |
| No. 36: Haapio prognostic model | Death within 2 years of dialysis initiation | Formula | C-statistic=0.74 | Calibration plot and Hosmer-Lemeshow test (p=0.015) | No | Not reported | No | ||
| No. 37: Schmidt prognostic model | Death within 1 year | Formula | Patients with CKD stage 4–5 | External sample | C-statistic=0.74 | Calibration plot and Hosmer-Lemeshow test (p=0.46) | No | Not reported | No |
| No. 38: Dialysis score (for patients with eGFR <15 mL/min/1.73 m2) |
Death within 1 year of dialysis initiation |
|
Patients on dialysis |
|
|
|
No | Not reported | No |
| No. 39: dialysis score (for patients with eGFR >15 mL/min/1.73 m2) |
Death within 1 year of dialysis initiation |
|
|
|
No | Not reported | No | ||
| No. 40: Lin random forest cost prediction model |
Medical costs 1 year after dialysis initiation | Computer software package | Patients >65 years of age on dialysis | Comparison of performance in training and testing datasets | Mean absolute error=0.51 | No | No | Not reported | No |
| No. 41: Lin random forest mortality prediction model |
Death within 1 year after dialysis initiation | Computer software package | C-statistic=0.66 | No | No | Not reported | No | ||
| No. 42: Lin artificial neural network model for costs |
Medical costs 1 year after dialysis initiation | Computer software package | Mean absolute error=1.85 | No | No | Not reported | No | ||
| No. 43: Lin artificial neural network model for mortality |
Death within 1 year after dialysis initiation | Computer software package | C-statistic=0.68 | No | No | Not reported | No | ||
| No. 44: Yoshida clinical nomogram |
|
Nomogram | Patients >80 years of age on dialysis | Bootstrapping |
|
|
No | Not reported | No |
| No. 45: Santos prognostic score | Death within 6 months of dialysis initiation | Point-based scoring system | Patients >65 years of age on dialysis (PD/HD) | Bootstrapping | C-statistic=0.79 | Calibration plot and Hosmer-Lemeshow test (p=0.58) | No | Not reported | No |
| No. 46: Ramspek basic dialysis prognostic model | Death within 2 years of dialysis initiation | Formula | Patients >70 years of age with CKD stage 4–5 | Bootstrapping | C-statistic=0.68 | Calibration plot and calibration-in-the-large (32.5% vs 32.6%) | No | Not reported | No |
| No. 47: Ramspek extended dialysis prognostic model | Death within 2 years of dialysis initiation | Formula | C-statistic=0.75 | Calibration plot and calibration-in-the-large (32.5% vs 32.6%) | No | Not reported | No | ||
| Start with CCM or not? | |||||||||
| No. 48: Ramspek basic CCM prognostic model | Death within 2 years of conservative care initiation | Formula | Patients >70 years of age with CKD stage 4–5 | Bootstrapping | C-statistic=0.68 | Calibration plot and calibration-in-the-large (56.3% vs 56.5%) | No | Not reported | No |
| No. 49: Ramspek extended CCM prognostic model | Death within 2 years of conservative care initiation | Formula | C-statistic=0.73 | Calibration plot and calibration-in-the-large (56.3% vs 56.3%) | No | Not reported | No | ||
| Transplantation or dialysis? | |||||||||
| No. 50: iChoose Kidney |
|
|
|
Split sample |
|
|
No | Yes | Yes |
| Accept or decline DDKT offer? | |||||||||
| No. 51: Kidney Donor Risk Index | Risk of deceased donor kidney graft failure |
|
DDKT recipients | Cross-validation | C-statistic=0.62 | No | Yes | Not reported | No |
| Accept or decline LDKT offer? | |||||||||
| No. 52: Living Kidney Donor Risk Index | Risk of living donor kidney graft failure |
|
LDKT recipients | Bootstrapping | C-statistic=0.59 | No | Yes | Not reported | No |
*Prediction formulated as reported in identified records.
†Population formulated as reported in identified records.
‡Discrimination describes how accurately a tool identifies a high probability of events in patients with the outcome of interest and is expressed as a slope or C-statistic. A C-statistic of 0.5 represents no predictive discrimination and a C-statistic of 1 represents perfect predictive discrimination. When the C-statistic is >0.7, a score has acceptable discriminatory power.
§Calibration describes the agreement between the observed and predicted outcomes and is generally expressed with a calibration plot, a calibration slope, as calibration-in-the-large or a goodness-of-fit test. A calibration plot compares the predicted risks with observed risks within subgroups of patients and provides the most information on calibration accuracy.
CCM, conservative care management; CKD, chronic kidney disease; C-statistic, concordance statistic; DDKT, deceased donor kidney transplantation; eGFR, estimated glomerular filtration rate; HD, haemodialysis; JAC, Japan cohort; KRT, kidney replacement therapy; LDKT, living donor kidney transplantation; n.a., not applicable; NPV, negative predictive value; PD, peritoneal dialysis; PPV, positive predictive value; PTs, prognostic tools; REIN, Renal Epidemiology and Information Network.
bmjopen-2021-055248supp004.pdf (478.9KB, pdf)
A relatively large proportion (n=19, 36,5%) of the identified PTs were developed to be used in elderly patients with AKD (no. 4, 25, 26, 28–31, 33, 34, 40–49). The remaining PTs can be used in the general population of patients with AKD.
The majority of PTs (n=32, 61.5%) are publicly available as formulas (no. 2–5, 7–19, 22, 24, 35–39, 46–52), eight of which (no. 2, 3, 19, 38, 39, 50–52) can be used on interactive websites. One of these PTs (no. 50) has been designed as a PtDA. Point-based scoring systems (no. 1, 20, 21, 25–34, 45) and nomograms (no. 44) were also used, although less frequently (n=14, 26.9%, n=1, 1.9%). A minority of PTs (n=6, 11.5%) are not publicly available (no. 6, 23, 40–43) and depend on computer software to be used.
Not all PTs were completely validated (assessed for performance) during development. About a quarter (n=11, 21.2%) were not evaluated on calibration outcomes (no. 22, 23, 26, 30, 34, 40–43, 51, 52), and some (n=2, 3.8%) were not validated at all (no. 20, 21). Most of them (n=37, 71,2%) were developed and validated with the same cohort of patients (no. 1, 6, 7, 9–18, 22–26, 28–33, 40–52). A quarter (n=13, 25.0%) were developed and validated with different patient cohorts (no. 2–5, 8, 19, 27, 34–39). The discriminatory power of the PTs was generally acceptable, one-fourth (n=13, 25.0%) had C-statistics below 0.7 on all, or a subset of, predictions (no. 9, 28–30, 34, 39, 41, 43, 46, 48, 50–52). The remaining PTs had better C-statistics.
Table 2 provides an overview of the PTs (no. 2–5, 19, 20, 25, 26, 30, 33, 51, 52) that were externally validated in independent external validation studies.31 32 46 55 60–69 Table S2 in online supplemental appendix 4 provides additional details on these external validation studies. One PT (no. 2) was externally validated in six different studies,32 60–63 67 two other PTs (no. 3, 25) were externally validated in three31 62 67 and four different studies,46 55 64 66 respectively. The other nine (no. 4, 5, 19, 20, 26, 30, 33, 51, 52) were externally validated less frequently.
Table 2.
Overview of PTs validated in independent external validation studies
| PT | Source | Population* | Prediction† | Discrimination‡ | Calibration§ |
| Start preparation for kidney failure? | |||||
| No. 2 | Retrospective cohort study60 | African-American patients with an eGFR between 20 and 65 mL/min/1.73 m2 |
|
|
No |
| No. 2 | Retrospective cohort study61 | Patients with CKD stage 2–5 | 3-year risk of kidney failure | C-statistic=0.91 | Calibration plot |
| No. 2 | Multicentre retrospective cohort study32 | Patients with an eGFR <60 mL/min/1.73 m2 | 5-year risk of kidney failure | C-statistic=0.95 | Calibration plot |
| No. 2 | Retrospective cohort study62 | Patients with CKD stage 3–5 | 5-year risk of kidney failure | C-statistic=0.88 | Calibration plot and Hosmer-Lemeshow test (p=0.05) |
| No. 3 | 5-year risk of kidney failure | C-statistic=0.89 | Calibration plot and Hosmer-Lemeshow test (p=0.03) | ||
| No. 2 | Multicentre retrospective cohort study67 | Patients with CKD stage 3–5 |
|
|
|
| No. 3 |
|
|
|
||
| No. 3 | Retrospective cohort study31 | Patients >65 years of age with an eGFR <30 mL/min/1.73 m2 | 1-year risk of kidney failure |
|
No |
| No. 2 | Multicentre prospective cohort study63 | Patients >75 years of age with an eGFR <20 mL/min/1.73 m2 |
|
|
|
| No. 4 | 1-year risk of kidney failure | C-statistic=0.66 | Calibration plot and Hosmer-Lemeshow test (p=0.47) | ||
| No. 5 | 5-year risk of kidney failure | C-statistic=0.65 | Calibration plot and Hosmer-Lemeshow test (p=0.72) | ||
| No. 19 |
|
|
|
||
| Start with dialysis or not? | |||||
| No. 25 | Multicentre retrospective cohort study64 | Patients >67 years of age on dialysis | Death within 6 months of dialysis initiation | C-statistic=0.63 | No |
| No. 25 | Multicentre retrospective cohort study46 | Patients >67 years of age on dialysis | Death within 6 months of dialysis initiation |
|
|
| No. 25 | Retrospective cohort study55 | Patients >65 years of age on dialysis (PD/HD) | Death within 6 months of dialysis initiation | C-statistic=0.70 | No |
| No. 26 | Multicentre retrospective cohort study65 | Patients on dialysis |
|
|
No |
| No. 20 | Retrospective cohort study66 | Patients >75 years of age on dialysis | Death within 6 months of dialysis initiation | C-statistic=0.67 | Calibration plot and Hosmer-Lemeshow test (p=0.004) |
| No. 25 | Death within 6 months of dialysis initiation | C-statistic=0.61 | Calibration plot and Hosmer-Lemeshow test (p=0.45) | ||
| No. 26 | Death within 3 months of dialysis initiation | C-statistic=0.62 | Calibration plot and Hosmer-Lemeshow test (p=0.03) | ||
| No. 30 |
|
|
|
||
| No. 33 | Death within 6 months of dialysis initiation | C-statistic=0.57 | Calibration plot and Hosmer-Lemeshow test (p=0.43) | ||
| Accept or decline DDKT offer? | |||||
| No. 51 | Multicentre retrospective cohort study68 | DDKT recipients |
|
|
|
| Accept or decline LDKT offer? | |||||
| No. 52 | Retrospective cohort study69 | LDKT recipients | Risk of living donor kidney graft failure | C-statistic=0.55 | Calibration plot |
*Population formulated as reported in identified records.
†Prediction formulated as reported in identified records.
‡Discrimination describes how accurately a tool identifies a high probability of events in patients with the outcome of interest and is expressed as a slope or C-statistic. A C-statistic of 0.5 represents no predictive discrimination and a C-statistic of 1 represents perfect predictive discrimination. When the C-statistic is >0.7, a score has acceptable discriminatory power.
§Calibration describes the agreement between the observed and predicted outcomes and is generally expressed with a calibration plot, a calibration slope, as calibration-in-the-large or a goodness-of-fit test. A calibration plot compares the predicted risks with observed risks within subgroups of patients and provides the most information on calibration accuracy.
CKD, chronic kidney disease; C-statistic, concordance statistic; DDKT, deceased donor kidney transplantation; eGFR, estimated glomerular filtration rate; HD, haemodialysis; LDKT, living donor kidney transplantation; n.a., not applicable; PD, peritoneal dialysis; PTs, prognostic tools.
The majority of the PTs (n=10, 83.3%) were externally validated in different patient populations (no. 2, 3, 4, 5, 19, 20, 25, 26, 30, 33) than the ones they were developed for. Three PTs had poor discriminatory power in these patient populations (no.2, 30, 33), with C-statistics between 0.5 and 0.6.63 66 Performance metrics were generally comparable between developmental and external validation studies when similar patient populations were used (see tables 1 and 2). Only one PT (no. 50) was reported to have been implemented in clinical practice. This PT was designed as a PtDA57 and is in that regard the only PT that has been evaluated for its effects on outcomes in the intended users.70
Educational programmes
We identified 41 EPs (excluding ten currently under investigation). Thirty-five were identified in peer-reviewed articles.71–104 Six were identified in meeting abstracts found in the grey literature.105–110
Table 3 provides an overview of the identified EPs and their characteristics. Table S3 in online supplemental appendix 4 provides additional details on the identified EPs (eg, sources for publicly available EPs). One EP was developed to promote peritoneal dialysis (PD) and helps patients choose whether to start with PD or not (no. 1). Eleven EPs help patients choose between dialysis options (no. 2–11), with two promoting a particular treatment modality (no. 5, 11). Ten help patients choose between dialysis and transplantation options (no. 12–21), with two promoting a particular treatment modality (no. 13, 14). Seven EPs were developed to promote LDKT (no. 2–28) and help patients decide whether to pursue LDKT or not. Two EPs help patients choose between dialysis and CCM options (no. 29, 30). Seven help patients choose between transplantation, dialysis and CCM options (no. 31–37), one of which promotes home therapy modalities (no. 37). Four EPs help patients choose between transplantation options (no. 38–41).
Table 3.
Overview of the identified EPs and their characteristics
| EP | Format | Treatment options* | Promotes treatment modality | Coaching | Patient participation in development | Reading level | Implemented | Evaluated |
| Start PD or not? | ||||||||
| No. 1: customised video counselling |
1 video (32 min) | PD | PD | No | Yes | n.a. | Not reported | Yes |
| What type of dialysis modality? | ||||||||
| No. 2: Toronto Hospital multidisciplinary predialysis programme |
|
|
No | No | No | n.a. | Yes | Yes |
| No. 3: Karolinska Hospital predialysis patient education |
|
|
No | Yes | No | n.a. | Yes | Yes |
| No. 4: Birmingham Heartlands Hospital predialysis counselling |
|
|
No | No | No | n.a. | Yes | Yes |
| No. 5: Two-phase educational intervention |
|
|
Self-care dialysis modalities | No | No | n.a. | Yes | Yes |
| No. 6: dialysis education |
Multiple outpatient consultations with clinicians (n.a.) | Dialysis | No | No | No | n.a. | Yes | Yes |
| No. 7: multimedia interactive patient education |
1 DVD (n.a.) |
|
No | No | No | n.a. | Not reported | Yes |
| No. 8: comprehensive PDEP |
Multiple sessions of individual education by nephrologists and experienced nurse (n.a.) |
|
No | No | No | n.a. | Yes | Yes |
| No. 9: physician-led CKD education programme |
1 session of group education (n.a.) | Dialysis | No | No | No | n.a. | Not reported | Yes |
| No. 10: patient-driven video educational tool |
1 video (50 min) |
|
No | No | Yes | n.a. | Not reported | Yes |
| No. 11: King Fahad Armed Forces Hospital PDEP |
Multiple outpatient consultations with clinicians (n.a.) |
|
PD | No | No | n.a. | Yes | Yes |
| Transplantation or dialysis? | ||||||||
| No. 12: Cliniques Universitaires St. Luc PDEP |
|
|
No | Yes | No | n.a. | Yes | Yes |
| No. 13: RTN |
|
|
Independent KRT modalities | Yes | No | n.a. | Yes | Yes |
| No. 14: In-hospital CKD education programme |
|
|
Home dialysis modalities | Yes | No | n.a. | Yes | Yes |
| No. 15: multidisciplinary predialysis education |
|
|
No | No | No | n.a. | Yes | Yes |
| No. 16: Faculty of Medicine Ataturk University PDEP |
|
|
No | No | No | n.a. | Yes | Yes |
| No. 17: Information on Dialysis (INDIAL) |
2 sessions of group education (4 hours total) |
|
No | No | No | n.a. | Yes | Yes |
| No. 18: renal school |
|
|
No | Yes | No | n.a. | Yes | Yes |
| No. 19: kidney team at home |
|
|
No | Yes | No | n.a. | Yes | Yes |
| No. 20: Infórmate Acerca de la Donación de Riñón en Vida (Infórmate) |
Website |
|
No | No | Yes | n.a. | Not reported | Yes |
| No. 21: Nissan Tamagawa Hospital multidisciplinary care |
4 outpatient consultations with clinicians (n.a.) |
|
No | No | No | n.a. | Yes | Yes |
| Pursue LDKT or not? | ||||||||
| No. 22: home-based educational intervention |
|
LDKT | LDKT | Yes | No | n.a. | Not reported | Yes |
| No. 23: promoting live donor kidney transplantation |
|
LDKT | LDKT | Yes | No | n.a. | Not reported | Yes |
| No. 24: increasing the pursuit of living kidney donation |
|
|
LDKT | No | No | n.a. | Not reported | Yes |
| No. 25: Talking about Live Kidney Donation |
|
LDKT | LDKT | Yes | No | Moderate to low health literacy | Not reported | Yes |
| No. 26: Living About Choices in Transplantation and Sharing |
|
LDKT | LDKT | No | Yes | n.a. | Yes | Yes |
| No. 27: Hispanic Kidney Transplant Programme |
|
|
LDKT | Yes | No | n.a. | Yes | Yes |
| No. 28: patient navigator and education programme |
Multiple sessions of individual education with patient navigators (n.a.) |
|
LDKT | Yes | No | n.a. | Yes | Yes |
| Dialysis or CCM? | ||||||||
| No. 29: St. Paul’s Hospital multidisciplinary predialysis clinic |
|
|
No | No | n.a. | n.a. | Yes | Yes |
| No. 30: Birmingham Heartlands Hospital predialysis education |
|
|
No | Yes | No | n.a. | Yes | Yes |
| Transplantation, dialysis or CCM? | ||||||||
| No. 31: Acute Start Dialysis Education and Support |
|
|
No | Yes | No | n.a. | Yes | Yes |
| No.32: Treatment Options Programme (TOP) |
|
|
No | Yes | No | n.a. | Yes | Yes |
| No.33: University of Michigan multidisciplinary and peer mentor education |
|
|
n.a. | Yes | No | n.a. | Yes | No |
| No. 34: comprehensive predialysis education |
|
|
No | Yes | No | n.a. | Yes | Yes |
| No. 35: digital modality decision programme |
Interactive website |
|
No | Yes | No | n.a. | Not reported | Yes |
| No. 36: options class |
1 session of individual education with a renal nurse (1–2 hours) |
|
No | No | No | n.a. | Yes | Yes |
| No. 37 individual chronic kidney disease education |
1 session of individual education with various experts (n.a.) |
|
Home therapy modalities | No | No | n.a. | Not reported | Yes |
| What type of transplantation? | ||||||||
| No. 38: communicating about choices in transplantation |
|
|
No | No | Yes | n.a. | Not reported | Yes |
| No. 39: explore transplant (ET) |
|
|
No | Yes | Yes | Low health literacy | Not reported | Yes |
| No. 40: ET at home |
|
|
No | Yes | Yes | Low health literacy | Not reported | Yes |
| No. 41: your path to transplant (YPT) |
|
|
No | Yes | No | n.a. | Not reported | Yes |
*Treatment options formulated as reported in the identified records.
APD, ambulatory peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; CCM, conservative care management; CKD, chronic kidney disease; DDKT, deceased donor kidney transplantation; EPs, educational programmes; HD, haemodialysis; KRT, kidney replacement therapy; LDKT, living donor kidney transplantation; n.a., not available; PD, peritoneal dialysis; PDEP, predialysis education programme; RTN, renal triage nurse.
Most EPs were developed for the general population of patients with AKD, however some (n=5, 12.2%) were specifically developed for Hispanic and African-American patients (no. 20, 26–28, 40), and some (n=3, 7.3%) were specifically developed for suboptimal dialysis initiation patients (no. 13, 14, 31).
About one-third of the EPs (n=14, 34.1%) consist of a single medium format (no. 1, 6–11, 17, 20, 21, 28, 35–37). The remaining programmes consist of a combination of different medium formats. About half of the EPs (n=20, 48.8%) use coaches to guide patients through the programme (no. 3, 12–14, 18, 19, 22, 23, 25, 27, 28, 30–35, 39–41). A minority (n=7, 17.0%) were developed with the input of patients (no. 1, 10, 20, 26, 38–40) and even less (n=3, 7.3%) describe a reading level (no. 25, 39, 40). Only a few EPs (n=2, 4.9%) are publicly available (no. 20, 32).
More than half of the EPs (n=26, 63.4%) were reported to have been implemented in clinical practice (no. 2–6, 8, 11–19, 21, 26–34, 36). All but one (no. 33) have been evaluated for their effects on outcomes in the intended users.
Table 4 provides an overview of the studies71–86 88–118 that evaluated the identified EPs. Table S4 in online supplemental appendix 4 provides additional details on these studies. The majority of these EPs (n=19, 47.5%) were evaluated in experimental studies (no.1, 3, 5, 7, 16, 19, 20, 22–27, 29, 35, 38–41), more than half of which (n=14, 73,7%) were randomised controlled trials (RCTs).71 74 86 88 90–92 101–103 111–113 115 Less (n=16, 40.0%) were evaluated in observational studies (no.2, 4, 6, 11–15, 17, 18, 21, 28, 30–32, 34), a minority of which (n=4, 25.0%) were prospective cohort studies.76 94 95 117 Five (n=5, 12.5%) EPs (no.8–10, 36, 37) were evaluated in studies presented in meeting abstracts.105–107 109 110
Table 4.
Overview of studies evaluating the identified EPs
| EP | Source | Population* | Sample size | Primary outcome(s)† | Secondary outcome(s)† | Main findings‡ |
| Start PD or not? | ||||||
| No. 1 | RCT74 | Patients with CKD stage 5 | Total=120
|
Pre-intervention and post-intervention/control: PD acceptance rate, PD catheter insertion on schedule |
|
There were no significant differences in PD acceptance rate, PD catheter insertion on schedule, patient knowledge or confidence in PD between the intervention and control groups |
| What type of dialysis modality? | ||||||
| No. 2 | Retrospective cohort study72 | Predialysis patients | Total=141 | Post-intervention: patient and clinical characteristics, initiation rates of dialysis access prior to the first dialysis session, rates of inpatient dialysis start | Post-intervention: length of in-hospital stay after dialysis initiation |
|
| No. 3 | Non-randomised controlled study75 | Patients with an eGFR <20 mL/min/1.73 m2 | Total=56
|
|
n.a. |
|
| No. 4 | Prospective cohort study76 | Patients on KRT | Total=33
|
|
n.a. |
|
| No. 5 | RCT71 | Patients with an eGFR <30 mL/min/1.73 m2 | Total=70
|
|
|
|
| RCT111 | Patients with an eGFR <30 mL/min/1.73 m2 | Total=70
|
|
n.a. |
|
|
| No. 6 | Multicentre retrospective cohort study77 | Patients on maintenance dialysis | Total=1504 | Post-intervention: patient and clinical characteristics, duration and type of nephrological care, number of medical visits in the year before dialysis start, education on dialysis modality options and CKD, time between education, permanent access creation and dialysis start, type of access for dialysis modality | n.a. |
|
| No. 7 | Quasi-experimental study78 | Patients with an eGFR <15 mL/min/1.73 m2 | Total=60
|
|
n.a. | There were significant differences in the improvement of knowledge (p<0.001), uncertainty (p<0.001) and decisional regret (p<0.001) between the intervention and control groups |
| No. 8 | Meeting abstract of retrospective cohort study105 | Patients on KRT | Total=209 | Post-intervention: patient and clinical characteristics, selection of dialysis modality, parameters of treatment outcome | n.a. |
|
| No. 9 | Meeting abstract of retrospective cohort study106 | Patients on dialysis | Total=1294 | Post-intervention: Patient and clinical characteristics, participation in the educational programme, differences in treatment outcomes based on educational programme attendance | n.a. |
|
| No. 10 | Meeting abstract of qualitative study107 |
|
Total=6 | Patient reported themes of experiences to be included in a video-educational tool, provider feedback on the tool, patient feedback on the tool | n.a. |
|
| No. 11 | Retrospective cohort study73 | Patients on dialysis | Total=213
|
Post-intervention: patient and clinical characteristics, choice of dialysis modality | n.a. |
|
| Transplantation or dialysis? | ||||||
| No. 12 | Retrospective cohort study79 | Patients on KRT | Total=242
|
Post-intervention: patient and clinical characteristics, influence of ESKD aetiology and age on the distribution of KRT modalities, the timing of dialysis initiation, the effects of late referral on the KRT modalities | n.a. |
|
| No. 13 | Retrospective cohort study81 | Suboptimal HD start patients | Total=178
|
180 days post-intervention: the likelihood of patients switching to independent KRT therapy | 180 days post-intervention/control: likelihood of independent KRT therapy after RTN education |
|
| No. 14 | Retrospective cohort study80 | Acute start dialysis patients | Total=228 | Post-intervention: patient and clinical characteristics, chosen dialysis modality at hospital discharge, comparison of patient characteristics between those choosing in-centre HD and HD home | n.a. |
|
| No. 15 | Retrospective cohort study82 | Patients with an eGFR <40 mL/min/1.73 m2 | Total=1218
|
Post-intervention: all-cause mortality, progression to ESKD, KRT initiation, cardiovascular outcomes, infectious events, hospitalisation rates | n.a. |
|
| No. 16 | Non-randomised controlled study83 | Kidney transplantation recipients | Total=88
|
Post-intervention: patient and clinical characteristics, pre-emptive LDKT rates | n.a. |
|
| No. 17 | Retrospective cohort study84 | Patients with an eGFR <15 mL/min/1.73 m2 | Total=227
|
Post-intervention: annual PD and HD incidence rates | n.a. | 54,3% of patients that received the intervention started with PD as compared with 28% of patients that did receive the intervention (p<0.001) |
| No. 18 | Retrospective cohort study85 | Patients with an eGFR <30 mL/min/1.73 m2 | Total=234
|
Post-intervention: emergency dialysis rates, acute catheter insertion rates, hospital length of stay, complication rates | n.a. |
|
| No. 19 | RCT86 |
|
Total=409 (163 patients)
|
Pre-intervention and 3 days post-intervention/control: knowledge, risk perception, self-efficacy, attitude towards communication, communication on KRT, willingness to accept a LDKT | Up to 6 months post-intervention/control: amount of living donor inquiries, mount of living donor evaluations, amount of actual LDKTs |
|
| RCT with cross-over113 |
|
Total=390 (80 patients)
|
Pre-intervention, 4 and 8 weeks post-intervention/control: knowledge, frequency of communication on each KRT option in the past 4 weeks, the extent to which the participant indented to communicate about each KRT option with loves ones or the patient |
|
|
|
| No. 20 | Multicentre pre-post study114 | Hispanic kidney transplantation candidates | Total=63 |
|
n.a. |
|
| Multicentre RCT115 |
|
Total=282 (112 patients)
|
|
n.a. |
|
|
| No. 21 | Retrospective cohort study104 | Patients with CKD | Total=112
|
Post-intervention: patient and clinical characteristics, annual decreases in eGFR values, time to dialysis initiation, urgent dialysis initiation rate, PD selection rates and PD retention rates | n.a. |
|
| Pursue LDKT or not? | ||||||
| No. 22 | RCT88 | Kidney transplant candidates | Total=132
|
Post-intervention/control: the proportion of patients with living donor inquiries, living donor evaluations and LDKT rates | Pre-intervention/control: LDKT knowledge, willingness and concerns regarding LDKT, the number of educated potential donors |
|
| RCT112 | Kidney transplant candidates | Total=132
|
1 year post-intervention/control: the proportion of patients with living donor inquiries, living donor evaluations and LDKT rates in both black and white patients |
|
|
|
| No. 23 | Non-randomised controlled study89 | HD patients eligible for kidney transplantation | Total=214
|
Pre-intervention and 1 week post-intervention/control: readiness to consider LDKT, readiness to talk about LDKT with friend or family, readiness to ask friends and family to donate a kidney | n.a. |
|
| No. 24 | RCT90 | Kidney transplant candidates | Total=100
|
3 months post-intervention/control: whether a potential living kidney donor contacted the living donor programme on behalf of a patient |
|
|
| No. 25 | Multicentre RCT91 | Patients with CKD stage 3–5 | Total=130
|
1–3 and 6 months post-intervention/control: self-reported achievement of at least one of the following five steps: discussing LDKT with a family member, discussing LDKT with their physician, initiating the clinical evaluation for LDKT recipients, completing the clinical evaluation for LDKT recipients and identifying a potential live kidney donor |
|
|
| No. 26 | RCT92 | African-American kidney transplant candidates | Total=268
|
Pre-intervention, immediately and 6 months post-intervention/control: knowledge of LDKT, willingness to talk to family members about LDKT, perceived benefits of LDKT | n.a. |
|
| No. 27 | Pre-post study93 |
|
Total=113 |
|
n.a. |
|
| Pre-post study116 |
|
Total=1286
|
Pre-intervention and post-intervention: patient and clinical characteristics, waiting lists as a proxy for patient referrals, the ratio of Hispanic to non-Hispanic white LDKTs and DDKTs | n.a. |
|
|
| No. 28 | Prospective cohort study94 | Kidney transplant candidates | Total=5571 | Post-intervention: patient and clinical characteristics, total potential living donors per patient, likelihood of receiving a DDKT or LDKT | n.a. |
|
| Dialysis or CCM? | ||||||
| No. 29 | Non-randomised controlled study72 | Predialysis patients | Total=76
|
Pre-intervention and post-intervention/control: number of urgent versus elective dialysis starts, percentage of patients training as outpatients, the number of admissions and hospital days during the first of dialysis | Pre-intervention and post-intervention/control: patient and clinical characteristics |
|
| No.30 | Prospective cohort study95 | Patients with an eGFR <25 mL/min/1.73 m2 | Total=118 | Post-intervention: patient and clinical characteristics, patient reported factors affecting modality choice, attendance rates at patient education day | n.a. |
|
| Transplantation, dialysis or CCM? | ||||||
| No. 31 | Mixed methods: opinion and retrospective cohort study97 | Acute start dialysis patients | Total=100 | Post-intervention: treatment modality decisions | n.a. | 44 patients decided to pursue a home dialysis modality after the intervention |
| No. 32 | Quality improvement report96 | Patients with CKD stage 3–4 | Total=30 217
|
Post-intervention: patient and clinical characteristics, patient dialysis modality selection, vascular access type, mortality rates in the first 90 days of dialysis | n.a. |
|
| Quality improvement report118 | Patients with CKD stage 3 and 4 | Total=73.500 | Post-intervention: patient dialysis modality selection, vascular access type | n.a. |
|
|
| No. 34 | Retrospective cohort study98 | Patients with CKD stage 4–5 | Total=108 | Post-intervention: patient and clinical characteristics, patient choice of dialysis modality, potential determinants for the choice of KRT modality | n.a. |
|
| Prospective cohort study117 | Patients with CKD stage 4–5 | Total=177 | Post-intervention: patient and clinical characteristics, patient choice of dialysis modality, potential determinants for the choice of KRT modality | n.a. |
|
|
| No. 35 | Pre-post study99 | Patients with an eGFR <30 mL/min/1.73 m2 | Total=25 |
|
n.a. |
|
| No. 36 | Meeting abstract of retrospective cohort study109 | Patients with an eGFR <20 mL/min/1.73 m2 | Total=460 | Post-intervention: patient and clinical characteristics, dialysis modality selection after options class, dialysis modality initiation after options class | n.a. |
|
| No. 37 | Meeting abstract of pre-post study110 | Patients with CKD | Total=39
|
Pre-intervention and post-intervention/control: self-assessed level of comprehension of KRT modalities | Pre-intervention and post-intervention/control: utilisation of written resources provided, an assessment of the factors that influence participants’ selection on their choice of modality |
|
| What type of transplantation? | ||||||
| No. 38 | Non-randomised controlled study100 | Kidney transplant candidates | Total=20
|
Pre-intervention, immediately and 1 month post-intervention/control: self-reported discussion of LDKT and/or DDKT |
|
|
| No. 39 | Multicentre RCT101 | Patients on dialysis | Total=253
|
Pre-intervention and 1 month post-intervention/control: patients’ readiness to allow someone to be a living donor, patients’ readiness to get on the DDKT wait list |
|
|
| No. 40 | Multicentre RCT102 | Black and low-income patients on dialysis | Total=561
|
Pre-intervention and post-intervention/control: DDKT and LDKT knowledge |
|
|
| No. 41 | RCT103 | Kidney transplant candidates | Total=802
|
Pre-intervention, 4 and 8 months post-intervention/control: patients’ readiness to pursue DDKT and LDKT |
|
|
*Population formulated as reported in the identified records.
†Outcomes formulated as reported in the identified records.
‡Main findings formulated as reported in the identified records.
ACTS, living about choices in transplantation and sharing; CAPD, continuous ambulatory peritoneal dialysis; CCM, conservative care management; CKD, chronic kidney disease; COACH, communicating about choices in transplantation; DDKT, deceased donor kidney transplantation; eGFR, estimated glomerular filtration rate; EPs, educational programmes; ESKD, end-stage kidney disease; HD, Haemodialysis; HKTP, Hispanic kidney transplant programme; KRT, Kidney replacement therapy; LDKT, Living donor kidney transplantation; MDC, multidisciplinary care; n.a., not applicable; PD, Peritoneal dialysis; PDEP, Predialysis education programme; RCT, Randomised controlled trial; SDM, shared decision-making; TALK, talking about live kidney donation; TOP, treatment options programme.
EPs were generally evaluated for their effects on health-related outcomes and on knowledge, communication and patient activation. None of the EPs were evaluated for their effects on SDM. Thirteen (n=13, 31.7%) EPs (no.1, 8, 9, 10, 11, 21, 34, 35, 36, 37, 39, 40, 41) were evaluated in studies published after the standardised outcome sets for CKD, dialysis and transplantation were published by ICHOM and SONG. None of these EPs were evaluated with these outcomes. EPs that promote particular treatment modalities (no.1, 5, 11, 13, 14, 22–28) appear to increase the number of patients planning to start with the promoted modalities (see table 4). Patients exposed to EPs generally had more favourable health-related outcomes than patients that were not exposed to EPs (see table 4). They were also more knowledgeable about their treatment options, better equipped to communicate about their treatment options and more active in choosing and requesting a preferred treatment modality (see table 4).
Patient decision aids
We identified 27 PtDAs (excluding two currently under investigation). Fourteen were identified in peer-reviewed articles.57 119–131 Thirteen were identified in the grey literature,132–141 seven of which in meeting abstracts.132–135
Table 5 provides an overview of the identified PtDAs and their characteristics. Table S5 in online supplemental appendix 4 provides additional details on the identified PtDAs (eg, sources for publicly available PtDAs). One PtDA helps patients choose whether or not they should start with dialysis (no. 1) and one helps them decide when to start with dialysis if they decide to do so (no. 2). Nine help patients choose between dialysis options (no. 3–11) and five help them choose between transplantation and dialysis options (no. 12–16). One PtDA helps patients decide whether or not they want to accept an infectious risk donor kidney donation offer (no. 17). Four help patients choose between dialysis and CCM options (no. 18–21). Six help patients choose between transplantation, dialysis and CCM options (no. 22–27).
Table 5.
Overview of the identified PtDAs and their characteristics
| PtDA | Format | Treatment options* | Values-clarification/preference elicitation exercise(s) | Patent participation in development | Reading level | Implemented | Evaluated |
| Start with dialysis or not? | |||||||
| No. 1: Kidney failure - should I start dialysis? |
Interactive website |
|
Yes | No | n.a. | Not reported | No |
| When to start dialysis? | |||||||
| No. 2: Kidney failure—when should I start dialysis? |
Interactive website |
|
Yes | No | n.a. | Not reported | No |
| What type of dialysis modality? | |||||||
| No. 3: My life, my dialysis choice |
Interactive website |
|
Yes | Yes | n.a. | Not reported | No |
| No. 4: Kidney failure— what type of dialysis should I have? |
Interactive website |
|
Yes | No | n.a. | Not reported | No |
| No. 5: Yorkshire Dialysis Decision Aid (YODDA) - web |
Interactive website |
|
Yes | Yes | Eighth to ninth grade level | Not reported | No |
| No. 6: YODDA - booklet |
PDF document (48 pages) |
|
Yes | Yes | Eighth to ninth grade level | Yes | Yes |
| No. 7: Shared end-stage renal patients decision-making (SHERPA-DM) option grid |
PDF document (1 page) |
|
No | Yes | n.a. | Yes | Yes |
| No. 8: SHERPA-DM decision aid |
PDF document (4 pages) |
|
Yes | Yes | n.a. | Yes | Yes |
| No. 9: Dialysis choice |
|
|
Yes | Yes | n.a. | Yes | Yes |
| No. 10: The dialysis guide |
Interactive application |
|
Yes | No | n.a. | Not reported | Yes |
| No. 11: Choosing dialysis —empowering patients on choices for renal replacement therapy (EPOCH-RRT) decision aid |
Interactive website |
|
Yes | Yes | n.a. | Not reported | Yes |
| Transplantation or dialysis? | |||||||
| No. 12: Kidney transplant P3 (patient provider partnerships) |
Interactive website |
|
No | Yes | n.a. | Not reported | No |
| No. 13: iChoose kidney | Interactive website |
|
No | Yes | n.a. | Yes | Yes |
| No. 14: My transplant coach |
Interactive website |
|
No | Yes | n.a. | Not reported | Yes |
| No. 15: To choose the treatment that suits you for kidney disease |
PDF document (16 pages) |
|
Yes | No | n.a. | Yes | No |
| No. 16: Option grid: KRT |
PDF document (1 page) |
|
No | Yes | Common European framework of reference level B1 | Yes | Yes |
| Accept or decline IRD kidney offer? | |||||||
| No. 17: Inform me: about increased risk donor kidneys |
Interactive website | DDKT | No | No | n.a. | Not reported | Yes |
| Dialysis or CCM? | |||||||
| No. 18: OPTIONS tool |
|
|
Yes | No | Eighth grade level | Not reported | Yes |
| No. 19: The conservative kidney management decision aid |
Interactive website |
|
Yes | Yes | n.a. | Not reported | No |
| No. 20: Yorkshire dialysis and conservative care decision aid |
PDF document (28 pages) |
|
Yes | Yes | n.a. | Not reported | No |
| No.21: Supportive kidney care video decision aid |
1 video (11.5 min) |
|
No | No | n.a. | Not reported | Yes |
| Transplantation, dialysis or CCM? | |||||||
| No. 22: The option grid - chronic kidney disease treatment options |
PDF document (1 page) |
|
No | Yes | n.a. | Not reported | Yes |
| No. 23: Patient decision aid—kidney failure treatment options |
PDF document (28 pages) |
|
Yes | No | n.a. | Not reported | No |
| No.24: Providing resources to enhance African-American patients' readiness to make decisions about kidney disease decision aid |
|
|
Yes | Yes | Fourth to sixth grade level | Not reported | Yes |
| No. 25: My kidneys, my choice |
|
|
Yes | Yes | n.a. | Yes | Yes |
| No. 26: The Dutch kidney guide |
Website |
|
No | Yes | n.a. | Yes | Yes |
| No.27: Option grid: KRT versus CCM | PDF document (1 page) |
|
No | Yes | Common European framework of reference level B1 | Yes | Yes |
*Treatment options formulated as reported in the identified records.
APD, ambulatory peritoneal dialysis; CAPD, continuous ambulatory peritoneal aialysis; CCM, conservative care management; DDKT, deceased donor kidney transplantation; HD, Haemodialysis; IRD, increased risk donors; KRT, kidney replacement therapy; LDKT, living donor kidney transplantation; n.a., not available; PD, peritoneal dialysis; PtDAs, patient decision aids; RRT, renal replacement therapy.
Most PtDAs were developed for the general population of patients with AKD, a minority (n=3, 11.1%) were specifically developed for elderly patients with AKD (no.18, 19, 21).
A large proportion of PtDAs (n=23, 85.2%) consist of a single medium format (no.1–8, 10–17, 19–23, 26, 27). Most of these PtDAs (n=11, 47.8%) are interactive websites (no.1–5, 11–14, 17). The remaining PtDAs consist of a combination of different medium formats. A majority of the PtDAs (n=17, 62.9%) contain values-clarification and preference-elicitation exercises (no.1–6, 8–11, 15, 18–20, 23–25).
Two-thirds of the PtDAs (n=18, 66.6%) were developed with the input of patients (no.3, 5–9, 11–14, 16, 19, 20, 22, 24–27), one of which was largely developed with the input of African-American patients (no.24). A minority (n=6, 22.2%) describe a reading level (no.5, 6, 16, 18, 24, 27). Only a few PtDAs (n=5, 18.5%) are not publicly available (no.7, 8, 18, 21, 24).
Ten PtDAs (n=10, 37.0%) were reported to have been implemented in clinical practice (no.6–9, 13, 15, 16, 25–27). The majority (n=17, 62.9%) have been evaluated for their effects on outcomes in the intended users (no.6–11, 13, 14, 16–18, 21, 22, 24–27).
Table 6 provides an overview of the IPDAS minimum standards component scores for 26 of the identified PtDAs. Table S6 in online supplemental appendix 4 provides these scores in greater detail. One PtDA (no. 21) could not be scored according to these criteria because the accompanying documentation did not provide enough information. Decision support interventions have to meet six qualifying criteria to qualify as PtDAs. Just about half (n=13, 48.1%) met all qualifying criteria (no.1, 4–7, 9, 11, 14, 18–20, 23–25). The sixth qualifying criterium (‘the PtDA describes what it is like to experience the consequences of the options’) was the least met criterium by the other PtDAs.
Table 6.
IPDAS minimum standards component scores of the identified PtDAs
| PtDA | Total score | IPDAS-Q1 | IPDAS-Q2 | IPDAS-Q3 | IPDAS-Q4 | IPDAS-Q5 | IPDAS-Q6 | IPDAS-C1 | IPDAS-C2 | IPDAS-C3 | IPDAS-C4 | IPDAS-C5 | IPDAS-C6 |
| No.1 | 9 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | Yes |
| No. 2 | 9 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | No | Yes |
| No. 3 | 8 | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes |
| No .4 | 9 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | Yes |
| No .5 | 10 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes |
| No .6 | 10 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes |
| No .7 | 9 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No |
| No .8 | 3 | No | Yes | Yes | No | No | No | No | No | Yes | No | No | No |
| No .9 | 8 | Yes | Yes | Yes | Yes | Yes | No | Yes | No | Yes | No | No | Yes |
| No .10 | 7 | Yes | Yes | Yes | Yes | Yes | No | Yes | No | Yes | No | No | No |
| No .11 | 10 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes |
| No .12 | 3 | No | No | Yes | Yes | Yes | No | No | No | No | No | No | No |
| No .13 | 5 | Yes | Yes | Yes | No | No | No | No | Yes | Yes | No | No | Yes |
| No .14 | 7 | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | No | No | Yes |
| No .15 | 5 | Yes | Yes | Yes | No | No | No | No | No | Yes | No | No | Yes |
| No .16 | 7 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | No | No | Yes |
| No .17 | 8 | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | No | Yes |
| No .18 | 10 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes |
| No .19 | 11 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| No .20 | 11 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| No .21 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| No .22 | 6 | No | No | Yes | Yes | Yes | No | No | Yes | Yes | Yes | No | No |
| No .23 | 8 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | No |
| No .24 | 11 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| No .25 | 8 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | No | Yes |
| No .26 | 8 | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | No | No | Yes |
| No .27 | 7 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | No | No | Yes |
IPDAS, International Patient Decision Aids Standards; n.a., not available; PtDAs, patient decision aids.
Table 7 provides an overview of the studies70 121–127 129 130 132 135 142–145 that evaluated 17 PtDAs (no.6–11, 13, 14, 16–18, 21, 22, 24–27). Table S7 in online supplemental appendix 4 provides additional details on these studies. Most (n=8, 47.0%) were evaluated in experimental studies (no.6, 11, 13, 17, 18, 21, 24, 25), three-quarter of which (n=6, 75.0%) were RCTs.70 124 126 127 129 145 Five (n=5, 29.4%) PtDAs (no.7, 8, 16, 26, 27) were evaluated in studies presented in meeting abstracts.132 135 The remaining four (n=4, 23,5%) PtDAs (no.9, 10, 14, 22) were evaluated in observational studies142 144 and mixed-methods studies,122 123 125 130 143 two of which included pilot evaluations.122 125
Table 7.
Overview of studies evaluating the identified PtDAs
| PtDA | Source | Population* | Sample size | Primary outcome(s)† | Secondary outcome(s)† | Main findings‡ |
| What type of dialysis modality? | ||||||
| No. 6 | Multicentre non-randomised controlled study121 | Patients with CKD referred for predialysis services | Total=189
|
|
n.a. |
|
| No. 7 | Meeting abstract of pilot study132 |
|
Total=38 (17 patients) |
Post-intervention: outcomes of acceptability, usability and feasibility of integrating the interventions into existing care models | n.a. |
|
| No. 8 | ||||||
| No.9 | Mixed methods: development and pilot study122 | Patients with an eGFR <20 mL/min/1.73 m2 | Total=137
|
Post-intervention: patient-reported SDM, decisional quality, the patient’s choice of dialysis modality, registration of the dialysis mode for patients starting dialysis | n.a. |
|
| Qualitative study: interviews142 | Patients with an eGFR <20 mL/min/1.73 m2 | Total=349
|
Post-intervention: patients’ experiences on the impact of SDM and Dialysis Choice (DC) on their involvement in the decision-making process | n.a. |
|
|
| Mixed methods: questionnaires and interviews143 | Patients with an eGFR <20 mL/min/1.73 m2 | Total=349
|
Post-intervention: patient-reported SDM, decisional quality, results of semi-structured interviews | n.a. |
|
|
| Qualitative study: interviews144 | Patients with an eGFR <20 mL/min/1.73 m2 | Total=349
|
3 months after dialysis initiation: results of semi-structured interviews | n.a. |
|
|
| No. 10 | Mixed methods: development and evaluation123 | Patients with an eGFR between 10 and 20 mL/min/1.73 m2 | Total=22 |
|
n.a. |
|
| No. 11 | RCT124 | Patients with an eGFR <25 mL/min/1.73 m2 | Total=133
|
|
|
|
| Transplantation or dialysis? | ||||||
| No. 13 | Multicentre RCT70 | Patients with ESKD and on dialysis for <1 year | Total=470
|
Pre-intervention and immediately post-intervention/control: transplant knowledge |
|
|
| No. 14 | Mixed methods: development and pilot study125 | Patients considering renal transplantation | Total=81 |
|
n.a. |
|
| No. 16 | Meeting abstract of prospective cohort study135 |
|
Total=293 (176 patients) | Post-intervention: patient-reported SDM, SDM awareness and use of the Option grid: KRT, the Dutch Kidney Guide and the Option grid: KRT versus CCM by healthcare professionals | n.a. |
|
| Accept or decline IRD kidney offer? | ||||||
| No. 17 | RCT126 | Kidney transplant candidates | Total=288
|
Immediately and at 1 week post-intervention/control: IRD knowledge kidneys, willingness to accept an IRD kidney offer, experiences with Inform Me | Pre-intervention/control: patient characteristics, health literacy, health numeracy |
|
| Dialysis or CCM? | ||||||
| No. 18 | RCT127 | Patients >70 years of age with AKD | Total=41
|
1 month and 3 months post-intervention/control: decisional regret, decisional conflict |
|
|
| No. 21 | Multicentre RCT129 | Patients >65 years of age with an eGFR <25 mL/min/1.73 m2 | Total=104
|
Pre-intervention and post-intervention/control: supportive kidney care knowledge |
|
|
| Transplantation, dialysis or CCM? | ||||||
| No. 22 | Mixed methods: development and evaluation130 | Patients with an eGFR <20 mL/min/1.73 m2 | Total=65 | Pre-intervention and 2 months post-intervention: decisional quality | n.a. |
|
| No. 24 | Multicentre RCT145 | Self-reported African-Americans with ESKD <2 years | Total=92
|
1, 3 and 6 months post-intervention/control: discussing LDKT with family members, discussing LDKT with their doctor, initiation of the recipient medical evaluation for LDKT, completion of the recipient evaluation for LDKT, identification of a potential live kidney donor, participants beliefs about kidney transplant and their concerns about LDKT |
|
|
| No. 25 | Multicentre pre-post study178 | Patients referred for ESKD education | Total=97 | Pre-intervention and post-intervention: patient characteristics, knowledge, worries, values and decision-making experience with the decision-aid, experienced education-methods, utilisation level of the decision-aid, whether decision-making involved significant others, ranking of preferred treatment options | n.a. |
|
| No. 26 | Meeting abstract of prospective cohort study135 |
|
Total=293 (176 patients) | Post-intervention: patient-reported shared decision-making, SDM awareness and use of the Option grid: KRT, the Dutch Kidney Guide and the Option grid: KRT versus CCM by healthcare professionals | n.a. |
|
| No. 27 | ||||||
*Population formulated as reported in the identified records.
†Outcomes formulated as reported in the identified records.
‡Main findings formulated as reported in the identified records.
AKD, advanced kidney disease; CCM, conservative care management; CKD, Chronic Kidney Disease; DDKT, deceased donor kidney transplantation; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; HD, Haemodialysis; IRD, increased risk donors; KRT, Kidney Replacement Therapy; LDKT, Living Donor Kidney Transplantation; n.a., not applicable; PD, peritoneal dialysis; PREPARED, providing resources to enhance African-American patients' readiness to make decisions about kidney disease; PtDAs, patient decision aids; RCT, randomised controlled trial; RRT, renal replacement therapy; SDM, shared decision-making; YODDA, Yorkshire dialysis decision aid.
PtDAs were generally evaluated for their effects on health-related outcomes, and on knowledge, decisional quality and patient activation. Only one PtDA (no.7) was evaluated for its effects on SDM.122 143 One meeting abstract presented a study that evaluated whether SDM scores differed among hospitals if PtDAs (no.16, 26, 27) were used or not.135 Ten (n=10, 37.0%) PtDAs (no.9, 10, 11, 13, 16, 18, 21, 24, 26, 27) were evaluated in studies published after the standardised outcome sets for CKD, dialysis and transplantation were published by ICHOM and SONG. None of these PtDAs were evaluated with these outcomes. Patients that used PtDAs were generally more knowledgeable about their treatment options, and had better scores of decisional quality and patient activation than patients that did not use PtDAs (see table 7). The two studies that evaluated the PtDA (no.7) on outcomes of SDM showed that patients experienced the intervention as SDM (see table 7). The meeting abstract that presented a study that evaluated whether SDM scores differed among hospitals if PtDAs were used or not showed that hospitals that used these PtDAs (no.16,26,27) generally had better scores for SDM compared with hospitals that did not use them (see table 7). However, these differences were not significant (see table 7).
Multicomponent initiatives
We identified nine MIs (excluding four currently under investigation). Eight were identified in peer-reviewed articles,146–155 one was identified in two meeting abstracts found in the grey literature.156 157
Table 8 provides an overview of the identified MIs and their characteristics. Table S8 in online supplemental appendix 4 provides additional details on the identified MIs (eg, sources for publicly available MIs). One MI was developed to promote PD (no.1) and helps patients choose whether to start with PD or not. One MI as developed to help patients choose between dialysis options (no.2). Two MIs help patients choose between transplantation and dialysis options (no.3,4), one of which promotes transplantation and strives to reduce racial disparities in access to kidney transplantation (no.3). Five help patients choose between transplantation, dialysis and CCM options (no.5–9), with three promoting a particular treatment modality (no.5,7,9). Almost all MIs were developed for the general population of patients with AKD, one (no.8) was specifically developed for suboptimal dialysis initiation patients.
Table 8.
Overview of the identified MIs and their characteristics
| MI | Format | Treatment options* | Values-clarification/preference elicitation exercise(s) | Promotes treatment modality | Coaching | Patient participation in development | Reading level | Implemented | Evaluated |
| Start PD or not? | |||||||||
| No. 1: Kidney Disease Therapy Society cooperative programme |
|
|
No | PD | No | No | n.a. | Yes | Yes |
| What type of dialysis modality? | |||||||||
| No. 2: structured modality information programme |
|
|
Yes | No | Yes | No | n.a. | Yes | Yes |
| Transplantation or dialysis? | |||||||||
| No. 3: Reducing Disparities in Access to KidNey Transplantation |
|
|
Yes | Transplantation | Yes | No | n.a. | Yes | Yes |
| No. 4: shared decision-making programme |
|
|
Yes | No | Yes | No | n.a. | Not reported | Yes |
| Transplantation, dialysis or CCM? | |||||||||
| No. 5: Haemodialysis Orientation Unit |
|
|
No | Self-care dialysis modalities | No | Yes | n.a. | Yes | Yes |
| No. 6: shared decision-making process for kidney replacement therapy choice |
|
|
Yes | No | Yes | No | n.a. | Yes | Yes |
| No. 7: GUIDE |
|
|
Yes | Transplantation | Yes | No | n.a. | Yes | Yes |
| No. 8: Unplanned dialysis start (UPS) |
|
|
Yes | No | Yes | No | n.a. | Yes | Yes |
| No. 9: shared decision-making for renal replacement therapy |
|
|
Yes |
|
Yes | No | n.a. | yes | Yes |
*Treatment options formulated as reported in the identified records.
ACTS, living about choices in transplantation and sharing; APD, ambulatory peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; CCM, conservative care management; CKD, chronic kidney disease; DDKT, deceased donor kidney transplantation; HD, Haemodialysis; KRT, kidney replacement therapy; LDKT, living donor kidney transplantation; n.a., not available; PD, peritoneal dialysis; RRT, renal replacement therapy.
All MIs include an educational component for patients. One MI also educates clinicians (no.3). Two-thirds (n=6, 66.7%) include decision support interventions or other tools that help patients make values-based and preferences-based decisions (no.2, 3, 6–9). One MI (no.3) includes a previously identified PtDA (no.13) and components of a previously identified EP (no.26). The majority (n=7, 77.8%) of MIs (no.2–4, 6–9) use coaches to support patients and guide them through the programme. Almost half of the MIs (n=4, 44.4%) were developed as quality improvement initiatives and include nationwide, or facility level, policy and protocol changes (no.1, 3, 5, 8). Some MIs (n=3, 33.3%) were specifically developed to implement SDM in clinical practice (no.4, 6, 9).
Only one MI (no.4) was developed with the input of patients. None contain reading level information. Only one MI (no.3) consists of components that are publicly available.
All but one MI (no.4) were reported to have been implemented in clinical practice. All MIs have been evaluated for their effects on outcomes in the intended users, most of which (n=8, 88.9%) were patients (no.1, 2, 4–9). One MI (no.3) was evaluated for its effects on a dialysis facility level.
Table 9 provides an overview of the studies146–157 that evaluated the identified MIs. Table S9 in online supplemental appendix 4 provides additional details on these studies. Two-thirds of MIs (n=6, 66.6%) were evaluated in observational studies (no.1, 5–9), three of which (n=3, 33.3%) were prospective cohort studies.146 149–153 155 A minority (n=2, 22.2%) were evaluated in experimental studies (no.3, 4), only one of which was an RCT.147 One MI (no. 2) was evaluated in two sequential studies presented in two meeting abstracts.156 157
Table 9.
Overview of studies evaluating the identified MIs
| MI | Source | Population* | Sample size | Primary outcome(s)† | Secondary outcome(s)† | Main findings‡ |
| Start PD or not? | ||||||
| No. 1 | Quality improvement report146 | Patients with CKD in the advanced stage | Total=63 | Post-intervention: PD selection rate | n.a. | After the intervention there was an increase in PD selection from 8.8% to 15% over 2 years |
| What type of dialysis modality? | ||||||
| No. 2 | Meeting abstract of retrospective cohort study156 |
|
Total=1141 | Post-intervention: patient clinical characteristics, use of decision-making tools by patients, KRT modality choice, KRT modality start | n.a. |
|
| Meeting abstract of retrospective cohort study157 | Patients with stage 4 and 5 CKD | Total=2012 | Post-intervention: patient clinical characteristics, use of decision-making tools by patients, KRT modality choice, KRT modality start | n.a. |
|
|
| Transplantation or dialysis? | ||||||
| No. 3 | Multicentre RCT147 | Dialysis facilities | Total=134
|
Pre-intervention and 12 months post-intervention: facility-level transplant referral |
|
|
| Multicentre prospective cohort study154 | Dialysis facility staff members | Total=94 |
|
n.a. |
|
|
| No. 4 | Quasi-experimental study148 | Patients with an eGFR <30 mL/min/1.73 m2 | Total=72
|
|
n.a. |
|
| Transplantation, dialysis or CCM? | ||||||
| No. 5 | Retrospective cohort study149 | Patients on HD | Total=93 | Post-intervention: number of patients treated in the HD orientation unit, patient and clinical characteristics, distribution of treatment modality 1 year after operation of the HD orientation unit | n.a. |
|
| No. 6 | Multicentre prospective cohort study150 | Patients with CKD | Total=1044
|
Post-intervention: patient and clinical characteristics, KRT choice and treatment initiation, chosen KRT modality and definitive KRT modality | n.a. |
|
| No. 7 | Retrospective cohort study151 | Patients with eGFR <15 mL/min/1.73 m2 | Total=102 |
|
n.a. |
|
| No. 8 | Multicentre prospective cohort study152 | Unplanned dialysis start patients | Total=270
|
Up to 12 months post-intervention: patient and clinical characteristics, the number of patients receiving and completing the programme, the number of patients making a dialysis modality decision, the final dialysis modality chosen, the number of patients receiving their chosen modality | n.a. |
|
| Multicentre prospective cohort study155 | Unplanned dialysis start patients | Total=270
|
Pre-intervention, 6 and 12 months post-intervention: patient and clinical characteristics, dialysis modality, details of when changed if changed, details of access procedures if changed, details of dialysis-related infectious events, number and length of hospitalisations, predictors of receiving PD or HD, flow of patients through the programme | n.a. |
|
|
| No. 9 | Retrospective cohort study153 | Patients with an eGFR <15 mL/min/1.73 m2 | Total=310
|
1 year post-intervention: patient- and clinical characteristics, evaluated for LDKT, receiving a LDKT, receiving PD or HD | n.a. |
|
*Population formulated as reported in the identified records.
†Outcomes formulated as reported in the identified records.
‡Main findings formulated as reported in the identified records.
CCI, Charlson Comorbidity Index; CCM, conservative care management; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HD, Haemodialysis; KRT, kidney replacement therapy; LDKT, living donor kidney transplantation; MIs, multicomponent initiatives; n.a., not applicable; PD, peritoneal dialysis; RADIANT, Reducing Disparities in Access to KidNey Transplantation; RCT, randomised controlled trial; RRT, renal replacement therapy; SDM, shared decision-making; UPS, unplanned dialysis start.
MIs were generally evaluated for their effects on health-related outcomes and on patient activation. None of the MIs were evaluated for their effects on SDM. Four (n=4, 44.4%) MIs (no.2, 3, 4, 9) were evaluated in studies published after the standardised outcome sets for CKD, dialysis and transplantation were published by ICHOM and SONG. None of these MIs were evaluated with these outcomes. Patients exposed to MIs generally had more favourable health-related outcomes (see table 9). They were also more active in choosing and requesting treatments (see table 9). MIs that promote particular treatment modalities (no.1, 3, 5, 7, 9) appear to increase the number of patients planning to start with these modalities (see table 9). The MI that was evaluated for its effects on a dialysis facility level (no.3) reported significant changes in the proportion of patients referred for transplantation147 and high fidelity to the intervention.154 It also appeared to reduce racial disparities in access to kidney transplantation.147
Interventions currently under development or investigation
We identified 16 interventions currently under investigation. The majority of these interventions (n=12, 75.0%) were identified in the grey literature,158–169 the remaining interventions (n=4, 25.0%) were identified in published protocol papers.170–173
Table 10 provides an overview of these interventions. Table S10 in online supplemental appendix 4 provides additional details on these interventions. More than half (n=10, 62.5%) are EPs (no.1, 2, 4, 5, 7–11, 16), one-quarter (n=4, 25.0%) are MIs (no.3, 6, 14, 15) and one-eighth (n=2, 12.5%) are PtDAs (no.12,13). Four interventions help patients choose between dialysis options (no.1–4), two of which promote a particular treatment modality (no.1, 4). Seven interventions promote LDKT and help patients choose whether or not to pursue LDKT (no.5–11). Two help patients choose between dialysis or CCM options (no.12, 13). Only one helps patients choose between transplantation, dialysis and CCM options (no.14). Two help patients choose between transplantation options (no.15, 16).
Table 10.
Overview of interventions currently under development or investigation
| Intervention | Type of intervention | Format | Treatment options* | Values-clarification/preference elicitation exercise(s) | Coaching | Promotes treatment modality |
| What type of dialysis modality? | ||||||
| No. 1: Web-based Interactive Health Communication Application for successful Home Dialysis (WISHED) |
EP | Interactive application (n.a.) |
|
No | No | Home dialysis |
| No. 2: Chronic Kidney Disease Enhanced Dialysis Education |
EP | Multiple sessions of individual education and coaching with coaches (n.a.) | Dialysis | No | Yes | No |
| No. 3: decision support intervention |
MI |
|
|
Yes | Yes | No |
| No. 4: comprehensive preESRD patient education |
EP | Three sessions of individual education with educators (n.a.) |
|
No | No | Home dialysis modalities |
| Pursue LDKT or not? | ||||||
| No. 5: house calls educational intervention |
EP |
|
|
No | Yes | LDKT |
| No. 6: house calls+web-based decision support |
MI |
|
|
Yes | Yes | LDKT |
| No. 7: house calls+peer mentorship |
EP |
|
|
No | Yes | LDKT |
| No. 8: destination transplant |
EP |
|
|
No | Yes | LDKT |
| No. 9: Talking about Live Kidney Donation (TALK) |
EP |
|
LDKT | No | Yes | LDKT |
| No. 10: communicating about choices in transplantation |
EP |
|
|
No | No | LDKT |
| No. 11: Living About Choices in Transplantation and Sharing (ACTS)—website |
EP |
|
LDKT | No | No | LDKT |
| Dialysis or CCM? | ||||||
| No. 12: Decision Aid for Renal Therapy (DART) |
PtDA | Interactive website |
|
Yes | No | No |
| No. 13: decision aid for elderly patients with kidney failure |
PtDA |
|
|
Yes | No | No |
| Transplantation, dialysis or CCM? | ||||||
| No. 14: Patient-centred kidney transition care |
MI |
|
|
Yes | Yes | No |
| What type of transplantation? | ||||||
| No. 15: Enhance Access to Kidney Transplantation and Living Kidney Donation |
MI |
|
Kidney transplantation | No | No | Kidney transplantation |
| No. 16: Explore transplant at home |
EP |
|
|
Yes | No | No |
*Treatment options formulated as reported in the identified records.
CCM, Conservative Care Management; DDKT, Deceased Donor Kidney Transplantation; EP, Educational Programme; ESRD, End-Stage Renal Disease; HD, haemodialysis; KFRE, Kidney Failure Risk Equation; LDKT, Living Donor Kidney Transplantation; MI, Multicomponent Initiative; PD, Peritoneal Dialysis; PtDA, Patient Decision Aid.
A quarter of these interventions (n=4, 25.0%) included components of previously identified interventions (no.8, 11, 14, 16), and two (n=2, 12.5%) are modifications (no.6, 7) of another intervention under investigation (no.5). Two interventions (n=2, 12.5%) have been previously evaluated and are undergoing additional evaluation (no.9, 10).
Half of the interventions (n=8, 50.0%) have been developed for specific patient populations, most of which (n=5, 62.5%) were developed for African-American, Hispanic or non-white race patients (no.5–8,11). Less (n=3, 37.5%) were developed for elderly patients (no.2, 12, 13). The remaining interventions can be used by the general population of patients with AKD.
Table 11 provides an overview of the studies158–173 evaluating these interventions. Table S10 in online supplemental appendix 4 provides additional details on these studies. Almost all of the interventions (n=15, 93.8%) will be evaluated in experimental studies, the majority (n=14, 93.3%) of which (no.1–9, 11, 12, 14–16) in RCTs.158–164 166 168–171 173 Selected outcomes include health-related outcomes, knowledge, communication, patient activation and decisional quality. None of the authors report evaluating these interventions for their effects on SDM. Nine (n=9, 56.3%) interventions (no.2, 3, 4, 8, 10, 11, 12, 14, 16) were identified in registries or protocol papers published after the standardised outcome sets for CKD, dialysis and transplantation were published by ICHOM and SONG. The authors for these interventions do not report evaluating their interventions with these outcomes. Six of the studies have completed their recruitment procedures,159 161 163 164 171 173 five are recruiting,158 160 162 165 172 three are active but not recruiting,166 168 169 one has an unknown recruitment status170 and one does not provide a recruitment status.167
Table 11.
Overview of current studies evaluating the novel interventions
| Intervention | Source | Population* | Study arms | Primary outcome(s)** | Secondary outcome(s)** | Recruitment status | Date of completion |
| What type of dialysis modality? | |||||||
| No. 1 | Multicentre RCT170 | Patients with an eGFR <20 mL/min/1.73 m2 | Intervention:
Control:
|
Within 3 months of dialysis initiation: the proportion of patients who receive any home-based dialysis modality | Pre-intervention, 6 and 12 months post-intervention/control: patient characteristics, the proportion of patients intending to perform home-based dialysis, dialysis knowledge, decisional conflict, level of social support | Not reported | Estimated study completion date: June 2017 |
| No. 2 | RCT158 | Patients >75 years of age with an eGFR <25 mL/min/1.73 m2 | Intervention:
Control:
|
Up to 24 months post-intervention/control: feasibility of the intervention, acceptability of the intervention | n.a. | Recruiting | Estimated completion date: May 2021 |
| No. 3 | RCT159 | Patients with CKD stage 5 | Intervention:
Control:
|
Post-intervention/control: control preference, knowledge, decisional self-efficacy, decisional conflict | Post-intervention/control: satisfaction with the decision, decisional regret | Completed | Actual study completion date: December 2019 |
| No. 4 | RCT160 | Veterans with CKD stage 4–5 | Intervention:
control:
|
Pre-intervention and 48 months post-intervention/control: home dialysis use |
|
Recruiting | Estimated completion date: September 2023 |
| Pursue LDKT or not? | |||||||
| No. 5 | RCT171 | Black kidney transplant candidates | Intervention:
Control:
|
Up to 2 years post-intervention/control: the occurrence of LDKT |
|
Completed | Actual completion date: June 2020 |
| No. 6 | RCT161 | Non-white race, Hispanic ethnicity or low-income kidney transplant candidates | Intervention:
Control:
|
Up to 2 years post-intervention/control: the occurrence of LDKT |
|
Completed | Actual completion date: May 2020 |
| No. 7 | Multicentre RCT162 | Black kidney transplant candidates | Intervention:
Control:
|
1 year post-intervention/control: the occurrence of LDKT |
|
Recruiting | Estimated completion date: January 2021 |
| No. 8 | RCT163 | Black kidney transplant candidates | Intervention:
Control:
|
Pre-intervention, 1 week and 9 months post-intervention/control: readiness to pursue LDKT |
|
Completed | Actual completion date: August 2018 |
| No. 9 | RCT164 | Patients with ESKD | Intervention:
Control:
|
Up to 43.6 months post-intervention/control: receipt of kidney transplant |
|
Completed | Actual completion date: August 2020 |
| No. 10 | Non-randomised controlled study165 | Patients on HD | Intervention:
Control:
|
Pre-intervention, 3 months and 1 year post-intervention/control: transplant knowledge, the number of transplant steps completed | 3 months and 1 year post-implementation/control: completion of transplant work-up, self-reported requests for living donation | Recruiting | Estimated completion date: July 2022 |
| No. 11 | Multicentre RCT172 | African-American kidney transplant candidates | Intervention:
Control:
|
12 months post-intervention/control: the proportion of patients with at least one living donor inquiry |
|
Recruiting | Estimated completion date: May 2022 |
| Dialysis or CCM? | |||||||
| No. 12 | Multicentre RCT166 |
|
Intervention:
Control:
|
Pre-intervention, 3, 6 and 18 months post-intervention/control: decisional conflict | Pre-intervention, 3, 6 and 18 months post-intervention/control: advance directives completion, healthcare evaluation, instability of patients’ preferences, patient/care-giver concordance on the goals of care | Active (not recruiting) | Estimated completion date: March 2022 |
| No. 13 | Mixed-methods study167 | Elderly patients with ESKD | Intervention:
Control:
|
Pre-intervention and post-intervention/control: treatment choice | n.a. | Not reported | Not reported |
| Transplantation, dialysis or CCM? | |||||||
| No. 14 | Multicentre RCT173 | Patients with AKD | Intervention:
Control:
|
Pre-intervention, 12, 24 and 36 months post-intervention/control: patient empowerment, confidence with self-care, proportion of patients deciding to initiate self-care treatment, hospitalisations, proportion of patients with advance care plans for kidney failure treatment preferences broadcast in electronic health record | Pre-intervention, 12, 24 and 36 months post-intervention/control: proportion of patients with self-care biomedical care plans, proportion of patients achieving values aligned care within 6 months of kidney failure treatment initiation, values and preferences documented in electronic health record, emergency dialysis initiation, time to kidney failure, vascular access at HD initiation, patient characteristics, financial distress/well-being, kidney function, presence and control of kidney disease progression risk factors, comorbid health conditions, depression, anxiety, need for mental health support, quality of life, self-management, diet, exercise, medication adherence, duration and frequency of care, patient centredness of care, health literacy, control preference, decisional conflict, decisional self-efficacy, patient activation, barriers to complex treatment plans, kidney transitions specialist adherence to protocol, sustainability of the intervention | Completed | Actual completion date: October 2020 |
| What type of transplantation? | |||||||
| No. 15 | Multicentre RCT168 | Dialysis facilities | Intervention:
Control:
|
Up to 2 years post-intervention/control: composite outcome of living kidney donor candidate referral and transplant recipient referral rate | Up to 2 years post-intervention/control: kidney transplantation rate, rate of pre-emptive kidney transplantation, rate of kidney transplant wait listing, average healthcare costs | Active (not recruiting) | Estimated completion date: March 2021 |
| No. 16 | Multicentre RCT169 | Patients with CKD stage 3–5 | Intervention:
Control:
|
Pre-intervention and 6 months post-intervention/control: DDKT and LDKT knowledge |
|
Active (not recruiting) | Estimated completion date: August 2020 |
*Study population formulated as reported in the identified records.
†Outcomes formulated as reported in the identified records.
AKD, advanced kidney disease; CKD, chronic kidney disease; CKD-EDU, chronic kidney disease enhanced dialysis education; COACH, communicating about choices in transplantation; DDKT, deceased donor kidney transplantation; eGFR, estimated glomerular filtration rate; ESKD, end stage kidney disease; ET, explore transplant; HD, haemodialysis; KRT, kidney replacement therapy; LDKT, living donor kidney transplantation; n.a., not applicable; PD, peritoneal dialysis; RCT, randomised controlled trial; SDM, shared decision-making; WISHED, web-based interactive health communication application for successful home dialysis.
Discussion
We identified a considerable number of interventions that can be used to support SDM for treatment modality decisions in AKD. We observed that there are interventions that support a decision between a limited number of treatment modalities, and that there are interventions that support a decision between all treatment modalities for kidney failure. Almost all PTs that we identified make predictions that support decisions encompassing a single action or a single treatment modality (eg, start or delay preparations for kidney failure, accept or reject LDKT offer, start or forego dialysis). One PT compares prognostic information of two treatment options (transplantation and dialysis), which can be used to help patients make a treatment modality decision based on their personal risks.57 Similarly, most of the EPs, PtDAs and MIs we identified either provide information on a single treatment modality (eg, dialysis) or on two different treatment modalities (eg, dialysis and transplantation). A minority of the identified interventions provide information on all treatment modalities for kidney failure, including CCM. We recommend that clinicians use interventions appropriate to the decisions their patients have to make. With regard to treatment modality decisions in AKD, patients go through a ‘hierarchy of nested decisions rather than a choice between discrete options’.19 For example, patients will first have to decide whether they would prefer KRT or CCM, after which those that opt for KRT have to choose between their treatment options (eg, transplantation or dialysis modalities). With the right tools, clinicians can help patients: (1) navigate this decision-making process and (2) make choices based on their values and preferences. Values-clarification and preference-elicitation are important in the deliberation phase of SDM, and may ultimately lead to making a shared decision based on what ‘matters most to patients’.9 10 Most of the PtDAs we identified contain exercises that help patients in this process, and in that regard offer more than just education alone. Ideally, clinicians should use a combination of EPs, PtDAs and PTs to support and engage their patients in the decision-making process and make treatment modality decisions according to the principles of SDM. MIs cater to this idea, but most of the MIs we identified combine education with decision aids or other tools that help patients make values-based and preference-based decisions. We identified one novel MI (no.14) that combines all three of these tools. This MI is currently being evaluated in an RCT, which may provide some of the first evidence on the effectiveness (on patient-reported, biomedical and health system outcomes) of an intervention that combines education with prognostic information and decision support for patients with AKD.173
We observed considerable variation in the level of detail provided regarding the content of the interventions we identified. Most researchers and developers gave information on the structure and the medium that were used for the interventions, but they generally left the topics that were discussed unmentioned. They also varied in how they called treatment modalities (eg, independent vs self-care dialysis), and in the level of detail in which they described treatment modalities (eg, PD vs CAPD and APD). We found most of this variation in EPs, which may be explained by a lack of standardisation to specify and report on their contents.20 On the contrary, for PtDAs the IPDAS criteria were developed to standardise and improve their contents, development, implementation and evaluation.174 These criteria are widely accepted, and researchers and developers use them to develop and score their PtDAs.121 126 131 We also scored the PtDAs we identified with these criteria and found that just about half officially qualified as PtDAs. There is a need for a similar set of criteria that can be used for the development, implementation and evaluation of EPs. The variation in the literature makes it difficult to understand causal relationships between the interventions and the reported outcomes. It also hampers the development of new and effective interventions because it limits the possibility of synthesising evidence and replicating effective interventions.20
About one-third of the interventions we identified were reported to have been implemented in clinical practice. We found that PTs were the interventions with the least information on implementation status, followed by PtDAs, EPs and MIs. Most of the PTs could not be used by both patients and clinicians because their developers only presented them as formulas. This has been noted before and limits their usability in clinical practice.21 In fact, the only PT that was reported to have been implemented was designed as a PtDA that can be used by both patients and clinicians. On the contrary, PtDAs are almost always either printed materials or interactive websites that patients can use at the convenience of their own time. This enhances their usability and makes them an important supplemental resource for patients to learn about their treatment options.175 EPs tend to vary in their components,20 but generally consist of multiple outpatient consultations or educational sessions that are supplemented with printed and/or audio-visual materials, websites and coaches that guide patients through the programme. Hospitals often develop their own proprietary EPs, which may explain why more than half of the EPs we identified were reported to have been implemented in clinical practice. Most of the MIs we identified were also reported to have been implemented in clinical practice, presumably because they were often part of quality improvement initiatives that used multifaceted implementation strategies to support the implementation process. Implementation is important because only this ultimately leads to patients actually using these interventions. It can also provide real-world evidence on their effects and on the effectiveness of different implementation strategies. The implementation of future interventions should be facilitated by developing them with usability in mind, and by offering them through implementation strategies that combine different approaches.176 In addition, involving stakeholders and end-users (eg, patients and clinicians) will also facilitate the implementation process.177 Overall, end-user participation was low in the development of the interventions we identified. It seems plausible that this might have affected their implementation rates, but we cannot support this with the available data.
There are significant knowledge gaps when it comes to the effects these interventions have on patients, on the decision-making process, on SDM, and on the effects that SDM has on both patients and the decision-making process. First of all, only about half of the interventions were evaluated for their effects. The majority of these interventions were MIs, EPs and PtDAs. The only PT that was evaluated was the PT that was designed as a PtDA.57 70 This PT was also evaluated as part of an MI, but its use was not mandatory so its contribution to the reported effects remains undetermined.147 More evidence is needed on how PTs can be used to support patients and clinicians in the decision-making process, and there are multiple validated PTs that can be used in future research projects.
Patients who were exposed to EPs, PtDAs and MIs generally had better outcomes than patients that were not exposed to these interventions. It is difficult to say which intervention was the most effective because the majority were evaluated in observational studies and the exact ‘ingredients’ that elicit the reported outcomes are often unknown.20 Moreover, there was considerable variation in the selection of outcomes used to evaluate these interventions. Also, none of the interventions that were evaluated in studies published after the standardised outcome sets for CKD, dialysis and transplantation from ICHOM and SONG were published were evaluated with these outcomes. This highlights a need for: (1) more experimental research and (2) a standardisation in the selection of outcome measures for health-related outcomes (eg, the ICHOM or SONG standard sets), as well as for knowledge, decisional quality and SDM.
Finally, there is a clear lack of evidence when it comes to the effects that these interventions have on SDM. Notably, none of the MIs that were explicitly developed to enhance SDM in clinical practice were evaluated for their effects on SDM.148 150 153 Only a minority of PtDAs were evaluated for their effects on SDM, and SDM outcomes were generally better in patients that used them.122 135 143 Interventions should be evaluated on outcomes of SDM, especially if the intention is to support and implement SDM through these interventions. It is unfortunate that none of the researchers and developers of the interventions currently under evaluation report evaluating these interventions for their effects on SDM.
This scoping review is unique in the fact that we did not limit ourselves to a certain type of intervention, or to interventions that were developed to support or promote a particular treatment modality decision. We also did not identify reviews that categorised interventions based on the treatment modality decision(s) they support like we did. Even though we limited our search query to records published in English, the proportion of excluded records published in another language was small. We did exclude a larger number of records due to the fact that they were not available to us, either because of: (1) subscription limitations, (2) internet protocol address geo-blocking or (3) stakeholders that did not reply to our emails requesting access to their contents. Nevertheless, we feel that the selection of interventions presented in this review is a realistic reflection of the current state of developments in this field. We hope it stimulates clinicians to use these interventions in clinical practice, and that it incentivises researchers and developers to address the knowledge gaps we identified.
Conclusions
This scoping review provides clinicians, researchers and other stakeholders with one comprehensive, but digestible source of information on interventions that can be used to support SDM for treatment modality decisions in AKD. The usability of these interventions for SDM largely depends on whether patients can use them to compare all their treatment options, and whether they contain questions or exercises that help patients make decisions based on their values and preferences. It also depends on whether patients can access them at the convenience of their own time, and on how easily they can be used during healthcare encounters. Clinicians interested in SDM can select interventions from this review based on these properties, and ideally combine interventions that complement each other.
The implementation of the identified interventions in clinical practice was moderate and most likely depended on usability and the presence or absence of an implementation strategy.
No conclusive advice can be given on which intervention is the most efficacious in supporting SDM for treatment modality decisions in AKD. Outcomes seem to be better in patients exposed to these interventions, but this is largely based on observational research. In addition, the effects of these interventions on SDM are under-reported. There is a definite need for more experimental research and a standardisation in the development, implementation and evaluation of these interventions.
Supplementary Material
Acknowledgments
The authors thank Dr J W Schoones of the Leiden University Medical Centre for his help in generating the search queries, and Drs J van Meel of the Maasstad Hospital for his help in managing the RefWorks database and in gaining access to paywalled articles. The authors would also like to thank Dr C F van Uden Kraan for her insight and recommendations regarding the design and content of this scoping review.
Footnotes
Twitter: @AMStiggelbout
Contributors: NE is the primary and corresponding author and was responsible for the first and all subsequent drafts of this scoping review. GNdG was the second reviewer and participated in the process of study selection and data extraction. MvdD, PvdN, WJB and AMS all participated in discussions on the study design, the design of data extraction forms and critically revised drafts for improvements before publication. The ICMJE criteria for authorship have been met, and all authors approved the final version to be published. NE is responsible as guarantor for the overal content of this scoping review.
Funding: This work was supported by ZonMW (registration no. 516007001) as part of the ‘Experiment Uitkomstindicatoren Santeon’.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.National Kidney Foundation . Global facts: about kidney disease. Available: https://www.kidney.org/kidneydisease/global-facts-about-kidney-disease [Accessed Apr 2022].
- 2.GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2020;395:709–33. 10.1016/S0140-6736(20)30045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kidney International Supplements . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Available: https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf [Accessed Apr 2022]. [DOI] [PubMed]
- 4.NICE guideline [NG203] . Chronic kidney disease: assessment and management. Available: https://www.nice.org.uk/guidance/ng203 [Accessed Apr 2022].
- 5.Mendelssohn DC, Malmberg C, Hamandi B. An integrated review of "unplanned" dialysis initiation: reframing the terminology to "suboptimal" initiation. BMC Nephrol 2009;10:22. 10.1186/1471-2369-10-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.RPA . Shared decision-making in the appropriate initiation of and withdrawal from dialysis. Available: https://cdn.ymaws.com/www.renalmd.org/resource/resmgr/Store/Shared_Decision_Making_Recom.pdf [Accessed Apr 2022].
- 7.Moss AH. Revised dialysis clinical practice guideline promotes more informed decision-making. Clin J Am Soc Nephrol 2010;5:2380–3. 10.2215/CJN.07170810 [DOI] [PubMed] [Google Scholar]
- 8.Winterbottom A, Bekker HL, Conner M, et al. Choosing dialysis modality: decision making in a chronic illness context. Health Expect 2014;17:710–23. 10.1111/j.1369-7625.2012.00798.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stiggelbout AM, Pieterse AH, De Haes JCJM. Shared decision making: concepts, evidence, and practice. Patient Educ Couns 2015;98:1172–9. 10.1016/j.pec.2015.06.022 [DOI] [PubMed] [Google Scholar]
- 10.Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision making: multistage consultation process. BMJ 2017;359:j4891. 10.1136/bmj.j4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Härter M, van der Weijden T, Elwyn G. Policy and practice developments in the implementation of shared decision making: an international perspective. Z Evid Fortbild Qual Gesundhwes 2011;105:229–33. 10.1016/j.zefq.2011.04.018 [DOI] [PubMed] [Google Scholar]
- 12.Härter M, Moumjid N, Cornuz J. Special Issue/Schwerpunkt: international accomplishments in shared decision making. Z Evid Fortbild Qual Gesundhwes 2017;123-124:1–5. [DOI] [PubMed] [Google Scholar]
- 13.Haesebaert J, Adekpedjou R, Croteau J, et al. Shared decision-making experienced by Canadians facing health care decisions: a web-based survey. CMAJ Open 2019;7:E210-E216. 10.9778/cmajo.20180202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Légaré F, Stacey D, Forest P-G, et al. Milestones, barriers and beacons: shared decision making in Canada inches ahead. Z Evid Fortbild Qual Gesundhwes 2017;123-124:23–7. 10.1016/j.zefq.2017.05.020 [DOI] [PubMed] [Google Scholar]
- 15.Durand M-A, Barr PJ, Walsh T, et al. Incentivizing shared decision making in the USA--where are we now? Healthc 2015;3:97–101. 10.1016/j.hjdsi.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 16.van Veenendaal H, van der Weijden T, Ubbink DT, et al. Accelerating implementation of shared decision-making in the Netherlands: an exploratory investigation. Patient Educ Couns 2018;101:2097–104. 10.1016/j.pec.2018.06.021 [DOI] [PubMed] [Google Scholar]
- 17.Engels N, de Graav G, van der Nat P, et al. Shared decision-making in advanced kidney disease: a scoping review protocol. BMJ Open 2020;10:e034142. 10.1136/bmjopen-2019-034142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raj R, Thiruvengadam S, Ahuja KDK, et al. Discussions during shared decision-making in older adults with advanced renal disease: a scoping review. BMJ Open 2019;9:e031427. 10.1136/bmjopen-2019-031427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winterbottom AE, Mooney A, Russon L, et al. Kidney disease pathways, Options and decisions: an environmental scan of international patient decision AIDS. Nephrol Dial Transplant 2020;35:2072–82. 10.1093/ndt/gfaa102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van den Bosch J, Warren DS, Rutherford PA. Review of predialysis education programs: a need for standardization. Patient Prefer Adherence 2015;9:1279–91. 10.2147/PPA.S81284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramspek CL, de Jong Y, Dekker FW, et al. Towards the best kidney failure prediction tool: a systematic review and selection aid. Nephrol Dial Transplant 2020;35:1527–38. 10.1093/ndt/gfz018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stacey D, Légaré F, Lewis K, et al. Decision AIDS for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2017;4:CD001431. 10.1002/14651858.CD001431.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.JBI . Welcome to the JBI manual for evidence synthesis. Available: https://jbi-global-wiki.refined.site/space/MANUAL [Accessed Apr 2022].
- 24.Preferred reporting items for systematic reviews and meta-analyses extension for scoping reviews (PRISMA-ScR) checklist. Available: http://www.prisma-statement.org/documents/PRISMA-ScR-Fillable-Checklist_11Sept2019.pdf [Accessed Apr 2022].
- 25.Durand M-A, Witt J, Joseph-Williams N, et al. Minimum standards for the certification of patient decision support interventions: feasibility and application. Patient Educ Couns 2015;98:462–8. 10.1016/j.pec.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 26.Sepucha KR, Abhyankar P, Hoffman AS, et al. Standards for universal reporting of patient decision aid evaluation studies: the development of SUNDAE checklist. BMJ Qual Saf 2018;27:380–8. 10.1136/bmjqs-2017-006986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ICHOM connect. Available: https://connect.ichom.org/standard-sets/chronic-kidney-disease/ [Accessed Apr 2022].
- 28.SONG. Available: https://songinitiative.org/ [Accessed Apr 2022].
- 29.Johnson ES, Thorp ML, Platt RW, et al. Predicting the risk of dialysis and transplant among patients with CKD: a retrospective cohort study. Am J Kidney Dis 2008;52:653–60. 10.1053/j.ajkd.2008.04.026 [DOI] [PubMed] [Google Scholar]
- 30.Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA 2011;305:1553–9. 10.1001/jama.2011.451 [DOI] [PubMed] [Google Scholar]
- 31.Drawz PE, Goswami P, Azem R, et al. A simple tool to predict end-stage renal disease within 1 year in elderly adults with advanced chronic kidney disease. J Am Geriatr Soc 2013;61:762–8. 10.1111/jgs.12223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marks A, Fluck N, Prescott GJ, et al. Looking to the future: predicting renal replacement outcomes in a large community cohort with chronic kidney disease. Nephrol Dial Transplant 2015;30:1507–17. 10.1093/ndt/gfv089 [DOI] [PubMed] [Google Scholar]
- 33.Norouzi J, Yadollahpour A, Mirbagheri SA, et al. Predicting renal failure progression in chronic kidney disease using integrated intelligent fuzzy expert system. Comput Math Methods Med 2016;2016:6080814. 10.1155/2016/6080814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tangri N, Inker LA, Hiebert B, et al. A dynamic predictive model for progression of CKD. Am J Kidney Dis 2017;69:514–20. 10.1053/j.ajkd.2016.07.030 [DOI] [PubMed] [Google Scholar]
- 35.Schroeder EB, Yang X, Thorp ML, et al. Predicting 5-year risk of RRT in stage 3 or 4 CKD: development and external validation. Clin J Am Soc Nephrol 2017;12:87–94. 10.2215/CJN.01290216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasegawa T, Sakamaki K, Koiwa F, et al. Clinical prediction models for progression of chronic kidney disease to end-stage kidney failure under pre-dialysis nephrology care: results from the chronic kidney disease Japan cohort study. Clin Exp Nephrol 2019;23:189–98. 10.1007/s10157-018-1621-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grams ME, Sang Y, Ballew SH, et al. Predicting timing of clinical outcomes in patients with chronic kidney disease and severely decreased glomerular filtration rate. Kidney Int 2018;93:1442–51. 10.1016/j.kint.2018.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foley RN, Parfrey PS, Hefferton D, et al. Advance prediction of early death in patients starting maintenance dialysis. Am J Kidney Dis 1994;23:836–45. 10.1016/s0272-6386(12)80137-5 [DOI] [PubMed] [Google Scholar]
- 39.Barrett BJ, Parfrey PS, Morgan J, et al. Prediction of early death in end-stage renal disease patients starting dialysis. Am J Kidney Dis 1997;29:214–22. 10.1016/s0272-6386(97)90032-9 [DOI] [PubMed] [Google Scholar]
- 40.Geddes CC, van Dijk PCW, McArthur S, et al. The ERA-EDTA cohort study--comparison of methods to predict survival on renal replacement therapy. Nephrol Dial Transplant 2006;21:945–56. 10.1093/ndt/gfi326 [DOI] [PubMed] [Google Scholar]
- 41.Mauri JM, Clèries M, Vela E, et al. Design and validation of a model to predict early mortality in haemodialysis patients. Nephrol Dial Transplant 2008;23:1690–6. 10.1093/ndt/gfm728 [DOI] [PubMed] [Google Scholar]
- 42.Couchoud C, Labeeuw M, Moranne O. French renal epidemiology and information network (REIN) registry. A clinical score to predict 6-month prognosis in elderly patients starting dialysis for end-stage renal disease. Nephrol Dial Transplant 2009;24:1553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Couchoud CG, Beuscart J-BR, Aldigier J-C, et al. Development of a risk stratification algorithm to improve patient-centered care and decision making for incident elderly patients with end-stage renal disease. Kidney Int 2015;88:1178–86. 10.1038/ki.2015.245 [DOI] [PubMed] [Google Scholar]
- 44.Dusseux E, Albano L, Fafin C, et al. A simple clinical tool to inform the decision-making process to refer elderly incident dialysis patients for kidney transplant evaluation. Kidney Int 2015;88:121–9. 10.1038/ki.2015.25 [DOI] [PubMed] [Google Scholar]
- 45.Weiss JW, Platt RW, Thorp ML, et al. Predicting mortality in older adults with kidney disease: a pragmatic prediction model. J Am Geriatr Soc 2015;63:508–15. 10.1111/jgs.13257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thamer M, Kaufman JS, Zhang Y, et al. Predicting early death among elderly dialysis patients: development and validation of a risk score to assist shared decision making for dialysis initiation. Am J Kidney Dis 2015;66:1024–32. 10.1053/j.ajkd.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doi T, Yamamoto S, Morinaga T, et al. Risk score to predict 1-year mortality after haemodialysis initiation in patients with stage 5 chronic kidney disease under predialysis nephrology care. PLoS One 2015;10:e0129180. 10.1371/journal.pone.0129180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wick JP, Turin TC, Faris PD, et al. A clinical risk prediction tool for 6-month mortality after dialysis initiation among older adults. Am J Kidney Dis 2017;69:568–75. 10.1053/j.ajkd.2016.08.035 [DOI] [PubMed] [Google Scholar]
- 49.Chen L-X, Josephson MA, Hedeker D, et al. A clinical prediction score to guide referral of elderly dialysis patients for kidney transplant evaluation. Kidney Int Rep 2017;2:645–53. 10.1016/j.ekir.2017.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haapio M, Helve J, Grönhagen-Riska C, et al. One- and 2-year mortality prediction for patients starting chronic dialysis. Kidney Int Rep 2017;2:1176–85. 10.1016/j.ekir.2017.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt RJ, Landry DL, Cohen L, et al. Derivation and validation of a prognostic model to predict mortality in patients with advanced chronic kidney disease. Nephrol Dial Transplant 2019;34:1517–25. 10.1093/ndt/gfy305 [DOI] [PubMed] [Google Scholar]
- 52.Obi Y, Nguyen DV, Zhou H, et al. Development and validation of prediction scores for early mortality at transition to dialysis. Mayo Clin Proc 2018;93:1224–35. 10.1016/j.mayocp.2018.04.017 [DOI] [PubMed] [Google Scholar]
- 53.Lin S-Y, Hsieh M-H, Lin C-L, et al. Artificial intelligence prediction model for the cost and mortality of renal replacement therapy in aged and Super-Aged populations in Taiwan. J Clin Med 2019;8:995. 10.3390/jcm8070995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshida M, Otsuka M, Watanabe Y, et al. A clinical nomogram for the prediction of early mortality in elderly patients initiating dialysis for end-stage renal disease. Ren Replace Ther 2020;6:11. 10.1186/s41100-020-0259-y [DOI] [Google Scholar]
- 55.Santos J, Oliveira P, Malheiro J, et al. Predicting 6-month mortality in incident elderly dialysis patients: a simple prognostic score. Kidney Blood Press Res 2020;45:38–50. 10.1159/000504136 [DOI] [PubMed] [Google Scholar]
- 56.Ramspek CL, Verberne WR, van Buren M, et al. Predicting mortality risk on dialysis and conservative care: development and internal validation of a prediction tool for older patients with advanced chronic kidney disease. Clin Kidney J 2021;14:189–96. 10.1093/ckj/sfaa021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patzer RE, Basu M, Larsen CP, et al. iChoose kidney: a clinical decision aid for kidney transplantation versus dialysis treatment. Transplantation 2016;100:630–9. 10.1097/TP.0000000000001019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation 2009;88:231–6. 10.1097/TP.0b013e3181ac620b [DOI] [PubMed] [Google Scholar]
- 59.Massie AB, Leanza J, Fahmy LM. A risk index for living donor kidney transplantation. Am J Transplant 2016;::324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grams ME, Li L, Greene TH, et al. Estimating time to ESRD using kidney failure risk equations: results from the African American study of kidney disease and hypertension (AASK). Am J Kidney Dis 2015;65:394–402. 10.1053/j.ajkd.2014.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lennartz CS, Pickering JW, Seiler-Mußler S, et al. External validation of the kidney failure risk equation and Re-Calibration with addition of ultrasound parameters. Clin J Am Soc Nephrol 2016;11:609–15. 10.2215/CJN.08110715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peeters MJ, van Zuilen AD, van den Brand JAJG, et al. Validation of the kidney failure risk equation in European CKD patients. Nephrol Dial Transplant 2013;28:1773–9. 10.1093/ndt/gft063 [DOI] [PubMed] [Google Scholar]
- 63.Prouvot J, Pambrun E, Couchoud C. Low performance of prognostic tools for predicting dialysis in elderly people with advanced CKD. J Nephrol 2021. [DOI] [PubMed] [Google Scholar]
- 64.Cheung KL, Montez-Rath ME, Chertow GM, et al. Prognostic stratification in older adults commencing dialysis. J Gerontol A Biol Sci Med Sci 2014;69:1033–9. 10.1093/gerona/glt289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peeters P, Van Biesen W, Veys N, et al. External validation of a risk stratification model to assist shared decision making for patients starting renal replacement therapy. BMC Nephrol 2016;17:41. 10.1186/s12882-016-0253-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thorsteinsdottir B, Hickson LJ, Giblon R, et al. Validation of prognostic indices for short term mortality in an incident dialysis population of older adults >75. PLoS One 2021;16:e0244081. 10.1371/journal.pone.0244081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tangri N, Grams ME, Levey AS, et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA 2016;315:164. 10.1001/jama.2015.18202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peters-Sengers H, Heemskerk MBA, Geskus RB. Validation of the prognostic kidney donor risk index (KDRI) scoring system of deceased donors for renal transplantation in the Netherlands: Erratum. Transplantation 2018;102:e359. 10.1097/TP.0000000000002297 [DOI] [PubMed] [Google Scholar]
- 69.Rehse G, Halleck F, Khadzhynov D, et al. Validation of the living kidney donor profile index in a European cohort and comparison of long-term outcomes with us results. Nephrol Dial Transplant 2019;34:1063–70. 10.1093/ndt/gfy118 [DOI] [PubMed] [Google Scholar]
- 70.Patzer RE, McPherson L, Basu M, et al. Effect of the iChoose kidney decision aid in improving knowledge about treatment options among transplant candidates: a randomized controlled trial. Am J Transplant 2018;18:1954–65. 10.1111/ajt.14693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Manns BJ, Taub K, Vanderstraeten C, et al. The impact of education on chronic kidney disease patients' plans to initiate dialysis with self-care dialysis: a randomized trial. Kidney Int 2005;68:1777–83. 10.1111/j.1523-1755.2005.00594.x [DOI] [PubMed] [Google Scholar]
- 72.Levin A, Lewis M, Mortiboy P, et al. Multidisciplinary predialysis programs: quantification and limitations of their impact on patient outcomes in two Canadian settings. Am J Kidney Dis 1997;29:533–40. 10.1016/s0272-6386(97)90334-6 [DOI] [PubMed] [Google Scholar]
- 73.Alghamdi AA, Almotairy KA, Aljoaid RM, et al. The impact of a pre-dialysis educational program on the mode of renal replacement therapy in a Saudi Hospital: a retrospective cohort study. Cureus 2020;12:e11981. 10.7759/cureus.11981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parapiboon W, Pitsawong W, Wongluechai L, et al. Customized versus conventional video counseling for peritoneal dialysis decision-making in patients with stage 5 chronic kidney disease under a PD-first policy: a randomized controlled study. Kidney Res Clin Pract 2020;39:451–9. 10.23876/j.krcp.20.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klang B, Björvell H, Berglund J, et al. Predialysis patient education: effects on functioning and well-being in uraemic patients. J Adv Nurs 1998;28:36–44. 10.1046/j.1365-2648.1998.00639.x [DOI] [PubMed] [Google Scholar]
- 76.Little J, Irwin A, Marshall T, et al. Predicting a patient's choice of dialysis modality: experience in a United Kingdom renal department. Am J Kidney Dis 2001;37:981–6. 10.1016/s0272-6386(05)80014-9 [DOI] [PubMed] [Google Scholar]
- 77.Marrón B, Ortiz A, de Sequera P, et al. Impact of end-stage renal disease care in planned dialysis start and type of renal replacement therapy--a Spanish multicentre experience. Nephrol Dial Transplant 2006;21 Suppl 2:ii51–5. 10.1093/ndt/gfl191 [DOI] [PubMed] [Google Scholar]
- 78.Chiou C-P, Chung Y-C. Effectiveness of multimedia interactive patient education on knowledge, uncertainty and decision-making in patients with end-stage renal disease. J Clin Nurs 2012;21:1223–31. 10.1111/j.1365-2702.2011.03793.x [DOI] [PubMed] [Google Scholar]
- 79.Goovaerts T, Jadoul M, Goffin E. Influence of a pre-dialysis education programme (PDEP) on the mode of renal replacement therapy. Nephrol Dial Transplant 2005;20:1842–7. 10.1093/ndt/gfh905 [DOI] [PubMed] [Google Scholar]
- 80.Rioux J-P, Cheema H, Bargman JM, et al. Effect of an in-hospital chronic kidney disease education program among patients with unplanned urgent-start dialysis. Clin J Am Soc Nephrol 2011;6:799–804. 10.2215/CJN.07090810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hanko J, Jastrzebski J, Nieva C, et al. Dedication of a nurse to educating suboptimal haemodialysis starts improved transition to independent modalities of renal replacement therapy. Nephrol Dial Transplant 2011;26:2302–8. 10.1093/ndt/gfq669 [DOI] [PubMed] [Google Scholar]
- 82.Cho EJ, Park HC, Yoon HB, et al. Effect of multidisciplinary pre-dialysis education in advanced chronic kidney disease: propensity score matched cohort analysis. Nephrology 2012;17:472–9. 10.1111/j.1440-1797.2012.01598.x [DOI] [PubMed] [Google Scholar]
- 83.Cankaya E, Cetinkaya R, Keles M, et al. Does a predialysis education program increase the number of pre-emptive renal transplantations? Transplant Proc 2013;45:887–9. 10.1016/j.transproceed.2013.02.075 [DOI] [PubMed] [Google Scholar]
- 84.Ribitsch W, Haditsch B, Otto R, et al. Effects of a pre-dialysis patient education program on the relative frequencies of dialysis modalities. Perit Dial Int 2013;33:367–71. 10.3747/pdi.2011.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alalawi F, Alnour H, Al Hadari A, et al. Renal school: Dubai experience. Saudi J Kidney Dis Transpl 2013;24:379–81. 10.4103/1319-2442.109612 [DOI] [PubMed] [Google Scholar]
- 86.Ismail SY, Luchtenburg AE, Timman R, et al. Home-based family intervention increases knowledge, communication and living donation rates: a randomized controlled trial. Am J Transplant 2014;14:1862–9. 10.1111/ajt.12751 [DOI] [PubMed] [Google Scholar]
- 87.Gordon EJ, Feinglass J, Carney P, et al. An interactive, bilingual, culturally targeted website about living kidney donation and transplantation for Hispanics: development and formative evaluation. JMIR Res Protoc 2015;4:e42. 10.2196/resprot.3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rodrigue JR, Cornell DL, Lin JK, et al. Increasing live donor kidney transplantation: a randomized controlled trial of a home-based educational intervention. Am J Transplant 2007;7:394–401. 10.1111/j.1600-6143.2006.01623.x [DOI] [PubMed] [Google Scholar]
- 89.Pradel FG, Suwannaprom P, Mullins CD, et al. Short-term impact of an educational program promoting live donor kidney transplantation in dialysis centers. Prog Transplant 2008;18:263–72. [DOI] [PubMed] [Google Scholar]
- 90.Barnieh L, McLaughlin K, Manns BJ, et al. Evaluation of an education intervention to increase the pursuit of living kidney donation: a randomized controlled trial. Prog Transplant 2011;21:36–42. 10.7182/prtr.21.1.e008266m25jtr77v [DOI] [PubMed] [Google Scholar]
- 91.Boulware LE, Hill-Briggs F, Kraus ES, et al. Effectiveness of educational and social worker interventions to activate patients' discussion and pursuit of preemptive living donor kidney transplantation: a randomized controlled trial. Am J Kidney Dis 2013;61:476–86. 10.1053/j.ajkd.2012.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arriola KRJ, Powell CL, Thompson NJ, et al. Living donor transplant education for African American patients with end-stage renal disease. Prog Transplant 2014;24:362–70. 10.7182/pit2014830 [DOI] [PubMed] [Google Scholar]
- 93.Gordon EJ, Reddy E, Gil S, et al. Culturally competent transplant program improves Hispanics' knowledge and attitudes about live kidney donation and transplant. Prog Transplant 2014;24:56–68. 10.7182/pit2014378 [DOI] [PubMed] [Google Scholar]
- 94.Marlow NM, Kazley AS, Chavin KD, et al. A patient navigator and education program for increasing potential living donors: a comparative observational study. Clin Transplant 2016;30:619–27. 10.1111/ctr.12728 [DOI] [PubMed] [Google Scholar]
- 95.Chanouzas D, Ng KP, Fallouh B, et al. What influences patient choice of treatment modality at the pre-dialysis stage? Nephrol Dial Transplant 2012;27:1542–7. 10.1093/ndt/gfr452 [DOI] [PubMed] [Google Scholar]
- 96.Lacson E, Wang W, DeVries C, et al. Effects of a nationwide predialysis educational program on modality choice, vascular access, and patient outcomes. Am J Kidney Dis 2011;58:235–42. 10.1053/j.ajkd.2011.04.015 [DOI] [PubMed] [Google Scholar]
- 97.Watson D. Acute start--chronic needs: education and support for adults who have had acute start dialysis. Semin Dial 2013;26:184–7. 10.1111/sdi.12060 [DOI] [PubMed] [Google Scholar]
- 98.Shukla AM, Easom A, Singh M, et al. Effects of a comprehensive predialysis education program on the home dialysis therapies: a retrospective cohort study. Perit Dial Int 2017;37:542–7. 10.3747/pdi.2016.00270 [DOI] [PubMed] [Google Scholar]
- 99.Dubin R, Rubinsky A. A digital modality decision program for patients with advanced chronic kidney disease. JMIR Form Res 2019;3:e12528. 10.2196/12528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Traino HM, West SM, Nonterah CW, et al. Communicating about choices in transplantation (coach). Prog Transplant 2017;27:31–8. 10.1177/1526924816679844 [DOI] [PubMed] [Google Scholar]
- 101.Waterman AD, Peipert JD. An explore transplant group randomized controlled education trial to increase dialysis patients' decision-making and pursuit of transplantation. Prog Transplant 2018;28:174–83. 10.1177/1526924818765815 [DOI] [PubMed] [Google Scholar]
- 102.Waterman AD, Peipert JD, McSorley A-M, et al. Direct delivery of kidney transplant education to black and low-income patients receiving dialysis: a randomized controlled trial. Am J Kidney Dis 2019;74:640–9. 10.1053/j.ajkd.2019.03.430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Waterman AD, Peipert JD, Cui Y, et al. Your path to transplant: a randomized controlled trial of a tailored expert system intervention to increase knowledge, attitudes, and pursuit of kidney transplant. Am J Transplant 2021;21:1186–96. 10.1111/ajt.16262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Imamura Y, Takahashi Y, Uchida S, et al. Effect of multidisciplinary care of dialysis initiation for outpatients with chronic kidney disease. Int Urol Nephrol 2021;53:1435–44. 10.1007/s11255-021-02787-w [DOI] [PubMed] [Google Scholar]
- 105.Papachristou E, Avramopoulou E, Kehagias I, et al. SP504PERITONEAL dialysis as first choice dialysis modality. Effects of a comprehensive predialysis patient educational program. Nephrology Dialysis Transplantation 2018;33:i518. 10.1093/ndt/gfy104.SP504 [DOI] [Google Scholar]
- 106.Fischbach BV, Gonzalez SA, Collazo Maldonado R. Physician-led CKD education improves quality and reduces cost of care in patients with ESRD [Abstract]. Journal of the American Society of Nephrology 2019;30:574–5. [Google Scholar]
- 107.Motz RG, Boyle J, Thakar CV. Patient driven video-educational tool in ESRD. Journal of the American Society of Nephrology 2019;30:185–6. [Google Scholar]
- 108.Crampton K, Adamowski T, Holewinski T. A multidisciplinary and peer mentor approach to educating CKD patients along the continuum [Abstract]. Blood Purif 2017;43:255. [Google Scholar]
- 109.Pravoverov L, Zheng S. Effect of renal replacement therapy options education class on patient outcomes. Abstract]. Journal of the American Society of Nephrology 2019;30:770–1. [Google Scholar]
- 110.Beavis Sobey J J, Holt S. Sat-477 individual versus group chronic kidney disease education [Abstract]. Kidney International Reports 2020. [Google Scholar]
- 111.McLaughlin K, Jones H, VanderStraeten C, et al. Why do patients choose self-care dialysis? Nephrol Dial Transplant 2008;23:3972–6. 10.1093/ndt/gfn359 [DOI] [PubMed] [Google Scholar]
- 112.Rodrigue JR, Cornell DL, Kaplan B, et al. A randomized trial of a home-based educational approach to increase live donor kidney transplantation: effects in blacks and whites. Am J Kidney Dis 2008;51:663–70. 10.1053/j.ajkd.2007.11.027 [DOI] [PubMed] [Google Scholar]
- 113.Massey EK, Gregoor PJHS, Nette RW, et al. Early home-based group education to support informed decision-making among patients with end-stage renal disease: a multi-centre randomized controlled trial. Nephrol Dial Transplant 2016;31:823–30. 10.1093/ndt/gfv322 [DOI] [PubMed] [Google Scholar]
- 114.Gordon EJ, Feinglass J, Carney P, et al. A website intervention to increase knowledge about living kidney donation and transplantation among Hispanic/Latino dialysis patients. Prog Transplant 2016;26:82–91. 10.1177/1526924816632124 [DOI] [PubMed] [Google Scholar]
- 115.Gordon EJ, Feinglass J, Carney P, et al. A culturally targeted website for Hispanics/Latinos about living kidney donation and transplantation: a randomized controlled trial of increased knowledge. Transplantation 2016;100:1149–60. 10.1097/TP.0000000000000932 [DOI] [PubMed] [Google Scholar]
- 116.Gordon EJ, Lee J, Kang R, et al. Hispanic/Latino disparities in living donor kidney transplantation: role of a culturally competent transplant program. Transplant Direct 2015;1:e29. 10.1097/TXD.0000000000000540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shukla AM, Hinkamp C, Segal E, et al. What do the US advanced kidney disease patients want? comprehensive pre-ESRD patient education (CPE) and choice of dialysis modality. PLoS One 2019;14:e0215091. 10.1371/journal.pone.0215091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mollicone D, Pulliam J, Lacson E. The culture of education in a large dialysis organization: informing patient-centered decision making on treatment options for renal replacement therapy. Semin Dial 2013;26:143–7. 10.1111/sdi.12053 [DOI] [PubMed] [Google Scholar]
- 119.Fortnum D, Smolonogov T, Walker R, et al. 'My kidneys, my choice, decision aid': supporting shared decision making. J Ren Care 2015;41:81–7. 10.1111/jorc.12100 [DOI] [PubMed] [Google Scholar]
- 120.Schatell D. A Paradigm Shift in Options, Education, and an Online Decision Aid: 'My Life, My Dialysis Choice'. Nephrol Nurs J 2015;154. [PubMed] [Google Scholar]
- 121.Winterbottom AE, Gavaruzzi T, Mooney A, et al. Patient acceptability of the Yorkshire dialysis decision aid (YoDDA) booklet: a prospective Non-Randomized comparison study across 6 predialysis services. Perit Dial Int 2016;;36:374–81. 10.3747/pdi.2014.00274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Finderup J, Jensen JKD, Lomborg K. Developing and pilot testing a shared decision-making intervention for dialysis choice. J Ren Care 2018. doi: 10.1111/jorc.12241. [Epub ahead of print: 17 Apr 2018]. [DOI] [PubMed] [Google Scholar]
- 123.Therkildsen SB, Hansen LH, Jensen LED, et al. A patient decision aid APP for patients with chronic kidney disease: questionnaire study. JMIR Form Res 2019;3:e13786. 10.2196/13786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Subramanian L, Zhao J, Zee J, et al. Use of a decision aid for patients considering peritoneal dialysis and In-Center hemodialysis: a randomized controlled trial. Am J Kidney Dis 2019;74:351–60. 10.1053/j.ajkd.2019.01.030 [DOI] [PubMed] [Google Scholar]
- 125.Axelrod DA, Kynard-Amerson CS, Wojciechowski D, et al. Cultural competency of a mobile, customized patient education tool for improving potential kidney transplant recipients' knowledge and decision-making. Clin Transplant 2017;31. doi: 10.1111/ctr.12944. [Epub ahead of print: 09 04 2017]. [DOI] [PubMed] [Google Scholar]
- 126.Gordon EJ, Sohn M-W, Chang C-H, et al. Effect of a mobile web APP on kidney transplant candidates' knowledge about increased risk donor kidneys: a randomized controlled trial. Transplantation 2017;101:1167–76. 10.1097/TP.0000000000001273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brown L, Gardner G, Bonner A. A randomized controlled trial testing a decision support intervention for older patients with advanced kidney disease. J Adv Nurs 2019;75:3032–44. 10.1111/jan.14112 [DOI] [PubMed] [Google Scholar]
- 128.Davison SN, Tupala B, Wasylynuk BA, et al. Recommendations for the care of patients receiving conservative kidney management: focus on management of CKD and symptoms. Clin J Am Soc Nephrol 2019;14:626–34. 10.2215/CJN.10510917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Eneanya ND, Percy SG, Stallings TL, et al. Use of a supportive kidney care video decision aid in older patients: a randomized controlled trial. Am J Nephrol 2020;51:736–44. 10.1159/000509711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Prichard A, Thomas N. The option grid: a shared decision-making tool for renal patients. Journal of Renal Nursing 2013;5:6–11. 10.12968/jorn.2013.5.1.6 [DOI] [Google Scholar]
- 131.Ameling JM, Auguste P, Ephraim PL, et al. Development of a decision aid to inform patients' and families' renal replacement therapy selection decisions. BMC Med Inform Decis Mak 2012;12:140. 10.1186/1472-6947-12-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Davis J, Murray M, Bissonnette J. Utilization of patient decision support tools and processes: the shared end-stage renal patients decision making (SHERPA-DM) project. Advances in Chronic Kidney Disease 2017;24:131. [Google Scholar]
- 133.Hart A, Bruin M, Chu S. A. development of an individualized online shared decision-making tool for kidney transplant candidates. Am J Transplant 2017;17. [Google Scholar]
- 134.Komatsu Y, Ishida M. Promoting shared decision-making practice for CKD patients through patient decision AIDS and nephrology staff education. Journal of the American Society of Nephrology 2018;29:1105. [Google Scholar]
- 135.Van Eck van der Sluijs A, Riemann A, Prantl K, et al. P1847IMPROVING shared decision making for end-stage renal disease patients in the Netherlands. Nephrology Dialysis Transplantation 2020;35:iii2158. 10.1093/ndt/gfaa142.P1847 [DOI] [Google Scholar]
- 136.Decision aid summary. Available: https://decisionaid.ohri.ca/AZsumm.php?ID=1649 [Accessed Apr 2022].
- 137.Decision aid summary. Available: https://decisionaid.ohri.ca/AZsumm.php?ID=1519 [Accessed Apr 2022].
- 138.Decision aid summary. Available: https://decisionaid.ohri.ca/AZsumm.php?ID=1109 [Accessed Apr 2022].
- 139.Yorkshire dialysis decision aid (YoDDA). Available: http://www.yodda.leeds.ac.uk/Survey/Introduction [Accessed Apr 2022].
- 140.A dialysis and conservative care decision aid: living with kidney disease. Available: https://www.kidneyresearchyorkshire.org.uk/wp-content/uploads/2020/09/Diaysis-Conservative-Care-FINAL-SEPT-2020.pdf [Accessed Apr 2022].
- 141.Kidney Failure Treatment Options. Available: https://www.bsuh.nhs.uk/wp-content/uploads/sites/5/2016/09/Patient-decision-aid.pdf [Accessed Apr 2022].
- 142.Finderup J, Dam Jensen J, Lomborg K. Evaluation of a shared decision-making intervention for dialysis choice at four Danish hospitals: a qualitative study of patient perspective. BMJ Open 2019;9:e029090. 10.1136/bmjopen-2019-029090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Finderup J, Lomborg K, Jensen JD, et al. Choice of dialysis modality: patients' experiences and quality of decision after shared decision-making. BMC Nephrol 2020;21:330. 10.1186/s12882-020-01956-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Finderup J, Jensen JD, Lomborg K. Shared decision-making in dialysis choice has potential to improve self-management in people with kidney disease: a qualitative follow-up study. J Adv Nurs 2021;77:1878–87. 10.1111/jan.14726 [DOI] [PubMed] [Google Scholar]
- 145.Boulware LE, Ephraim PL, Ameling J, et al. Effectiveness of informational decision AIDS and a live donor financial assistance program on pursuit of live kidney transplants in African American hemodialysis patients. BMC Nephrol 2018;19:107. 10.1186/s12882-018-0901-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Okada K, Abe M, Soma M. Implementation of a cooperative program for peritoneal dialysis. Contrib Nephrol 2012;177:84–92. 10.1159/000336939 [DOI] [PubMed] [Google Scholar]
- 147.Patzer RE, Paul S, Plantinga L. Southeastern kidney transplant coalition. A randomized trial to reduce disparities in referral for transplant evaluation. J Am Soc Nephrol 2017;28:935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ho Y-F, Chen Y-C, Huang C-C, et al. The effects of shared decision making on different renal replacement therapy decisions in patients with chronic kidney disease. J Nurs Res 2020;28:e109. 10.1097/jnr.0000000000000386 [DOI] [PubMed] [Google Scholar]
- 149.Eschbach JW, Seymour M, Potts A, et al. A hemodialysis orientation unit. Nephron 1983;33:106–10. 10.1159/000182922 [DOI] [PubMed] [Google Scholar]
- 150.Prieto-Velasco M, Quiros P, Remon C. Spanish group for the implementation of a shared decision-making process for RRT choice with patient decision aid tools. The concordance between patients' renal replacement therapy choice and definitive modality: is it a utopia? PLoS One 2015;10:e0138811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.de Maar JS, de Groot MAJ, Luik PT, et al. Guide, a structured pre-dialysis programme that increases the use of home dialysis. Clin Kidney J 2016;9:826–32. 10.1093/ckj/sfw037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Machowska A, Alscher MD, Reddy Vanga S, et al. Factors influencing access to education, decision making, and receipt of preferred dialysis modality in unplanned dialysis start patients. Patient Prefer Adherence 2016;10:2229–37. 10.2147/PPA.S119243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee C-T, Cheng C-Y, Yu T-M, et al. Shared decision making increases living kidney transplantation and peritoneal dialysis. Transplant Proc 2019;51:1321–4. 10.1016/j.transproceed.2019.02.025 [DOI] [PubMed] [Google Scholar]
- 154.Hamoda RE, Gander JC, McPherson LJ, et al. Process evaluation of the radiant community study: a dialysis facility-level intervention to increase referral for kidney transplantation. BMC Nephrol 2018;19:13. 10.1186/s12882-017-0807-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Machowska A, Alscher MD, Vanga SR, et al. Offering patients therapy options in unplanned start (options): implementation of an educational program is feasible and effective. BMC Nephrol 2017;18:18. 10.1186/s12882-016-0419-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Marrón B, Ostrowski J, Timofte D, et al. SP831ROUTINE use of decision making tools increases peritoneal dialysis choice and take on in an international setting. Nephrology Dialysis Transplantation 2017;32:iii423. 10.1093/ndt/gfx159.SP831 [DOI] [Google Scholar]
- 157.Marrón B, Pearce S, Ostrowski J, et al. P1853FIVE years prospective, observational, International study on the impact of decision-making tools for choice of renal replacement therapy modality. Nephrology Dialysis Transplantation 2020;35:iii2164. 10.1093/ndt/gfaa142.P1853 [DOI] [Google Scholar]
- 158.Feasibility of enhanced dialysis education (EDU) intervention for chronic kidney disease (CKD) patients (CKD-EDU). Available: https://clinicaltrials.gov/ct2/show/NCT03465449 [Accessed Apr 2022].
- 159.Decision support for the renal replacement therapy with end-stage renal disease. Available: https://clinicaltrials.gov/ct2/show/NCT03921437 [Accessed Apr 2022].
- 160.Trial to evaluate and assess the effect of comprehensive Pre-ESKD education on home dialysis use in veterans (TEACH-VET). Available: https://clinicaltrials.gov/ct2/show/study/NCT04064086 [Accessed Apr 2022].
- 161.House calls and decision support: improving access to live donor transplantation. Available: https://clinicaltrials.gov/ct2/show/study/NCT01786525 [Accessed Apr 2022].
- 162.House calls and peer mentorship (HC+PM). Available: https://clinicaltrials.gov/ct2/show/NCT03354910 [Accessed Apr 2022].
- 163.Increasing kidney transplant among blacks on the transplant waiting list. Available: https://clinicaltrials.gov/ct2/show/NCT02319447 [Accessed Apr 2022].
- 164.Increasing equity in transplant evaluation and living donor kidney transplantation. Available: https://www.clinicaltrials.gov/ct2/show/NCT02342119 [Accessed Apr 2022].
- 165.Social networks and renal education: promoting transplantation (SNARE). Available: https://clinicaltrials.gov/ct2/show/NCT03536858 [Accessed Apr 2022].
- 166.Decision aid for renal therapy (dart). Available: https://clinicaltrials.gov/ct2/show/NCT03522740 [Accessed Apr 2022].
- 167.DukeNUS medical school. Available: https://www.duke-nus.edu.sg/lcpc/research/projects-by-themes/retreat [Accessed Apr 2022].
- 168.Enhance access to kidney transplantation and living kidney donation (EnAKT LKD). Available: https://clinicaltrials.gov/ct2/show/NCT03329521 [Accessed Apr 2022]. [DOI] [PMC free article] [PubMed]
- 169.Explore Transplant @Home Within Kaiser Permanente Southern California. Available: https://clinicaltrials.gov/ct2/show/NCT03389932 [Accessed Apr 2022].
- 170.Harvey A, Walsh M, Jain AK, et al. The WISHED trial: implementation of an interactive health communication application for patients with chronic kidney disease. Can J Kidney Health Dis: 2016;3:29. 10.1186/s40697-016-0120-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rodrigue JR, Pavlakis M, Egbuna O, et al. The "House Calls" trial: a randomized controlled trial to reduce racial disparities in live donor kidney transplantation: rationale and design. Contemp Clin Trials 2012;33:811–8. 10.1016/j.cct.2012.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Patzer RE, McPherson L, Redmond N, et al. A culturally sensitive web-based intervention to improve living donor kidney transplant among African Americans. Kidney Int Rep 2019;4:1285-1295. 10.1016/j.ekir.2019.05.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Green JA, Ephraim PL, Hill-Briggs FF, et al. Putting patients at the center of kidney care transitions: prepare now, a cluster randomized controlled trial. Contemp Clin Trials 2018;73:98–110. 10.1016/j.cct.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.International patient decision aid standards (IPDAS) collaboration. Available: http://ipdas.ohri.ca/index.html [Accessed Apr 2022].
- 175.Malkina A, Tuot DS. Role of telehealth in renal replacement therapy education. Semin Dial 2018;31:129–34. 10.1111/sdi.12663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Boaz A, Baeza J, Fraser A. European implementation score Collaborative Group (EIS). effective implementation of research into practice: an overview of systematic reviews of the health literature. BMC Res Notes 2011;4:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.O'Cathain A, Croot L, Duncan E, et al. Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open 2019;9:e029954. 10.1136/bmjopen-2019-029954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fortnum D, Grennan K, Smolonogov T. End-stage kidney disease patient evaluation of the Australian 'My Kidneys, My Choice' decision aid. Clin Kidney J 2015;8:469–75. 10.1093/ckj/sfv050 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-055248supp001.pdf (158.3KB, pdf)
bmjopen-2021-055248supp002.pdf (4.8MB, pdf)
bmjopen-2021-055248supp003.pdf (163.9KB, pdf)
bmjopen-2021-055248supp004.pdf (478.9KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.